Abstract
Androgens play a pivotal role in the regulation of body fat distribution. Adipogenesis is a process whereby multipotent adipose stem cells (ASCs) initially become preadipocytes (ASC commitment to preadipocytes) before differentiating into adipocytes. Androgens inhibit human (h) subcutaneous (SC) abdominal preadipocyte differentiation in both sexes, but their effects on hASC commitment to preadipocyte formation is unknown. We therefore examined whether androgen exposure to human (h) ASCs, isolated from SC abdominal adipose of nonobese women, impairs their commitment to preadipocyte formation and/or subsequent differentiation into adipocytes. For this, isolated hASCs from SC abdominal lipoaspirate were cultured in adipogenesis-inducing medium for 0.5–14 days in the presence of testosterone (T, 0–100 nM) or dihydrotestosterone (DHT, 0–50 nM). Adipogenesis was determined by immunofluorescence microscopy and by quantification of adipogenically relevant transcriptional factors, PPARγ, C/EBPα and C/EBPβ. We found that a 3-day exposure of hASCs to T (50 nM) or DHT (5 nM) in adipogenesis-inducing medium impaired lipid acquisition and decreased PPARγ, C/EBPα and C/EBPβ gene expression. The inhibitory effects of T and DHT at this early-stage of adipocyte differentiation, were partially and completely reversed by flutamide (F, 100 nM), respectively. The effect of androgens on hASC commitment to a preadipocyte phenotype was examined via activation of BMP4 signaling. T (50 nM) and DHT (5nM) significantly inhibited the stimulatory effect of BMP4-induced ASC commitment to the preadipocyte phenotype, as regards PPARγ and C/EBPα gene expression. Our findings indicate that androgens, in part through androgen receptor action, impair BMP4-induced commitment of SC hASCs to preadipocytes and also reduce early-stage adipocyte differentiation, perhaps limiting adipocyte numbers and fat storage in SC abdominal adipose.
Keywords: testosterone, dihydrotestosterone, adipogenesis, cell commitment
INTRODUCTION
Adipocytes are derived from multipotent adipose stem cells (ASCs), which have the capacity to differentiate into not only adipocytes but also other messenchymal cell lineages including myocytes, chondrocytes, and osteocytes [1]. Under appropriate stimuli, ASCs are restricted to produce adipocyte progenitors or preadipocytes (i.e., ASC commitment to preadipocytes) [2–4]. These preadipocytes then differentiate into adipocytes (i.e., cell differentiation) [5–9]. Sex steroids influence this adipogenic progression, as evidenced by a sexual dimorphism of body fat distribution [10–12] beginning at puberty and promoting preferential accumulation of subcutaneous (SC) abdominal and visceral adipose in women and men, respectively [10,13–15]. Many of these sexually dimorphic changes in body fat appear mediated through androgens, since physiological androgen administration to women down-regulates SC abdominal adipose hormone-sensitive lipase, the final step in lipolysis; and diminishes in vivo lipolysis [16,17]. In addition, androgen regulates insulin-mediated glucose uptake in SC adipose tissue [18].
Earlier in adipogenesis, moreover, androgens also inhibit SC abdominal preadipocyte differentiation into adipocytes in both men and women, implying that this particular androgen action differs by sex in magnitude of androgen exposure [19]. The effect of androgens on antecedent hASC commitment to preadipocytes, however, is unknown.
The present study, therefore, examines whether androgen exposure of hASCs from SC abdominal adipose of nonobese women impairs their commitment to the preadipocyte phenotype in vitro, with subsequent effects on preadipocyte differentiation into adipocytes. Our utilization of hASCs from SC abdominal adipose retrieved from nonobese women through elective liposuction [1], unlike other studies [19,20–21], eliminates the confounding effects of obesity on these cells during in vivo adipogenesis.
Our data show that androgens, partially acting through the androgen receptor, impair BMP4-induced commitment of SC abdominal hASCs to the preadipocyte phenotype and also reduce early-stage preadipocyte differentiation to adipocytes. These results support the hypothesis that excess of androgens in women may diminish lipid storage capacity within SC abdominal adipose, promoting ectopic lipid deposition and lipotoxicity.
EXPERIMENTAL
Subcutaneous abdominal hASC isolation
Lipoaspirates (100–200 g per aspirate) were obtained from SC abdominal adipose of 10 nonobese Caucasian women (mean BMI, 23.4 ± 4.2 [SD] kg/m2; mean age, 52 ± 11 [SD] years) undergoing elective liposuction. None of the medications used by these individuals were known to affect adipogenesis. All studies were approved by the University of California Los Angeles, Institutional Review Board. Lipoaspirates were obtained from the lower SC abdomen using standard procedures and aspirated fat was washed with phosphate buffered saline (PBS) and digested at 37°C in PBS containing 0.075% collagenase for 30 min on a shaker. Adipocytes (floating cells) were separated from pellets by centrifugation (800×g, 10 min). Pellets containing stromal-vascular cells were resuspended in DMEM/5% FCS, filtered through 150-mm mesh and washed with DMEM/5% FCS. Cells were plated in 6 well clusters containing DMEM/10% FCS, 0.05 U/ml penicillin, 0.05 mg/ml streptomycin, 1.25 mg/ml fungizone (regular DMEM medium) and cultured at 37°C until cells reached confluency. hASCs from each patient were cultured and processed in a uniform manner for the purpose of three independent experiments, as described below.
Cell Culture
The first set of in vitro experiments examined the entire process of adipogenesis, including 1) hASC commitment to preadipocytes and 2) their subsequent progression through early and late stages of preadipocyte differentiation. Confluent hASCs were incubated in an established adipogenesis-inducing medium (containing 0.5 mM isobutylmethylxanthine, 1 μM dexamethasone, 10 μM insulin, 200 μM indomethacin and peroxisome proliferator-activated receptor γ [PPARγ] agonist, according with standard protocol [ZenBio, Inc, Research Triangle Park, NC]) [19,20,22] at 37°C in 5% CO2 for 0.5, 3, 7 or 14 days in the presence of vehicle alone (control) (see below), testosterone (T, 50 nM) or dihydrotestosterone (DHT, 5nM). In some experiments, hASCs were incubated under similar conditions with T (50 nM) or DHT (5nM) and/or the antiandrogen flutamide (F, 100 nM). These concentrations were chosen to elicit androgen effects at the lowest T and DHT concentrations and reversal of such effects with a maximal flutamide concentration, based upon previous dose-response studies (0–100 nM T), (0–50 nM DHT) and (0–100 nM F) (data not shown). T, DHT and F were initially diluted in ethanol to prepare a 10 mM stock solution and stored at 4°C. Further dilutions of these reagents were performed in PBS and culture medium leading to a final ethanol concentration of 0.0001 % in 20 μl PBS and culture medium. Twenty μl of the same concentration of ethanol-containing PBS and culture medium was added to hASCs as vehicle control. Culture medium was changed every two days throughout the entire incubation period.
The second set of experiments examined in vitro commitment of hASCs to the preadipocyte phenotype. Confluent hASCs were preincubated for 2 hours in regular DMEM medium (see above) with vehicle alone (control), BMP4 (5 nM) (R & D Systems, Minneapolis, MN), T (50 nM), DHT (5 nM) and/or F (100 nM). This BMP4 concentration was chosen based upon initial dose-response studies (0–25 nM) (data not shown) confirming its ability to stimulate hASC commitment to the preadipocyte phenotype, as previously described [2,3]. Cells were then incubated for an additional 3 days in adipogenesis-inducing medium alone (without BMP4, androgen or flutamide).
Immunofluorescence studies
Following all experiments, cells were fixed in 4% paraformaldehyde, rinsed twice in PBS, and then incubated with the free fatty acid immunofluorescent marker, BODIPY-C1,12 (Invitrogen, Carslbad, CA); and the nuclear staining marker, 4′, 6-diamidino-2-phenoylidole (DAPI) (Invitrogen, Carslbad, CA) following standard conditions. ASCs are typical spindle-shape cells, while preadipocytes are more rounded cells with lipid inclusions in the cytoplasm. ASCs were initially characterized by immunofluorescent markers, CD105 and CD146, specific to multipotent mesenchymal stem cells (MSC) (BD Biosciences, San Diego, CA) (Fig. 1A–B). All immunofluorescent experiments were conducted in a blinded manner and were initially performed as time-course studies over 14 days or 3 days (see below), with androgen-induced differences in cell morphology and lipid content compared using semi-quantitative Likert Scale analysis.
Fig. 1.
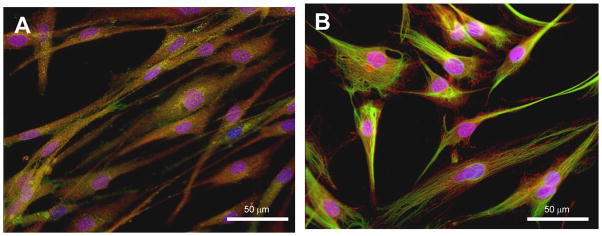
Phenotype of hASCS isolated from non-obese healthy women. hASCs were cultured in regular DMEM medium up to 70–80 % confluency. Immunofluorescence was performed using specific markers for mesenchymal stem cells to characterize the mesenchymal origin of the hASCs). A, CD105 and DAPI; B, CD-146 and DAPI.
Quantification of number of cells
Fluorescent images were acquired using an Evos imunofluorescence inverted microscope (Advanced Microscopy, Mill Creek, WA). BODIPY-C1, 12/DAPI positive cells were quantified using ImageJ software (http://rsb.info.nih.gov/ij/). The results were expressed as fold changes (treated vs. vehicle control per total single image).
RNA isolation and quantitative real-time PCR (qRT-PCR)
Total cellular RNA was isolated using an RNeasy kit (Qiagen, Hilden, Germany) according to manufacturer’s protocol. First strand cDNA was synthesized using first strand RT2 kit (Qiagen, Hilden, Germany). mRNA was quantified by qRT-PCR using RT2 qPCR Master Mix according to manufacturer’s protocol (Qiagen, Hilden, Germany). qRT-PCR was performed on an ABI 7300 (A&B Applied Biosystems, Foster City, CA) using standard temperature cycling conditions. Human PPARγ, C/EBPα, and C/EBPβ primers were provided by A&B Applied Biosystems, Foster City, CA. Glyceraldehyde 3-phosphate dehydrogenase (GADPH) was used as internal control. The relative expression of target genes was measured using the comparative critical threshold (Ct) method. The results were expressed as fold changes (treated vs. vehicle control) obtained from triplicate values. GADPH expression did not change across treatments or cell progression through adipogenesis.
Statistical analyses
All results were shown as mean ± S.E.M. hASC isolated from each patient was subjected to at least three independent experiments performed in triplicates. Data were analyzed by using an ANOVA model (for multiple groups) following Tukey HSD or Students’ t-test (for two independent samples). Statistical significance was determined as p< 0.05.
RESULTS
Effect of androgens at different stages of adipogenesis
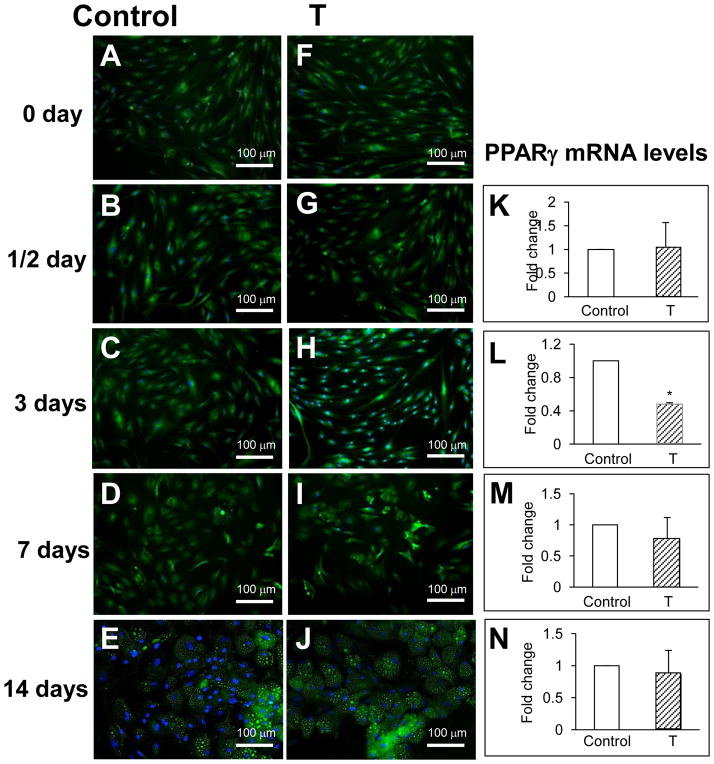
To determine the effect of T at different stages of adipogenesis, hASCs were exposed to T for 0–14 days in adipogenesis-inducing medium. Changes in cell morphology and lipid content were assessed using BODIPY-C1,12 (green) co-labeled with the nuclear stain DAPI (blue). Exposure of hASCs to T (50 nM) for 0.5 day did not alter cell morphology or PPARγ mRNA levels (Figs. 2B vs. 2G and Fig. 2K). Three-day exposure of hASCs to T (50 nM) in adipogenesis-inducing medium maintained typical hASC morphology in most of these cells as they exhibited a spindle-shape and contained fewer lipids in the cytoplasm in comparison to vehicle control (Figs. 2C vs. 2H). PPARγ mRNA levels were also suppressed in these T-exposed hASCs (0.44 ± 0.05 fold change vs. vehicle control, p<0.001) (Fig. 2L and Fig. 3A). Longer time intervals of hASC exposure to T (7 or 14 days) did not affect cell morphology, lipid accumulation (Figs. 2D–E vs I–J) or PPARγ mRNA levels (Figs. 2M–N). Furthermore, 3-day T (50 nM) treatment of hASCs in adipogenesis-inducing medium also suppressed mRNA expression for C/EBPα (0.39 ± 0.06 fold change vs. vehicle control, p<0.001) and C/EBPβ (0.30 ± 0.06 fold change vs. vehicle control, p<0.001) (Figs. 3B–C). These results indicate that T inhibits adipogenesis at early (3 days) but not late stages of preadipocyte differentiation into adipocytes. Exposure of hASCs to DHT (5 nM) for 3 days also suppressed this early-stage of preadipocyte differentiation to adipocytes, as evidenced by decreased mRNA expression for PPARγ (0.59 ± 0.08 fold change vs. vehicle control, p<0.001), C/EBPα (0.51 ± 0.08 fold vs. vehicle control, p<0.001) and C/EBPβ γ (0.26 ± 0.06 fold change vs. vehicle control, p<0.001) (Figs. 3A–C).
Fig. 2.
Effect of T on adipogenesis over time. Confluent hASCs from non-obese healthy women were cultured over time (0.5 to 14 days) in adipogenesis-inducing medium with vehicle alone (control) or with T (50 nM). Immunofluorescence with BODIPY-C1,12 and DAPI was used to assess cell morphology and lipid content, respectively: A–E, vehicle control and F–J, treated with T (50 nM). Effect of T on PPARγ gene expression: K–N. PPARγ gene expression was quantified by qRT-PCR. Results represent fold changes treated vs. vehicle control samples. * p<0.001 vs. vehicle control.
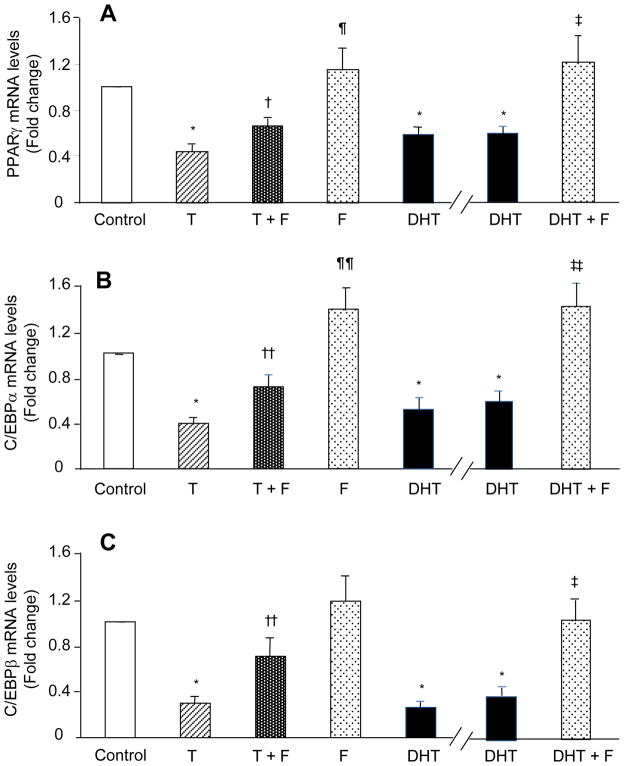
Fig. 3.
Effects of 3-day T or DHT exposure in the presence and absence of flutamide (F) on hASC commitment through early-stage preadipocyte differentiation. Confluent hASCs were cultured for 3 days in adipogenesis-inducing medium with vehicle alone (control), T (50 nM), DHT (5 nM) and or F (100 nM). Two sets of experiments are separated by the break in the x-axis. A, PPARγ; B, CEBPα; and C, CEBPβ mRNA expressions were quantified by qRT-PCR. Results represent fold changes treated vs. vehicle control samples. *p < 0.001 vs. vehicle control; †p < 0.02, ††p <0.01, T vs. T+F; ¶p <0.01, ¶¶p < 0.003, F vs. T+F; ‡p <0.05, ‡‡p < 0.005 DHT vs. DHT+F.
Effect of F on androgen inhibition of early stage preadipocyte differentiation
To determine whether T inhibition of early-stage preadipocyte differentiation is mediated through the androgen receptor (AR), hASCs were treated with T and the androgen antagonist F for 3 days in adipogenesis-induced DMEM medium. Coincubation of hASCs with F (100 nM) and T (50 nM) for 3 days partially reversed T inhibition of mRNA expression for PPARγ(0.66 ± 0.08 fold change vs. vehicle control; T vs. T + F, p<0.020; F vs. T + F, p< 0.003), C/EBPα (0.71 ± 0.10 fold change; T vs. T + F, p<0.01; F vs. T + F, p< 0.003) and CEBP-β (0.70 ± 0.16 fold change vs. vehicle control; T vs. T+ F, p< 0.01; F vs. F +T, p< 0.1) (Fig. 3A–C).
In a separate set of experiments, coincubation of hASCs with F (100 nM) and DHT (5 nM) for 3 days reversed DHT inhibition of mRNA expression for PPARγ (1.26 ± 0.26 fold change vs. vehicle control; DHT vs. DHT + F, p<0.03); C/EBPα (1.40 ± 0.21 fold vs. vehicle control, DHT vs. DHT + F, p<0.01) and C/EBPβ γ (0.99 ± 0.15 fold change vs. vehicle control, DHT vs. DHT + F, p<0.005), restoring all mRNA levels to those of hASCs treated with F alone (Figs. 3A–C, right panel). These results indicate that androgen inhibition of early-stage adipocyte differentiation is mediated in part through AR action.
Effect of androgens on hASC commitment to preadipocytes
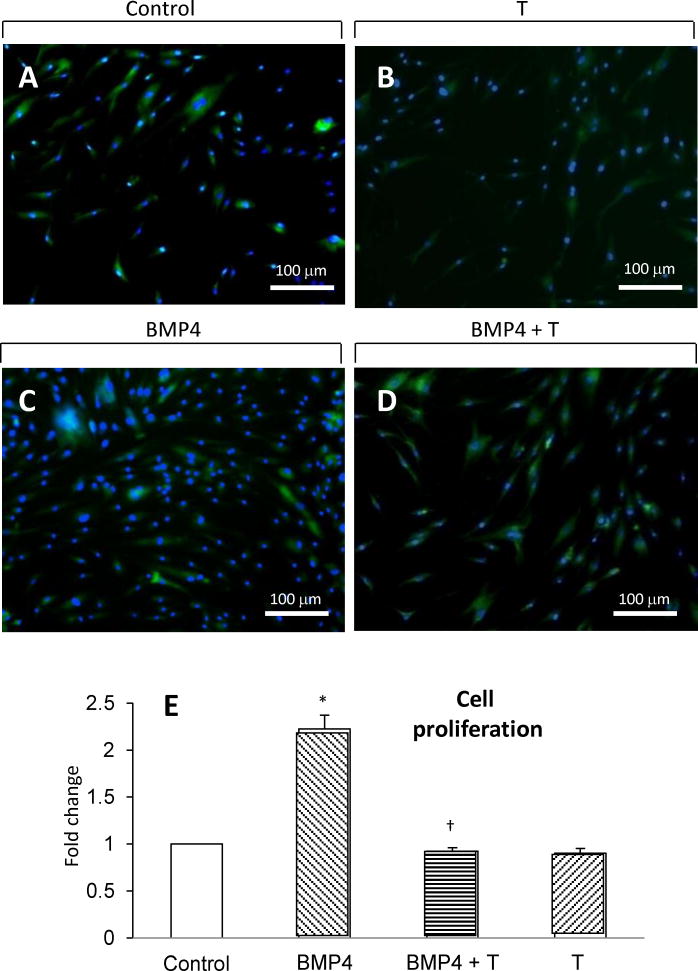
hASC commitment to the preadipocyte phenotype is regulated via activation of BMP4 signaling [2–4]. Therefore, hASCs were initially preincubated with BMP4 in the presence or absence of T for two hours in regular medium, after which hASCs were cultured in adipogenesis-inducing DMEM medium alone for an additional 3 days. As expected, 2-hour BMP4 (5 nM) preincubation induced hASC commitment to the preadipocyte phenotype, as evidenced by increased numbers of newly formed preadipocytes after 3-day incubation in adipogenesis-inducing medium (2.23 ± 0.15 fold change vs. vehicle control, p<0.001) (Figs. 4A vs. 4C and Fig. 4E). In the presence of 2-hour BMP4 (5 nM) preincubation, simultaneous T (50 nM) preincubation significantly inhibited BMP4-induced ASC commitment to the preadipocyte phenotype by reducing the numbers of preadipocytes (0.92 ± 0.04 fold change vs. vehicle control; BMP4 vs. BMP4 + T, p<0.001) (Figs. 4C vs. 4D and Fig. 4E). Two-hour exposure of hASC to T (50 nM) alone did not alter cell proliferation (Figs. 4A vs. B and Fig. 4E).
Fig. 4.
Effect of 2-hour T preincubation on BMP4-induced hASC commitment to the preadipocyte phenotype. Confluent hASCs were first incubated for 2 hours in regular DMEM medium with vehicle alone (control), BMP4 (5 nM) and/or T (50 nM),. Cells were then incubated in adipogenesis-inducing medium for another 3 days without BMP4 or T. Immunocytochemistry with BODIPY-C1,12 and DAPI was used to assess cell morphology and lipid content: A, vehicle control; B, 50 nM T; C, 5 nM BMP4; D, 5 nM BMP4 + 50 nM T. BODIPY-C1, 12/DAPI positive cells were quantified using ImageJ software. E, Cell proliferation was determined by counting number of BODIPY-C1, 12/DAPI positive cells (see Methods). The results were expressed as fold changes treated vs. vehicle control samples. *p< 0.001 vs. vehicle control.
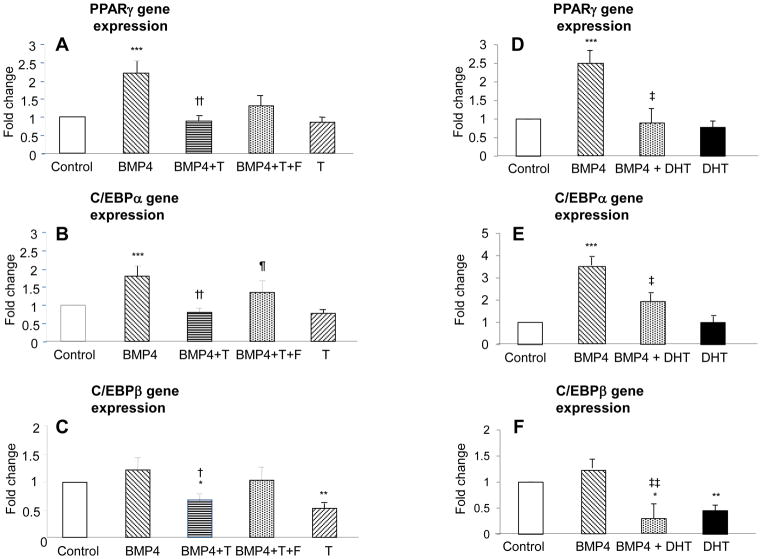
Two-hour BMP4 (5 nM) preincubation also increased mRNA expression for PPARγ (2.20 ± 0.31 fold change vs. vehicle control, p<0.001) and C/EBPα (1.80 ± 0.27 fold change vs. vehicle control, p<0.001) (Figs. 5A–B), but did not affect C/EBPβ mRNA expression (1.22 ± 0.22 fold change vs. vehicle control; p=0.3) (Fig. 5C). Two-hour BMP4 (5 nM) preincubation with T (50 nM) significantly inhibited BMP4-induced mRNA expression of PPARγ (0.89 ± 0.13 fold change vs. vehicle control; BMP4 vs. BMP4 + T, p<0.001) and C/EBPα (0.80 ±0.13 fold change vs. vehicle control; BMP4 vs. BMP4 + T, p<0.001) (Figs. 5A–B). Independent of 2-hour BMP4 preincubation, simultaneous T pretreatment for 2 hours significantly decreased C/EBPβ mRNA expression (without BMP4: T, 0.53 ± 0.09 fold change vs. vehicle control, p<0.005; with BMP4: T, 0.67± 0.13 fold change vs. vs. vehicle control, p<0.05) (Fig. 5C).
Fig. 5.
Effect of 2-hour androgen preincubation of hASCs with or without flutamide (F) on BMP4-induced mRNA expression of PPARγ, CEBP-α, and CEBP-β. Confluent hASCs were first preincubated for 2 hours in regular DMEM medium with or without BMP4 (5 nM), T (50 nM), DHT (5 nM) and F (100 nM). Cells were then incubated in adipogenesis-inducing medium for another 3 days without BMP4, androgen or F. mRNA expression of PPARγ, CEBP-α, and CEBP-β were determined by qRT-PCR: A and D, PPARγ; B and E, CEBP-α; and C and F, CEBP-β. Results represent fold changes treated vs. vehicle control: *p< 0.05, **p <0.005, ***p<0.001 vs. vehicle control; †p< 0.05, †† p< 0.001 BMP4 vs. BMP4 + T; ¶p < 0.05, BMP4 + T vs. BMP4 + T + F; ‡p <0.05, ‡‡p < 0.005 BMP4 vs. BMP4 + DHT.
In replicate experiments substituting DHT for T, 2-hour BMP4 (5 nM) preincubation again increased in mRNA expression for PPARγ (2.49 ± 0.36 fold change vs. vehicle control, p<0.001) and C/EBPα (3.99 ± 0.49 fold change vs. vehicle control, p<0.008) (Figs. 5D–E), but not C/EBPβ mRNA expression (1.27 ± 0.17 fold change vs. vehicle control; p=0.5) (Fig. 5F). Two-hour BMP4 (5 nM) preincubation with DHT (5 nM) significantly inhibited BMP4-induced mRNA expressions of PPARγ (0.89 ± 0.40 fold change vs. vehicle control; BMP4 vs. BMP4 + DHT, p<0.01) and C/EBPα (2.37 ±0.39 fold change vs. vehicle control; BMP4 vs. BMP4 + D, p<0.05) (Figs. 5D–E). Again independent of 2-hour BMP4 preincubation, simultaneous DHT pretreatment for 2 hours significantly reduced C/EBPβ mRNA expression (without BMP4: DHT, 0.45 ± 0.11 fold change vs. vehicle control, p<0.03; with BMP4: DHT, 0.31± 0.27 fold change vs. vs. vehicle control; p<0.005) (Fig. 5C). These results suggest that androgen inhibition of PPARγ and C/EBPα, but not necessarily C/EBPβ, mRNA expression during hASC commitment to preadipocytes is mediated through BMP4 signaling.
Effect of F on T inhibition of hASC commitment to preadipocytes
Two-hour preincubation of hASCs with BMP4 (5 nM) and T (50 nM) plus F (100 nM) tended to reverse T inhibition of BMP4-induced PPARγ mRNA expression (1.36 ± 0.30 fold change vs. vehicle control; BMP4 + T vs. BMP4 + T + F, p=0.1) and significantly reversed T inhibition of BMP4-induced C/EBPα mRNA expression (1.34 ± 0.30 fold change vs. vehicle control; BMP4 + T vs. BMP4 + T + F p<0.05) (Fig. 5A and 5B). Two-hour preincubation of hASCs with BMP4 (5 nM) and T (50 nM) plus F (100 nM) did not significantly alter C/EBPβ gene expression (1.05 ± 0.25 change vs. vehicle control; BMP4 + T vs. BMP4 + T + F, p=0.2) (Fig. 5C).
DISCUSSION
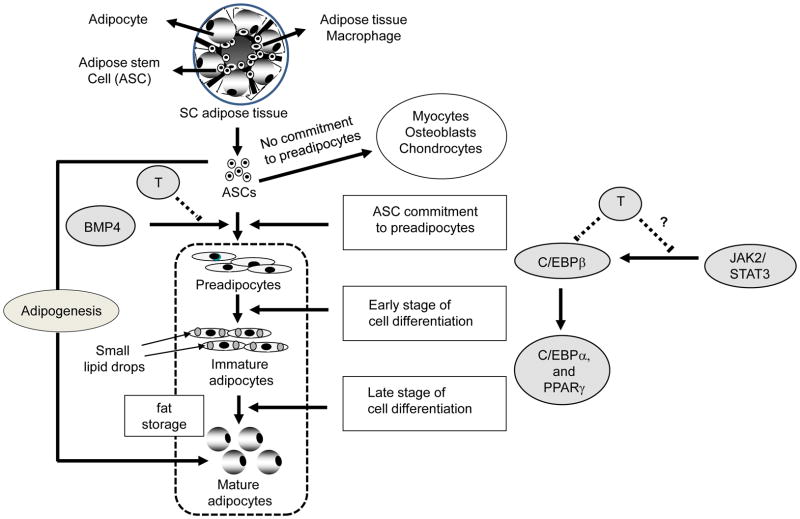
Adipose consists of a complex population of cells, including adipocytes, macrophages, endothelial cells, vascular tissue and hASCs all of which co-interact to affect reproductive and metabolic function. hASCs are multipotent stem cells that can differentiate along adipocyte, osteoblast, chondrocyte and other mesenchymal cell lineages [26–28]. Under appropriate stimulus, however, hASCs undergo commitment to the adipocyte lineage. Adipogenesis involves three major stages: (i) hASC commitment to the preadipocyte phenotype via activation of BMP4 [2,3], characterized by conversion of typical spindle-shape cells to more rounded cells containing cytoplasmic lipid inclusions; (ii) early-stage preadipocyte differentiation to adipocytes, characterized by proliferation of preadipocytes via activation of C/EBPβ and C/EBPδ to induce PPARγ and C/EBPα; and (iii) late-stage preadipocyte differentiation into adipocytes, characterized by cell-cycle arrest with further activation of PPARγ and C/EBPα. These pro-adipogenic events are further modulated by other transcriptional factors to promote gradual intracellular lipid accumulation and maturation into adipocytes [4–7,22].
Using morphological and mRNA expression criteria to stage adipogenesis [2,3], our data show that 3-day exposure of hASCs to T maintains their elongated fibroblast-like appearance characteristic of hASCs and reduces PPARγ, C/EBPα and C/EBPβ mRNA expression, confirming previous reports of androgen inhibition of human preadipocyte differentiation [19,20,22]. Furthermore, androgen action on early-stage preadipocyte differentiation is mediated, in part, through the AR, since T inhibition of PPARγ, C/EBPα and C/EBPβ mRNA expression is partially reversed by F (an antiandrogen), while similar inhibitory effects of DHT (a nonaromatizable androgen) on these genes appear more completely reversed by F. In support of this, AR overexpression of mesenchymal stem cells in transgenic mice reduces SC fat and diminishes adipocyte size [29]. That androgen inhibition of hASC commitment to preadipocytes might also involve aromatization is further suggested by the ability of estradiol to impair both in vitro adipogenesis in mice and mesenchymal stem cell preadipocyte differentiation in men [20,30]. This hypothesis requires further investigation beyond the scope of the present study since exposure of our hASCs to either an aromatase inhibitor (Formestane, 100 nM) or an estrogen receptor antagonist (Fulvestrant, 100 nM) alone induced intrinsic effects on gene expression (data not shown), which complicate interpretation of steroid interactions on these cells.
More specifically our data show, for the first time to our knowledge, that androgens inhibit hASC commitment to the preadipocyte phenotype as an antecedent to preadipocyte differentiation into adipocytes. Two-hour preincubation of hASCs with BMP4 promoted hASC commitment to preadipocytes, as previously described [2,3] and also induced PPARγ and C/EBPα gene expression during subsequent 3-day exposure to adipogenic culture conditions. Coincubation of hASCs with BMP4 and T during this 2-hour preincubation maintained cells in an elongated fibroblast-like appearance and impaired BMP4-induced cell commitment to preadipocytes over the next 3 days, as evidenced by T inhibition of BMP4-induced PPARγ and C/EBPα gene expression. Flutamide reversed T-inhibition of BMP4-induced C/EBPα gene expression, suggesting AR-mediated action, perhaps controlled via the zinc-finger protein, Zfp423, as a critical transcriptional regulator of BMP4-induced ASC commitment to preadipocytes [31]. These effects of T, mediated through its cognate receptor, are also supported by previous studies indicating the presence of AR in human mesenchymal stem cells maintained in regular DMEM medium [20].
Conversely, 2-hour preincubation of hASCs with BMP4 before 3-day exposure to adipogenesis-induced DMEM medium did not affect C/EBPβ gene expression, which has been shown to regulate early-stage preadipocyte differentiation through the JAK2/STAT3 pathway [32]. The ability of T to therefore inhibit C/EBPβ mRNA expression over this same time interval, regardless of BMP4, could represent its inhibitory action on preadipocyte differentiation, mediated through the JAK2/STAT3 pathway rather than BMP4 signaling (Fig. 6). Whether the inhibitory effects of androgens on PPARγ, C/EBPα and C/EBPβ mRNA expression involve transcriptional regulation and/or stability of mRNA levels remains to be determined.
Fig. 6.
Schematic representation of T action on adipogenesis. Testosterone inhibits hASC commitment through preadipocyte phenotype via impairment of BMP4-induced PPARγ and C/EBPα mRNA expression. An additional inhibitory effect of T on early-stage preadipocyte differentation and C/EBPβ mRNA expression may occur through the JAK2/STAT3 pathway.
The present study shows effects of androgens on hASCs isolated only from SC adipose tissue. These cells display a more homogeneous fibroblast like morphology characteristic of messenchymal stem cells, have a higher rate of growth, and generate more functional mature adipocytes than hASCs isolated from other fat depots (e.g. visceral fat) (Chazenbalk et al, 2012, unpublished data) [33]. hASCs isolated from SC adipose tissue, therefore are likely the optimal cells to study the effect of androgens on adipogenesis.
The strength of our study is the use of hASCs from SC abdominal adipose of non-obese healthy women, which avoids the confounding effect of obesity as a modulator of adipogenesis [9,21,34] Our choice of normal perimenopausal women for study participation was designed to minimize any endogenous exposure of hASCs to hyperandrogenism and/or athero-inflammatory processes from adiposity-dependent insulin resistance [35]. Another strength of our study is its capability to determine androgen action on different stages of adipogenesis with the combined used of immunofluorescence microscopy with qRT-PCR analysis of critical genes governing hASC commitment and preadipocyte differentiation.
One limitation of our study, however, is the use of adipogenic media over 14 days to successfully culture hASCs through their commitment to preadipocyte differentiation and adipocyte development. These cell culture conditions may have overshadowed androgen inhibition of late-stage preadipocyte differentiation, as shown by other investigators [19,20], and may explain why T and DHT effects were not observed beyond the 3-day interval used to study early-stage preadipocyte differentiation. Alternatively use of hASCs from women in their fifth decade of life may not be representative of hASC function in younger reproductive-aged women.
Nevertheless, our findings may have implications for women with polycystic ovary syndrome (PCOS), [36], who manifest hyperandrogenism, ovulatory dysfunction and polycystic ovaries in its complete phenotype [37]. In such PCOS women, an in vivo action of androgen excess on SC abdominal hASCs, if similar to our in vitro data, could decrease adipocyte numbers in SC abdominal adipose and thereby reduce the capacity of this adipose to safely store fat [38,39]. Based upon the concept of impaired “adipose tissue expandability” [38,39], if energy intake exceeds this capacity, SC abdominal adipocytes would likely overfill with lipid and promote ectopic lipid deposition accompanied by insulin resistance, agreeing with the findings of enlarged SC abdominal adipocytes and insulin resistance in PCOS women [40].
In conclusion, androgen acts in part through its own receptor to impair BMP4-induced commitment of hASCs to preadipocytes and to reduce early-stage preadipocyte differentiation into adipocytes in SC abdominal adipose of nonobese women. These inhibitory effects of androgen on early aspects of human SC abdominal adipogenesis could alter the numbers of adipocytes in this adipose depot and thereby affect its capacity to safely store fat in women with androgen excess.
Highlights.
Androgens play a critical role in the regulation of human adipogenesis
Testosterone impairs human adipose stem cell commitment to preadipocytes through BMP4
Androgens reduce early but not late stage preadipocyte differentiation to adipocytes
Inhibitory effects of testosterone are mediated in part by androgen receptor action
Excess of androgens could affect subcutaneous adipose tissue to safely store fat
Acknowledgments
The authors thank Ariela Simerman, Erica Keller and Vanessa Madrigal for their collaboration on these studies.
Funding: This study was funded by the UCLA Department of Obstetrics and Gynecology, and in part by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the NIH through cooperative agreement U54 HD071836.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowers RR, Kim JW, Otto TC, Lane MD. Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: role of the BMP-4 gene. Proc Natl Acad Sci U S A. 2006;103:13022–13027. doi: 10.1073/pnas.0605789103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang H, Song TJ, Li X, Hu L, He Q, et al. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A. 2009;106:12670–12675. doi: 10.1073/pnas.0906266106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227–246. doi: 10.1194/jlr.R021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 7.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 8.Siersbaek R, Mandrup S. Transcriptional networks controlling adipocyte differentiation. Cold Spring Harb Symp Quant Biol. 2011;76:247–255. doi: 10.1101/sqb.2011.76.010512. [DOI] [PubMed] [Google Scholar]
- 9.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allan CA, McLachlan RI. Androgens and obesity. Curr Opin Endocrinol Diabetes Obes. 2010;17:224–232. doi: 10.1097/MED.0b013e3283398ee2. [DOI] [PubMed] [Google Scholar]
- 11.Ross MR, Schomer DF, Chappell P, Enzmann DR. MR imaging of head and neck tumors: comparison of T1-weighted contrast-enhanced fat-suppressed images with conventional T2-weighted and fast spin-echo T2-weighted images. AJR Am J Roentgenol. 1994;163:173–178. doi: 10.2214/ajr.163.1.8010208. [DOI] [PubMed] [Google Scholar]
- 12.Lemieux S, Prud’homme D, Bouchard C, Tremblay A, Despres JP. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am J Clin Nutr. 1993;58:463–467. doi: 10.1093/ajcn/58.4.463. [DOI] [PubMed] [Google Scholar]
- 13.Chrzanowska M, Suder A. Ontogenesis changes and sex dimorphism of subcutaneous fat distribution: 12-year longitudinal study of children and adolescents from Cracow, Poland. Am J Hum Biol. 2008;20:424–430. doi: 10.1002/ajhb.20749. [DOI] [PubMed] [Google Scholar]
- 14.Nedungadi TP, Clegg DJ. Sexual dimorphism in body fat distribution and risk for cardiovascular diseases. J Cardiovasc Transl Res. 2009;2:321–327. doi: 10.1007/s12265-009-9101-1. [DOI] [PubMed] [Google Scholar]
- 15.Van Eerden P. Obesity in pregnancy. S D Med. 2011;(Spec No):46–50. [PubMed] [Google Scholar]
- 16.Zang H, Carlstrom K, Arner P, Hirschberg AL. Effects of treatment with testosterone alone or in combination with estrogen on insulin sensitivity in postmenopausal women. Fertility and Sterility. 2006;86:136–144. doi: 10.1016/j.fertnstert.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 17.Zang H, Ryden M, Wahlen K, Dahlman-Wright K, Arner P, et al. Effects of testosterone and estrogen treatment on lipolysis signaling pathways in subcutaneous adipose tissue of postmenopausal women. Fertility and Sterility. 2007;88:100–106. doi: 10.1016/j.fertnstert.2006.11.088. [DOI] [PubMed] [Google Scholar]
- 18.Varlamov O, White AE, Carroll JM, Bethea CL, Reddy A, et al. Androgen effects on adipose tissue architecture and function in nonhuman primates. Endocrinology. 2012;153:3100–3110. doi: 10.1210/en.2011-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blouin K, Nadeau M, Perreault M, Veilleux A, Drolet R, et al. Effects of androgens on adipocyte differentiation and adipose tissue explant metabolism in men and women. Clin Endocrinol (Oxf) 2010;72:176–188. doi: 10.1111/j.1365-2265.2009.03645.x. [DOI] [PubMed] [Google Scholar]
- 20.Gupta V, Bhasin S, Guo W, Singh R, Miki R, et al. Effects of dihydrotestosterone on differentiation and proliferation of human mesenchymal stem cells and preadipocytes. Mol Cell Endocrinol. 2008;296:32–40. doi: 10.1016/j.mce.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Harmelen V, Skurk T, Rohrig K, Lee YM, Halbleib M, et al. Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women. Int J Obes Relat Metab Disord. 2003;27:889–895. doi: 10.1038/sj.ijo.0802314. [DOI] [PubMed] [Google Scholar]
- 22.Singh R, Artaza JN, Taylor WE, Braga M, Yuan X, et al. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with beta-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology. 2006;147:141–154. doi: 10.1210/en.2004-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshimura K, Suga H, Eto H. Adipose-derived stem/progenitor cells: roles in adipose tissue remodeling and potential use for soft tissue augmentation. Regen Med. 2009;4:265–273. doi: 10.2217/17460751.4.2.265. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56:1517–1526. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- 25.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- 26.Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Moseley TA, Zhu M, Hedrick MH. Adipose-derived stem and progenitor cells as fillers in plastic and reconstructive surgery. Plast Reconstr Surg. 2006;118:121S–128S. doi: 10.1097/01.prs.0000234609.74811.2e. [DOI] [PubMed] [Google Scholar]
- 28.Charriere G, Cousin B, Arnaud E, Andre M, Bacou F, et al. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003;278:9850–9855. doi: 10.1074/jbc.M210811200. [DOI] [PubMed] [Google Scholar]
- 29.Semirale AA, Zhang XW, Wiren KM. Body composition changes and inhibition of fat development in vivo implicates androgen in regulation of stem cell lineage allocation. J Cell Biochem. 2011;112:1773–1786. doi: 10.1002/jcb.23098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar A, Ruan M, Clifton K, Syed F, Khosla S, et al. TGF-beta mediates suppression of adipogenesis by estradiol through connective tissue growth factor induction. Endocrinology. 2012;153:254–263. doi: 10.1210/en.2011-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, et al. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464:619–623. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang K, Guo W, Yang Y, Wu J. JAK2/STAT3 pathway is involved in the early stage of adipogenesis through regulating C/EBPbeta transcription. J Cell Biochem. 2012;112:488–497. doi: 10.1002/jcb.22936. [DOI] [PubMed] [Google Scholar]
- 33.Baglioni S, Cantini G, Poli G, Francalanci M, Squecco R, et al. Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell. PLoS One. 2012;7:e36569. doi: 10.1371/journal.pone.0036569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, et al. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab. 2007;92:430–436. doi: 10.1210/jc.2006-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord. 2007;8:127–141. doi: 10.1007/s11154-007-9046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 38.de Zegher F, Lopez-Bermejo A, Ibanez L. Adipose tissue expandability and the early origins of PCOS. Trends Endocrinol Metab. 2009;20:418–423. doi: 10.1016/j.tem.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochim Biophys Acta. 2010;1801:338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Manneras-Holm L, Leonhardt H, Kullberg J, Jennische E, Oden A, et al. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab. 2011;96:E304–311. doi: 10.1210/jc.2010-1290. [DOI] [PubMed] [Google Scholar]