Abstract
Inflammation is an important pathogenic mechanism in many neurodegenerative disorders. Activated microglia play a pivotal role in releasing proinflammatory factors including interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), and cyclooxygenase-2 (COX-2) for inducing inflammation. While microglia mediated inflammation is essential in maintaining CNS homeostasis, chronic inflammation results in activation of proteases for cell death. Here, we examined the effect of PPT (estrogen receptor α agonist), DPN (estrogen receptor β agonist), and estrogen on rat primary microglia following exposure to lipopolysaccharide (LPS). Exposure of microglia to LPS (200 ng/ml) for 24 h induced cell death. After LPS toxicity for 15 min, microglia were treated with 25 nM PPT, 25 nM DPN, or 100 nM estrogen that prevented cell death by attenuating the release of IL-1α, IL-1β, TNF-α, and COX-2. Treatment of cells with 100 nM fulvestrant (estrogen receptor antagonist) prior to addition of PPT, DPN, or estrogen significantly decreased their ability to prevent cell death, indicating involvement of estrogen receptor (ER) in providing PPT, DPN, or estrogen mediated cytoprotection. Reverse transcriptase polymerase chain reaction (RT–PCR) analyses showed alterations in mRNA expression of Bax, Bcl-2, calpain, and calpastatin during apoptosis. We also examined mRNA expression of ERβ and ERα following exposure of microglia to LPS and subsequent treatment with PPT, DPN, or estrogen. We found that estrogen or estrogen receptor agonists upregulated expression of ERs. Overall, results indicate that estrogen receptor agonist or estrogen uses a receptor mediated pathway to protect microglia from LPS toxicity.
Keywords: Estrogen, Microglia, Lipopolysaccharide estrogen receptor alpha, Estrogen receptor beta
Introduction
The pathology of neurodegenerative disorders such as multiple sclerosis (MS), Alzheimer’s disease (AD), Parkinson’s disease (PD), spinal cord injury (SCI), and traumatic brain injury (TBI) involves the progressive loss of neurons and subsequent impairment of central nervous system (CNS) function in the affected region. Experimental evidence suggests neuroinflammation plays a critical role in the progression of neurodegenerative disorders. Patients suffering from neurodegenerative disorders present with elevated cerebrospinal fluid (CSF) levels of pro-inflammatory mediators including inducible-nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) [1]. In addition, long-term use of nonsteroidal anti-inflammatory drugs (NSAIDs) appears to decrease the risk of developing AD and PD [2, 3]. Given the critical role of inflammation in neurodegenerative disorders, elucidation of the mechanisms underlying induction of CNS inflammation is important.
Microglia are the primary inflammatory cells of the CNS. In healthy adults, microglia are considered to be in a ramified state and play an important role in maintaining neuronal function and survival by producing a variety of neurotrophic factors and removing cellular debris during synaptic remodeling [4, 5]. However, following insult to the CNS, chronic activation of microglia results in the release of pro-inflammatory agents including cytokines, reactive oxygen species, free radicals, nitric oxide, proteases and complement factors [6, 7]. Inflammation induced by microglial activation has been associated with neuronal damage and death characteristic of neurodegenerative disorders [8, 9]. In addition, post-mortem studies suggest activated microglia are present in disease-associated brain regions of patients with neurodegenerative disorders including AD, MS, PD, and ALS [10, 11]. Because of the role of inflammation in neurodegenerative disorders, effective therapeutic strategies may involve inhibition of microglial activation and release of pro-inflammatory factors.
Estrogen has been proposed as a potential therapeutic agent in the treatment of neurodegenerative disorders. Numerous studies have shown that estrogen confers neuroprotection in animal models of disorders including stroke, multiple sclerosis, and PD [12–14]. Experimental findings also suggest gender differences in markers for Huntington’s disease, amyotrophic lateral sclerosis, and TBI [15–18]. Estrogen may provide neuroprotection through its actions as a potent anti-oxidant, anti-apoptotic, and anti-inflammatory agent [19, 20]. Evidence suggests 17β-estradiol treatment inhibits microglial activation following exposure to inflammatory stimuli including lipopolysaccharide (LPS) and 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropryridine (MPTP) [21, 22]. In addition, other groups have reported that estrogen treatment of insulted microglia attenuates release of cytokines, reactive oxygen species, free radicals, and complement proteins [23–25]. However, debate remains about the mechanisms by which estrogen inhibits microglial activation and the roles of estrogen receptor subtypes α (ERα) and β (ERβ) in this process [26–28].
The current study was designed to further investigate the effects of estrogen treatment on microglial activation. We hypothesized that estrogen or estrogen receptor agonist treatment of microglia following exposure to LPS would increase cell viability, and attenuate release of proinflammatory agents including nitric oxide, tumor necrosis factor α (TNFα), COX-2, interleukin-1α (IL-1α), and interleukin-1β (IL-1β). We further hypothesized that estrogen would attenuate expression of apoptotic mediators including bax and calpains. Finally, we believed the effects of estrogen treatment on microglia exposed to LPS would be due in part to an increase in ERβ expression.
Materials and Methods
To test our hypotheses, we obtained commercially available rat microglia isolated from primary rat brain cell culture (ScienCell Research Laboratories, Carlsbad, CA, USA). Microglia were cultured to sub-confluency in 75-cm2 flasks with 10 ml of microglia medium (MM) (ScienCell Research Laboratories) containing 5% fetal bovine serum (FBS), 1% microglia growth supplement (MGS) and 1% penicillin/streptomycin in a fully humidified incubator containing 5% CO2 at 37°C. Prior to treatment, stock solutions of 25 nM PPT, 25 nM DPN, 100 nM β-estradiol, and 200 ng/ml LPS were prepared. Microglia in control groups were exposed to PPT (an ERα agonist), DPN (an ERβ agonist), β-estradiol, or LPS alone. Microglia in treatment groups were exposed to LPS for 15 min before treatment. Following LPS toxicity, cells were treated with PPT, DPN, or estrogen for 24 h. One treatment group for each of the ER agonists used was also treated with the ER antagonist fulvestrant. Cells were harvested immediately following the 24 h treatment period.
Cell viability was measured using the trypan blue dye exclusion test, which has been described previously [29]. Briefly, this test utilizes attached and detached cell populations to estimate cell viability. Cells maintaining membrane integrity were counted as viable and did not take up trypan blue. Compromised cells took up trypan blue and were counted as dead. A minimum of 800 cells were counted in four different fields and cell viability was calculated as a percentage of total cell population.
Nitric oxide (NO) production by microglia was examined using a previously described method [30, 31]. Briefly, nitric oxide production was measured by quantifying the amount of nitrite, a stable oxidation product of NO, using the spectrophotometric Greiss reaction. An aliquot of each cell culture medium was mixed with an equal volume of 1% sulfanilamide in water and 0.1% N-1-naphthylethylene dihydrochloride in 5% phosphoric acid. The absorbance was measured at 550 nm. Sodium nitrite was dissolved and diluted in culture medium to obtain concentrations of 1–100 µM and subsequently used to generate a standard curve. Nitric oxide production was presented graphically as a ratio of nitrite concentration determined spectrophotometrically in each treatment group over nitrite concentration in the control group.
Levels of mRNA expression of pro-inflammatory and pro-apoptotic agents were measured using reverse transcriptase polymerase chain reaction (RT–PCR). Extraction of total RNA and RT–PCR were performed according to standard procedure [32]. All rat primers were for RT–PCR experiments were designed using Oligo software (National Biosciences, Plymouth, MN, USA) and custom synthesized (Operon Technologies, Alameda, CA, USA). AmpliTaqGold™ DNA polymerase (Perkin Elmer Applied Biosystems, Foster City, CA, USA) was used in all RT–PCR experiments to ensure specific amplification. The number of amplification cycles was altered depending on the specific gene to achieve detection. The level of β-actin expression served as an internal control in all experiments. Product (10 µl) from each RT–PCR reaction was resolved on a 1.4% agarose gel by electrophoresis at 2 V/cm in 40 mM Tris–HCl, pH 8.3, 40 mM Na-acetate, 1 mM EDTA. The gel was stained with 1 µg/ml ethidium bromide and destained in water to ensure a clear background. Following staining, the gel was photographed using a Kodak camera and digitalized using Photoshop software (Adobe Systems, Seattle, WA).
Results from each treatment group were analyzed using StatView software (Abacus Concepts, Berkeley, CA, USA). Data were expressed as a mean ± standard error of mean of separate experiments (n ≥ 3). Results from each treatment were compared using one-way analysis of variance (ANOVA) followed by Fisher’s post hoc testing. The level of significance for all analyses was set at P < 0.05.
Results
Viability of Microglia
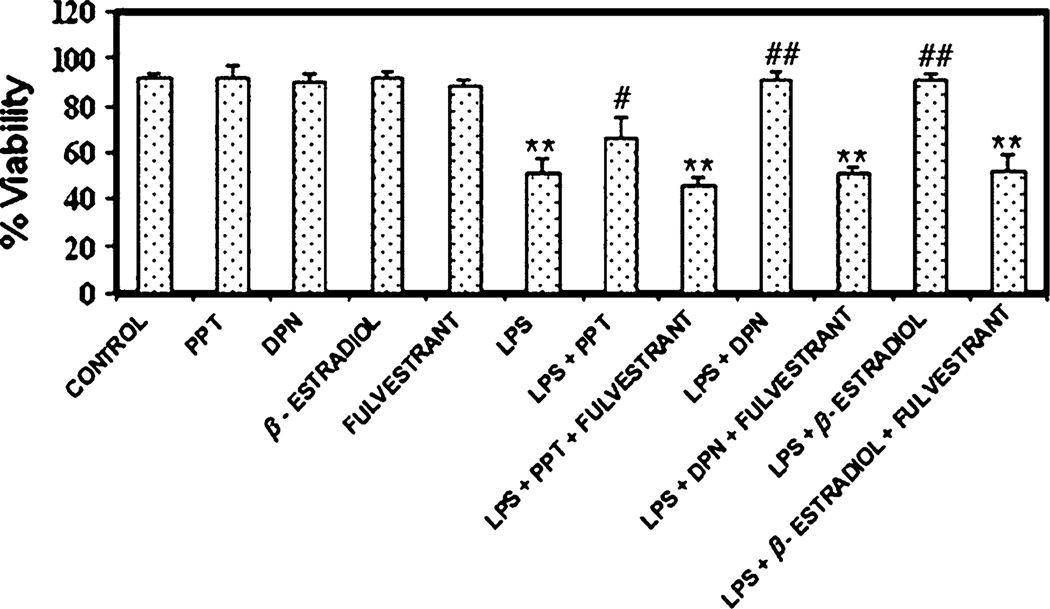
Microglial viability was measured using the trypan blue dye exclusion test (Fig. 1). Exposure of microglia to LPS for 24 h significantly decreased cell viability (~40%), as noted in other studies [33]. Treatment with non-specific 17β-estradiol, or the ERβ agonist DPN provided the greatest attenuation of LPS-induced microglial death, restoring cell viability to levels similar to that of cells not exposed to LPS. Quantitatively, the ERα agonist PPT appeared to provide less significant cytoprotection. These findings suggest a greater role for ERβ than ERα in protection of microglia following LPS toxicity, and is consistent with findings in microglia expressing only ERβ [2]. Attenuation of cell death by all three ER agonists was reversed by the use of the non-specific ER antagonist fulvestrant. Treatment of control microglia not exposed to LPS with ER agonist and antagonist had no appreciable effect on cell viability.
Fig. 1.
Measurement of microglia viability using the trypan blue dye exclusion test following LPS exposure and estrogen treatment. Cell viability was measured as a percentage of total cell population. LPS = lipopolysaccharide; PPT = ERα agonist; DPN = ERβ agonist. **P ≤ 0.01 compared to control; #P < 0.05 compared to LPS exposure; ##P ≤ 0.01 compared to LPS exposure
Attenuation of Nitric Oxide Production
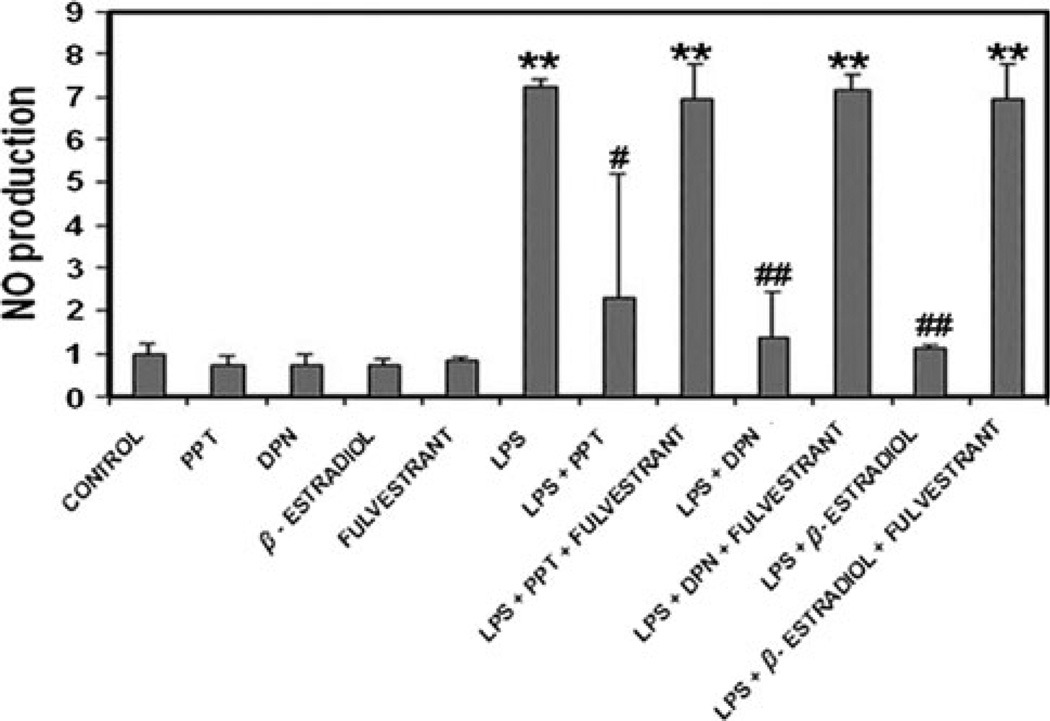
Production of nitric oxide by microglia was examined using the spectrophotometric Greiss reaction to measure nitrite, a stable oxidation product of NO (Fig. 2). Microglia exposed to LPS for 24 h had significantly higher NO production than control cells. Production of NO by LPS-induced microglia was significantly inhibited by both 17β-estradiol and the ERβ agonist DPN. PPT, an ERα agonist, attenuated microglial NO production to a lesser degree than the other ER agonist, suggesting this effect of estrogen treatment on activated microglia may be mediated by ERβ. The inhibitory effects of ER agonists on microglial production of NO was reversed by the use of fulvestrant, a non-specific ER antagonist, suggesting a receptor mediated mechanism may be involved.
Fig. 2.
Measurement of relative NO production by primary rat microglia across all treatment groups. Production of NO was measured by quantifying nitrite concentration spectrophotometrically. Relative NO production for each group was determined as a ratio to NO production in the control group. **P ≤ 0.01 compared to control; #P < 0.05 compared to LPS exposure; ##P ≤ 0.01 compared to LPS exposure
Effect of LPS and Estrogen Treatment on Microglial Estrogen Receptors
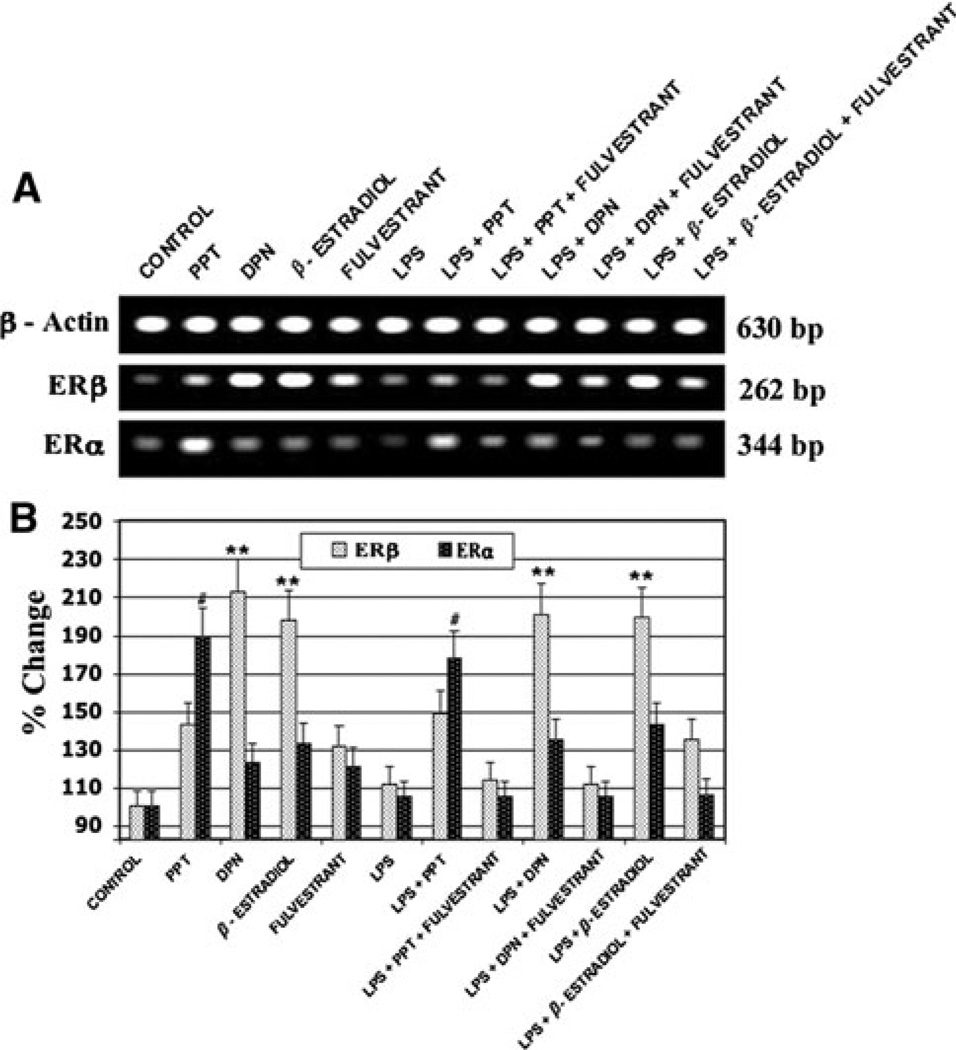
Expression of ERα and ERβ mRNA among primary rat microglia was measured using RT–PCR (Fig. 3). Exposure of microglia to LPS had no significant effect on the expression of ERβ, but appeared to decrease expression of ERα. Treatment with 17β-estradiol or the ERβ agonist DPN lead to a significant increase in mRNA levels of ERβ, but had no effect on ERα levels. Conversely, use of the ERα agonist PPT caused up-regulation of ERα with no significant effect on ERβ expression. These findings indicate an increase in the expression of ERβ may be responsible for the effects of estrogen or DPN treatment on cell viability and NO production following LPS-induced activation of primary rat microglia.
Fig. 3.
Quantification of ERα and ERβ mRNA levels following LPS toxicity and subsequent treatment with estrogen or estrogen receptor agonist using RT–PCR. #P ≤ 0.01 compared to control ERα expression; **P ≤ 0.01 compared to control ERβ expression
Effect of LPS and Estrogen or Estrogen Receptor Agonist Treatment on The Expression of Microglial Pro-Inflammatory Cytokines
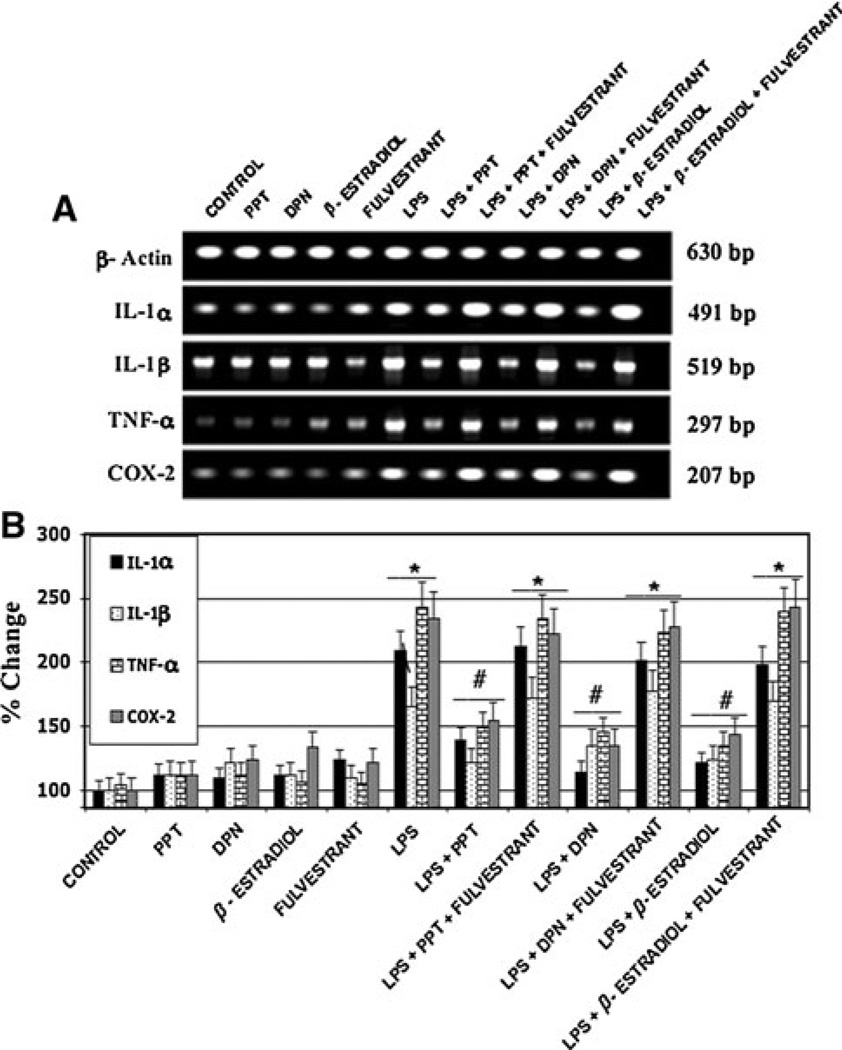
RT–PCR was also utilized in order to quantify the expression of pro-inflammatory agents in microglia exposed to LPS (Fig. 4). Specifically, we examined mRNA levels of IL-1α, IL-1β, TNF-α, and COX-2 following LPS toxicity and subsequent estrogen treatment. Generally, exposure to LPS significantly increased microglial production of all inflammatory mediators investigated in this study. The greatest increases were noted in mRNA levels of IL-1α, TNF-α, and COX-2 following LPS toxicity in primary microglia. A less vigorous up-regulation of IL-1β was also noted due to LPS exposure. Although ER agonist treated microglia exhibited increased expression of cytokines and COX-2 over control cells, use of 17β-estradiol, the ERα agonist PPT, and the ERβ agonist DPN all significantly attenuated production of these inflammatory agents. In particular, treatment of microglia with 17β-estradiol lead to the most significant inhibition of microglial up-reguluation of IL-1α, IL-1β, TNF-α, and COX-2. These findings are consistent with other studies and suggest both ERα and ERβ may play a role in the anti-inflammatory actions of estrogen following exposure of microglia to LPS [26]. Fulvestrant, the ER antagonist, inhibited the anti-inflammatory effects of all three ER agonists, suggesting a receptor mediated pathway is involved (Table 1).
Fig. 4.
mRNA levels of pro-inflammatory peptides expressed by activated primary rat microglia calculated using RT–PCR. *P < 0.05 compared to control; #P < 0.05 compared to LPS exposure
Table 1.
Sequences and sizes of primers used for reverse transcriptase polymerase chain reaction (RT–PCR)
| Gene | Primer sequences | Product size (bp) |
|---|---|---|
| β-actin | F: 5′-TAC AAC CTC CTT GCA GCT CC-3′ | 630 |
| R: 5′-GGA TCT TCA TGA GGT AGT CTG TC-3′ | ||
| ERβ | F: 5′-TCC CTC TTT GCG TTT GGA CTA-3′ | 262 |
| R: 5′-TTC CCG GCA GCA CCA GTA ACC-3′ | ||
| ERα | F: 5′-AAT TCT GAC AAT CGA CGC CAG-3′ | 344 |
| R: 5′-GTG CTT CAA CAT TCT CCC TCC TC-3′ | ||
| IL-1α | F: 5′-AAGATGTCCAACTTCACCTTCAAGGAGAGCCG-3′ | 491 |
| R: 5′-AAGATGTCCAACTTCACCTTCAAGGAGAGCCG-3′ | ||
| IL-1β | F: 5′-CCAGGATGAGGACCCAAGCA-3′ | 519 |
| R: 5′-TCCCGACCATTGCTGTTTCC-3′ | ||
| TNF-α | F: 5′-TACTGAACTTCGGGGTGATCGGTCC-3′ | 297 |
| R: 5′-CAGCCTTGTCCCTTGAAGAGAACC-3′ | ||
| COX-2 | F: 5′-CCT GAG TGG GAT GAC GA-3′ | 207 |
| R: 5′-CGG ATG CCA GTG ATA GAG-3′ | ||
| m-calpain | F: 5′-GGG CAG ACC AAC ATC CAC CTC AGC AAA AAC-3′ | 404 |
| R: 5′-GTC TCG ATG CTG AAG CCA TCT GAC TTG AT-3′ | ||
| Calpastatin | F: 5′-AGT AGT TCT GGA CCC AAT G-3′ | 230 |
| R: 5′-CCC CAG TAG ACT TCT CTT TC-3′ | ||
| Bax | F: 5′-GCA GGG AGG ATG GCT GGG GAG A-3′ | 352 |
| R: 5′-TCC AGA CAA GCA GCC GCT CAC G-3′ | ||
| Bcl-2 | F: 5′-GGA TGA CTT CTC TCG TCG CTA C-3′ | 255 |
| R: 5′-TGC AGA TGC CGG TTC AG-3′ |
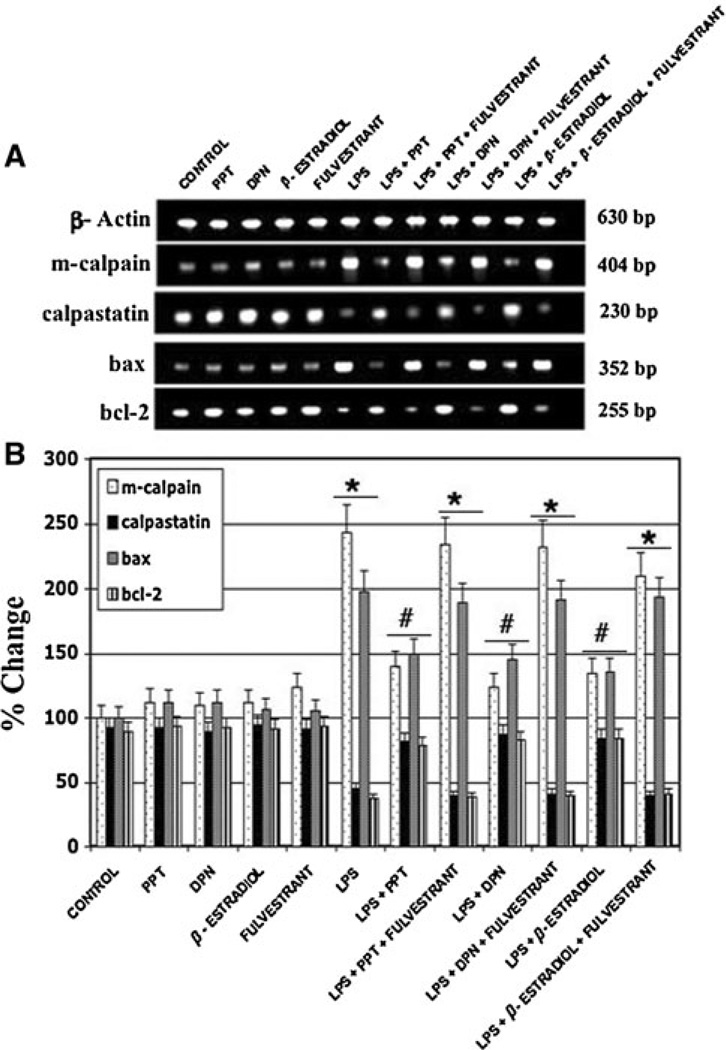
LPS Treatment Upregulates Calapin and Pro-Apoptotic Protein Production in Microglia
Expression of mRNA levels of both pro- and anti-apoptotic proteins following microglial exposure to LPS was also quantified using RT–PCR (Fig. 5). The pro-apoptotic proteins investigated in this study included bax and the calcium activated neutral proteases or calpains. We also examined expression of the anti-apoptotic proteins bcl-2 and calpastatin, an endogenous inhibitor of calpain. Microglia exposed to LPS for 24 h showed increased expression of bax and calpain and decreased mRNA levels of bcl-2 and calpastatin. Treatment of activated microglia with estrogen or ER agonists attenuated expression of the apoptotic proteins bax and calpain and up-regulated the anti-apoptotic proteins bcl-2 and calpastatin. No significant differences were found in the expression of apoptotic mediators between treatment with 17β-estradiol, PPT, and DPN. These findings indicate both ERα and ERβ activation may play a role in attenuation of microglial apoptosis following LPS toxicity.
Fig. 5.
Expression of pro-apoptotic and anti-apoptotic proteins by microglia following exposure to LPS and subsequent treatment with estrogen or ER agonist. *P < 0.05 compared to control; #P < 0.05 compared to LPS exposure
Discussion
Estrogen has been proposed as a potential treatment for neurodegenerative disorders due to the potent anti-oxidant, anti-inflammatory, and anti-apoptotic properties of this steroid hormone [34]. Experimental evidence also suggests estrogen has a neuroprotective effect in animal models of ischemia, multiple sclerosis, and PD [12–14, 35]. Additionally, estrogen receptor agonists have shown potential as neuroprotective agents in in vivo experimental autoimmune encephalomyelitis [36, 37]. The current study expands upon our understanding of the mechanisms by which estrogen treatment attenuates LPS-induced microglial activation and release of inflammatory agents. To our knowledge, the treatment of activated microglia with both ERα and ERβ agonists has not been reported until now. Utilization of specific agonists allowed us to further isolate the roles different ER sub-types play in attenuation of the microglia-mediated inflammatory response.
Numerous clinical and experimental findings suggest microglia-mediated inflammation may play a significant role in the progressive loss of neurons characteristic of neurodegenerative disorders [1–3]. Overall, our studies confirm the findings of other reports that LPS toxicity causes activation of microglia and subsequent production of pro-inflammatory agents including NO, IL-1α, IL-1β, TNF-α, and COX-2 [19, 27] However, treatment with estrogen or estrogen agonists appears to attenuate the actions of microglia following LPS exposure. The specific effects of estrogen or estrogen agonists may be dependent on which ER sub-type is activated. Specifically, we have shown that an increase in the expression of ERβ may play a significant role in cytoprotection of activated microglia. Estrogen-mediated microglial protection may play an essential role in maintaining proper homeostasis following CNS insult [4, 5]. Furthermore, in our study, an increase in the ERβ expression following treatment with 17β-estradiol or DPN provided greater attenuation of NO production. Attenuation of microglial NO release by estrogen or DPN may be effective in reducing neuronal damage by lowering free-radical concentrations in the CNS.
We have also shown that activation and up-regulation of ERα plays an important role in attenuation of the release of cytokines and COX-2. Binding of ERα by PPT lead to the greatest inhibition of microglial production of IL-1α, IL-1β, TNF-α, and COX-2. Thus, it appears that ERα may play a more significant role in the inflammatory response of microglia than ERβ. This finding indicates an effective therapeutic strategy to combat the characteristic neuroinflammation underlying neurodegenerative disorders may involve the use of an agent which activates ERα. Such a role for ERα predominantly in inflammation has been implicated in experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS). In some studies ERβ has been attributed to neurodegeneration.
Finally, findings generated in the current study indicate that estrogen treatment of microglia following LPS toxicity inhibits activation of apoptosis. Estrogen, the ERα agonist PPT, and the ERβ agonist DPN all decreased mRNA levels of the pro-apoptotic proteins bax and calpain in LPS-exposed microglia. Furthermore, activation of both ERα and ERβ increased levels of bcl-2 and calpastatin, an endogenous peptide calpain inhibitor. No significant differences were noted between the use of estrogen, DPN, or PPT in relation to attenuation of apoptosis and increased expression of the anti-apoptotic proteins bcl-2 and calpastatin. These results indicate that both ERα and ERβ may play a role in estrogen-mediated attenuation of microglial apoptosis following exposure to LPS. These findings are consistent with the increase in cell viability among LPS-induced microglia treated with estrogen or DPN and suggest loss of microglia due to LPS toxicity occurs via apoptosis. The action of estrogen and ER agonists in inhibition of microglial apoptosis could potentially maintain proper CNS homeostasis after insult.
In summary, the present study expands upon current understanding of the role of microglia in mediating neuroinflammation. Activation of microglia by LPS toxicity caused increased production of NO, cytokines, COX-2 and apoptotic proteins, along with decreased microglial viability. However, we have shown here that, through the actions of both ERα and ERβ, treatment with estrogen or ER agonists attenuates activation of microglia following LPS exposure. Activated microglia treated with estrogen or ER agonists showed a significantly decreased inflammatory response and cell death. Because neuroinflammation plays a significant role in neurodegenerative disorders, estrogen or estrogen agonists may represent an effective new treatment strategy to decrease the neuronal damage and subsequent function losses associated with neurodegeneration. More work is needed to understand how estrogen or estrogen agonists mediate neuroprotection in in vivo settings.
Acknowledgments
Dr. Yu’s work has formed an essential foundation upon which future research and clinical pursuits may build.
Contributor Information
Joshua A. Smith, Department of Neurosciences, Medical University of South Carolina, 96 Jonathan Lucas Street, 310 Clinical Sciences Building, Charleston, SC 29425, USA
Arabinda Das, Department of Neurosciences, Medical University of South Carolina, 96 Jonathan Lucas Street, 310 Clinical Sciences Building, Charleston, SC 29425, USA.
Jonathan T. Butler, Department of Neurosciences, Medical University of South Carolina, 96 Jonathan Lucas Street, 310 Clinical Sciences Building, Charleston, SC 29425, USA
Swapan K. Ray, Department of Pathology, Microbiology and Immunology, University of South Carolina School of Medicine, Columbia, SC, USA
Naren L. Banik, Email: baniknl@musc.edu, Department of Neurosciences, Medical University of South Carolina, 96 Jonathan Lucas Street, 310 Clinical Sciences Building, Charleston, SC 29425, USA.
References
- 1.Calabrese V, Scapagnini G, Ravagna A, et al. Nitric oxide synthase is present in the cerebrospinal fluid of patients with active multiple sclerosis and is associated with increases in cerebrospinal fluid protein nitrotyrosine and S-nitrosothiols and with changes in glutathione levels. J Neurosci Res. 2002;70:580–587. doi: 10.1002/jnr.10408. [DOI] [PubMed] [Google Scholar]
- 2.McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: epidemiological, animal model, and clinical studies. Neurobiol Aging. 2007;28:639–647. doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Zandi PP, Anthony JC, Hayden KM, et al. Reduced incidence of AD with NSAID but not H2 receptor antagonists: the Cache County Study. Neurology. 2002;59:880–886. doi: 10.1212/wnl.59.6.880. [DOI] [PubMed] [Google Scholar]
- 4.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 5.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 6.Dheen ST, Kaur C, Ling EA. Microglia activation and its implications in the brain diseases. Curr Med Chem. 2007;14:1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- 7.McGeer PL, Kawamata T, Walker DG. Microglia in degenerative neurological disease. Glia. 1993;7:84–92. doi: 10.1002/glia.440070114. [DOI] [PubMed] [Google Scholar]
- 8.Aktas O, Smorodchenko A, Brocke S. Neuronal damage in autoimmune neuroinflammation mediated by the death ligand TRAIL. Neuron. 2005;46:421–432. doi: 10.1016/j.neuron.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Purisai MG, McCormack AL, Cumine S. Microglia activation as a priming event lead to paraquat-induced dopaminergic cell degeneration. Neurobiol Dis. 2007;25:392–400. doi: 10.1016/j.nbd.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickson DW, Lee SC, Mattiace SH, et al. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer’s disease. Glia. 1993;7:75–83. doi: 10.1002/glia.440070113. [DOI] [PubMed] [Google Scholar]
- 11.Henkel JS, Engelhardt JI, Siklos L. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol. 2004;55:221–235. doi: 10.1002/ana.10805. [DOI] [PubMed] [Google Scholar]
- 12.Garay L, Gonzalez Deniselle MC, Gierman L, et al. Steroid protection in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Neuroimmunomodulation. 2008;15:76–83. doi: 10.1159/000135627. [DOI] [PubMed] [Google Scholar]
- 13.Jia J, Guan D, Zhu W, et al. Estrogen inhibits Fas-mediated apoptosis in experimental stroke. Exp Neurol. 2009;215:48–52. doi: 10.1016/j.expneurol.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morissette M, Al Sweidi S, Callier S, et al. Estrogen and SERM neuroprotection in animal models of Parkinson’s disease. Mol Cell Endocrinol. 2008;290:60–69. doi: 10.1016/j.mce.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Bayir H, Marion DW, Puccio AM, et al. Marked gender effect on lipid peroxidation after severe traumatic brain injury in adult patients. J Neurotrauma. 2004;21:1–8. doi: 10.1089/089771504772695896. [DOI] [PubMed] [Google Scholar]
- 16.Bode FJ, Stephan M, Suhling H, et al. Sex differences in a transgenic rat model of Huntington’s disease: decreased 17 beta-estradiol levels correlate with reduced numbers of DARPP32 + neurons in males. Hum Mol Gen. 2008;17:2595–2609. doi: 10.1093/hmg/ddn159. [DOI] [PubMed] [Google Scholar]
- 17.Frutiger K, Lukas TJ, Gorrie G, et al. Gender differences in levels of Cu/Zn superoxide dismutase (SOD1) in cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2008;9:184–187. doi: 10.1080/17482960801984358. [DOI] [PubMed] [Google Scholar]
- 18.Kupina NC, Detloff MR, Bobrowski WF, et al. Cytoskeletal protein degradation and neurodgeneration evolves differently in males and females following experimental head injury. Exp Neurol. 2003;180:55–73. doi: 10.1016/s0014-4886(02)00048-1. [DOI] [PubMed] [Google Scholar]
- 19.Dimayuga FO, Reed JL, Carnero GA, et al. Estrogen and brain inflammation: effects on microglial expression of MHC, costimulatory molecules and cytokines. J Neuroimmunol. 2005;161:123–136. doi: 10.1016/j.jneuroim.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Moosmann B, Behl C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc Natl Acad Sci USA. 1999;96:8867–8872. doi: 10.1073/pnas.96.16.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tripanichkul W, Sripanichkulchai K, Finkelstein DI. Estrogen down-regulates glial activation in male mice following 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine intoxication. Brain Res. 2006;1084:28–37. doi: 10.1016/j.brainres.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 22.Vegeto E, Bonincontro C, Pollio G. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J Neurosci. 2001;21:1809–1818. doi: 10.1523/JNEUROSCI.21-06-01809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruce-Keller AJ, Keeling JL, Keller JN. Antiinflammatory effects of estrogen on microglial activation. Endocrinology. 2000;141:3646–3656. doi: 10.1210/endo.141.10.7693. [DOI] [PubMed] [Google Scholar]
- 24.Drew PD, Chavis JA. Female sex steroids: effects upon microglial cell activation. J Neuroimmunol. 2000;111:77–85. doi: 10.1016/s0165-5728(00)00386-6. [DOI] [PubMed] [Google Scholar]
- 25.Zemlyak I, Brooke S, Sapolsky R. Estrogenic protection against gp120 neurotoxicity: role of microglia. Brain Res. 2005;1046:130–136. doi: 10.1016/j.brainres.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 26.Baker AE, Brautigam VM, Watters JJ. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor beta. Endocrinology. 2004;145:5021–5032. doi: 10.1210/en.2004-0619. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Fan XL, Zhao Y, et al. Estrogen provides neuroprotection against activated microglia-induced dopaminergic neuronal injury through both estrogen receptor-alpha and estrogen receptor-beta in microglia. J Neurosci Research. 2005;81:653–665. doi: 10.1002/jnr.20583. [DOI] [PubMed] [Google Scholar]
- 28.Tapia-Gonzalez S, Carrero P, Pernia O, et al. Selective estrogen receptor (ER) modulators reduce microglia reactivity in vivo after peripheral inflammation: potential role of microglial ERs. J Endocrinol. 2008;198:219–230. doi: 10.1677/JOE-07-0294. [DOI] [PubMed] [Google Scholar]
- 29.Das A, Sribnick EA, Wingrave JM, et al. Calpain activation in apoptosis of ventral spinal cord 4.1 (VSC4.1) motoneurons exposed to glutamate: calpain inhibition provides functional neuroprotection. J Neurosci Res. 2005;81:551–562. doi: 10.1002/jnr.20581. [DOI] [PubMed] [Google Scholar]
- 30.Das A, Banik NL, Ray SK. Molecular mechanisms of the combination of retinoid and interferon-gamma for inducing differentiation and increasing apoptosis in human glioblastoma T98G and U87MG cells. Neurochem Res. 2009;34:87–101. doi: 10.1007/s11064-008-9669-x. [DOI] [PubMed] [Google Scholar]
- 31.Davidge ST, Baker PN, Laughlin MK, et al. Nitric oxide produced by endothelial cells increases production of eicosanoids through activation of prostaglandin H synthase. Circ Res. 1995;77:274–283. doi: 10.1161/01.res.77.2.274. [DOI] [PubMed] [Google Scholar]
- 32.Ray SK, Neuberger TJ, Deadwyler G, et al. Calpain and calpastatin expression in primary oligodendrocyte culture: preferential localization of membrane calpain in cell processes. J Neurosci Res. 2002;70:561–569. doi: 10.1002/jnr.10414. [DOI] [PubMed] [Google Scholar]
- 33.Pan XD, Chen XC, Zhu YG, et al. Neuroprotective role of tripchloride on inflammatory neurotoxicity induced by lipopolysaccharide-activated microglia. Biochem Pharmacol. 2008;76:362–372. doi: 10.1016/j.bcp.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 34.Sribnick EA, Ray SK, Banik NL. Estrogen as a multiactive neuroprotective agent in traumatic injuries. Neurochem Res. 2004;29:2007–2014. doi: 10.1007/s11064-004-6874-0. [DOI] [PubMed] [Google Scholar]
- 35.Sawada H, Shimohama S. Neuroprotective effects of estradiol in mesencephalic dopaminergic neurons. Neurosci Biobehav Rev. 2000;24:143–147. doi: 10.1016/s0149-7634(99)00059-7. [DOI] [PubMed] [Google Scholar]
- 36.Tiwari-Woodruff S, Morales LB, Lee R, et al. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER) alpha and ERbeta ligand treatment. Proc Natl Acad Sci USA. 2007;104:14813–14818. doi: 10.1073/pnas.0703783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crawford DK, Mangiardi M, Song B, et al. Oestrogen receptor beta ligand: a novel treatment to enhance endogenous functional remyelination. Brain. 2010;133:2999–3016. doi: 10.1093/brain/awq237. [DOI] [PMC free article] [PubMed] [Google Scholar]