Abstract
RasGRP3, an activator for H-Ras, R-Ras and Rap1/2, has emerged as an important mediator of signaling downstream from receptor coupled phosphoinositide turnover in B and T cells. Here, we report that RasGRP3 showed a high level of expression in multiple human melanoma cell lines as well as in a subset of human melanoma tissue samples. Suppression of endogenous RasGRP3 expression in these melanoma cell lines reduced Ras-GTP formation as well as c-Met expression and Akt phosphorylation downstream from HGF or EGF stimulation. RasGRP3 suppression also inhibited cell proliferation and reduced both colony formation in soft agar and xenograft tumor growth in immunodeficient mice, demonstrating the importance of RasGRP3 for the transformed phenotype of the melanoma cells. Reciprocally, overexpression of RasGRP3 in human primary melanocytes altered cellular morphology, markedly enhanced cell proliferation, and rendered the cells tumorigenic in a mouse xenograft model. Suppression of RasGRP3 expression in these cells inhibited downstream RasGRP3 responses and suppressed cell growth, confirming the functional role of RasGRP3 in the altered behavior of these cells. The identification of the role of RasGRP3 in melanoma highlights its importance, as a Ras activator, in the phosphoinositide signaling pathway in human melanoma and provides a new potential therapeutic target.
Keywords: Ras activator, Akt, HGF, melanoma, cell transformation, guanine nucleotide exchange factor
Introduction
There is abundant evidence to suggest that Ras is a critical cell signaling element impacting the development, maintenance, and progression of human melanoma, as it is in many other cancers. N-Ras is mutated in 15–20% of human melanomas (Haluska and Ibrahim 2006). The overexpression of mutated constitutively activated N-Ras in melanocytes of INK4a−/− background transgenic mice resulted in abnormal proliferation of the melanocytes and development of metastatic cutaneous melanoma (Ackermann et al 2005). Although H-Ras is less frequently mutated in melanoma, the expression of a mutated constitutively activated H-Ras in wild type melanocytes induced melanocytic hyperplasia and contributed to their susceptibility to develop melanoma in vivo (Broome Powell et al 1999). Activation of M-Ras was involved in MGSA/GRO-mediated melanocyte transformation (Wang et al 2000). Activated R-Ras promoted integrin-mediated melanoma cell migration (Gawecka et al). In addition, even when not mutated, wild-type Ras showed constitutive activation in many melanoma cell lines but not in normal melanocytes (Satyamoorthy et al 2003).
The importance of the Ras signaling pathway in melanoma is further emphasized by the frequency with which mutations are detected in the effector pathways downstream of Ras signaling. Thus, mutations in B-Raf are found in some 60% of melanomas and mutations in PTEN, an inhibitor of Akt activity, are found in 30% (Ikediobi et al 2006, Pollock et al 2003, Wu et al 2003). Likewise, suppression of the Ras/PI3K signaling pathway reduced melanoma cell metastasis (Huang et al 2008).
Functionally, the RasGRPs act as immediate upstream activators of Ras isoforms. The RasGRPs are regulated both by diacylglycerol binding to the C1 domains as well as through phosphorylation by protein kinase C, which itself is regulated by diacylglycerol (Aiba et al 2004, Stone 2006, Zheng et al 2005). They should thus respond to the many receptor tyrosine kinases and G-protein coupled receptors which activate phospholipase C isoforms, among other responses, leading to phospholipase C breakdown of phosphatidylinositol 3,5-bisphosphate and generation of diacylglycerol. Therefore RasGRPs can physiologically and simultaneously activate multiple downstream wild-type Ras family members and link them to signals from the many upstream growth factors or cytokines which drive diacylglycerol turnover, including the B receptor, T-cell receptor, hepatocyte growth factor and epidermal growth factor (Aiba et al 2004, Brodie et al 2004, Lorenzo et al 2000, Lorenzo et al 2001, Oh-hora et al 2003, Stone 2006, Stope et al 2004, Zheng et al 2005).
Emerging evidence indicates that RasGRPs can function as oncogenes in multiple cancers. Overexpression of RasGRPs caused Ras activation coincident with cell transformation and tumorigenesis in both mice and human (Oki-Idouchi and Lorenzo 2007, Suzuki et al 2002). Highly expressed RasGRP3 has been found in human Burkitt's lymphoma, human pre–B-cell leukemia and human natural killer (NK)–like T-cell leukemia (Teixeira et al 2003). Additionally, re-expression of RasGRP3 enhanced angiogenesis during pregnancy and tumorigenesis (Roberts et al 2004). We recently reported that RasGRP3 expression was an important contributor to the cancer phenotype in several human prostate cancer cell lines (Yang et al 2010).
These observations have motivated a more extensive examination of RasGRP3 expression and function in solid tumors. Here, we examine the role of RasGRP3 in melanoma.
Results
RasGRP3 is expressed in human melanoma tissues and multiple melanoma cell lines
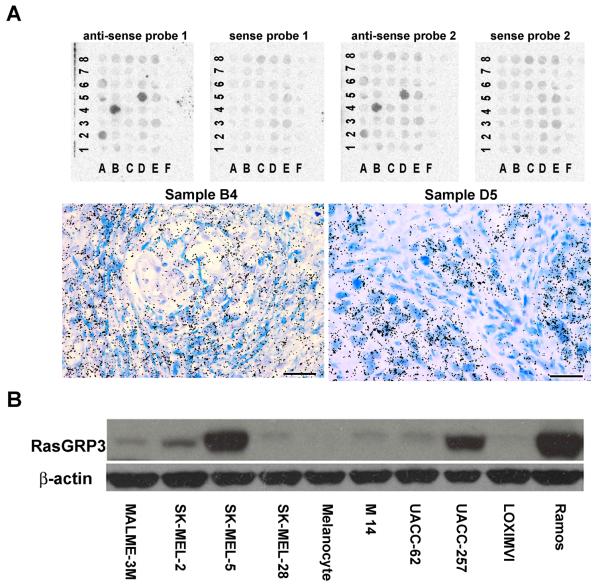
We examined the RasGRP3 mRNA expression level in a human melanoma tissue array by mRNA in situ hybridization. Five out of forty melanoma tissue samples (samples A2, A5, A6, B4 and D5 in Figure 1A) (12.5% of the melanomas) showed RasGRP3 expression (Figure 1A and Supplementary Figure 1A). None of the eight normal skin samples showed expression (Figure 1A, columns labeled F). Using Q-PCR, we also examined a human melanoma cDNA array for RasGRP3 expression. Marked variation (65-fold) in inter-individual RasGRP3 expression was observed in the cDNA array (Supplementary Figure 1B). While we were unable to address how these levels of RasGRP3 expression in the melanoma tissues compared to normal melanocytes in skin, we could, however, detect the expression of RasGRP3 in most of the tested melanoma cell lines both by immunoblotting (Figure 1B) and by RT-PCR (Supplementary Figure 1C). In the human primary adult melanocytes, the endogenous RasGRP3 expression was not detectable by immunoblotting. The RasGRP3 was also unable to be detected by RT-PCR in the human primary adult melanocytes (not shown). However, melanin binds and inhibits thermostable DNA polymerase (Eckhart et al 2000, Sutlovic et al 2005) and the much higher levels of melanin present in the melanocytes than in the melanoma cells lines caused interference, as evidenced by reduced detection of GAPDH as well, necessitating caution in interpretation. For comparison with the melanoma cell lines, we included the Ramos B-lymphoblastoid cell line as a positive control in both assays, since the Ramos cells have been described as expressing the highest endogenous level of RasGRP3 (Teixeira et al 2003). RasGRP3 protein was highly expressed in the SK-MEL-5 and UACC-257 cell lines (Figure 1B). It was expressed at a lower level in the SK-MEL-2, UACC-62, M14, MALME-3M and SK-MEL-28 cell lines (Figure 1B for protein and Supplementary Figure 1C for RNA levels). In the human adult primary melanocytes, RasGRP3 expression was not detectable under these conditions (Figure 1B). It should be noted that there was not exact correspondence between the levels of RasGRP3 protein detected by immunoblotting (Figure 1B) and the levels of message as detected by RT-PCR (Supplementary Figure 1C). Thus, whereas SK-MEL-5 showed the highest expression by both methods, the M14 cell line showed relatively lower expression by immunoblotting than by RT-PCR. Since we know, for example, that the RasGRP3 antibody response is greater for phosphorylated RasGRP3 (data not shown) than for unphosphorylated RasGRP3, the relative levels should be viewed as approximate.
Figure 1.
RasGRP3 expression in melanoma tumors and tumor cell lines. (A) Detection of RasGRP3 expression in human melanoma tissues by in situ hybridization. Tissue microarrays (Biomax Inc) were hybridized with antisense probes 1 and 2 generated against two non-overlapping regions of the RasGRP3 mRNA. Sense fragments were used as negative controls. A positive autoradiographic signal is detected in samples # A2, A5, A6, B4 and D5, by both probes. There is no detectable specific signal in the normal skin samples (F columns). Higher magnification pictures (lower panel left and right) show labeled cells within the tissue samples #B4 and #D5 of the array. Silver grains (black dots) deposited over the cells indicate the localization of radiolabeled molecules hybridized to endogenous mRNA. The tissue is stained with Giemsa solution. Scale bars: 100 μm. (B) Expression of endogenous RasGRP3 in multiple cell lines. The RasGRP3 levels in the total cell lysates of individual cell lines containing 40 μg total protein were detected by immunoblotting. Ramos cells were used as positive control. The results are representative of 3 individual experiments.
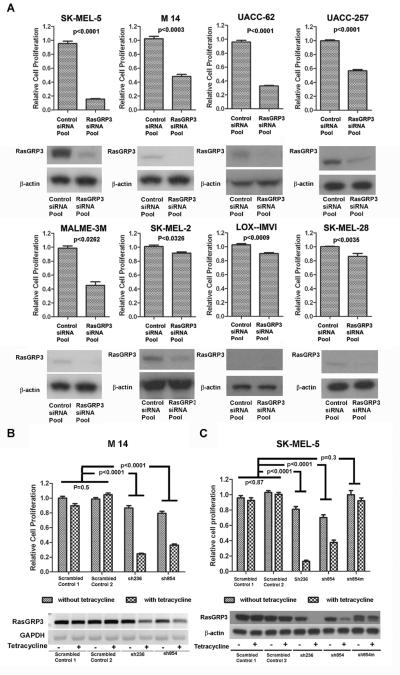
Down regulation of RasGRP3 suppressed proliferation of multiple human melanoma cell lines
In five of eight melanoma cell lines, transient inhibition of endogenous RasGRP3 expression (Figure 2A) was accompanied by substantial inhibition of cell proliferation; less (but still significant) inhibition was observed in the LOX-IMVI, SK-MEL-2 and SK-MEL-28 cell lines (Figure 2A). While the basis for the reduced response is not known, the LOX-IMVI cells expressed the lowest level of RasGRP3 expression of the cell lines examined. The SK-MEL-2 cells express a constitutively active N-Ras, meaning that this major Ras family member no longer requires an upstream activator, and the SK-MEL-28 cell line is mutated in the EGF receptor (see Supplementary Table 3 for a listing of mutations in each of the cell lines examined) (Ikediobi et al 2006). To achieve long term suppression of RasGRP3 expression, we established cell lines derived from M14 and SK-MEL-5 cells expressing tet-on inducible RasGRP3 shRNA. The specific shRNAs were selected based on their ability to knockdown the exogenous RasGRP3-V5 in RasGRP3-LNCaP cells (Supplementary Figure 2A) and the endogenous RasGRP3 in M14 cells (Supplementary Figure 2B).
Figure 2.
Inhibition of endogenous RasGRP3 expression retarded cell proliferation in multiple human melanoma cell lines. (A) Individual cell lines were transiently transfected with RasGRP3 siRNA pool and Control siRNA pool 1 at 80 nM final concentration. After 96 hours, cell proliferation was determined by using the CyQuant NF cell proliferation assay, with values normalized to the levels of untreated cells. Values represent the mean ± SEM for four independent experiments. Confirmation of the extent of suppression of RasGRP3 was determined by immunoblotting. Results are representative of 3 experiments. (B and C)The Tet-on stable cell lines created from the M14 and SK-MEL-5 cell lines were seeded at a density of 104 cells/ml in complete growth medium. Cells were treated with/without 1 μg/ml tetracycline for 120 hours and proliferation was determined by using the CyQuant NF cell proliferation assay, with values normalized to the levels of parental M14 or SK-MEL-5 cells. Values represent the mean ± SEM of four independent experiments. Endogenous RasGRP3 expression was determined by RT-PCR (M14) or immunoblot (SK-MEL-5) 120 hours after induction. Results are representative of three independent experiments.
Because of promoter leakage with the tet-on system, the level of endogenous RasGRP3 expression in the absence of tetracycline was already somewhat reduced in the M14 derivative sh854 cell line and in both of the SK-MEL-5 derivative cell lines, sh236 and sh854 (Figure 2B and C). To confirm the specificity of the effects of the RasGRP3 shRNAs, we created a mutated variant of RasGRP3 retaining the amino acid sequence of RasGRP3 but altered in its coding sequence to diminish the homology with shRNA854. Stable cell lines were prepared by introducing this RasGRP3 mutant into the SK-MEL-5 derivative sh854 cell line. After inducing shRNA expression, RasGRP3 expression was detected by either RT-PCR in the M14 cells or by immunoblotting in the SK-MEL-5 cells (Figure 2B and C). Inhibition of cell proliferation mirrored the suppression of RasGRP3 expression. A reduction in cell growth was detected in the cells that already expressed a reduced amount of RasGRP3 as a result of promoter leakage of the shRNA, as compared with the cell lines expressing a normal level of RasGRP3 (Figure 2B and C). A further reduction in cell proliferation accompanied the further reduction of RasGRP3 caused by induction of the shRNA expression. The control shRNAs had little effect on cell growth (Figure 2B and C). Expression of the shRNA854 resistant RasGRP3 mutant maintained RasGRP3 expression and rendered the cell growth rate insensitive to expression of the shRNA854 (Figure 2C). Expression of the RasGRP3 mutant by itself did not affect cell proliferation compared to the control (p = 0.62).
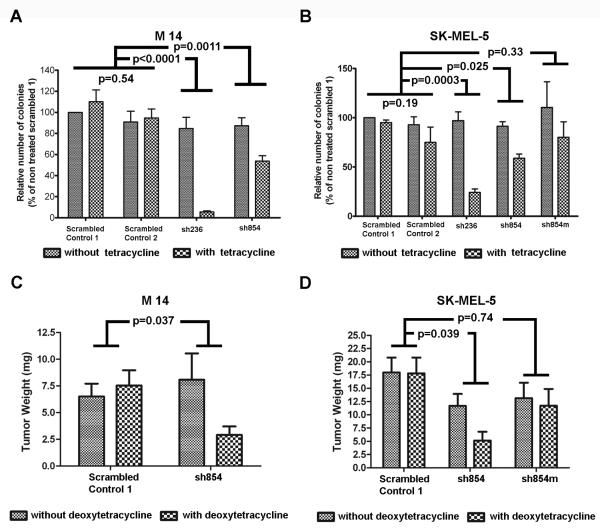
Inhibition of endogenous RasGRP3 expression inhibited anchorage independent growth and suppressed xenograft tumor growth of both the M14 and SK-MEL-5 cell lines
For both M14 and SK-MEL-5 cells, inhibition of endogenous RasGRP3 expression decreased both the total number and size of colonies in soft agar (Figure 3A, B and Supplementary Figure 3A and B) compared to controls. Expressing the mutant RasGRP3 partially blocked the inhibition of the growth in soft agar caused by the shRNA854 (Figure 3B and Supplementary Figure 3B). There was no significant difference in number of colonies between the control cell line and the cell line expressing the RasGRP3 mutant (p = 0.58) in the absence of shRNA854 induction (Figure 3B).
Figure 3.
Reduction of endogenous RasGRP3 expression inhibited anchorage independent growth and rate of tumor growth of both M14 and SK-MEL-5 cell lines. (A and B) Colonies were imaged 10 days after plating the cells in soft agar. The number of colonies larger than the median size of the corresponding control (colonies of the cells expressing the scrambled control shRNA 1 in the absence of tetracycline) were counted and expressed as a percent of the colony number of the control. Values represent mean +/− SEM of the three independent experiments. (C and D) NOD.SCID/NCr mice were injected subcutaneously in the flanks with 1×107 cells/injection of the indicated M14 and SK-MEL-5 derived lines. On the eighth day after injection, half the animals in each treatment group were shifted to food containing deoxytetracycline. The animals were sacrificed 12 weeks (M14) or 8 weeks (SK-MEL-5) after injection, and the tumors were excised and weighed.
The involvement of endogenous RasGRP3 in tumorigenesis was assessed in a mouse xenograft model, using the cell lines expressing either control shRNA or the shRNA854. Expression of the shRNA854 caused a marked reduction in tumor growth as measured by weight of excised tumors both of the M14 and SK-MEL-5 cell lines (Figure 3C, D, Table 1 and Table 2). Deoxytetracycline induction had no effect on tumor growth in control cells. Likewise, in the shRNA854 derived SK-MEL-5 cells expressing the mutated RasGRP3 deoxytetracycline treatment failed to affect tumor growth (Figure 3D and Table 2). Expression of the RasGRP3 mutant did not significantly affect tumor growth, compared with control cells (p = 0.27), in the absence of the shRNA854 induction (Figure 3D and Table 2).
Table 1.
Reduction of endogenous RasGRP3 expression inhibited tumor formation of M14 cell lines.
| Cell Line (M14) | Total tumor/Total injection | Tumor weight (mg) | ||
|---|---|---|---|---|
|
| ||||
| Deoxytetracycline | Deoxytetracycline | |||
| − | + | − | + | |
| Control | 14/20 | 14/20 | 6.5±1.2 | 7.5±1.4 |
| sh854 | 12/20 | 10/20 | 8.1±2.5 | 2.9±0.8 |
Table 2.
Reduction of endogenous RasGRP3 expression inhibited tumor formation of SK-MEL-5 cell lines.
| Cell Line (SK-MEL-5) | Total tumor/Total injection | Tumor weight (mg) | ||
|---|---|---|---|---|
|
| ||||
| Deoxytetracycline | Deoxytetracycline | |||
| − | + | − | + | |
| Control | 14/16 | 14/16 | 18.0±2.8 | 17.5±3.0 |
| sh854 | 11/16 | 9/16 | 11.7±2.3 | 5.2±1.6 |
| sh854m | 12/16 | 10/16 | 13.1±2.9 | 11.7±3.1 |
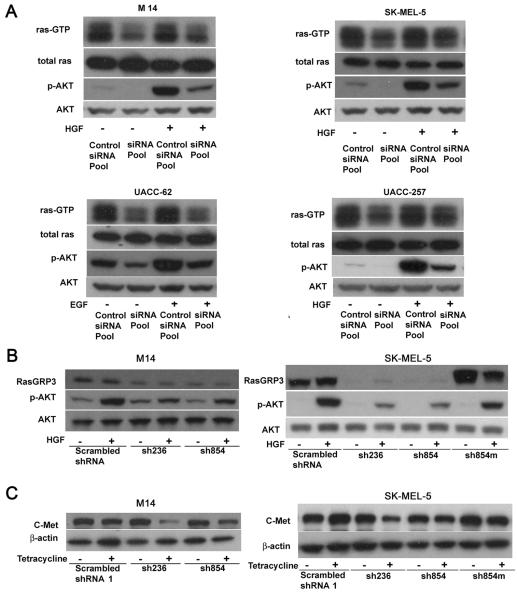
Endogenous RasGRP3 contributes to Ras and Akt1/2/3 activation as well as c-Met expression in multiple melanoma cell lines
RasGRP3 has been reported to activate H-Ras, R-Ras, and Rap1/2, among Ras family members (Yamashita et al 2000). To clarify the role of RasGRP3 in melanoma cell signaling, its effects on activation of Ras and downstream effectors were investigated. Inhibition of RasGRP3 expression was accompanied by a reduced level of Ras-GTP in M14, SK-MEL-5, UACC-62 and UACC-257 cells (Figure 4A), confirming that RasGRP3 was indeed an important contributor to the state of Ras activation in these cells. These results were also confirmed in M14 and SK-MEL-5 cell lines expressing inducible RasGRP3 shRNAs (Supplementary Figure 5C).
Figure 4.
RasGRP3 is involved in Ras and Akt activation. (A) Individual cell lines were transiently transfected with RasGRP3 siRNA pool and Control siRNA pool 1 at 80 nM final concentration. After 72 hours, cells were treated with or without HGF (20 ng/mL) or EGF (100 pg/ml) as indicated for 20 minutes. Ras-GTP levels were detected by pull-down assay from 200 μg total protein. Levels of total Ras were used as control. Akt and phosphorylated Akt were detected by immunoblotting of cell lysates. (B) The Tet-on stable cell lines created from the M14 and SK-MEL-5 cell lines were treated with tetracycline (1 μg/ml) for120 hours. The cells were then treated with/without HGF (20 ng/mL) for 20 minutes. The Akt and phosphorylated Akt were detected by immunoblotting of cell lysates. (C) The Tet-on stable cell lines created from the M14 and SK-MEL-5 cell lines were treated with tetracycline (1 μg/ml) for 120 hours as indicated. Expression of c-Met was detected by immunoblotting of cell lysates. All results in this figure were representative of 3 independent experiments.
It has been reported that activation of the EGF receptor and the B-cell receptor trigger PKC or Src mediated RasGRP3 activation through its phosphorylation followed by activation of the Ras pathway (Aiba et al 2004, Stope et al 2004, Zheng et al 2005). Moreover, activated H-Ras in turn activates PI3K (Pacold et al 2000, Rodriguez-Viciana et al 1994). Thus we have evaluated involvement of RasGRP3 in the response of the melanoma cells to multiple growth factors and cytokines including HGF, EGF, V-EGF, TGF-β and TNF-α (Supplementary Figure 4A). HGF treatment led to Akt phosphorylation at Ser473 in M14, SK-MEL-5, UACC-257 (Figure 4A), SK-MEL-2, SKMEL-28, and MALME-3M cells (Supplementary Figure 4A). In the UACC-62 cells, HGF was without effect but instead EGF enhanced Akt phosphorylation (Supplementary Figure 4A). Transient suppression of endogenous RasGRP3 decreased both basal and HGF induced Akt phosphorylation in the M14, SK-MEL-5, and UACC-257 cells (Figure 4A and Supplementary Figure 5A), as well as the SK-MEL-28 cells (Supplementary Figure 5B). In the UACC-62 cells, suppression of endogenous RasGRP3 expression reduced both the basal and EGF induced Akt phosphorylation (Figure 4A and Supplementary Figure 5A). In the SK-MEL-2 cells down-regulation of RasGRP3 expression decreased HGF induced Akt phosphorylation but did not affect the basal level of Akt phosphorylation (Supplementary Figure 5B). Consistent with a modest effect of RasGRP3 knockdown on proliferation of the SK-MEL-2 cells, which bear a constitutive N-Ras mutation, this result may reflect the influence of other family members in these cells. Additionally, down regulation of RasGRP3 by inducible shRNA also reduced the HGF-induced Akt phosphorylation in both the M14 and SK-MEL-5 cell lines (Figure 4B). Once again, the exogenous RasGRP3 mutant enhanced the level of phosphorylated Akt in the SK-MEL-5 cells (Figure 4B). Consistent with the central role of the Akt pathway in the survival of these cells, decreasing the level of p-Akt using an Akt inhibitor resulted in significant inhibition of cell proliferation in all the cell lines (Supplementary Figure 4B). Expression of c-Met, the receptor for HGF, was also affected by the level of RasGRP3. Inhibition of RasGRP3 expression led to reduction of c-Met expression in both M14 and SK-MEL-5 cell lines (Figure 4C).
Transformation of human adult melanocytes by overexpressing RasGRP3
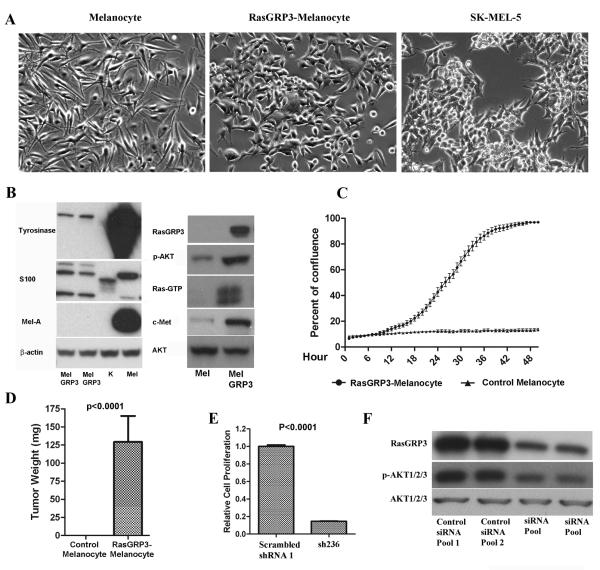
To further explore the biological role of RasGRP3 in human melanoma, we overexpressed RasGRP3-V5 in human adult primary melanocytes (Pu et al 2005). Relative to the control melanocytes, the RasGRP3 overexpressing melanocytes showed a cuboidal shape with shorter, less numerous processes resembling SK-MEL-5 cells (Figure 5A). The RasGRP3-melanocytes lost or showed greatly diminished expression of the melanocyte markers tyrosinase and melanin A (Figure 5B). Their S100 expression was detected (Figure 5B). Opposite to the effects on cell signaling caused by inhibition of endogenous RasGRP3 in melanoma cells, overexpression of RasGRP3 in melanocytes was accompanied by an elevated level of Ras activation, Akt activation and c-Met expression (Figure 5B). These results further support the role of RasGRP3 in the HGF/c-Met and PI3K/Akt signaling pathways in melanoma.
Figure 5.
Effect of RasGRP3 expression on normal melanocytes. (A) Phase constrast imaging of normal human adult melanocytes and of the RasGRP3-melanocytes. SK-MEL-5 cells are shown for comparison. Images are representative of 3 independent experiments. (B) Cell lysates of RasGRP3-melanocytes (MelGRP3), keratinocytes (K), and primary adult melanocytes (Mel) were assayed by immunoblotting as indicated (melanin A, MelA). Results are representative of 3 independent experiments. (C) Control adult melanocytes and RasGRP3-melanocytes were cultured in RPMI 1640 with 10% normal FBS for 5 days. The plates were scanned in the IncuCyte™ at one hour intervals for 60 hours. The data were analyzed by the IncuCyte™ cell proliferation assay software. Results are representative of 3 independent experiments. (D) NOD.SCID/NCr mice (7 mice for each group) were injected subcutaneously in the flanks with RasGRP3-Melanocytes or control melanocytes (plenti/V5-GW/lacZ), 5×106 cells/injection. The injected mice were fed with a regular diet and sacrificed after 5 weeks. The tumors were then excised and weighed. (E) RasGRP3-melanocytes were transduced with virus containing scrambled shRNA control or sh236 respectively. Cell proliferation was determined 120 hours after transduction using the CyQuant NF cell proliferation assay. The values are normalized to that of untreated group. Values are mean ± SEM of 3 independent experiments. (F) Cells were treated as in panel E. RasGRP3 expression, Akt and p-Akt were detected by immunoblotting. Results are representative of three independent experiments.
After 3 weeks antibiotic selection, the stable RasGRP3-melanocytes showed a markedly enhanced growth rate comparing to control melanocytes, without melanocyte growth medium. The doubling time of the cells is around 12 hours (Figure 5C). Furthermore, following subcutaneous injection of these cells (after three weeks of antibiotic selection), they formed tumors in immunodeficient mice, whereas no detectable growth was observed for control melanocytes (Figure 5D). As a further control in this system, reduction in the level of exogenous RasGRP3 expression markedly retarded the RasGRP3-melanocyte's proliferation (Figure 5E), accompanied by a reduced level of basal Akt phosphorylation (Figure 5F).
Discussion
It is has been clearly identified that the Ras pathway plays a crucial role in human melanoma. However, only 15% of human melanomas contain a Ras gene mutation. The cases containing a constitutively activated Ras mutation are even less (Ball et al 1994, van Elsas et al 1995). More frequent in melanoma are mutations in signaling elements downstream of Ras, such as B-Raf and PTEN (Ikediobi et al 2006, Pollock et al 2003, Wu et al 2003). But none of them is sufficient to initiate melanoma by itself (Cully et al 2006, Dankort et al 2009, Sumimoto et al 2004). Changes in upstream signaling elements provide a complementary approach to influence the Ras pathway. As Ras activators, RasGRPs could be candidate molecules to play such a role. Our data show substantial RasGRP3 expression in multiple human melanoma cell lines as well as in subset of human melanoma tissue samples. Our findings provide evidence that RasGRP3 expression has broader tissue distribution than has been generally recognized (Coughlin et al 2005, Roberts et al 2004, Teixeira et al 2003). The elevated RasGRP3 level does not appear to result from increased copy number of RasGRP3 in either melanoma tumors or melanoma cell lines (Bastian et al 2003, Bauer and Bastian 2006, Stark and Hayward 2007). On the other hand, RasGRP3 is subject both to developmental silencing as well as induction by growth factors (Roberts et al 2004).
Suppression of endogenous RasGRP3 inhibited cell proliferation and anchorage independent growth and xenograft tumor growth of melanoma cell lines. Thus, the endogenous RasGRP3 contributes to the phenotype of multiple melanoma cell lines. This finding was strongly supported by the effects of overexpressing RasGRP3 in normal human adult melanocytes, which caused greatly enhanced cell growth and conferred tumorigenicity. Suppression of endogenous RasGRP3 expression in melanoma cell lines reduced Ras-GTP formation as well as c-Met expression and Akt phosphorylation downstream from HGF or EGF stimulation. We conclude that the regulation of wild-type Ras at its physiological expression level by upstream growth factors is mediated in part through endogenous RasGRP3 in human melanoma cells. Thus, RasGRP3/Ras represents an important node linking the HGF/c-Met and PI3K/Akt signaling pathways in melanoma. These findings help fill out our current understanding of the HGF/C-Met/Ras/PI3K/AKT signaling networks in melanoma (Furge et al 2001, Khwaja et al 1997, Orian-Rousseau et al 2007, Takayama et al 1997). As described by other researchers, a BRAF mutation (V600E) is the most common mutation in human melanoma (Haluska and Ibrahim 2006). Compared to wild type BRAF, BRAFV600E shows around 500-fold higher activity, causing sustained activation of ERK signaling (Hingorani et al 2003, Houben et al 2004, Ikenoue et al 2003, Ikenoue et al 2004, Karasarides et al 2004). Seven out of the eight tested melanoma cell lines contain a BRAF mutation (V600E) (Supplementary Table 3). Highly activated ERK1/2 was detected in all tested melanoma cell lines. Treatment of HGF and EGF did not lead to further activation of ERK1/2, except in the SK-MEL-5 cells. Inhibition of RasGRP3 affected neither the basal level of ERK1/2 activation nor HGF induced ERK1/2 activation (data not show).
Ras-associated protein-1 (Rap1) is another member of the Ras family of small GTPases and a target of RasGRP3 (Yamashita et al 2000). Along with Ras, Rap1 has been shown to influence melanoma tumorigenesis and metastasis through regulating the activation of MAPK pathway and integrin activation (Gao et al 2006). Its activity in human melanoma is regulated not only by activators but also an inactivator, Rap1 GTPase-activating protein (GAP). Rap1GAP expression was decreased in human melanoma tumors and cell lines, but not in benign nevus cells, reflecting promoter hypermethylation (Zheng et al 2009). This down-regulation of Rap1Gap caused marked elevation of Rap1 activation and promoted melanoma cell proliferation, survival, and migration (Zheng et al 2009). In preliminary experiments, we did not observe a change in Rap1-GTP levels upon suppression of RasGRP3 expression in the SK-MEL-5 cells (data not shown). If correct, this may suggest that the rate limiting regulator of Rap1, at least in this cell line, is either RasGAP or one or more of the other known RapGEFs in addition to RasGRP3 (Guo et al 2001, Ohba et al 2001).
Serrano and Chodosh reported that highly expressed constitutive H-Ras induced senescence in human or rodent primary cells (Sarkisian et al 2007, Serrano et al 1997). But Chodosh and colleagues also showed that a lower level of oncogenic RAS expression did not caused cellular senescence. In contrast, it promoted continuous cell proliferation in vitro and tumor formation in vivo (Sarkisian et al 2007). In addition, endogenous expression of constitutive active K-RAS is sufficient to initiate epithelial cells transformation and promote tumourigenesis without detectable senescence (Tuveson et al 2004). These studies support the idea that oncogenic effects of Ras family members are critically dependent on the intensity of the Ras signal and the type of Ras isoform. In our study, introduction of RasGRP3 caused outgrowth of transformed human primary melanocytes. As an activator of multiple Ras isoforms, the downstream biological effects of overexpressed RasGRP3 should be more complex than biological response triggered by overexpression of a single a Ras isoform. Compared to the uncontrolled constitutively active Ras, RasGRP3 introduced into melanocytes still performed as a normal cellular signal transducer which was able to respond to extracellular stimulation. The intensity of the downstream Ras signal would thus depend on the intensity of biological extracellular stimulation rather than on the expression level of single mutated activated Ras. Additionally, the outgrowing RasGRP3 overexpressing cells will necessarily reflect selection against senescence, to the degree that it might have occurred in some cells.
In summary, our findings further highlight the importance of the RasGRP3/Ras pathway in melanoma, as already clear from analysis of mutations in B-Raf and PTEN associated with melanoma, but also emphasize the complexity of this pathway. Since it was expressed at a high level in a subset of human melanomas, contributing to melanoma tumorigenicity and involved in the phosphoinositide signaling pathway, RasGRP3 represents a novel potential therapeutic target for certain human melanomas.
Materials and Methods
Cell lines, reagents and antibodies
The MALME-3M, M14, SK-MEL-5, SK-MEL-2, SK-MEL-28, LOX-IMVI, UACC-257 and UACC-62 cell lines were obtained from Jackson Laboratory (Bar Harbor, ME). Complete medium for all these cell lines consisted of RPMI-1640 medium supplemented with 10% fetal bovine serum (ATCC, Manassas, VA). The human epidermal melanocytes (adult) were purchased from Invitrogen and cultured in Medium 254 supplemented with PMA-Free Human Melanocyte Growth Supplement-2 (Invitrogen). RasGRP3 rabbit monoclonal antibody was from Cell Signaling (Beverly, MA), mouse anti-Ras monoclonal antibody was purchased from Upstate (Lake Placid, NY). Primary antibodies for p-AKT1/2/3, AKT1/2/3, S100 and the anti-H-Ras polyclonal antibody were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-V5 monoclonal antibody, the mouse anti-tyrosinase monoclonal antibody, the Virapower Lentiviral expression system, CyQuant NF cell proliferation assay kit, Lipofectamine 2000, blasticidin and precast Tris-glycine gels were from Invitrogen (Carlsbad, CA). Recombinant human HGF, VEGF, EGF, TGF-beta and TNF-alpha were from R&D Systems (Minneapolis, MN).
In situ hybridization histochemistry
Two published probes were generated and inserted into a Bluescript II SK vector (Stratagene, La Jolla, CA) (Roberts et al 2004, Yamashita et al 2000). Slides containing paraffin embedded human multiple melanoma tissue arrays (US Biomax Inc, Rockville, MD) were labeled with the two probes. For detailed experimental procedures, see Supplementary Materials and Methods.
Generation of tetracycline inducible H1 lentiviral shRNA constructs
The sequences encoding the shRNAs for this study are listed in Supplementary Table 2. The pLenti4/BLOCK-iT™-DEST vectors containing specific shRNA were constructed according to the manufacturer's instructions. All the constructs were verified by DNA sequencing. The lentiviral constructs were then produced and titered.
Establishment of tetracycline-regulated shRNA expressing stable cell lines and the cell lines stably overexpressing wild type RasGRP3 or its shRNA854 resistant mutant
TetR-expressing host cell lines derived from M14 and SK-MEL-5 cells were generated following the manufacturer's instructions. These two cell lines are heterogeneous populations of blasticidin-resistant cells stably expressing the Tet repressor. Subsequently the TetR-expressing host cell lines were infected with the lentiviral vectors as indicated and subjected to Zeocin™ (500 μg/ml) and blasticidin (10 μg/ml) selection for 3 weeks. Studies were carried out on the pooled, antibiotic-resistant cells.
Cell proliferation assay
Cell proliferation was measured using the CyQuant NF cell proliferation assay or using the IncuCyte™ instrument (Essen Instruments Inc., Ann Harbor, MI) as described in detail in the Supplementary information.
Analysis of Ras activation using the Raf1-RBD-GST pull-down assay
Activation of Ras was evaluated as described by Teixeira and coworkers (Teixeira et al 2003).
Western blot assay
The samples containing 40 μg total protein were separated by electrophoresis and transferred onto Immobilon-P membranes (Millipore Corporation, Bedford, MA). After the membranes were blocked and labeled with the appropriate primary and secondary antibodies, the signal was developed by ECL (Amersham, Piscataway, NJ) and imaged on BioMax XAR or MR films (Kodak, Rochester, NJ).
Growth of cell lines in mouse xenograft system
Mouse studies were performed under a protocol approved by the National Cancer Institute (NCI) and NIH Animal Care and Use Committee. NOD.SCID/NCr male mice (NIH, Frederick, MD) were injected subcutaneously in the flanks with 5× 106 or 1×107 cells/injection. In the case of those cell lines containing tetracycline inducible expression systems, on the eighth day after injection half the animals in each treatment group were shifted to food containing deoxytetracycline. The animals were sacrificed after 8 weeks for mice injected with the SK-MEL-5 derived cell lines, RasGRP3-melanocytes, or the control melanocytes, and after 12 weeks for mice injected with the M14 derived cell lines.
Anchorage-independent growth assay in soft agar
Cells were plated and cultured for 10 days as described in details in Supplementary information. Phase contrast images were taken and the number of colonies greater than the median size of the non-treated controls was determined using Image J software.
Supplementary Material
Acknowledgments
This research was supported in part by the intramural program of the NIH, Center for Cancer Research, National Cancer Institute (project number Z1A BC 005270) and in part by grant ETT 495/09. ZE Toth is supported by the Bolyai fellowship. We thank Glenn Merlino for helpful comments.
Footnotes
Conflict of interest The authors declare no conflict of interest.
References
- Ackermann J, Frutschi M, Kaloulis K, McKee T, Trumpp A, Beermann F. Metastasizing melanoma formation caused by expression of activated N-RasQ61K on an INK4a-deficient background. Cancer Res. 2005;65:4005–4011. doi: 10.1158/0008-5472.CAN-04-2970. [DOI] [PubMed] [Google Scholar]
- Aiba Y, Oh-hora M, Kiyonaka S, Kimura Y, Hijikata A, Mori Y, et al. Activation of RasGRP3 by phosphorylation of Thr-133 is required for B cell receptor-mediated Ras activation. Proc Natl Acad Sci U S A. 2004;101:16612–16617. doi: 10.1073/pnas.0407468101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball NJ, Yohn JJ, Morelli JG, Norris DA, Golitz LE, Hoeffler JP. Ras mutations in human melanoma: a marker of malignant progression. J Invest Dermatol. 1994;102:285–290. doi: 10.1111/1523-1747.ep12371783. [DOI] [PubMed] [Google Scholar]
- Bastian BC, Olshen AB, LeBoit PE, Pinkel D. Classifying melanocytic tumors based on DNA copy number changes. Am J Pathol. 2003;163:1765–1770. doi: 10.1016/S0002-9440(10)63536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J, Bastian BC. Distinguishing melanocytic nevi from melanoma by DNA copy number changes: comparative genomic hybridization as a research and diagnostic tool. Dermatol Ther. 2006;19:40–49. doi: 10.1111/j.1529-8019.2005.00055.x. [DOI] [PubMed] [Google Scholar]
- Brodie C, Steinhart R, Kazimirsky G, Rubinfeld H, Hyman T, Ayres JN, et al. PKCdelta associates with and is involved in the phosphorylation of RasGRP3 in response to phorbol esters. Mol Pharmacol. 2004;66:76–84. doi: 10.1124/mol.66.1.76. [DOI] [PubMed] [Google Scholar]
- Broome Powell M, Gause PR, Hyman P, Gregus J, Lluria-Prevatt M, Nagle R, et al. Induction of melanoma in TPras transgenic mice. Carcinogenesis. 1999;20:1747–1753. doi: 10.1093/carcin/20.9.1747. [DOI] [PubMed] [Google Scholar]
- Coughlin JJ, Stang SL, Dower NA, Stone JC. RasGRP1 and RasGRP3 regulate B cell proliferation by facilitating B cell receptor-Ras signaling. J Immunol. 2005;175:7179–7184. doi: 10.4049/jimmunol.175.11.7179. [DOI] [PubMed] [Google Scholar]
- Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr., et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart L, Bach J, Ban J, Tschachler E. Melanin binds reversibly to thermostable DNA polymerase and inhibits its activity. Biochem Biophys Res Commun. 2000;271:726–730. doi: 10.1006/bbrc.2000.2716. [DOI] [PubMed] [Google Scholar]
- Furge KA, Kiewlich D, Le P, Vo MN, Faure M, Howlett AR, et al. Suppression of Ras-mediated tumorigenicity and metastasis through inhibition of the Met receptor tyrosine kinase. Proc Natl Acad Sci U S A. 2001;98:10722–10727. doi: 10.1073/pnas.191067898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Feng Y, Bowers R, Becker-Hapak M, Gardner J, Council L, et al. Ras-associated protein-1 regulates extracellular signal-regulated kinase activation and migration in melanoma cells: two processes important to melanoma tumorigenesis and metastasis. Cancer Res. 2006;66:7880–7888. doi: 10.1158/0008-5472.CAN-06-0254. [DOI] [PubMed] [Google Scholar]
- Gawecka JE, Griffiths GS, Ek-Rylander B, Ramos JW, Matter ML. R-Ras regulates migration through an interaction with filamin A in melanoma cells. PLoS One. 5:e11269. doi: 10.1371/journal.pone.0011269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo FF, Kumahara E, Saffen D. A CalDAG-GEFI/Rap1/B-Raf cassette couples M(1) muscarinic acetylcholine receptors to the activation of ERK1/2. J Biol Chem. 2001;276:25568–25581. doi: 10.1074/jbc.M101277200. [DOI] [PubMed] [Google Scholar]
- Haluska FG, Ibrahim N. Therapeutic targets in melanoma: map kinase pathway. Curr Oncol Rep. 2006;8:400–405. doi: 10.1007/s11912-006-0065-x. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Jacobetz MA, Robertson GP, Herlyn M, Tuveson DA. Suppression of BRAF(V599E) in human melanoma abrogates transformation. Cancer Res. 2003;63:5198–5202. [PubMed] [Google Scholar]
- Houben R, Becker JC, Kappel A, Terheyden P, Brocker EB, Goetz R, et al. Constitutive activation of the Ras-Raf signaling pathway in metastatic melanoma is associated with poor prognosis. J Carcinog. 2004;3:6. doi: 10.1186/1477-3163-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HP, Shih YW, Chang YC, Hung CN, Wang CJ. Chemoinhibitory effect of mulberry anthocyanins on melanoma metastasis involved in the Ras/PI3K pathway. J Agric Food Chem. 2008;56:9286–9293. doi: 10.1021/jf8013102. [DOI] [PubMed] [Google Scholar]
- Ikediobi ON, Davies H, Bignell G, Edkins S, Stevens C, O'Meara S, et al. Mutation analysis of 24 known cancer genes in the NCI-60 cell line set. Mol Cancer Ther. 2006;5:2606–2612. doi: 10.1158/1535-7163.MCT-06-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenoue T, Hikiba Y, Kanai F, Tanaka Y, Imamura J, Imamura T, et al. Functional analysis of mutations within the kinase activation segment of B-Raf in human colorectal tumors. Cancer Res. 2003;63:8132–8137. [PubMed] [Google Scholar]
- Ikenoue T, Hikiba Y, Kanai F, Aragaki J, Tanaka Y, Imamura J, et al. Different effects of point mutations within the B-Raf glycine-rich loop in colorectal tumors on mitogen-activated protein/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase and nuclear factor kappaB pathway and cellular transformation. Cancer Res. 2004;64:3428–3435. doi: 10.1158/0008-5472.CAN-03-3591. [DOI] [PubMed] [Google Scholar]
- Karasarides M, Chiloeches A, Hayward R, Niculescu-Duvaz D, Scanlon I, Friedlos F, et al. B-RAF is a therapeutic target in melanoma. Oncogene. 2004;23:6292–6298. doi: 10.1038/sj.onc.1207785. [DOI] [PubMed] [Google Scholar]
- Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo PS, Beheshti M, Pettit GR, Stone JC, Blumberg PM. The guanine nucleotide exchange factor RasGRP is a high -affinity target for diacylglycerol and phorbol esters. Mol Pharmacol. 2000;57:840–846. [PubMed] [Google Scholar]
- Lorenzo PS, Kung JW, Bottorff DA, Garfield SH, Stone JC, Blumberg PM. Phorbol esters modulate the Ras exchange factor RasGRP3. Cancer Res. 2001;61:943–949. [PubMed] [Google Scholar]
- Oh-hora M, Johmura S, Hashimoto A, Hikida M, Kurosaki T. Requirement for Ras guanine nucleotide releasing protein 3 in coupling phospholipase C-gamma2 to Ras in B cell receptor signaling. J Exp Med. 2003;198:1841–1851. doi: 10.1084/jem.20031547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba Y, Ikuta K, Ogura A, Matsuda J, Mochizuki N, Nagashima K, et al. Requirement for C3G-dependent Rap1 activation for cell adhesion and embryogenesis. Embo J. 2001;20:3333–3341. doi: 10.1093/emboj/20.13.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki-Idouchi CE, Lorenzo PS. Transgenic overexpression of RasGRP1 in mouse epidermis results in spontaneous tumors of the skin. Cancer Res. 2007;67:276–280. doi: 10.1158/0008-5472.CAN-06-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orian-Rousseau V, Morrison H, Matzke A, Kastilan T, Pace G, Herrlich P, et al. Hepatocyte growth factor-induced Ras activation requires ERM proteins linked to both CD44v6 and F-actin. Mol Biol Cell. 2007;18:76–83. doi: 10.1091/mbc.E06-08-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacold ME, Suire S, Perisic O, Lara-Gonzalez S, Davis CT, Walker EH, et al. Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase gamma. Cell. 2000;103:931–943. doi: 10.1016/s0092-8674(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- Pu Y, Perry NA, Yang D, Lewin NE, Kedei N, Braun DC, et al. A novel diacylglycerol-lactone shows marked selectivity in vitro among C1 domains of protein kinase C (PKC) isoforms alpha and delta as well as selectivity for RasGRP compared with PKCalpha. J Biol Chem. 2005;280:27329–27338. doi: 10.1074/jbc.M414132200. [DOI] [PubMed] [Google Scholar]
- Roberts DM, Anderson AL, Hidaka M, Swetenburg RL, Patterson C, Stanford WL, et al. A vascular gene trap screen defines RasGRP3 as an angiogenesis-regulated gene required for the endothelial response to phorbol esters. Mol Cell Biol. 2004;24:10515–10528. doi: 10.1128/MCB.24.24.10515-10528.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- Sarkisian CJ, Keister BA, Stairs DB, Boxer RB, Moody SE, Chodosh LA. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat Cell Biol. 2007;9:493–505. doi: 10.1038/ncb1567. [DOI] [PubMed] [Google Scholar]
- Satyamoorthy K, Li G, Gerrero MR, Brose MS, Volpe P, Weber BL, et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 2003;63:756–759. [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Stark M, Hayward N. Genome-wide loss of heterozygosity and copy number analysis in melanoma using high-density single-nucleotide polymorphism arrays. Cancer Res. 2007;67:2632–2642. doi: 10.1158/0008-5472.CAN-06-4152. [DOI] [PubMed] [Google Scholar]
- Stone JC. Regulation of Ras in lymphocytes: get a GRP. Biochem Soc Trans. 2006;34:858–861. doi: 10.1042/BST0340858. [DOI] [PubMed] [Google Scholar]
- Stope MB, Vom Dorp F, Szatkowski D, Bohm A, Keiper M, Nolte J, et al. Rap2B-dependent stimulation of phospholipase C-epsilon by epidermal growth factor receptor mediated by c-Src phosphorylation of RasGRP3. Mol Cell Biol. 2004;24:4664–4676. doi: 10.1128/MCB.24.11.4664-4676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumimoto H, Miyagishi M, Miyoshi H, Yamagata S, Shimizu A, Taira K, et al. Inhibition of growth and invasive ability of melanoma by inactivation of mutated BRAF with lentivirus-mediated RNA interference. Oncogene. 2004;23:6031–6039. doi: 10.1038/sj.onc.1207812. [DOI] [PubMed] [Google Scholar]
- Sutlovic D, Definis Gojanovic M, Andelinovic S, Gugic D, Primorac D. Taq polymerase reverses inhibition of quantitative real time polymerase chain reaction by humic acid. Croat Med J. 2005;46:556–562. [PubMed] [Google Scholar]
- Suzuki T, Shen H, Akagi K, Morse HC, Malley JD, Naiman DQ, et al. New genes involved in cancer identified by retroviral tagging. Nat Genet. 2002;32:166–174. doi: 10.1038/ng949. [DOI] [PubMed] [Google Scholar]
- Takayama H, LaRochelle WJ, Sharp R, Otsuka T, Kriebel P, Anver M, et al. Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc Natl Acad Sci U S A. 1997;94:701–706. doi: 10.1073/pnas.94.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira C, Stang SL, Zheng Y, Beswick NS, Stone JC. Integration of DAG signaling systems mediated by PKC-dependent phosphorylation of RasGRP3. Blood. 2003;102:1414–1420. doi: 10.1182/blood-2002-11-3621. [DOI] [PubMed] [Google Scholar]
- Tuveson DA, Shaw AT, Willis NA, Silver DP, Jackson EL, Chang S, et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell. 2004;5:375–387. doi: 10.1016/s1535-6108(04)00085-6. [DOI] [PubMed] [Google Scholar]
- van Elsas A, Zerp S, van der Flier S, Kruse-Wolters M, Vacca A, Ruiter DJ, et al. Analysis of N-ras mutations in human cutaneous melanoma: tumor heterogeneity detected by polymerase chain reaction/single-stranded conformation polymorphism analysis. Recent Results Cancer Res. 1995;139:57–67. doi: 10.1007/978-3-642-78771-3_5. [DOI] [PubMed] [Google Scholar]
- Wang D, Yang W, Du J, Devalaraja MN, Liang P, Matsumoto K, et al. MGSA/GRO-mediated melanocyte transformation involves induction of Ras expression. Oncogene. 2000;19:4647–4659. doi: 10.1038/sj.onc.1203820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Goel V, Haluska FG. PTEN signaling pathways in melanoma. Oncogene. 2003;22:3113–3122. doi: 10.1038/sj.onc.1206451. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Mochizuki N, Ohba Y, Tobiume M, Okada Y, Sawa H, et al. CalDAG-GEFIII activation of Ras, R-ras, and Rap1. J Biol Chem. 2000;275:25488–25493. doi: 10.1074/jbc.M003414200. [DOI] [PubMed] [Google Scholar]
- Yang D, Kedei N, Li L, Tao J, Velasquez JF, Michalowski AM, et al. RasGRP3 contributes to formation and maintenance of the prostate cancer phenotype. Cancer Res. 2010;70:7905–7917. doi: 10.1158/0008-5472.CAN-09-4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Gao L, Feng Y, Yuan L, Zhao H, Cornelius LA. Down-regulation of Rap1GAP via promoter hypermethylation promotes melanoma cell proliferation, survival, and migration. Cancer Res. 2009;69:449–457. doi: 10.1158/0008-5472.CAN-08-2399. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Liu H, Coughlin J, Zheng J, Li L, Stone JC. Phosphorylation of RasGRP3 on threonine 133 provides a mechanistic link between PKC and RAS signaling systems in B cells. Blood. 2005 doi: 10.1182/blood-2004-10-3916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.