Abstract
The parasympathetic control of heart rate arises from premotor cardiac vagal neurons (CVNs) located in the nucleus ambiguus. Previous microinjection studies in nucleus ambiguus show that dopamine evokes a decrease in heart rate, but the underlying mechanisms responsible for these responses were not identified. This study tested whether dopamine modulates inhibitory GABAergic and glycinergic and/or excitatory glutamatergic neurotransmission to CVNs. Retrogradely labeled CVNs were identified in an in-vitro rat brainstem slice preparation and synaptic events were recorded using whole cell voltage clamp techniques.
Bath application of dopamine (100 M) had no effect on excitatory synaptic events, but reversibly inhibited the frequency (but not amplitude) of GABAergic inhibitory postsynaptic currents (IPSCs) in CVNs. Similarly, dopamine (10 M & 100 M) inhibited glycinergic IPSC frequency by ~ 50% and 70% respectively. The reduction in inhibitory neurotransmission to CVNs by dopamine was prevented by the sodium channel blocker TTX (1μM) indicating that the dopamine mediated effects were action potential dependent. Dopamine evoked responses were mimicked by the D2-like receptor agonist, Quinpirole but not D1-like receptor agonist, SKF 38393. In addition, the dopamine mediated depression of inhibitory synaptic responses were prevented by the D2 receptor antagonist sulpiride, but not by D1-like or adrenergic or serotonergic receptor antagonists, suggesting that these responses were D2-like receptor mediated and not D1-like or adrenergic or 5-HT receptor mediated. These data suggest that dopamine acts via disinhibition, and diminishes inhibitory GABAergic and glycinergic neurotransmission to CVNs, which would be predicted to increase parasympathetic activity to the heart and evoke a bradycardia.
Keywords: Dopamine, Parasympathetic, Heart rate, Antidepressants, Depression
1.0 Introduction
Parasympathetic activity to the heart originates from the cardiac vagal neurons (CVNs) located in the nucleus ambiguus (NA) of the brainstem (Mendelowitz and Kunze, 1991). CVNs are intrinsically silent and receive numerous synaptic inputs including those from GABAergic, glycinergic, glutamatergic, serotonergic and purinergic pathways (Neff et al., 1998, Mendelowitz, 1999, Wang et al., 2003, Dergacheva et al., 2010). Synaptic activity to CVNs is modulated by catecholaminergic pathways and receptors (Philbin et al., 2010, Boychuk et al., 2011, Bateman et al., 2012) and these targets likely act as links between depression and cardiovascular disease. However one still unstudied catecholamine that has strong potential to modulate the neurotransmission to CVNs is dopamine.
Dopamine neurotransmission is mediated by G-protein coupled receptor groups, D1-like (comprised of D1 and D5 receptors) and D2-like (D2, D3 and D4 receptors) (Missale et al., 1998). Previous studies have identified tyrosine hydroxylase (TH, the rate- limiting enzyme in the synthesis of the catecholamines) immunoreactive neurons in ventral (A1 and C1) and dorsomedial (C2) regions with projections to CVNs (Boychuk et al., 2011), nerve terminals innervating CVNs (Massari et al., 1998) and D2-like receptor localization in various brainstem regions including the nucleus of the solitary tract (NTS), dorsal motor nucleus of the vagus, motor nucleus of the trigeminal nerve, hypoglossal nucleus, locus coerulus and NA (Yokoyama et al., 1994). Other immunohistochemical studies reported the location of dopaminergic neurons (Kalia et al., 1985, Zheng and Travagli, 2007) and fibers (Maqbool et al., 1993) in dorsomedial (NTS, DMNX and area postrema) and the ventrolateral regions of medulla oblongata that send projections to CVNs (Neff et al., 1998, Frank et al., 2009). These studies indicate CVNs are a likely potential target for dopaminergic pathways.
Dopamine modulates cardiorespiratory functions by acting on peripheral carotid body chemoreceptors (Gonzalez et al., 1994) as well as centrally in the brainstem. For instance, dopamine presynaptically inhibited both spontaneous and evoked excitatory glutamatergic excitatory postsynaptic currents (EPSCs) between chemoreceptor sensory afferents and secondary neurons of the caudal NTS, thereby regulating blood pressure and respiration (Kline et al., 2002). Administration of bromocriptine, a D2-like receptor agonist in healthy human subjects reduced plasma norepinephrine levels and blood pressure (Franchi et al., 2001). Dopamine microinjected in to the NA caused a dose dependent decrease in heart rate in artificially ventilated spinal rats (Chitravanshi and Calaresu, 1992). However, there is a paucity of information concerning the mechanisms underlying dopamine induced alterations in CVN activity that dominates the neural control of heart rate. The aim of this study was to investigate whether dopamine can modulate the essential excitatory glutamatergic, inhibitory GABAergic and glycinergic neurotransmission to CVNs in the NA.
2.0 Experimental procedures
All animal procedures carried out were in compliance with The George Washington University institutional guidelines and in accordance with the recommendations of the panel on Euthanasia of the American Veterinary Medical Association and the NIH publication (85-23, revised 1996) “Guide for the care and Use of Laboratory Animals”. The minimal number of animals was used and care was taken to reduce any possible discomfort.
2.1 Labeling
In an initial surgery, 2-5 day old Sprague-Dawley rats (Hilltop Laboratory Animals Inc., scottdale, PA, USA) were anesthetized with hypothermia by cooling to approximately 4°C. After the heart rate was reduced and no pain reflex was observed, a right thoractomy was performed to expose the heart. As described earlier (Mendelowitz and Kunze, 1991), the retrograde tracer X-rhoda-mine-5-(and-6)-isothiocyanate (Invitrogen, USA) was then injected in to the fat pads at the base of the heart to retrogradely label CVNs. The animals were monitored for 1-3 days for complete recovery.
2.2 In-vitro Brainstem slice preparation
On the day of the experiment, the pups were anesthetized with isoflurane and sacrificed by cervical dislocation. The brain tissue was separated and placed in a physiological saline solution (PSS) maintained at 4°C and continuously bubbled with 100% Oxygen. The composition of PSS is as follows (in mM): NaCl (140), KCl (5), CaCl2 (2), glucose (5) and HEPES (10). With the aid of a dissection microscope, the hindbrain was isolated from the brain tissue, glued on to a stage and placed in a slicing chamber of a vibratome containing PSS. Brain slices of 450-500 m thickness, containing NA were cut. The slices were then mounted in a recording chamber that is constantly perfused with a physiological buffer solution with the following composition (in mM): NaCl (125), KCl (3), CaCl2 (2), NaHCO3 (26), glucose (5) and HEPES (5) and oxygenated with carbogen (95% O2-5% CO2). The osmolality of the solutions was 285-290 mOsm and pH was maintained at 7.4.
2.3 Electrophysiological recordings
CVNs in the NA were identified by the presence of the fluorescence tracer rhodamine. CVNs were studied by whole cell voltage clamp technique using Axopatch 200B and pClamp 8 software (Axon Instruments, Union City, CA), with the voltage being clamped at −80 mV at room temperature. The patch pipettes (2.5-5 MΩ) were filled with a solution consisting of KCl (150 mM), MgCl2 (4 mM), EGTA (10 mM), Na-ATP (2 mM) and HEPES (10 mM) or K-gluconic acid (150 mM), HEPES (10 mM), EGTA (10 mM), MgCl2 (1 mM) and CaCl2 (1 mM) at a pH of 7.3 for recording inhibitory and excitatory events respectively.
Glutamatergic EPSCs were isolated by continuous focal application of a solution containing strychnine (1 M), a glycinergic receptor antagonist and gabazine (25 M), a GABA (A) receptor antagonist in a puffer pipette positioned near the patched neuron. GABAergic inhibitory postsynaptic currents (IPSCs) were isolated by including 6-cyano-7-nitroquinoxaline (CNQX, 50 M), non-NMDA receptor antagonist, 2-amino-5-phosphonopentanoic acid (DL-AP5, 50 M), NMDA receptor antagonist and strychnine (1 M) in the puffer solution. Glycinergic IPSCs were isolated by including CNQX (50 M), AP5 (50 M) and gabazine (25 M) in the puffer solution.
2.4 Drugs
The respective excitatory or inhibitory postsynaptic currents were recorded in control conditions for 10 minutes followed by drug treatments for 5 minutes. All the drugs used in this study were bath applied and each slice was limited to one experiment only. To prevent the oxidation of dopamine, the antioxidant sodium metabisulfite (75 M) was included in the solutions containing dopamine (Smythies and Galzigna, 1998, Kline et al., 2002). Sodium metabisulfite (75 M) alone had no effect on GABAergic, glycinergic or glutamatergic IPSC frequency, amplitude and whole cell holding currents. Dopamine was prepared freshly every time before use and the solutions were light protected to prevent oxidation. Dopamine was bath applied at increasing doses (0.1 M-100 M) and each neuron received the full range of concentrations of dopamine or its agonists. Tetrodotoxin (TTX, 1 M) was added to the perfusate to prevent action potential generation and isolate spontaneous miniature postsynaptic events. Dopamine, sodium metabisulfite, gabazine, strychnine, CNQX, AP5, prazosin, propranolol, atipamezole, ketanserin were obtained from Sigma-Aldrich (St.Louis, MO, USA). WAY-100635, ondansetron, SCH-23390, SKF 38393, Quinpirole and sulpiride were obtained from R & D systems, Inc (Minneapolis, MN, USA).
2.5 Data Analysis
The synaptic events recorded from CVNs were analysed using MiniAnalysis (version 5.6.12; Synaptosoft, Decatur, GA) and whole cell holding currents were detected using pClamp 8 software. Threshold value was set to the root mean square of noise levels multiplied by 5. The frequency and amplitude of synaptic events were grouped in 10 sec bins and were averaged during the last 2 minute period in control conditions, drug application and washout conditions. The cumulative probability curves were plotted for inter event interval and amplitude in 1sec bins. The data are presented as mean ± SEM and statistically compared by student’s paired t-test or repeated measures-ANOVA followed by Dunnetts or Tukeys Kramer post-test using the software Graph pad Prism 5 (La Jolla, CA). Data with p<0.05 were considered significant. *p<0.05; **p<0.01 and ***p<0.001.
3.0 Results
3.1 Dopamine inhibits GABAergic and glycinergic neurotransmission to CVNs
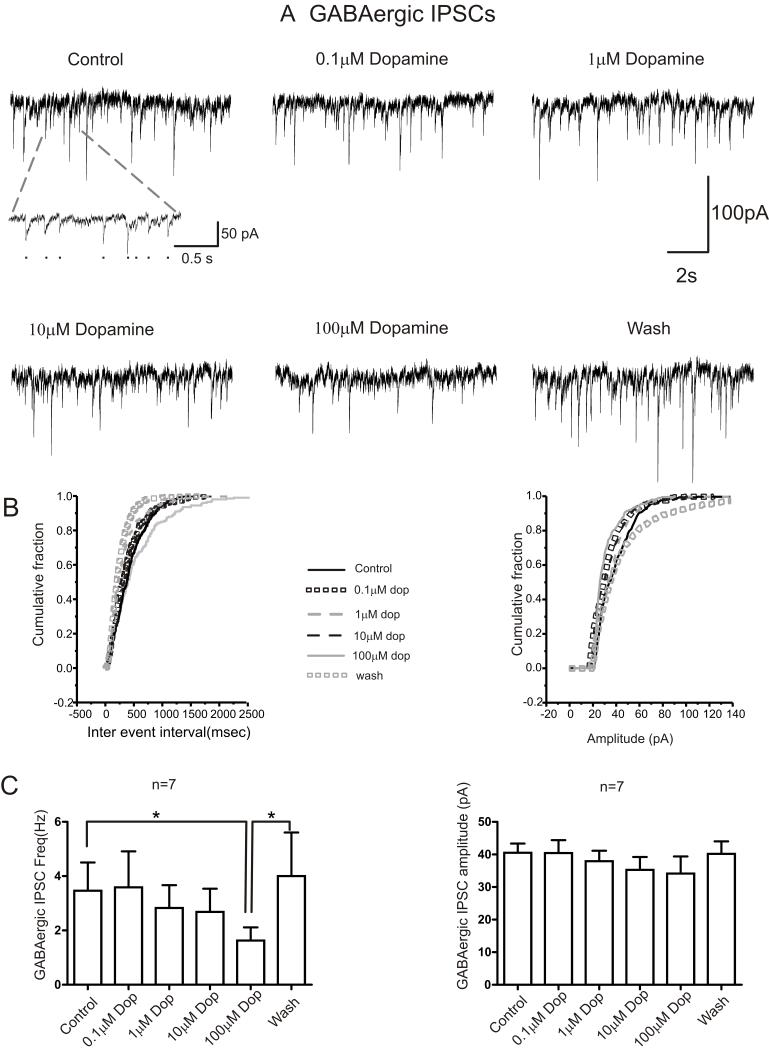
To investigate the actions of dopamine on GABAergic, glycinergic and glutamatergic neurotransmission to CVNs, dopamine was bath applied at concentrations ranging from 0.1 M to100 M followed by a washout period of 15 minutes. Dopamine at concentrations of 0.1-10 M did not affect GABA IPSC frequency, amplitude or holding currents, Table 1 & Figure 1. However, a higher concentration of dopamine (100 M) significantly and reversibly inhibited the frequency of GABAergic IPSCs to ~ 50% compared to control (3.4 ± 1.0 Hz in control and 1.6 ± 0.5 Hz in dopamine [100 M], 4.0 ± 1.6 Hz in wash; p<0.05; n=7). The amplitude of GABA IPSCs were not significantly altered by dopamine (40.5 ± 2.8 pA in control and 34.1 ± 5.2 pA in dopamine [100 M], p=0.18, n=7), Table 1 & Figure 1A, B & C. Figure 1B shows the cumulative probability distributions of inter event interval and amplitude of GABA IPSCs recorded from a typical neuron (as shown in Figure 1A) plotted in control, dopamine (0.1-100 M) and washout conditions. The cumulative probability distribution of inter event interval shifted towards the right in dopamine (100 M) indicating an increase in the inter event interval (i.e., decreased frequency), with no effect on the amplitude distribution. Whole cell holding currents were not affected by dopamine (100 M, p>0.05), Table 1.
Table 1.
The effects of dopamine (0.1-100 M) on GABA, glycine and glutamatergic IPSC frequency, amplitude and holding currents.
| GABAergic IPSCs | Control |
Dopamine,
0.1μM (5 min) |
Dopamine,
1μM (5 min) |
Dopamine,
10μM (5 min) |
Dopamine,
100μM(5 min) |
Washout
(15 min) |
|---|---|---|---|---|---|---|
| Frequency (Hz) | 3.4 + 1.0 (7) | 3.6 + 1.3 (7) | 2.8 + 0.8 (7) | 2.7 + 0.8 (7) | 1.6 + 0.5 (7)* | 4.0 + 1.6 (7)$ |
| Amplitude (pA) | 40.5 + 2.8 (7) | 40.4 + 3.9 (7) | 38.0 + 3.2 (7) | 35.3 + 4.0 (7) | 34.2 + 1.2 (7) | 40.2 + 3.8 (7) |
| Holding current (pA) | −241.4 + 35.2 (7) |
−224.3 + 33.7 (7) |
−198.6 + 33.2 (7) |
−194.3 + 35.7 (7) |
−198.6 + 40.2 (7) |
−234.3 + 49.6 (7) |
| Glycinergic IPSCs | ||||||
| Frequency (Hz) | 2.9 + 0.7 (9) | 2.6 + 0.7 (9) | 1.8 + 0.5 (9) | 1.4 + 0.4 (9)** | 1.0 + 0.3 (9)* | 2.7 + 0.6 (9)$ |
| Amplitude (pA) | 44.3 + 6.0 (9) | 39.9 + 4.7 (9) | 35.3 + 3.5 (9) | 36.6 + 2.4 (9) | 34.6 + 3.0 (9) | 47.0 +5.3 (9) |
| Holding current (pA) | −169.0 + 20.5 (9) |
−183.3 + 24.0 (9) |
−188.9 + 36.5 (9) |
−169 + 20.2 (9) |
−186.7 + 21.7 (9) |
−172.2 + 18.8 (9) |
| Glutamatergic EPSCs | ||||||
| Frequency (Hz) | 2.9 + 0.7 (8) | 3.4 + 0.9 (8) | 2.9 + 0.7 (8) | 2.6 + 0.6 (8) | 2.5 + 0.6 (8) | 2.1 + 0.6 (8) |
| Amplitude (pA) | 13.4 + 1.8 (8) | 13.7 + 1.9 (8) | 13.6 + 2.0 (8) | 13.8 + 2.1 (8) | 13.3 + 2.0 (8) | 12.7 + 2.2 (8) |
| Holding current (pA) | −56.2 + 16.8 (8) |
−48.1 + 13.0 (8) |
−33.7 + 11.2 (8) |
−33.7 + 12.0 (8) |
−30.0 + 11.2 (8) |
−26.2 + 11.0 (8) |
Control vs. dopamine; p<0.05
dopamine (100 M) vs. Wash.
p<0.01
***p<0.001.
Number of neurons indicated in parentheses.
Figure 1. Dopamine inhibits frequency of GABAergic IPSCs to cardiac vagal neurons.
A. Representative traces from a typical experiment isolating for GABAergic activity to CVNs is shown in control conditions and while subsequently bath applying various doses of dopamine (0.1 M – 100 M) for 5 minutes followed by a washout of dopamine for 15 minutes. Inset shows an expanded trace of synaptic activity in control condition, black dots representing individual synaptic events. B. Cumulative probability distribution of inter-event interval and amplitude of GABA IPSCs from the same neuron shown in A in control, dopamine (0.1-100 M) and washout conditions. The synaptic events were averaged during the last 2 minute period of exposure in each condition. The inter event interval was increased, whereas the IPSC amplitude remained unaffected in dopamine (100 M). C. Histogram showing the dose dependent responses of dopamine on GABAergic IPSC frequency and amplitude (n=7). * p<0.05.
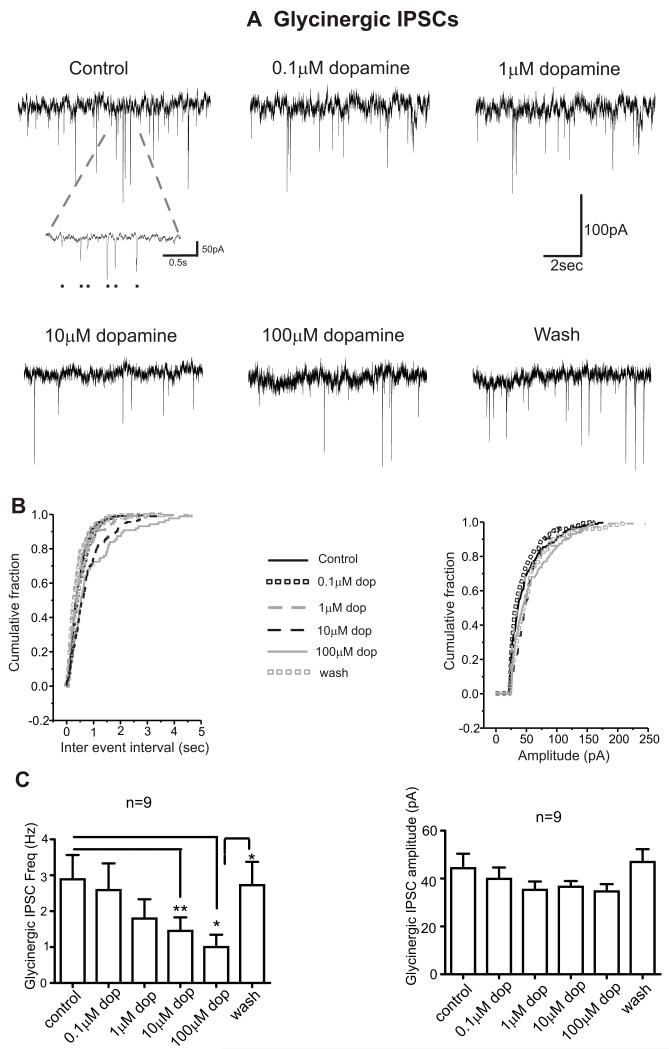
Low concentrations (0.1 M & 1 M) of dopamine had no effect on glycinergic IPSC frequency, amplitude and holding currents, Table 1 & Figure 2. However, dopamine at both 10 M and 100 M reversibly inhibited the frequency of glycinergic IPSCs by ~ 50% and ~ 70% respectively (2.9 ± 0.7 Hz in control, 1.4 ± 0.4 Hz in dopamine [10 M] and 1.0 ± 0.3 Hz in dopamine [100 M] and 2.7 ± 0.6 Hz in wash; p<0.05 for control vs. dopamine [100μM] and dopamine [100μM] vs. wash; p<0.01 for control vs. dopamine [10μM]; n=9), Figure 2. The amplitude of glycinergic IPSCs and whole cell holding currents were not affected by dopamine at any concentration (10 M & 100 M, p>0.05), Table 1 & Figure 2A, B & C. Figure 2B shows the cumulative probability distributions of inter event interval and amplitude of glycinergic IPSCs recorded from a typical neuron (as shown in Figure 2A) plotted in control, dopamine (0.1-100 M) and washout conditions. The cumulative probability distribution of inter event interval shifted towards the right in dopamine (10 M & 100 M) indicating an increase in the inter event interval (i.e., decreased frequency), with no effect on amplitude distribution.
Figure 2. Dopamine inhibits the frequency of glycinergic IPSCs to CVNs.
A. Representative traces from a typical experiment isolating for glycinergic events to CVNs is shown in control conditions and while application of various doses of dopamine (0.1 M- 100 M) for 5 minutes followed by a washout period for 15 minutes. Inset shows an expanded trace of synaptic activity in control condition, black dots representing individual synaptic events. B. Cumulative fraction plotted against inter-event interval and amplitude of glycinergic IPSCs in control, dopamine (0.1-100 M) and washout conditions. C. Histogram showing the summary data of dose dependent responses of dopamine on glycinergic IPSC frequency and amplitude (n=9). * p<0.05; **p<0.01.
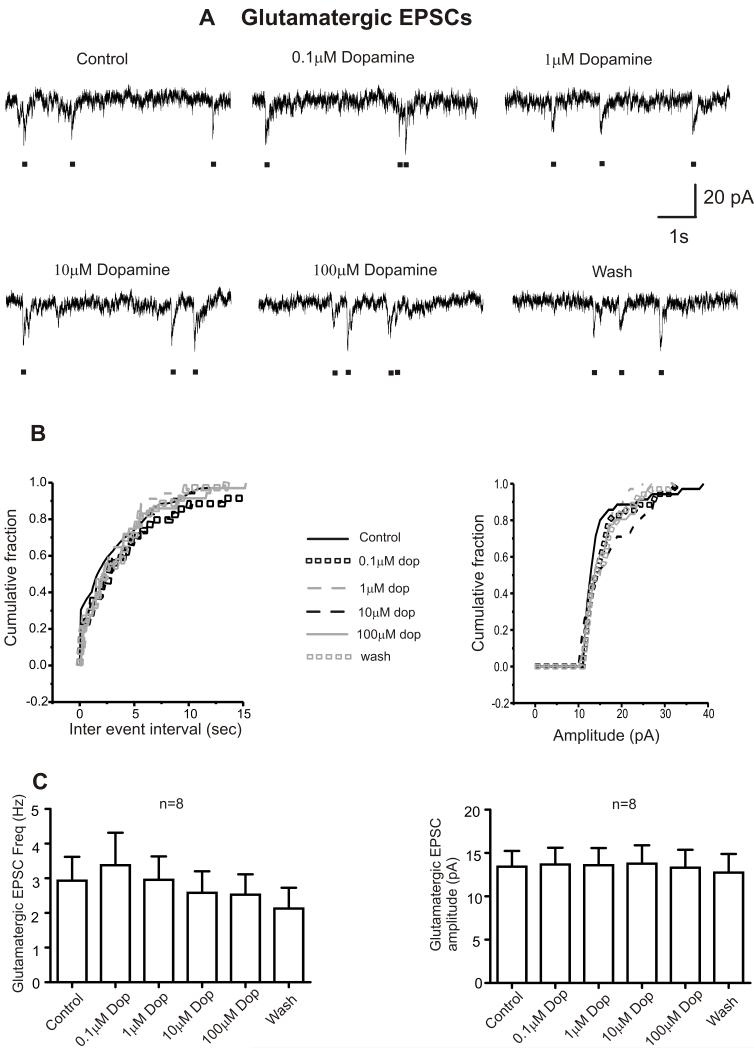
In contrast to its actions on inhibitory neurotransmission, dopamine, at all tested concentrations (0.1-100 M) had no effect on the frequency or amplitude of glutamatergic excitatory post synaptic currents or holding currents, Table 1 & Figure 3.
Figure 3. Dopamine has no effect on glutamatergic transmission to CVNs.
A. Representative traces from a typical experiment isolating for glutamatergic events to CVNs is shown in control conditions and while application of various doses of dopamine (0.1 M- 100 M) for 5 minutes followed by a washout period for 15 minutes. Black dots represents glutamatergic synaptic currents which can be clearly distinguished from noise levels. B. Cumulative fraction plotted against inter-event interval and amplitude of glycinergic IPSCs in control, dopamine (0.1-100 M) and washout conditions. C. Histogram showing the dose dependent responses of dopamine on glutamatergic EPSC frequency and amplitude (n=8).
3.2 Dopamine mediated actions on GABA and glycinergic IPSCs are action potential dependent
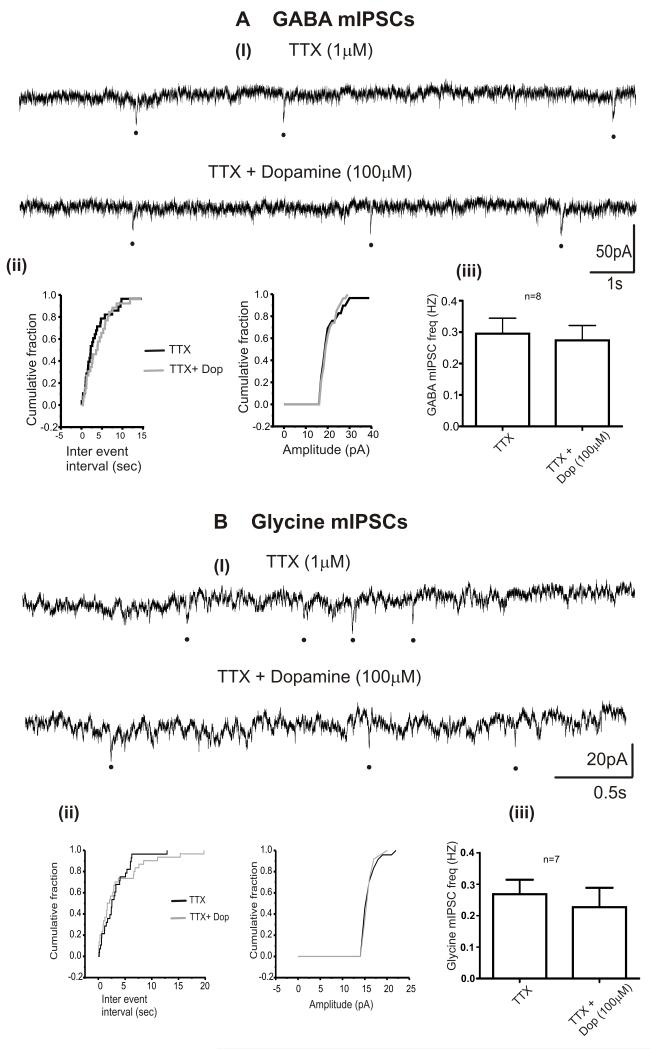
To identify the sites of action of dopamine on inhibitory neurotransmission to CVNs, dopamine was applied in presence of the voltage gated sodium channel blocker, TTX. GABA and glycinergic miniature IPSCs were isolated by bath application of TTX for 5 min. As shown in Figure 4, TTX (1 M) prevented the dopamine (100 M) induced inhibition of GABAergic (0.3 ± 0.05 Hz in TTX and 0.3 ± 0.05 Hz in TTX + dopamine [100 M]; n=8) and glycinergic miniature IPSC frequency (0.6 ± 0.1 Hz in TTX and 0.5 ± 0.1 Hz in TTX + dopamine [100 M]; n=7). The amplitudes of GABAergic (23 ± 1.6 pA in TTX and 21.3 ± 1.3 pA in TTX + dopamine [100μM]; n=8) and glycinergic miniature IPSCs (16.3 ± 1.3 pA in TTX and 17.4 ± 2.3 pA in TTX + dopamine [100 M]; n=7) were unaltered by dopamine [100μM].
Figure 4. Dopamine induced effects on inhibitory neurotransmission to CVNs are action potential mediated.
A, B. Representative traces from a typical experiment isolating for GABAergic (Ai) and glycinergic (Bi) miniature IPSCs in the presence of TTX and TTX + dopamine (100 M). A, ii and B, ii. The cumulative probability distribution curves plotted for inter event interval and amplitude of GABA and glycinergic IPSCs respectively while superfusing TTX (1 M, black line) and TTX + Dopamine (grey line, 100 M). A, iii and B, iii. The summary data showing the effect of dopamine on the frequency of GABAergic (n=8). and glycinergic miniature IPSCs (n=7). Black dots represents miniature IPSCs.
3.3 Dopamine induced inhibition of GABA & glycinergic neurotransmission to CVNs is mediated by activation of D2 – like receptors
The type of receptors involved in dopamine mediated effects on GABA and glycinergic activity to CVNs were further examined in the presence of a D1-like receptor antagonist SCH-23390 and the D2-like receptor antagonist, Sulpiride. Superfusion of SCH-23390 (10μM) for 5 minutes did not affect either frequency or amplitude of both GABAergic and glycinergic IPSCs. Dopamine in the presence of SCH-23390 continued to inhibit the frequency of GABA (2.4 ± 0.6 Hz in control, 2.7 ± 0.5 Hz in SCH-23390 [10 M] and 0.7 ± 0.2 Hz in SCH-23390 + Dopamine [100 M]; p < 0.05 for SCH-23390 vs. SCH-23390 + Dopamine [100 M]; n= 5) and glycinergic IPSCs (2.6 ± 0.4 Hz in control, 3.4 ± 0.6 Hz in SCH-23390 [10 M] and 1.3 ± 0.3 Hz in SCH-23390 + Dopamine [10 M]; p < 0.001; n=7). Application of sulpiride (20 M) had no effect on either the frequency, amplitude or baseline currents of GABAergic or glycinergic IPSCs. However, sulpiride (20 M) prevented the inhibition by dopamine (100 M) on GABAergic IPSC frequency (2.3 ± 0.7 Hz in control, 2.0 ± 0.7 Hz in sulpiride [20 M] and 2.4 ± 0.4 Hz in sulpiride + dopamine [100 M]; n=7). Similarly, sulpiride prevented the effects of dopamine (10μM) on glycinergic IPSC frequency (2.9 ± 1.0 Hz in control, 2.6 ± 0.9 Hz in sulpiride [20 M] and 2.2 ± 1.0 Hz in sulpiride + dopamine [10 M]; n=7). In addition, sulpiride also abolished the effects of dopamine (100 M) on glycinergic IPSC frequency (1.5 ± 0.2 Hz in sulpiride and 1.0 ± 0.5 Hz in sulpiride + dopamine [100 M]; n=6; p=0.11).
3.4 Actions of Dopamine on GABA and Glycinergic neurotransmission to CVNs are mimicked by D2-like receptor agonist but not D1-like receptor agonist
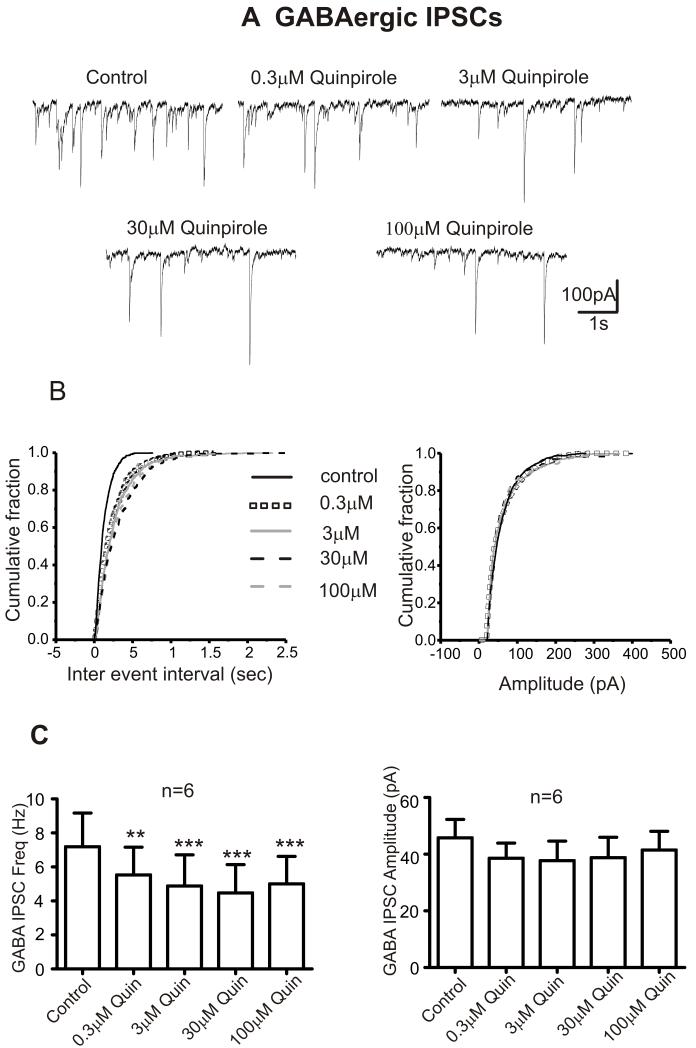
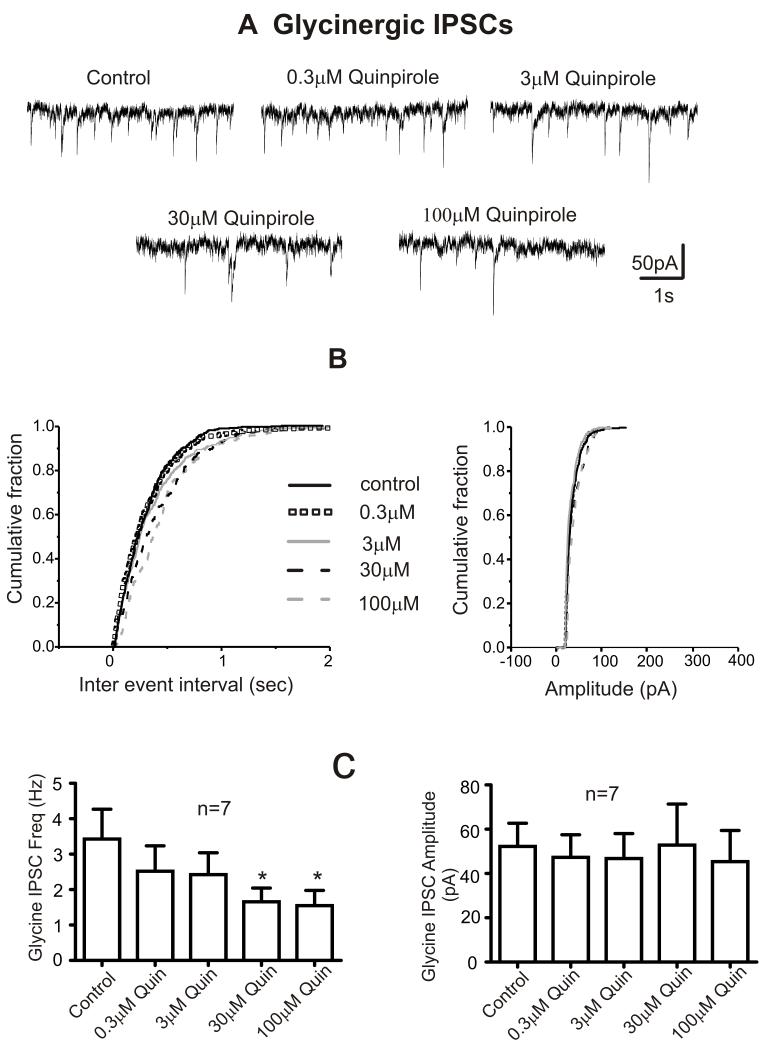
To further investigate if the effects of dopamine on GABA and glycinergic neurotransmission to CVNs are mediated by D2-like receptors, CVNs were exposed subsequently to various doses (0.3 M-100 M) of D1 like or D2 like receptor agonists. The D1-like receptor agonist, SKF 38393 had no effect on the frequency, amplitude or holding currents of GABA and glycinergic IPSCs, Table 2. In contrast, the D2-like receptor agonist, Quinpirole (0.3-100 M) inhibited the frequency of both GABA and glycinergic IPSCs without affecting their amplitudes or holding currents, Figures 5, 6 & Table 2. There is shift towards the right of the probability distribution for inter event interval in the presence of Quinpirole (0.3-100 M) indicating a decrease in the frequency of synaptic events. The distribution of amplitude with quinpirole remained unchanged, Figures 5B & 6B.
Table 2.
The effects of D1- like (SKF 38393) and D2-like (Quinpirole) agonist (0.3-100 M) on GABA and glycinergic IPSC frequency, amplitude and holding currents.
| GABA IPSCs | Control | SKF 38393 (0.3μM) | SKF 38393 (3μM) | SKF 38393 (30μM) | SKF 38393 (100μM) |
|---|---|---|---|---|---|
| Frequency (Hz) | 4.2 + 0.5 (7) | 4.5 + 0.6 (7) | 4.9 + 0.6 (7) | 4.5 + 0.5 (7) | 4.3 + 0.5 |
| Amplitude (pA) | 51.6 + 7.9 (7) | 45.7 + 5.9 (7) | 44.6 + 5.2 (7) | 41.5 + 4.5 (7) | 41.5 + 3.3 (7) |
| Holding current (pA) | −185.7 + 55.3 (7) | −221.4 + 74.7 (7) | −224.3 + 66.1 (7) | −217.1 + 59.4 (7) | −217.1 + 56.1 (7) |
| Glycine IPSCs | |||||
| Frequency (Hz) | 2.7 + 1.1 (7) | 3.0 + 1.0 (7) | 3.3 + 1.2 (7) | 3.4 + 1.1 (7) | 3.0 + 1.0 (7) |
| Amplitude (pA) | 42.4 + 6.4 (7) | 44.5 + 7.0 (7) | 41.7 + 6.0 (7) | 41.2 + 5.8 (7) | 38.0 + 5.7 (7) |
| Holding current (pA) | −107.1 + 29.6 (7) | −137.1 + 39.4 (7) | −151.4 + 43.2 (7) | −178.6 + 63.2 (7) | −190.0 + 64.0 (7) |
| GABA IPSCs | Control | Quinpirole (0.3μM) | Quinpirole (3μM) | Quinpirole (30μM) | Quinpirole (100μM) |
|---|---|---|---|---|---|
| Frequency (Hz) | 6.8 + 1.7 (6) | 5.3 + 1.4 (6)** | 5.0 + 1.5 (6)*** | 4.3 + 1.4 (6)*** | 5.0 + 1.6 (6)*** |
| Amplitude (pA) | 45.8 + 6.5 (6) | 38.6 + 5.3 (6) | 37.7 + 6.9 (6) | 38.7 + 7.2 (6) | 41.5 + 6.6 (6) |
| Holding current (pA) | −113.3 + 28.5 (6) | −150.0 + 42.8 (6) | −183.3 + 54.3 (6) | −191.7 + 67.6 (6) | −161.7 + 56.3 (6) |
| Glycine IPSCs | |||||
| Frequency (Hz) | 3.4 + 0.8 (7) | 2.5 + 0.7 (7) | 2.4 + 0.6 (7) | 1.6 + 0.4 (7)* | 1.5 + 0.4 (7)* |
| Amplitude (pA) | 52.2 + 10.5 (7) | 47.3 +10.3 (7) | 46.8 + 11.3 (7) | 52.8 + 18.6 (7) | 45.4 + 14.0 (7) |
| Holding current (pA) | −200.0 + 32.7 (7) | −193.0 + 33.5 (7) | −193.0 + 29.7 (7) | −207.1 + 41.4 (7) | −228.6 + 67.1 (7) |
Control vs. D1-like or D2-like agonist, p<0.05
p<0.01
p<0.001.
Number of neurons indicated in parentheses.
Figure 5. Dopamine induced changes in GABAergic neurotransmission to CVNs are mediated by D2-like receptors.
A. Representative traces from a typical experiment isolating for GABAergic activity to CVNs is shown in control conditions and while subsequently bath applying various doses of D2 like agonist Quinpirole (0.3 M – 100 M) for 5 minutes.
B. Cumulative probability distribution curves plotted for inter-event interval and amplitude of GABA IPSCs in control and Quinpirole (0.3-100 M). The synaptic events were averaged during the last 2 minute period of exposure in each condition. There is shift towards right for the probability distribution of inter vent interval in the presence of Quinpirole (0.3-100 M) suggesting a decrease in the frequency of synaptic events. However, the distribution of amplitude in quinpirole remains unchanged.
C. Histogram showing the dose dependent responses of Quinpirole on GABAergic (n=6) IPSC frequency and amplitude. **p<0.01; ***p<0.001.
Figure 6. Dopamine induced changes in glycinergic neurotransmission to CVNs are mediated by D2-like receptors.
A. Representative traces from a typical experiment isolating for glycinergic activity to CVNs is shown in control conditions and while subsequently bath applying various doses of D2 like agonist Quinpirole (0.3 M – 100 M) for 5 minutes.
B. Cumulative probability distribution curves plotted for inter-event interval and amplitude of GABA IPSCs in control and Quinpirole (0.3-100 M). The synaptic events were averaged during the last 2 minute period of exposure in each condition. There is shift towards right for the probability distribution of inter vent interval in the presence of Quinpirole (0.3-100 M) suggesting a decrease in the frequency of synaptic events. However, the distribution of amplitude in quinpirole remains unchanged.
C. Histogram showing the dose dependent responses of Quinpirole on glycinergic (n=7) IPSC frequency and amplitude. *p < 0.05.
3.5 Dopamine effects are not mediated by the activation of adrenergic and serotonergic receptors
Dopamine is known to activate adrenergic receptors (Rey et al., 2001, Cornil et al., 2002, Cornil and Ball, 2008) and serotonin (5-HT) receptors such as 5-HT1A, 5-HT2A/2C and 5-HT3 (Woodward et al., 1992, Oz et al., 2003, Bhattacharyya et al., 2006) in addition to dopamine receptors. To test if the dopamine (100 M) induced effects on inhibitory neurotransmission to CVNs are due to dopamine activation of adrenergic or serotonin receptors, dopamine (100 M) was applied in presence of a cocktail containing adrenergic and serotonergic receptor antagonists. The cocktail was comprised of the 1 adrenergic receptor blocker prazosin (3 M), 2 adrenergic receptor blocker atipamezole (1 M), - adrenergic blocker propranolol (10 M), 5-HT1A receptor antagonist WAY-100635 (100 M), 5-HT2A/2C receptor antagonist ketanserin (1 M) and 5-HT3 receptor antagonist ondansetron (100 M). Bath application of these receptor antagonists had no effect on the responses to dopamine as dopamine (100 M) continued to significantly inhibit GABAergic IPSC frequency in the presence of the adrenergic and serotonergic receptor antagonists (2.8 ± 0.7 Hz in control, 2.3 ± 0.8 Hz in cocktail and 1.0 ± 0.4 Hz in cocktail + dopamine [100 M]; n=7; p<0.05 for cocktail vs. cocktail + dopamine [100 M]).
Application of the adrenergic receptor antagonists prazosin (3 M), propranolol (10 M) and atipamezole (1 M) (2.1 ± 0.5 Hz in control, 1.4 ± 0.3 Hz in adrenergic receptor antagonists and 0.4 ± 0.07 Hz in adrenergic receptor antagonists + dopamine [100 M]; n=8; p<0.05), as well as co-application of the 5-HT receptor antagonists WAY-100635 (100 M), ketanserin (1 M) and ondansetron (100 M) did not inhibit the dopamine evoked depression of glycinergic IPSC frequency (2.5 ± 0.5 Hz in control, 1.8 ± 0.3 Hz in 5-HT receptor antagonists and 0.6 ± 0.2 Hz in 5-HT receptor antagonists + dopamine [100 M]; n=8; p<0.05 5-HT receptor antagonists vs. 5-HT receptor antagonists + dopamine [100 M]).
4.0 Discussion
The major finding from this study is that dopamine, acting via D2-like receptors, inhibits the GABAergic and glycinergic input to CVNs in the NA but has no direct postsynaptic actions at CVNs themselves.
4.1 Dopamine depresses spontaneous inhibitory neurotransmission to CVNs
Dopamine inhibited the frequency of GABA and glycinergic IPSCs in CVNs without affecting glutamatergic transmission to CVNs. Glycinergic transmission was more sensitive to lower concentrations of dopamine (10 M) compared to GABA (100 M). Similar actions of dopamine on spontaneous GABA release have been reported in striatal cholinergic interneurons (Momiyama and Koga, 2001). The amplitudes or holding currents of GABA and glycinergic events were unaltered by dopamine suggesting that dopamine has no effect on the sensitivity of postsynaptic GABA and glycine receptors, but acts on preceding inhibitory neurons. The consequences of reducing GABA and glycinergic transmission to CVNs would predict an increase in vagal output to the heart and a bradycardia, in accordance with prior microinjection studies that found injections of dopamine into the nucleus ambiguus lowered heart rate (Chitravanshi and Calaresu, 1992).
GABA is the major inhibitory neurotransmission to the CVNs which is endogenously active and plays a role in tonic control of heart rate and regulating cardio-respiratory interactions such as respiratory sinus arrhythmia (Neff et al., 2003). In addition, other work has shown that glycine receptors are also involved in tonic control of the heart rate (DiMicco et al., 1979, Chitravanshi et al., 1991). However, a recent study in rats reported that strychnine sensitive glycine receptors are not involved in tonic or reflex control of cardiac vagal output (Hildreth and Goodchild, 2010) but are needed for 5H1A receptor activation induced facilitation of bradycardia and changes in heart rate (Hildreth and Goodchild, 2010). In support of the current findings differential sensitivity of GABA and glycinergic neurons and inputs projecting to CVNs has been reported previously in which spontaneous glycinergic IPSCs were more sensitive to neuromodulators such as orexin-A (Wang et al., 2005) and 5HT1A/7 receptor agonists (Chen et al., 2008) compared to GABA IPSCs. These reports indicate that GABA and glycine inputs to CVNs are likely differentially regulated, including their modulation by dopamine.
Previous studies from this lab has identified, using photo uncaging and electrical stimulation approaches, that GABAergic neurons projecting to CVNs are located in RVLM region (including pre-botzinger complex), two regions medial to NA i.e., lateral paragigangtocellular nucleus and magnocellular reticular nucleus (Frank et al., 2009) and the NTS (Wang et al., 2001). Although the presence of glycinergic fibers and receptors surrounding CVNs has been supported by both anatomical (Batten, 1995) and microinjection studies (Chitravanshi et al., 1991), the origin of glycinergic neurons that project to CVNs remains unknown.
4.2 Dopamine induced effects are mediated by D2-like receptors
Dopamine evoked inhibitory effects on GABA and glycinergic IPSCs were abolished by D2-like receptor antagonist, sulpiride but not by D1-like receptor antagonist, SCH-23390 indicating the inhibition of GABA and glycine neurotransmission to CVNs was due to activation of D2-like receptors by dopamine. The D2-like receptor agonist, Quinpirole but not D1-like receptor agonist, SKF 38393 mimicked the inhibitory effects of dopamine on GABA and glycinergic neurotransmission to CVNs; consistent with the activation of D2-like, but not D1-like, receptors by dopamine. The attenuation of dopamine induced responses by D2-like receptor antagonist has been observed in other brain regions (Momiyama and Koga, 2001, Kline et al., 2002). The localization of D2-like receptors in GABAergic neurons in the NTS (Yokoyama et al., 1994, Kline et al., 2002), suggests a population of GABAergic neurons in the NTS may be a likely target of dopamine and be responsible for the dis-inhibition of CVNs. However it should be noted that D1-like and D2-like receptors are not endogenously active in this study as sulpiride and SCH-23390 alone had no effect on GABA and glycinergic IPSC frequency or amplitude.
Functional interactions between dopamine, 5-HT and noradrenergic neurons and receptors have been reported earlier (Guiard et al., 2008). Dopamine has been shown to interact with various 5-HT receptors such as 5-HT1A/1C, 5-HT2A/2C and 5-HT3 (Woodward et al., 1992, Oz et al., 2003, Bhattacharyya et al., 2006) and adrenergic receptors (Cornil et al., 2002, Cornil and Ball, 2008, Alberto et al., 2011). Activation of 5-HT1A/7 and 5-HT2 receptors (Dergacheva et al., 2007, Wang et al., 2007) and adrenergic receptors (Philbin et al., 2010, Bateman et al., 2012) inhibits the frequency of spontaneous GABAergic IPSCs to CVNs. Hence it was important to test whether dopamine induced effects on inhibitory neurotransmission to CVNs were mediated due to the activation of 5-HT and noradrenergic receptors. The concentrations of 5-HT antagonists and adrenergic receptor antagonists used in this study are consistent with the previous studies (Dergacheva et al., 2007, Dergacheva et al., 2009, Gorini et al., 2009, Philbin et al., 2010, Boychuk et al., 2011) and the data in this study indicates that the effects of dopamine in this study were mediated independent from adrenergic or serotonergic receptor activation and involved D2 –like, but not D1 –like, receptor activation.
4.3 The actions of dopamine on GABA and glycine IPSCs is presynaptic
To test whether the dopamine evoked actions were presynaptic or postsynaptic; the effects of dopamine on TTX isolated miniature IPSCs were examined. These miniature IPSCs are caused by action potential independent spontaneous presynaptic release of neurotransmitters. Dopamine had no effect on miniature IPSCs (mIPSCs) in the presence of TTX indicating dopamine alters inhibitory neurotransmission to CVNs via an action potential dependent mechanism likely modulating preceding neurons in the inhibitory pathways to CVNs. A presynaptic action of dopamine has been reported previously in other brain areas such as NTS and striatal neurons (Momiyama and Koga, 2001, Kline et al., 2002).
4.4 Likely sources of dopamine pathways to inhibitory neurons projecting to CVNs
Dopamine may be released not only from dopaminergic neurons but also from noradrenergic neurons, where dopamine acts as a precursor for noradrenaline and is often a cotransmitter (Devoto et al., 2001, Devoto et al., 2003). The likely sources for noradrenergic neurons and pathways that synapse upon CVNs include the catecholaminergic neurons in the rostro ventrolateral medulla (RVLM, A1 and C1 cell groups), locus coerulus (LC) and adrenergic neurons in the NTS (Kalia et al., 1985, Neff et al., 1998, Samuels and Szabadi, 2008, Frank et al., 2009).
The LC plays an important role in controlling arousal and autonomic functions (Samuels and Szabadi, 2008). There is ample evidence for the co-release of dopamine with noradrenaline in the cortical regions (Devoto et al., 2003, Smith and Greene, 2012) from LC noradrenergic nerve terminals. LC also projects to brainstem areas including the NA and RVLM (Westlund and Coulter, 1980, Samuels and Szabadi, 2008). In accordance with the results of this study, it is hypothesized that dopamine co-released from the LC terminals acts on the preceding GABAergic neurons of RVLM that project to CVNs. In addition, dopaminergic neurons and fibers have been immunohistochemically identified to be located in dorsomedial (NTS, DMNX) and ventrolateral (A1) regions of the brainstem (Kalia et al., 1985, Maqbool et al., 1993, Kitahama et al., 2000). Therefore a likely mechanism consistent with the results in this study and others is that dopamine disinhibits CVNs by inhibiting GABAergic pathways originating from the NTS (Frank et al., 2009), a neuronal population that expresses D2 receptors (Yokoyama et al., 1994).
4.5. Methodological limitations
It should be noted that the current studies were performed on brainstem slices of 2-5 day old rat pups, an immature age at which synaptogenesis is incomplete. Tonic cardiac vagal motor activity is active at birth but reaches maturity only after the second or third week of postnatal age in rats (Adolph, 1967, Tucker and Johnson, 1984, Kasparov and Paton, 1997). It is possible that the modulation by dopamine seen in this study is different in adult animals, or in diseases with alterations in dopamine function, such as depression. The site of action of dopamine was not identified in the current study. However, the coronal section of brainstem slices used in this study was 450-500 m in thickness, thus encompassing ~60% of the medulla at this age of the animal. This limits the potential identity of the sites of action of dopamine, although the targets of dopamine could include the dendrites, soma or synaptic terminals of the preceding neurons in the cardiorespiratory network that generate inhibitory neurotransmission to CVNs.
Dopamine was applied at various concentrations ranging from 0.1-100 M. Because the cardiac vagal neurons and the neurons that project to them are often located deep within the brainstem slice, the actual concentration of dopamine near the neurons is likely to be significantly less than that of the dopamine concentration in the bath solution. Unfortunately given the variable depth of CVNs within the tissue, and the unknown penetration of the dopamine we cannot accurately determine the minimal concentration necessary to activate the receptors responsible for the synaptic responses observed in this study. However the goal of this study was to ascertain the most likely targets for dopamine to alter CVN activity. In future studies additional work will be necessary to examine when dopamine pathways are activated and how dopamine neurons alter synaptic pathways to CVNs and the cardiorespiratory network in the intact animal, but this work provides an important foundation for our understanding of this field.
5.0 Conclusion
The present study demonstrates that dopamine suppresses spontaneous inhibitory GABA and glycinergic transmission to CVNs, via action on preceding inhibitory neurons. Such dopamine induced actions were mediated by D2-like receptors but not by D1-like or adrenergic or 5-HT receptors. This dis-inhibition would excite CVNs and would be predicted to increase cardio-protective vagal output to the heart, resulting in bradycardia.
Highlights.
Dopamine, via D2-like receptors, suppresses GABA and glycinergic synaptic activity to CVNs.
Dopamine effects on inhibitory neurotransmission were action potential dependent.
Dis-inhibition of CVNs by dopamine would be predicted to increase vagal activity to the heart.
Acknowledgements
This study was supported by NIH grants HL 49965, HL 59895 and HL 72006 awarded to D. Mendelowitz.
Abbreviations
- DL-AP5
2-amino-5-phosphonopentanoic acid
- CVN
cardiac vagal neuron
- CNQX
6-cyano-7-nitroquinoxaline
- DMNX
dorsal motor nucleus of the vagus
- EPSC
excitatory postsynaptic current
- IPSC
inhibitory postsynaptic current
- LC
locus coerulus
- mIPSC
miniature inhibitory postsynaptic current
- NA
nucleus ambiguus
- NE
norepinephrine
- NTS
nucleus tractus solitarius
- PSS
physiological salt solution
- RVLM
rostroventrolateral medulla
- TTX
Tetrodotoxin
- 5-HT
serotonin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship contributions:
Participated in research design: J.D, P.B and D.M.
Conducted experiments: J.D. and P.B.
Performed data analysis: J.D. and D.M.
Wrote or contributed to writing of the manuscript: J.D. and D.M.
References
- Adolph EF. Ranges of heart rates and their regulations at various ages (rat) The American journal of physiology. 1967;212:595–602. doi: 10.1152/ajplegacy.1967.212.3.595. [DOI] [PubMed] [Google Scholar]
- Alberto CO, Trask RB, Hirasawa M. Dopamine acts as a partial agonist for alpha2A adrenoceptor in melanin-concentrating hormone neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:10671–10676. doi: 10.1523/JNEUROSCI.6245-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Boychuk CR, Philbin KE, Mendelowitz D. beta adrenergic receptor modulation of neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Neuroscience. 2012;210:58–66. doi: 10.1016/j.neuroscience.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batten TF. Immunolocalization of putative neurotransmitters innervating autonomic regulating neurons (correction of neurones) of cat ventral medulla. Brain research bulletin. 1995;37:487–506. doi: 10.1016/0361-9230(95)00029-e. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Raote I, Bhattacharya A, Miledi R, Panicker MM. Activation, internalization, and recycling of the serotonin 2A receptor by dopamine. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15248–15253. doi: 10.1073/pnas.0606578103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boychuk CR, Bateman RJ, Philbin KE, Mendelowitz D. alpha1-adrenergic receptors facilitate inhibitory neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Neuroscience. 2011;193:154–161. doi: 10.1016/j.neuroscience.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Hou LL, Wang JJ. 5-HT1A/7 receptor agonist excites cardiac vagal neurons via inhibition of both GABAergic and glycinergic inputs. Acta pharmacologica Sinica. 2008;29:529–538. doi: 10.1111/j.1745-7254.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC, Agarwal SK, Calaresu FR. Microinjection of glycine into the nucleus ambiguus elicits tachycardia in spinal rats. Brain research. 1991;566:290–294. doi: 10.1016/0006-8993(91)91711-9. [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC, Calaresu FR. Dopamine microinjected into the nucleus ambiguus elicits vagal bradycardia in spinal rats. Brain research. 1992;583:308–311. doi: 10.1016/s0006-8993(10)80040-x. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Ball GF. Interplay among catecholamine systems: dopamine binds to alpha2-adrenergic receptors in birds and mammals. The Journal of comparative neurology. 2008;511:610–627. doi: 10.1002/cne.21861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Balthazart J, Motte P, Massotte L, Seutin V. Dopamine activates noradrenergic receptors in the preoptic area. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:9320–9330. doi: 10.1523/JNEUROSCI.22-21-09320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O, Griffioen KJ, Neff RA, Mendelowitz D. Respiratory modulation of premotor cardiac vagal neurons in the brainstem. Respir Physiol Neurobiol. 2010;174:102–110. doi: 10.1016/j.resp.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O, Griffioen KJ, Wang X, Kamendi H, Gorini C, Mendelowitz D. 5-HT(2) receptor subtypes mediate different long-term changes in GABAergic activity to parasympathetic cardiac vagal neurons in the nucleus ambiguus. Neuroscience. 2007;149:696–705. doi: 10.1016/j.neuroscience.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O, Kamendi H, Wang X, Pinol RM, Frank J, Jameson H, Gorini C, Mendelowitz D. The role of 5-HT3 and other excitatory receptors in central cardiorespiratory responses to hypoxia: implications for sudden infant death syndrome. Pediatr Res. 2009;65:625–630. doi: 10.1203/PDR.0b013e3181a16e9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto P, Flore G, Longu G, Pira L, Gessa GL. Origin of extracellular dopamine from dopamine and noradrenaline neurons in the medial prefrontal and occipital cortex. Synapse. 2003;50:200–205. doi: 10.1002/syn.10264. [DOI] [PubMed] [Google Scholar]
- Devoto P, Flore G, Pani L, Gessa GL. Evidence for co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex. Molecular psychiatry. 2001;6:657–664. doi: 10.1038/sj.mp.4000904. [DOI] [PubMed] [Google Scholar]
- DiMicco JA, Gale K, Hamilton B, Gillis RA. GABA receptor control of parasympathetic outflow to heart: characterization and brainstem localization. Science. 1979;204:1106–1109. doi: 10.1126/science.451556. [DOI] [PubMed] [Google Scholar]
- Franchi F, Lazzeri C, Barletta G, Ianni L, Mannelli M. Centrally mediated effects of bromocriptine on cardiac sympathovagal balance. Hypertension. 2001;38:123–129. doi: 10.1161/01.hyp.38.1.123. [DOI] [PubMed] [Google Scholar]
- Frank JG, Jameson HS, Gorini C, Mendelowitz D. Mapping and identification of GABAergic neurons in transgenic mice projecting to cardiac vagal neurons in the nucleus ambiguus using photo-uncaging. Journal of neurophysiology. 2009;101:1755–1760. doi: 10.1152/jn.91134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Gorini C, Jameson HS, Mendelowitz D. Serotonergic modulation of the trigeminocardiac reflex neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Journal of neurophysiology. 2009;102:1443–1450. doi: 10.1152/jn.00287.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiard BP, El Mansari M, Merali Z, Blier P. Functional interactions between dopamine, serotonin and norepinephrine neurons: an in-vivo electrophysiological study in rats with monoaminergic lesions. Int J Neuropsychopharmacol. 2008;11:625–639. doi: 10.1017/S1461145707008383. [DOI] [PubMed] [Google Scholar]
- Hildreth CM, Goodchild AK. Role of ionotropic GABA, glutamate and glycine receptors in the tonic and reflex control of cardiac vagal outflow in the rat. BMC neuroscience. 2010;11:128. doi: 10.1186/1471-2202-11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia M, Fuxe K, Goldstein M. Rat medulla oblongata. II. Dopaminergic, noradrenergic (A1 and A2) and adrenergic neurons, nerve fibers, and presumptive terminal processes. The Journal of comparative neurology. 1985;233:308–332. doi: 10.1002/cne.902330303. [DOI] [PubMed] [Google Scholar]
- Kasparov S, Paton JF. Changes in baroreceptor vagal reflex performance in the developing rat. Pflugers Archiv: European journal of physiology. 1997;434:438–444. doi: 10.1007/s004240050418. [DOI] [PubMed] [Google Scholar]
- Kitahama K, Nagatsu I, Geffard M, Maeda T. Distribution of dopamine-immunoreactive fibers in the rat brainstem. Journal of chemical neuroanatomy. 2000;18:1–9. doi: 10.1016/s0891-0618(99)00047-2. [DOI] [PubMed] [Google Scholar]
- Kline DD, Takacs KN, Ficker E, Kunze DL. Dopamine modulates synaptic transmission in the nucleus of the solitary tract. Journal of neurophysiology. 2002;88:2736–2744. doi: 10.1152/jn.00224.2002. [DOI] [PubMed] [Google Scholar]
- Maqbool A, Batten TF, Berry PA, McWilliam PN. Distribution of dopamine-containing neurons and fibres in the feline medulla oblongata: a comparative study using catecholamine-synthesizing enzyme and dopamine immunohistochemistry. Neuroscience. 1993;53:717–733. doi: 10.1016/0306-4522(93)90619-q. [DOI] [PubMed] [Google Scholar]
- Massari VJ, Dickerson LW, Gray AL, Lauens tein JM, Blinder KJ, Newsome JT, Rodak DJ, Fleming TJ, Gatti PJ, Gillis RA. Neural control of left ventricular contractility in the dog heart: synaptic interactions of negative inotropic vagal preganglionic neurons in the nucleus ambiguus with tyrosine hydroxylase immunoreactive terminals. Brain research. 1998;802:205–220. doi: 10.1016/s0006-8993(98)00613-1. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D. Advances in Parasympathetic Control of Heart Rate and Cardiac Function. News Physiol Sci. 1999;14:155–161. doi: 10.1152/physiologyonline.1999.14.4.155. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D, Kunze DL. Identification and dissociation of cardiovascular neurons from the medulla for patch clamp analysis. Neurosci Lett. 1991;132:217–221. doi: 10.1016/0304-3940(91)90305-d. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Momiyama T, Koga E. Dopamine D(2)-like receptors selectively block N-type Ca(2+) channels to reduce GABA release onto rat striatal cholinergic interneurones. The Journal of physiology. 2001;533:479–492. doi: 10.1111/j.1469-7793.2001.0479a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff RA, Mihalevich M, Mendelowitz D. Stimulation of NTS activates NMDA and non-NMDA receptors in rat cardiac vagal neurons in the nucleus ambiguus. Brain research. 1998;792:277–282. doi: 10.1016/s0006-8993(98)00149-8. [DOI] [PubMed] [Google Scholar]
- Neff RA, Wang J, Baxi S, Evans C, Mendelowitz D. Respiratory sinus arrhythmia: endogenous activation of nicotinic receptors mediates respiratory modulation of brainstem cardioinhibitory parasympathetic neurons. Circ Res. 2003;93:565–572. doi: 10.1161/01.RES.0000090361.45027.5B. [DOI] [PubMed] [Google Scholar]
- Oz M, Zhang L, Rotondo A, Sun H, Morales M. Direct activation by dopamine of recombinant human 5-HT1A receptors: comparison with human 5-HT2C and 5-HT3 receptors. Synapse. 2003;50:303–313. doi: 10.1002/syn.10273. [DOI] [PubMed] [Google Scholar]
- Philbin KE, Bateman RJ, Mendelowitz D. Clonidine, an alpha 2-receptor agonist, diminishes GABAergic neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Brain research. 2010;1347:65–70. doi: 10.1016/j.brainres.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey E, Hernandez-Diaz FJ, Abreu P, Alonso R, Tabares L. Dopamine induces intracellular Ca2+ signals mediated by alpha1B-adrenoceptors in rat pineal cells. European journal of pharmacology. 2001;430:9–17. doi: 10.1016/s0014-2999(01)01250-x. [DOI] [PubMed] [Google Scholar]
- Samuels ER, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Curr Neuropharmacol. 2008;6:235–253. doi: 10.2174/157015908785777229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Greene RW. CNS dopamine transmission mediated by noradrenergic innervation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:6072–6080. doi: 10.1523/JNEUROSCI.6486-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythies J, Galzigna L. The oxidative metabolism of catecholamines in the brain: a review. Biochimica et biophysica acta. 1998;1380:159–162. doi: 10.1016/s0304-4165(97)00131-1. [DOI] [PubMed] [Google Scholar]
- Tucker DC, Johnson AK. Development of autonomic control of heart rate in genetically hypertensive and normotensive rats. The American journal of physiology. 1984;246:R570–577. doi: 10.1152/ajpregu.1984.246.4.R570. [DOI] [PubMed] [Google Scholar]
- Wang J, Irnaten M, Mendelowitz D. Characteristics of spontaneous and evoked GABAergic synaptic currents in cardiac vagal neurons in rats. Brain research. 2001;889:78–83. doi: 10.1016/s0006-8993(00)03112-7. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang X, Irnaten M, Venkatesan P, Evans C, Baxi S, Mendelowitz D. Endogenous acetylcholine and nicotine activation enhances GABAergic and glycinergic inputs to cardiac vagal neurons. Journal of neurophysiology. 2003;89:2473–2481. doi: 10.1152/jn.00934.2002. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Chen YH, Li KY, Sun FY. Differential sensitivity of GABAergic and glycinergic inputs to orexin-A in preganglionic cardiac vagal neurons of newborn rats. Acta pharmacologica Sinica. 2005;26:1442–1447. doi: 10.1111/j.1745-7254.2005.00231.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Dergacheva O, Kamendi H, Gorini C, Mendelowitz D. 5-Hydroxytryptamine 1A/7 and 4alpha receptors differentially prevent opioid-induced inhibition of brain stem cardiorespiratory function. Hypertension. 2007;50:368–376. doi: 10.1161/HYPERTENSIONAHA.107.091033. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Coulter JD. Descending projections of the locus coeruleus and subcoeruleus/medial parabrachial nuclei in monkey: axonal transport studies and dopamine-beta-hydroxylase immunocytochemistry. Brain research. 1980;2:235–264. doi: 10.1016/0165-0173(80)90009-0. [DOI] [PubMed] [Google Scholar]
- Woodward RM, Panicker MM, Miledi R. Actions of dopamine and dopaminergic drugs on cloned serotonin receptors expressed in Xenopus oocytes. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:4708–4712. doi: 10.1073/pnas.89.10.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama C, Okamura H, Nakajima T, Taguchi J, Ibata Y. Autoradiographic distribution of [3H]YM-09151-2, a high-affinity and selective antagonist ligand for the dopamine D2 receptor group, in the rat brain and spinal cord. The Journal of comparative neurology. 1994;344:121–136. doi: 10.1002/cne.903440109. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Travagli RA. Dopamine effects on identified rat vagal motoneurons. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1002–1008. doi: 10.1152/ajpgi.00527.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]