Abstract
Objective
This study sought to determine the impact of fat gain and its distribution on endothelial function in lean healthy humans.
Background
Endothelial dysfunction has been identified as an independent predictor of cardiovascular events. Whether fat gain impairs endothelial function is unknown.
Methods
A randomized controlled study to assess the effects of fat gain on endothelial function. We recruited 43 normal weight healthy volunteers (mean age 29 years; 18 women). Subjects were assigned to gain weight (approximately 4 kg) (n=35) or to maintain weight (n=8). Endothelial function (brachial artery flow mediated dilation -FMD) was measured at baseline, after fat gain (8 weeks) and after weight loss (16 weeks) for fat-gainers and at baseline and follow-up (8 weeks) for weight-maintainers. Body composition was measured by DXA and abdominal CT scans.
Results
After an average weight gain of 4.1 kg, fat-gainers significantly increased their total, visceral and subcutaneous fat. Blood pressure and overnight polysomnography did not change after fat gain or loss. FMD remained unchanged in weight-maintainers. FMD decreased in fat-gainers (9.1 ± 3% vs. 7.8 ± 3.2%, p =0.003), but recovered to baseline when subjects shed the gained weight. There was a significant correlation between the decrease in FMD and the increase in visceral fat gain (rho = −0.42, p=0.004), but not with subcutaneous fat gain (rho = −0.22, p=0.15).
Conclusions
In normal weight healthy young subjects, modest fat gain results in impaired endothelial function, even in the absence of changes in blood pressure. Endothelial function recovers after weight loss. Increased visceral rather than subcutaneous fat predicts endothelial dysfunction.
Keywords: weight gain, endothelial function, visceral fat
Introduction
Endothelial dysfunction is considered a systemic process and an early event in the atherosclerotic process (1). Brachial artery flow mediated dilation (FMD) reflects coronary endothelial function (2,3) and has been associated with a higher prevalence of coronary artery disease (1), and as an independent predictor of cardiovascular events in patients with and without established atherosclerosis (4,5).
Increased body fat has been linked to a higher risk for cardiovascular disease. Previous studies assessing obesity and endothelial dysfunction have been cross-sectional in nature and thus no causal interaction can be defined (6,7). Although visceral obesity is predictive of increased cardiovascular risk, there are no data examining the interactions between visceral obesity and endothelial function. We tested the hypothesis that endothelial function is impaired after weight gain, and recovers after reversal of weight gain. We also tested the hypothesis that impaired endothelial function is linked to visceral or subcutaneous fat accumulation.
Methods
We recruited 43 healthy volunteers with a baseline body mass index (BMI) between 18.5 to 24.9 kg/m2. Subjects were excluded if they were smokers, were pregnant, were taking any medication or had any acute or chronic illness. This study was approved by the Mayo Clinic Institutional Review Board, and all subjects signed a written informed consent.
Fat-gainer and weight-maintainer protocols
After a weight maintenance period of 3 days, subjects were randomly assigned to be in the fat-gainer or weight-maintainer group, with a 20% chance of being a weight-maintainer. For the fat-gainer group, for the first 8 weeks, each subject received 1000 kcal/d (40% carbohydrate, 40% fat, and 20% protein) in addition to weight maintenance requirements. The goal was to gain 3 to 4 kg of total body fat (5% increase in weight). After the fat gain, subjects underwent a diet program to return to their basal weight. Dietary counseling was available to all participants and their weight was monitored by a dietitian throughout the study. Exercise treadmill testing was conducted to assess changes in levels of physical fitness during the study.
Body composition
We measured height by wall stadiometer, weight by electronic scale, and waist and hip circumferences by non-elastic tape. The volunteers underwent computed tomography (CT) measures of visceral fat area (single slice CT at the L2–3 interspace) and dual-energy x-ray absorptiometry (DXA) (Lunar Radiation, Madison, WI) at baseline and after 8 weeks for both groups and after weight loss (16 weeks) for fat-gainers. For logistic reasons, CT and DXA measures were obtained in all 35 subjects before and after weight gain and in 16 subjects after weight loss.
Vascular Studies
All subjects were asked to abstain from alcohol and caffeine for 24 hours before the study. Subjects underwent complete overnight polysomnography to exclude development of sleep apnea using the apnea-hypopnea index (events/hour) 8 with weight gain. FMD was measured in the morning with the subjects fasting, with high resolution ultrasound following a standard protocol as previously described (9).
Blood measurements
Blood samples were obtained at the three time points. Leptin was measured using a RIA kit (Linco, St Louis, MO), insulin was measured using a two-site immuno enzymatic assay (Beckman Instruments, Chaska, MN), adiponectin was measured using an ELISA kit (Mediagnost, Reutlingen, Germany), glucose was measured using hexokinase reagent using Hitachi 912 chemistry analyzer (Boehringer Mannheim, Indianapolis, IN) and lipid profile (Cholesterol, triglycerides, and HDL) was measured using the standard turbidometry method on Hitachi 912 (Roche Diagnostic). High sensitivity CRP was measured on a Hitachi 912 chemistry analyzer using a polystyrene particle enhanced immunoturbidometric assay (DiaSorin).
Statistical analysis
Data were summarized by means and standard deviation for quantitative variables and number and percentages for categorical variables. The changes between baseline and after 8 weeks were compared using paired t-test analyses. An additional analysis was performed for fat-gainers after weight loss. We used an unpaired t test to compare fat-gainers and weight-maintainers at baseline and at 8 weeks (non-parametric test showed similar results). Due to the non-linearity of the data, we used Spearman correlation coefficients between the changes in FMD with total, visceral and subcutaneous fat gain, and changes in cardiometabolic biomarkers.
Finally, we divided into tertiles the amount of visceral and subcutaneous fat gained to assess the relationship between magnitude of fat gain and changes in FMD among fat-gainers. Data were analyzed with JMP (SAS Institute, Cary, NC). Two-tail p-values <0.05 were considered significant. Bonferroni correction was used to adjust for multiple comparisons and a two-tail p-value <0.016 was considered significant.
Results
We recruited 43 lean healthy volunteers, 35 fat-gainers and 8 weight-maintainers. Mean age was 29 ± 6 years and 18 (42%) were women. Fat-gainers were slightly older than weight-maintainer (26 ± 3 vs. 29 ± 7 years, p = 0.05), but there were no other significant differences between the two groups (Table 1).
Table 1.
Subjects characteristics at baseline and during the fat-gainer and weight-maintainer protocols
| Variable | Fat-gainers (n=35) | Weight-maintainers (n=8) | |||
|---|---|---|---|---|---|
| Baseline | Fat-Gain | Recovery § | Baseline | Follow-up | |
| Age, years | 29.6 ± 7.1 | - | - | 26 ± 3.5 | - |
| Female, % | 13 (37.1) | - | - | 5 (62.5) | - |
| Systolic blood pressure, mmHg | 116.2 ± 13 | 117.4 ± 16.7 | 116.5 ± 13.5 | 114.2 ± 11 | 111.7 ± 7.6 |
| Diastolic blood pressure, mmHg | 70.3 ± 9.7 | 69.4 ± 9.9 | 70.0 ± 8.1 | 69 ± 6.2 | 2.8 ± 10.1 |
| Heart rate, beats per minute | 62.2 ± 10 | 66.7 ± 13.4 | 64.3 ± 12.0 | 67.3 ± 11 | 67.5 ± 13.5 |
| Weight, kg | 70.8 ± 13 | 75.1 ± 13.5* | 72.1 ± 12.9 | 66.6 ± 13 | 66.6 ± 13.5 |
| BMI, kg/m2 | 23.1 ± 2.9 | 24.6 ± 3.1* | 23.5 ± 2.7 | 22.3 ± 1.9 | 22.3 ± 1.8 |
| Waist circumference, cm | 83.2 ± 7.5 | 86.2 ± 7.6* | 84.0 ± 8.1 | 76.8 ± 8.1 | 77.5 ± 8.5 |
| Hip circumference, cm | 97.2 ± 4.7 | 100.7 ± 5.7† | 98.1 ± 6.2 | 95.5 ± 4.0 | 95.8 ± 4.2 |
| Body fat, percent | 25.7 ± 9.5 | 28.3 ± 9.4* | 26.3 ± 9.5 | 25.9 ± 5.8 | 26.2 ± 5.4 |
| Total body fat area, cm2 | 164.0 ± 78 | 206.8 ± 87* | 171 ±1.83 | 129.9 ± 43 | 127.0 ± 31 |
| Subcutaneous fat area, cm2 | 116.7 ± 57 | 146.6 ± 64* | 122.5 ± 60 | 93.8 ± 23 | 94.2 ± 17 |
| Visceral fat area, cm2 | 47.3 ± 31.4 | 60.1 ± 35* | 48.5 ± 33.2 | 36.0 ± 27 | 32.8 ± 20 |
| Apnea-hypopnea index | 0.64 ± 1.1 | 0.56 ± 0.83 | 0.62 ± 0.98 | 0.43 ± 0.6 | 1.75 ± 2.5 |
| Insulin, uU/mL | 4.5 ± 1.8 | 6.2 ± 3.2 ‡ | 4.7 ± 3.1 | 4.3 ± 1.1 | 4.3 ± 1.7 |
| Glucose, mg/dL | 94 ± 8 | 95 ± 11 | 90 ± 9 ‡ | 88 ± 6.4 | 88 ± 4.2 |
| Leptin, ng/mL | 6.8 ± 5.5 | 11.4 ± 8.9 * | 6.7 ± 6.9 | 5.9 ± 3.4 | 6.4 ± 4.2 |
| Adiponectin, ng/mL | 8376 ± 3772 | 9179 ± 4281 ‡ | 9451 ± 4662 | 8829 ± 3601 | 8167 ± 3019 |
| CRP, mg/dL # | 0.03 ± 0.02 | 0.06 ± 0.08 | 0.03± 0.01 | 0.26 ± 0.54 | 0.09 ± 0.11 |
| Cholesterol, mg/dL | 155± 24 | 160 ± 34 | 151 ± 20 | 160 ± 20 | 162 ± 17 |
| HDL, mg/dL | 37 ± 10 | 36 ± 9.3 | 43 ± 10 ‡ | 45.2 ± 7.7§ | 44.8 ± 6.7 |
| Triglycerides, mg/dL | 82 ± 29 | 95 ± 54 | 90 ± 33 | 78 ± 17 | 69 ± 15 |
| FMD % change | 9.02 ± 2.8 | 7.86 ±3.24 ‡ | 9.19 ± 3.80 | 8.2 ± 2.1 | 8.4 ± 4.2 |
BMI = body mass index; FMD = flow mediated dilation.
Between group comparisons:
P-value <0.05.
Within group comparisons:
P-value <0.0001,
P-value <0.001
P-value <0.05 when compared to baseline
CRP levels were obtained in 14 of the 35 subjects. Some patients had CRP levels < 0.02, and for the statistical analysis we considered the CRP level as 0.02.
CT and DXA measures were obtained only in 16 of the 35 subjects.
Weight-maintainers
There were no differences in any variable measured between baseline and 8 weeks after the weight maintenance period (Table 1).
Fat-gainers
After an average weight gain of 4.1 ± 0.20 kg of which > 80% was fat, total body, visceral, and subcutaneous fat area and total body fat percent increased significantly (all p-values <0.0001). After an average of 3.6 kg weight loss at recovery, anthropometric and body composition measures recovered to baseline values (all p-values >0.05). Neither weight gain nor weight loss resulted in significant changes in resting blood pressure or heart rate (Table 1).
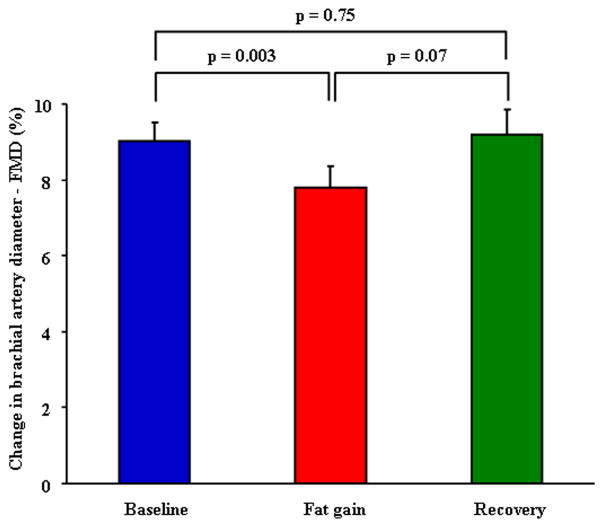
For the fat-gain group, FMD diminished significantly (9.1 ± 3.0 vs. 7.6 ± 3.2, p = 0.003), and recovered to baseline (9.0 ± 3.8) after losing the gained weight (figure 1). For the weight-maintainer group, FMD remained unchanged during the study (p = 0.45, Table 1).
Figure 1. Endothelial Function in Fat Gainers.
Comparison of brachial artery FMD at baseline, after fat gain and subsequent fat loss (recovery) (n=35).
Changes in endothelial function as measured by percent change in FMD after weight gain were not significantly correlated with changes of cardiometabolic biomarkers (Table 2).
Table 2.
Correlation coefficients (rho) between percentage change in FMD and changes in cardiometabolic biomarkers.
| % Change in FMD | ||
|---|---|---|
| rho | P-value | |
| Leptin | 0.08 | 0.66 |
| Adiponectin | −0.16 | 0.39 |
| Insulin | 0.05 | 0.77 |
| C-reactive Protein | −0.12 | 0.68 |
| Glucose | −0.12 | 0.53 |
| HOMA-IR | −0.14 | 0.47 |
| Total cholesterol | −0.12 | 0.51 |
| LDL | −0.17 | 0.34 |
| HDL | 0.07 | 0.70 |
| Triglycerides | 0.18 | 0.33 |
LDL = Low density lipoprotein; HDL = High density lipoprotein.
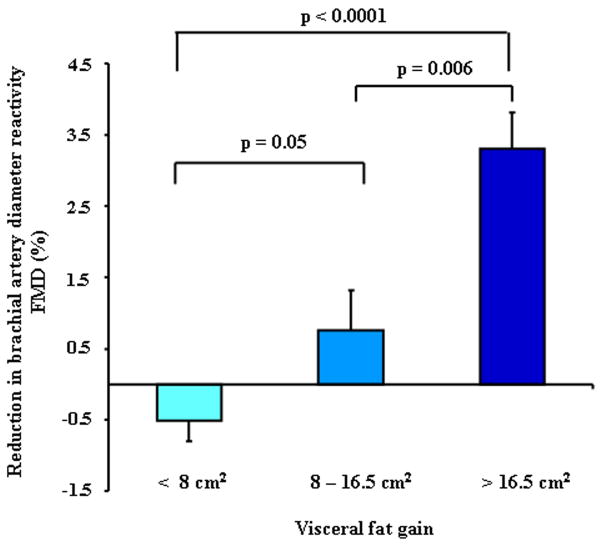
We found a significant and negative correlation between percent changes in FMD with total fat gain (rho = −0.45, p = 0.002) in the abdominal area. After analyzing visceral and subcutaneous fat separately, visceral (rho = −0.42, p = 0.004), but not subcutaneous fat gain (rho = −0.22, p = 0.15) was significantly related to reduction of FMD. Furthermore, the magnitude of impairment in FMD was significantly higher in the subjects who had greater increases in visceral fat (figure 2), Total body fat, BMI, waist circumference and waist-to-hip ratio increments were not related to changes in FMD.
Figure 2. Endothelial Dysfunction by Tertiles of Visceral Fat Gain in the Fat Gain Group.
Endothelial dysfunction (percent reduction in FMD) by tertiles of visceral fat gain in fat-gainers (n=35).
Discussion
The important and novel finding of this study is that modest fat gain in normal weight healthy young subjects under standardized conditions of diet and activity is associated with attenuated endothelial function, even in the absence of changes in blood pressure. Endothelial function recovers after reversal of the fat gain. Importantly, endothelial dysfunction is significantly linked to visceral but not to subcutaneous fat gain.
To our knowledge, this is the first randomized controlled longitudinal study to demonstrate that in normal weight healthy young subjects, modest visceral fat gain is associated with blunted FMD. Endothelial dysfunction is considered an early marker of atherosclerotic disease, with important clinical implications (1, 4, 10–12). We found that FMD was blunted after weight gain, and recovered after restoration of normal weight, suggesting that changes in endothelial function are reversible, at least in the short term. Moreover, our data suggest that the development of endothelial dysfunction precedes any blood pressure increase.
Interestingly, the magnitude of attenuation in endothelial function in healthy subjects, who gained mainly visceral fat, is very similar to that observed with smoking, diabetes and aging (13, 14). Modest short-term weight gain is often an accepted consequence of the holiday season. Our study provides evidence that modest fat gain affects endothelial function, arguing against our cultural permissiveness towards weight gain or “going up a clothing size” as a “normal” phenomenon and strengthens the case for weight control as a means of attenuating cardiovascular risk.
The selective interaction between endothelial dysfunction and visceral fat gain observed is consistent with epidemiologic studies linking cardiovascular risk to central measures of obesity (15,16). Mechanisms linking visceral adiposity to increased risk include higher levels of adipokines and pro-inflammatory molecules (17,18). Moreover, free fatty acid flux of visceral fat is biochemically distinct, and may be more deleterious to the liver, predisposing to insulin resistance (18), and perhaps endothelial dysfunction. Previous studies have shown an association between adiponectin and endothelial function (19,20). However, in our study population FMD was not associated with changes in leptin, adiponectin, and CRP, which is in accordance with a recent study that did not find a significant association between FMD and adiponectin levels in obese subjects (21).
Potential limitations of the study include that the intentional weight gain may not fully reflect the long-term gradual changes occurring in uncontrolled conditions in the general population, suggesting some caution in interpreting the results.
Conclusions
Modest fat gain causes endothelial dysfunction in normal weight healthy young adults, even in the absence of changes in blood pressure and heart rate. Endothelial function recovers after reversal of fat gain. Visceral fat gain is significantly correlated with impaired FMD. Endothelial dysfunction secondary to visceral fat gain may be an important mechanism linking central obesity to increased cardiovascular morbidity and mortality.
Acknowledgments
Funding Sources: ARC was supported by a Postdoctoral Fellowship from the American Heart Association. VKS was supported by NIH Grants R01 HL73211 and R21 DK81014. JSJ was partially supported by faculty funds from the Board of Post-Graduate Education of the Karolinska Institute (KID Award) and by the European Foundation for the Study of Diabetes through a Research Fellowship. FHSK was supported by AHA grant 09-20069G. PS was supported by AHA grant 0725787Z. FLJ is a recipient of a Clinical Scientist Development Award from the American Heart Association.
The authors gratefully acknowledge Ms. Debra L. Pfeifer and Ms. Ann B. Peterson for their superb secretarial and administrative assistance, and the expertise of the CRU Nutrition Research team, specially Ms. Kim L. Edens and Ms. Sunita Nayar.
Footnotes
Conflicts of interest include the following: Abel Romero-Corral, Virend K Somers and Francisco Lopez-Jimenez are recipients of a grant from Select Research for separate work related to measurement of obesity.
References
- 1.Cox DA, Vita JA, Treasure CB, et al. Atherosclerosis impairs flow-mediated dilation of coronary arteries in humans. Circulation. 1989;80:458–465. doi: 10.1161/01.cir.80.3.458. [DOI] [PubMed] [Google Scholar]
- 2.Widlansky ME, Gocke N, Keaney JF. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 3.Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108:2054–2059. doi: 10.1161/01.CIR.0000089191.72957.ED. [DOI] [PubMed] [Google Scholar]
- 4.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–7. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 5.Shimbo D, Grahame-Clarke C, Miyake Y, et al. The association between endothelial dysfunction and cardiovascular outcomes in a population-based multi-ethnic cohort. Atherosclerosis. 2007;192:197–203. doi: 10.1016/j.atherosclerosis.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Sivitz WI, Wayson SM, Bayless ML, Sinkey CA, Haynes WG. Obesity impairs vascular relaxation in human subjects: hyperglycemia exaggerates adrenergic vasoconstriction arterial dysfunction in obesity and diabetes. J Diabetes Complications. 2007;21:149–57. doi: 10.1016/j.jdiacomp.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Hogikyan RV, Galecki AT, Pitt B, Halter JB, Greene DA, Supiano MA. Specific impairment of endothelium-dependent vasodilation in subjects with type 2 diabetes independent of obesity. J Clin Endocrinol Metab. 1998;83:1946–52. doi: 10.1210/jcem.83.6.4907. [DOI] [PubMed] [Google Scholar]
- 8.American Sleep Disorders Association. EEG arousals: scoring rules and examples; a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–184. [PubMed] [Google Scholar]
- 9.Otto ME, Svatikova A, Barretto R, et al. Early morning attenuation of endothelial function. Circulation. 2004;109:2507–2510. doi: 10.1161/01.CIR.0000128207.26863.C4. [DOI] [PubMed] [Google Scholar]
- 10.Neunteufl T, Heher S, Katzenschlager R, et al. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000;86:207–210. doi: 10.1016/s0002-9149(00)00857-2. [DOI] [PubMed] [Google Scholar]
- 11.Gokce N, Keaney JFJ, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 12.Chan SY, Mancini GB, Kuramoto L, Schulzer M, Frohlich J, Ignaszewski A. The prognostic importance of endothelial dysfunction and carotid atheroma burden in patients with coronary artery disease. J Am Coll Cardiol. 2003;42:1037–43. doi: 10.1016/s0735-1097(03)00927-6. [DOI] [PubMed] [Google Scholar]
- 13.Lavi S, Prasad A, Yang EH, et al. Smoking is associated with epicardial coronary endothelial dysfunction and elevated white blood cell count in patients with chest pain and early coronary artery disease. Circulation. 2007;115:2621–7. doi: 10.1161/CIRCULATIONAHA.106.641654. [DOI] [PubMed] [Google Scholar]
- 14.Vehkavaara S, Seppala-Lindroos A, Westerbacka J, Groop PH, Yki-Jarvinen H. In vivo endothelial dysfunction characterizes patients with impaired fasting glucose. Diabetes Care. 1999;22:2055–60. doi: 10.2337/diacare.22.12.2055. [DOI] [PubMed] [Google Scholar]
- 15.Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368(9536):666–78. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 16.de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007;28(7):850–6. doi: 10.1093/eurheartj/ehm026. [DOI] [PubMed] [Google Scholar]
- 17.Guo Z, Hensrud DD, Johnson CM, Jensen MD. Regional postprandial fatty acid metabolism in different obesity phenotypes. Diabetes. 1999;48:1586–92. doi: 10.2337/diabetes.48.8.1586. [DOI] [PubMed] [Google Scholar]
- 18.Votruba SB, Jensen MD. Regional Fat Deposition as a Factor in FFA Metabolism. Annu Rev Nutr. 2007;27:149–63. doi: 10.1146/annurev.nutr.27.061406.093754. [DOI] [PubMed] [Google Scholar]
- 19.Tan KC, Xu A, Chow WS, et al. Hypoadiponectinemia is associated with impaired endothelium-dependent vasodilation. J Clin Endocrinol Metab. 2004;89:765–769. doi: 10.1210/jc.2003-031012. [DOI] [PubMed] [Google Scholar]
- 20.Ouchi N, Ohishi M, Kihara S, et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003;42:231–234. doi: 10.1161/01.HYP.0000083488.67550.B8. [DOI] [PubMed] [Google Scholar]
- 21.Sturm W, Sandhofer A, Engl J, et al. Influence of visceral obesity and liver fat on vascular structure and function in obese subjects. Obesity (Silver Spring) 2009;17(9):1783–8. doi: 10.1038/oby.2009.81. [DOI] [PubMed] [Google Scholar]