Abstract
Drugs of abuse exert their effects by exploiting natural neurobiological reward mechanisms, especially the mesolimbic dopamine (DA) system. However, the mesolimbic system does not operate in isolation, and input from other reward-relevant structures may play a role in cocaine’s rewarding effects. The medial preoptic area (mPOA) of the hypothalamus is involved in the regulation of two essential and naturally rewarding behaviors, sexual and maternal behaviors. It also makes strong neuroanatomical connections with areas of the mesolimbic system, particularly the ventral tegmental area (VTA). As such, the mPOA is a logical candidate for a neuroanatomical locus modulating activity in the mesolimbic system and emergent behavioral expressions of drug reward, yet the role of this structure is largely unexplored. Here, using a female rat model, we show that the mPOA innervates the VTA in a region-specific manner, lesions of the mPOA augment cocaine-induced Fos expression in the nucleus accumbens and cocaine-induced conditioned place preference. We also show that approximately 68% of mPOA-VTA efferents release γ-aminobutyric acid (GABA), over 75% are sensitive to DA as evidenced by co-localization with DA receptors, and nearly 60% of these contain both DA receptors and GABA, which suggests a novel key role for the mPOA in the inhibition of the mesolimbic DA circuit. Combined, these results reveal the mPOA as a critical modulating structure in cocaine-induced mesolimbic activity and behavioral manifestation of reward, at least in part via GABAergic output that is sensitive to DA input.
Keywords: medial preoptic area, reward, cocaine, addiction, dopamine
INTRODUCTION
Appropriate behavioral responses to natural reward are necessary for survival and reproductive success. To this end, neurobiological reward mechanisms have evolved to reinforce behaviors such as sexual interactions and parental care. However, drugs of abuse like cocaine subvert this natural reward/reinforcement system. In particular, the mesolimbic system is recognized as playing a critical role in reward, and cocaine exerts its psychostimulant effects by producing activity in this pathway (Kalivas & Duffy, 1990; Koob, Sanna, & Bloom, 1998; Nestler, 2005; Wise, 2002). However, this system does not operate in isolation, and input from other reward-relevant structures may play a critical role in cocaine-induced activity.
An area that is vital for natural reward but has received little attention as a potential modulator of cocaine-induced activity is the medial preoptic area (mPOA) of the hypothalamus. The hypothalamus is well-known for its role in motivated behaviors and reinforcement, and the mPOA in particular is critically involved in the regulation of two essential and naturally rewarding functions-- sexual (Hull & Dominguez, 2007; Hull, Dominguez, & Muschamp, 2007) and maternal (J. S. Lonstein, 2002; Numan & Insel, 2003) behaviors. It also strongly interacts with areas of the mesolimbic system, particularly the ventral tegmental area (VTA) (Simerly & Swanson, 1988; Zahm et al., 2011). As such, the mPOA is a logical candidate for a neuroanatomical locus modulating cocaine-induced activity, yet surprisingly little is known about the role of this structure in the context of drug abuse. This is a critical gap in knowledge because the mPOA is also a central integrative region for gonadal hormone stimulation, and as such, studies elucidating on its role in drug response will shed light on mechanisms through which hormones might impact drug activity.
The purpose of the present study was to determine if and how the mPOA modulates cocaine-induced neural and behavioral response. To this end, a series of four experiments investigated in female rats: (1) the extent and distribution of anatomical interactions between the mPOA and VTA; (2) whether the mPOA modulates cocaine-induced activity in the nucleus accumbens (NAc); (3) whether the mPOA modulates behavioral expression of cocaine reward. Because GABA in the VTA regulates dopaminergic function and a large number of GABA-containing neurons reside in the mPOA, we also examined (4) whether cells in the mPOA that project to the VTA contain GABA and/or dopamine (DA) receptors. Results obtained in these experiments will help resolve whether the mPOA modulates neural and behavioral responses to cocaine.
MATERIALS AND METHODS
Subjects
Adult female Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) were singly housed in a climate-controlled room (22° C; 40–50% humidity) on a reverse light-dark cycle (14:10; lights off at 10 A.M) with food and water freely available. All rats were ovariectomized and implanted with an intrascapular estrogen silastic capsule (5% 17-β-estradiol benzoate, 95% cholesterol; 12 mm in length; 1.98 mm I.D.×3.18 mm O.D.; Dow Corning, Midland, MI). Procedures were in accordance with the National Institutes of Health Guidelines for the Use of Animals in Research and were approved by the University of Texas at Austin Institutional Animal Care and Use Committee.
Lesions of the mPOA
All lesions or sham lesions (n = 93) were performed using a Radionics radio-frequency lesion generator, with a TCZ thermo-coupled electrode (0.25 mm exposed tip). Surgeries were performed with the animals situated in a stereotaxic apparatus while still under general anesthesia directly after the ovariectomy. Bilateral lesions were aimed at the rostro-central mPOA (AP, −0.25 mm; ML, ±0.6 mm; DV, −8.3 mm; according to coordinates from Paxinos and Watson (2007)). Once the electrode reached the mPOA, the temperature was raised to, and maintained at, +80°C (±3°C) for 20 seconds. This procedure was also done in a bilateral sham-treatment group, without introduction of radio frequencies. Animals were allowed a three-week recovery before beginning procedures for cocaine-induced conditioned place preference or cocaine-induced cellular activity.
Ablations produced with radiofrequency-lesion generators are more reproducible in size and shape than are those produced with electrolytic or chemical techniques. For example, electrolytic lesions can vary up to a factor of four in terms of size and geometry, after fixed lesion current and time were applied (Sweet & Mark, 1953). The reason for this variability is that electrolytic lesions are influenced by electrolysis action, gas formation, anatomy of tissue planes, and nearby vascular interruption, which can vary between animals. Lesions produced with radio frequencies do not present these problems, thus the radiofrequency technique was employed for our experiments.
After all tests were completed, lesion placements were verified histologically. Coronal brain sections including the mPOA were processed using methyl green, a Nissl stain that allows visualization of all cell nuclei. Any animal that did not have an mPOA lesion was removed from further analyses.
Conditioned Place Preference
We utilized a fully biased place-conditioning paradigm using automated two-chambered CPP apparatuses (San Diego Instruments Place Preference System, San Diego, CA). The inner dimensions of each conditioning chamber were 35 cm wide×21 cm deep×34.5 cm high. The left chamber featured a smooth black plastic floor whereas the right chamber featured a haircell textured black plastic floor. Each individual chamber featured an overhead array of experimenter-operated switchable and dimmable white and red LED lights. The red LED light was at maximum intensity on the side with the smooth floor. Each apparatus contained a 16×4 photobeam array for recording time spent in each chamber (sec), number of chamber transitions, exploratory approaches to adjacent chambers, gross locomotor activity (consecutive breaks of adjacent beams), and fine motor activity (repeated breaks of the same beam).
All animals (n = 44) were allotted 20 minutes free access to the entire apparatus as a baseline pre-conditioning test (PRE test session). For conditioning, drug-paired chamber assignments were based on the animals’ initial non-preferred side during the PRE. For the conditioning cycles, all animals received an intraperitoneal injection of saline (1mL/kg) and were secluded to their initially preferred chamber for 30 minutes. Directly thereafter, they received either a 10 mg/kg intraperitoneal (i.p.) injection of cocaine, or equivolume saline for control animals, followed by confinement to their respective drug-paired chambers for 30 min. We repeated this pattern for two conditioning cycles, and then provided each animal 20 minutes of access to the entire apparatus to assess preferences for the drug-paired chamber (POST test session). The four groups consisted of those having sham lesions and vehicle injection (SV; n = 12), sham lesions and cocaine injection (SC; n = 13), mPOA lesions and vehicle injection (LV; n = 10), or mPOA lesions and cocaine injection (LC; n = 9).
Retrograde and Anterograde Tract-Tracer Injections
Anatomical connections between the mPOA and the VTA were examined using the retrograde tract-tracer Fluorogold (Fluorochrome, LLC, Denver, CO) injected into the VTA (AP, −6.2 mm; ML, +0.7 mm; DV, −8.2 mm; according to Paxinos and Watson (2007)) or the anterograde tract-tracer biotinylated dextran amine 10000 MW (BDA; Sigma-Aldrich, St. Louis, MO) injected into the mPOA (AP, −0.25 mm; ML −0.5 mm; DV, −8.5 mm). Surgeries were performed with assistance of a stereotaxic apparatus while animals were still under general anesthesia immediately after the ovariectomy. The skull was exposed via a medial incision and the brain exposed directly above the regions of interest. A glass electrode (opening, 30 µm diameter) containing the tract tracer was lowered to the predetermined coordinates. The tract-tracer was iontophoretically introduced (+7 µA; 7 seconds on, 1 second off for 5 minutes) through a silver wire inserted into the electrode with the tract-tracer solution using an iontophoretic pump (Midgard Precision Current Source, Stoelting Co., Wood Dale, IL). The electrode was left in place for one minute after the injection and then slowly removed from the brain.
Tissue Collection
To assess cocaine-induced neural activity in the mesolimbic system, animals were injected with cocaine (10mg/kg; i.p.) one hour before being euthanized. As for the other experiments, one to three days after the CPP POST or seven to ten days after the tract-tracer injections, animals were euthanized with a lethal dose of Euthasol (0.3 mL/animal, Virbac Animal Health, Inc., Fort Worth, TX). The thoracic and abdominal cavities were exposed and the descending aorta was clamped. Rats were then perfused with 50 ml of 0.1M PBS followed by 250 ml of 4% paraformaldehyde in 0.1M PB via transcardial puncture. The brain was quickly removed, post-fixed for 1 hour in the same fixative. Thereafter, the brains were transferred to a solution containing 30% sucrose in 0.1M PB. Brains were stored and maintained at 4 °C for at least 48 hrs until sectioning. Coronal sections, cut at 35 μm, were stored at −20 °C in cryoprotectant solution containing 30% ethylene glycol, 30% sucrose, and 0.0002% sodium azide in 0.1 Μ PB.
Immunohistochemistry
Free-floating sections containing the NAc, mPOA, or superior gray of the colliculus (SuG) were extensively rinsed in 0.1M PB between incubations. All incubations were performed at room temperature with gentle agitation. Sections were blocked with 1% H2O2 for 10 min and then soaked for 1-hr in incubation-block solution (0.1% BSA, 0.4% Triton-X, in 0.1M PB). Sections were then incubated overnight (16-hrs) with a polyclonal antibody recognizing Fos (rabbit anti-Fos, 1:5000; Santa Cruz Biotechnology, Santa Cruz, CA). After incubation with primary antibody, sections were exposed to biotin-conjugated goat anti-rabbit IgG (1:500 in incubation solution) for one hour. Secondary antibody was followed by incubation in avidin-biotin complex (Vector Laboratories, Burlingame, CA) for one hour; tissue was then transferred to a solution containing 0.02% 3,3’-diaminobenzidine (DAB), 0.1% H202 in 0.1M PB, and 2% nickel sulfate. Extensive washing terminated this reaction. Fos-positive cells were counted in the regions of interest using NIH freeware Image J. Four sections for both the NAc core and shell [two rostral and two caudal; 0.65 mm2 area for core (NAcc) and 0.50 mm2 area for the shell (NAcs)] were arbitrarily chosen from each animal, and the immunoreactive cells were counted for each section.
Immunofluorescence was employed to examine colocalization of FG tract-tracer, GABA, and dopamine D2 receptor (D2R) in the mPOA, or BDA, the nuclear protein DAPI, and VGAT in the VTA. Colocalization of all antigens was obtained using a procedure similar to the one described above, with the exception of DAPI and Fluorogold due to its autofluorescent properties (a blue 461 nm wavelength as its emission maximum) and BDA. Visualization of GABA and D2R were visualized after blocking sections for 10 min with 1% H2O2 and incubating the tissue overnight in blocking solution with a primary antibody recognizing GABA raised in mouse (1:16,000; Fluorochrome, LLC, Denver, CO). Sections were then exposed for 60 min to biotin-conjugated goat anti-mouse IgG (1:200 in incubation solution; Vector Laboratories, INS, Burlingame, CA) and for 60 min to an avidin-biotin complex (1:1000 in 0.1M PB; ABC-elite; Vector Laboratories, Burlingame, CA). Thereafter the tissue was incubated in a solution containing biotinylated tyramide (1:1000; in 0.1M PB; Perkin Elmer, Waltham, MA) for 10 minutes. An Alexa Fluor 488-tagged streptavidin (1:400 in 0.1M PB; Life Technologies, Grand Island, NY) was then introduced for one hour. The second fluorescence complex was visualized with the same procedure described above but without the enhancement steps. An anti-rabbit D2R antibody (1:800; EMD Millipore, Billerica, MA) was introduced followed by an Alexa Fluor 555-tagged goat anti-rabbit secondary antibody (1:200; Life Technology Corporation, Carlsbad, CA). The anterograde tract-tracer BDA was visualized in coronal sections containing the VTA using the same enhancement steps. The tissue was incubated in Alexa Fluor 488-tagged streptavidin for 30 minutes. Primary antibodies used were rabbit anti-VGAT (1:2400; EMD Millipore, Billerica, MA) followed by secondary antibodies (Alexa Fluor 555-tagged goat anti-rabbit, 1:100, Life Technology Corporation, Carlsbad, CA). Sections were mounted and coverslipped. Control sections for all antibodies included omission of the primary antibody, which resulted in no staining.
Statistical Analyses
All data were analyzed using PASW statistical package (18th Ed). The numbers of immuno-positive single- and double-labeled cells were analyzed with an ANOVA or Kruskal-Wallis non-parametric test with Mann-Whitney U used to determine between group differences. The conditioned place preference post-test was analyzed via ANCOVA with total ambulations as the covariate. Tukey post-hoc analyses were used to test between group differences if the interaction between the independent variables were significant. Statistical significance was set at α = 0.05 for all analyses.
RESULTS
Experiment 1: The mPOA innervates the VTA in a region-specific manner
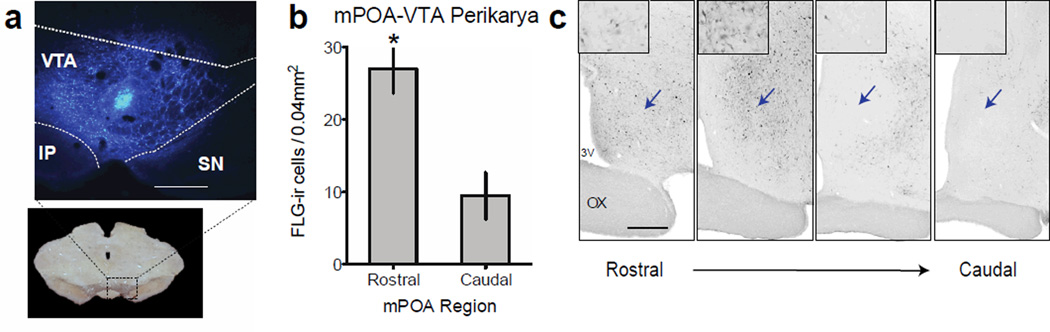
After injections of the retrograde tract-tracer Fluorogold (FG) into the VTA or the NAc followed by analysis of FG-positive cells in the mPOA, we found dense projections from the mPOA to the VTA but very few projections to the NAc, consistent with previous reports (Simerly & Swanson, 1988). A closer analysis of regions within the mPOA revealed a significantly greater number of FG-positive cells projecting to the VTA in the rostral compared to the caudal mPOA (paired-samples t(13)= 3.939, p < 0.001, Figure 1). The discovery of this anatomical profile is especially interesting because the rostral regions have been previously linked to the appetitive facets of motivated behavior, particularly mating behavior, while the more caudal regions are involved in consummatory behaviors (Balthazart & Ball, 2007). This finding also suggests that modulation of reward by the mPOA is contingent on activity in its rostral region.
Figure 1.
Distribution of afferents to the VTA coming from the mPOA. (a) Representative injection site of Fluorogold in the VTA (bar, 500µm). (b) Graph demonstrating that the number of mPOA-VTA efferents is higher in the rostral mPOA than in the caudal mPOA. (c) Photomicrographs of coronal sections profiling the rostro-caudal concentration of efferents in the mPOA, indicated by arrows. Scale bar, 250µm; values, mean ± SEM; * p < 0.001. Abbreviations: SN = substantia nigra; IP = interpeduncular nucleus; OX = optic chiasm; 3V = Third ventricle.
Experiment 2: Lesions of the mPOA increase cocaine-induced activity in the NAc
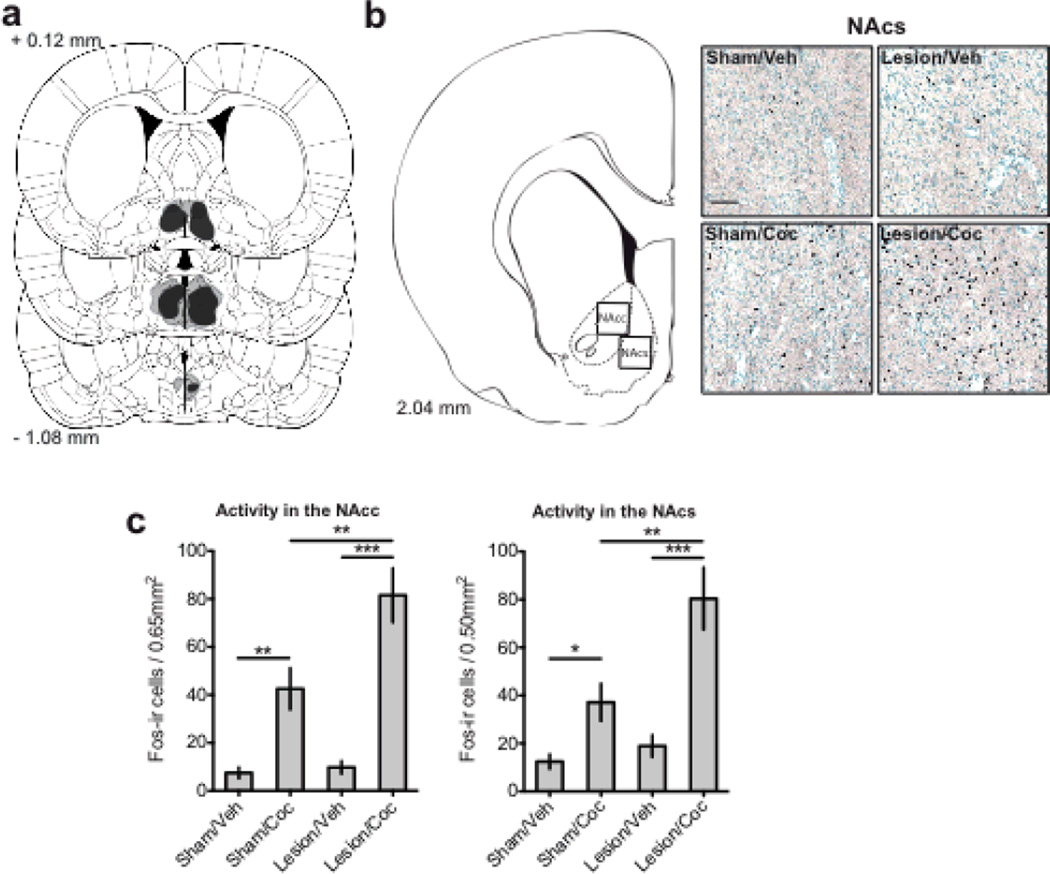
To help establish a functional connection between the mPOA and cocaine-induced mesolimbic activity, we compared Fos-positive immunoreactivity in the NAc of animals with or without radiofrequency lesions of their mPOA, following intraperitoneal cocaine (10 mg/kg) or vehicle injections. Consistent with previous findings (Graybiel, Moratalla, & Robertson, 1990; Young, Porrino, & Iadarola, 1991; Zahm et al., 2010), cocaine administration in intact animals activated the NAc as evidenced by an increased number of Fos-positive cells versus vehicle controls. However, animals with lesions of their mPOA had a significantly greater response to cocaine versus animals with sham lesions. Specifically, removal of the mPOA augmented cocaine-induced activity in the NAc (Figure 2); this effect was observed in both the NAc core (NAcc; F(1,50)= 8.978, p < 0.01) and shell regions (NAcs; F(1,50)= 9.105, p < 0.01).
Figure 2.
Lesions of the mPOA increased cocaine-induced activity in the NAc. (a) Representative perimeters of smallest (black) and largest (gray lines) lesion for animals with radiofrequency lesions of their mPOA, drawn from Paxinos and Watson (2007). (b) Representative photomicrographs of Fos-ir in the NAcs of animals receiving cocaine or vehicle injections and lesion or sham-lesion of their mPOA. (c) Graph demonstrating the number of Fos-positive cells in the NAcc and NAcs across treatment conditions. Scale bar, 50 µm; values are expressed as mean ± SEM; * p < 0.05, ** p < 0.01, *** p < 0.001. Coronal plates were adapted from The Rat Brain in Stereotaxic Coordinates (6th ed.), Fig 2a from pages 75, 80, and 84, Fig 2b from page 58, by G. Paxinos & C. Watson, 2007, New York, NY: Academic Press. Copyright 2007 by Elsevier Academic Press; adapted with permission.
Experiment 3: Lesions of the mPOA increase cocaine-induced conditioned place preference
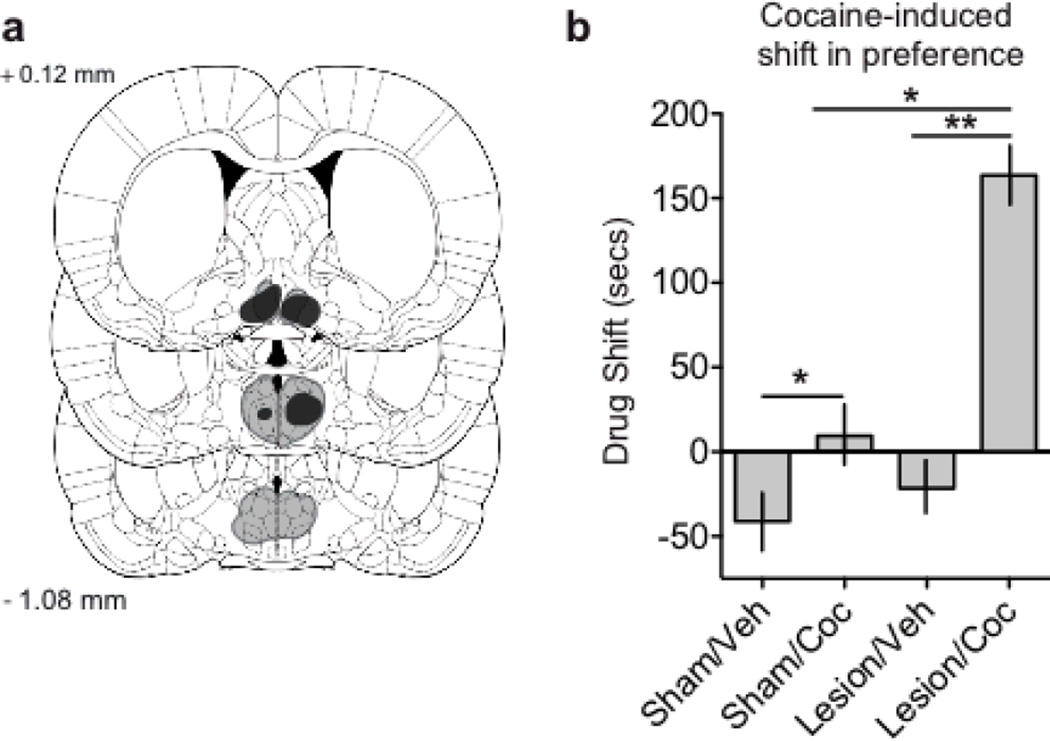
To address the issue of whether the increased neural activity described above translates into behavioral expression of reward, we utilized cocaine-induced conditioned place preference (CPP). Results showed that lesions of the mPOA indeed increased the effects of cocaine on reward, as evidenced by a greater cocaine-induced preference in animals with mPOA lesions versus both their vehicle-treated lesioned counterparts and their cocaine-treated sham-lesioned controls (F(1.50)= 9.105, p < 0.01; Figure 3). Together these three experiments reveal a modulatory role for neural mechanisms in the mPOA on cocaine responses upstream in the mesolimbic system and subsequent behavioral manifestation of reward.
Figure 3.
Lesions of the mPOA increased cocaine-induced conditioned place preference. (a) Representative perimeters of smallest (black) and largest (gray lines) lesion for animals with radiofrequency lesions of their mPOA, drawn from Paxinos and Watson (2007). (b) Graph demonstrating the shift in chamber preference across treatment conditions, cocaine treated animals displayed a cocaine-induced shift in time spent in an initially non-preferred chamber, whereas animals receiving lesions of the mPOA displayed an even greater shift. Values are expressed as mean ± SEM; * p < 0.05, ** p < 0.01. Coronal plates were adapted from The Rat Brain in Stereotaxic Coordinates (6th ed.), Fig 3a from pages 75, 80, and 84, by G. Paxinos & C. Watson, 2007, New York, NY: Academic Press. Copyright 2007 by Elsevier Academic Press; adapted with permission.
Experiment 4: mPOA-VTA efferents are GABAergic and receptive to DA
While the mPOA appears to play a modulatory role in cocaine response, the phenotypic characteristics of the mPOA-VTA efferents governing this relationship have not been fully investigated. Given (1) that a large number of GABA-containing neurons reside in the mPOA (Gao & Moore, 1996), (2) the importance of VTA GABA on cocaine-induced dopaminergic function (Jhou, Fields, Baxter, Saper, & Holland, 2009; Kaufling, Veinante, Pawlowski, Freund-Mercier, & Barrot, 2010; Lecca, Melis, Luchicchi, Muntoni, & Pistis, 2012), and (3) the fact that our lesions increased rather than decreased cocaine reward and neural activity, suggesting removal of an inhibitory mechanism, our initial efforts focused on whether mPOA-VTA efferents arise from GABAergic neurons and, furthermore, whether these neurons are sensitive to DA. This experiment will show whether the mPOA contributes to GABA activity in the VTA, which is recognized as a tonic modulator of DA release in the NAc (Jhou et al., 2009; Johnson & North, 1992; Laviolette & van der Kooy, 2001; Nestler, 2005; Shank, Seitz, Bubar, Stutz, & Cunningham, 2007; Stobbs et al., 2004; van Zessen, Phillips, Budygin, & Stuber, 2012). This experiment would also show whether DA acting in the mPOA influences this output.
After injecting FG in the VTA (n = 6), we examined FG-positive, GABA-positive, and D2R-positive cells in the rostral mPOA and discovered that a large percent of mPOA-VTA efferents contained GABA (67.6 ± 6.5%) and D2R (76.3 ± 6.5%), while 58.3% ± 7.3% contained both GABA and D2R (Figure 4A). To further validate the apparent GABAergic profile of mPOA-VTA efferents, we injected the anterograde tract tracer biotinylated dextran amine 10000 MW (BDA) in the mPOA followed by analysis of BDA and the vesicular transporter protein for GABA (VGAT) in the VTA. Qualitative analyses revealed that many of these fibers contained VGAT (Figure 4B), further demonstrating that many mPOA-VTA efferents are GABAergic. We note that the neurotransmitter content associated with mPOA-VTA efferents was heretofore unknown, so our initial analysis of exclusively GABA-containing neurons does not discount possible influences of other neurotransmitters. Nonetheless, our results indicate that the rostral mPOA modulates mesolimbic activity via GABAergic output, which may then regulate DA release in the NAc. Moreover, this mPOA output itself is receptive to DA activity, as evidenced by DA receptors co-localized with efferent neurons.
Figure 4.
mPOA-VTA efferents contain GABA and DA receptors. (a) Confocal photomicrograph demonstrating co-localization of mPOA-VTA efferents (Fluorogold, blue), GABA (green), and D2R (red). Analyses of mPOA-VTA efferents revealed that 67.6 ± 6.5% contained GABA, 76.3 ± 6.5% contained D2R, while 58.3% ± 7.3% contained both GABA and D2R, indicated by arrows. (b) Following injection of the anterograde tract tracer biotinylated dextran amine (BDA) into the mPOA, qualitative analyses of BDA (green) and the vesicular transporter protein for GABA (VGAT, red) in the VTA revealed that most BDA-positive fibers contained VGAT, further validating the GABAergic profile of this pathway. Also shown here is DAPI nuclear labeling in blue; scale bars are 10 µm.
DISCUSSION
The findings presented here reveal for the first time that the mPOA modulates cocaine-induced neural and behavioral activity. We discovered a dense concentration of efferent GABAergic neurons residing in the rostral mPOA that project to the VTA and are receptive to DA. A functional link between the mPOA and mesolimbic system was established with lesions of the mPOA, which enhanced cocaine-induced neural activity in the NAc and enhanced behavioral expression of cocaine reward, presumably due to the removal of inhibitory mPOA efferents to the VTA. Taken together, the present study reveals a robust modulatory role of the mPOA in cocaine-induced brain activity and behavior.
It is well established that DA in the NAc, which arises from neurons residing in the VTA, plays a vital role in the regulation of drug and natural reward, including reproductive and maternal behaviors. Studies demonstrating that mating activates cells in the NAc and increases DA levels support this conclusion (Hull & Dominguez, 2007; Hull et al., 2007). For example, NAc DA and Fos activity increase in anticipation of sexual activity and further increases during mating in males and females (Bradley & Meisel, 2001; Damsma, Pfaus, Wenkstern, Phillips, & Fibiger, 1992; Jenkins & Becker, 2003; Pfaus & Phillips, 1991). As with sex, DA is also integral to the expression of maternal behaviors. To this point, consider studies showing that presenting pups to a lactating dam increases levels of DA (Hansen, Bergvall, & Nyiredi, 1993) and Fos activity (Fleming, Suh, Korsmit, & Rusak, 1994) in the NAc, whereas removal of DA fibers or pharmacological blockage of DA receptors in the NAc impairs maternal behaviors (Hansen, 1994; Keer & Stern, 1999). These and other studies establish mesolimbic activity as a critical component in the regulation of these two naturally rewarding behaviors.
By artificially stimulating these same neuronal mechanisms, drugs of abuse like cocaine subvert the natural reward system. Supporting this conclusion is evidence that rats will self-administer amphetamine (Hoebel et al., 1983; Phillips, Robbins, & Everitt, 1994), DA reuptake inhibitors (Carlezon, Devine, & Wise, 1995), and DA receptor agonists directly into the NAc (Ikemoto, Glazier, Murphy, & McBride, 1997). A similarly rewarding effect is observed using CPP; when given a choice between an environment previously paired with microinjections of DA agonists into the NAc or an environment paired with vehicle injections, animals prefer the drug-paired environment (Carr & White, 1983, 1986; White, Packard, & Hiroi, 1991). Given that cocaine increases DA in the NAc, and lesions of DA fibers in the NAc severely inhibit cocaine self-administration and CPP (Lyness, Friedle, & Moore, 1979; Pettit, Ettenberg, Bloom, & Koob, 1984), it has been concluded that activity in the mesolimbic system serves as a critical mechanism responsible for the psychostimulant effects of the drug, much in the same way as it does for natural reward.
Activity of VTA DA neurons is governed by inhibitory input acting on GABAA and GABAB receptors, excitatory input acting on NMDA and non-NMDA receptors, and dopaminergic input acting on primarily D2 receptors (Johnson & North, 1992). Of neurons residing in the VTA, approximately 65% are dopaminergic (Margolis, Lock, Hjelmstad, & Fields, 2006; Nair-Roberts et al., 2008), which give rise to DA in the NAc (Björklund & Lindvall, 1984; Fallon & Moore, 1978; Phillipson & Griffiths, 1985; Swanson, 1982). Consequently, modulation of these cells leads to dynamic changes in DA in the NAc and ensuing reward responses following a natural or drug stimulus. The prominence of GABA’s influence on DA neurons in the VTA for NAc DA and stimulus reinforcement is now well established (Jhou et al., 2009; Johnson & North, 1992; Laviolette & van der Kooy, 2001; Shank et al., 2007; Stobbs et al., 2004), as several studies illustrate the importance of these mechanisms. Studies using intracranial self-administration show that rats will self-administer a GABAA antagonist (picrotoxin) into the anterior VTA, but not the substantia nigra or other areas near the VTA (Ikemoto, Murphy, & McBride, 1997). Ikemoto and colleagues also showed increased DA in the NAc of rats that received picrotoxin into the anterior VTA (Ikemoto, Kohl, & McBride, 1997). Co-infusion of muscimol, a GABAA agonist, reduced both the intracranial self-administration of picrotoxin and the increase release of DA in the NAc (Ikemoto, Kohl, et al., 1997; Ikemoto, Murphy, et al., 1997). A more recent study performed in mice showed that optogenetic activation of VTA GABA neurons suppresses DA neurons in the VTA and the release of DA in the NAc, while also disrupting sucrose consumption (van Zessen et al., 2012). These and other findings establish that disinhibition of VTA DA neurons increases extracellular DA in the NAc and is reinforcing.
It is understood that GABA coming into the VTA arises locally in the VTA and in the rostromedial tegmental nucleus. Here, however, we showed for the first time that at least a portion of the GABA entering the VTA originates in the mPOA (Experiment 4). This link may help explain why lesions of the mPOA augmented cocaine-induced activity in the NAc (Experiment 2) and the ensuing cocaine-reward response as evidenced by CPP (Experiment 3), since the lesions effectively removed a source of GABAergic input into the VTA, which allowed for the disinhibition of its output into the NAc. These findings point to a modulatory role for the mPOA in cocaine-induced mesolimbic activity, through GABAergic connections coming primarily from its rostral region (Experiment 1).
The existence of this connection should not be surprising, given that the mPOA regulates naturally rewarding sexual and maternal behaviors (Stolzenberg & Numan, 2011). The mPOA also contains robust connections with the mesolimbic system (Simerly & Swanson, 1988). The importance of the mPOA for mating is evidenced in several studies showing that lesions significantly impair both male and female sexual behavior, whereas stimulation facilitates both consummatory and appetitive aspects of behavior (Guarraci & Clark, 2006; Hull & Dominguez, 2007; Numan & Insel, 2003; Yang & Clements, 2000). Conversely, neuronal activity in the mPOA increases with mating and in the presence of sexually relevant stimuli (Hull & Dominguez, 2007; Hull et al., 2007; Pfaus, 1999; Pfaus & Heeb, 1997). It is interesting to note the anatomical specificity of mPOA-VTA efferents, which were seen primarily in the rostral regions of the mPOA, because studies point to the rostral mPOA as important for the appetitive aspects of sexual behavior, whereas the caudal region is involved in primarily consummatory behaviors (Balthazart & Ball, 2007). Moreover, with regard to the mPOA and maternal behavior, in rat dams, pre- and post-partum lesions of this area severely disrupt maternal behaviors; by contrast, neuronal activity increases in the mPOA of dams when exposed to pups and while expressing behaviors (J.S. Lonstein & Morrell, 2007; Numan & Insel, 2003). Finally, disruption of connections between the mPOA and VTA attenuate retrieval behavior (Numan & Stolzenberg, 2009) and impede operant responses to obtain access to pups (Lee, Clancy, & Fleming, 2000). Together these data have led to the proposal that mPOA efferents activate VTA DA input to the NAc (Numan & Stolzenberg, 2009). Initially our findings appear to counter this proposed model, as we found that a large number of cells projecting from the mPOA to the VTA are GABA-producing neurons. However, a more recent view of the mPOA and its function suggests varying roles for different subregions within the mPOA in regulating different aspects of behaviors (Balthazart & Ball, 2007). In fact, large-bilateral lesions of the mPOA are required to impact behavior, whereas smaller or unilateral lesions have little, if any, impact (Hull & Dominguez, 2007; Numan & Stolzenberg, 2009). Our experiments focused on the rostral mPOA, where we discovered that 68% of mPOA-VTA efferents were GABA-producing neurons, albeit most, other non-GABAergic cells comprise this efferent tract and may also impact mesolimbic activity in different ways.
A wealth of pharmacological, neurochemical, and endocrinological evidence demonstrates a very important role for gonadal hormones in modulating rewarding stimuli, including cocaine (Carroll, Lynch, Roth, Morgan, & Cosgrove, 2004; Evans, Haney, & Foltin, 2002; Larson, Anker, Gliddon, Fons, & Carroll, 2007; Lynch, 2006; Lynch, Roth, & Carroll, 2002; Russo et al., 2003). However, the neuroanatomical locus of gonadal hormone influences on cocaine effects remains uncertain. Although our experiments did not explicitly examine hormonal action, it is still possible to speculate on the neuroendocrine implications of our findings. The highest concentration of estrogen (Shughrue, Lane, & Merchenthaler, 1997; Simerly, Chang, Muramatsu, & Swanson, 1990), progesterone (Hagihara, Hirata, Osada, Hirai, & Kato, 1992; Quadros, Pfau, & Wagner, 2007), and androgen receptors (Simerly et al., 1990) in the CNS are found primarily in the mPOA, which is a known neuroanatomical locus through which gonadal hormones act to modulate natural reward (Hull et al., 1999; J.S. Lonstein & Morrell, 2007). Our results now show that the mPOA also modulates cocaine reward, at least partly via GABA into the VTA. Given its abundant receptivity to gonadal hormones, ongoing and future experiments will examine whether the mPOA is a window via which gonadal hormones impact cocaine reward responses, in much the same way that it influences other naturally rewarding processes.
Finally, we should like to comment on cocaine and gender-sensitive differences. Cocaine affects females differently than males (e.g. Lynch et al., 2002; Becker et al., 2001). In light of our results, this is significant because the mPOA contains the first discovered sexually dimorphic nucleus (SDN); the SDN of the preoptic area (SDN-POA) is structurally five to eight times larger in male rats than in female rats (Gorski et al., 1978). Sexual dimorphism persists in the MPOA even to the level of the synapse, as female rats have more synapses on dendritic spines and fewer on shafts than do males (Raisman and Field, 1971). A homologous SDN exists in the human hypothalamus (Swaab et al., 2001), which is also larger in men than in women. This dimorphism is important to note because females have an increased response to cocaine after estrogen replacement, whereas males do not have this same response. Here, we showed a modulatory role for the mPOA in cocaine-induced activity; therefore it is reasonable to expect that in this capacity, the mPOA may also influence gender-sensitive differences in cocaine activity. Ongoing and future experiments will examine whether the mPOA modulates these gender differences.
CONCLUSIONS
In conclusion, we discovered a dense concentration of efferent GABAergic neurons residing in the rostral mPOA of female rats that project to the VTA and are receptive to DA. A functional link between the mPOA and mesolimbic system was established with lesions of the mPOA, which enhanced cocaine-induced neural activity in the NAc and enhanced behavioral expression of cocaine reward, presumably due to the removal of the inhibitory mPOA efferents to the VTA. Taken together, the present data suggest a modulatory role of the mPOA in cocaine-induced neural and behavioral activity.
Acknowledgments
Funding for this project was provided by NIDA grants R21-DA023604 and R01-DA032789 to Juan M. Dominguez. We wish to thank Dr. Andrea Gore (Univ of Texas at Austin), Dr. Elaine Hull (Florida State Univ), and Dr. Timothy Schallert (Univ of Texas at Austin) for helpful insight and important perspectives. We also wish to thank Ms. Julie Hayes at the Microscopy and Imaging Facility of the Institute for Cellular and Molecular Biology at The University of Texas at Austin for assistance with confocal microscopy, and members of the Dominguez Lab for general assistance.
Contributor Information
Daniel J. Tobiansky, Department of Psychology, The University of Texas at Austin
Peter G. Roma, Institutes for Behavior Resources, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine
Tomoko Hattori, Department of Psychology, The University of Texas at Austin.
Ryan G. Will, Department of Psychology, The University of Texas at Austin
Victoria L. Nutsch, The Institute for Neuroscience, The University of Texas at Austin
Juan M. Dominguez, Department of Psychology, The University of Texas at Austin The Institute for Neuroscience, The University of Texas at Austin; The Waggoner Center for Alcohol and Addiction Research, The University of Texas at Austin.
REFERENCES
- Balthazart J, Ball GF. Topography in the preoptic region: Differential regulation of appetitive and consummatory male sexual behaviors. Front Neuroendocrinol. 2007 doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann N Y Acad Sci. 2001;937:172–187. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Björklund A, Lindvall O. Dopamine-containing systems in the CNS. In: Björklund A, Hökfelt T, editors. Classical Transmitters in the CNS. Vol. 2. Amsterdam: Elsevier; 1984. pp. 55–122. [Google Scholar]
- Bradley KC, Meisel RL. Sexual behavior induction of c-Fos in the nucleus accumbens and amphetamine-stimulated locomotor activity are sensitized by previous sexual experience in female Syrian hamsters. The Journal of neuroscience. 2001;21(6):2123–2130. doi: 10.1523/JNEUROSCI.21-06-02123.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Devine DP, Wise RA. Habit-forming actions of nomifensine in nucleus accumbens. Psychopharmacology (Berl) 1995;122(2):194–197. doi: 10.1007/BF02246095. [DOI] [PubMed] [Google Scholar]
- Carr GD, White NM. Conditioned place preference from intra-accumbens but not intra-caudate amphetamine injections. Life Sci. 1983;33(25):2551–2557. doi: 10.1016/0024-3205(83)90165-0. [DOI] [PubMed] [Google Scholar]
- Carr GD, White NM. Anatomical disassociation of amphetamine's rewarding and aversive effects: an intracranial microinjection study. Psychopharmacology (Berl) 1986;89(3):340–346. doi: 10.1007/BF00174372. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25(5):273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behav Neurosci. 1992;106(1):181–191. doi: 10.1037//0735-7044.106.1.181. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159(4):397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180(3):545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Suh EJ, Korsmit M, Rusak B. Activation of Fos-like immunoreactivity in the medial preoptic area and limbic structures by maternal and social interactions in rats. Behav Neurosci. 1994;108(4):724–734. doi: 10.1037//0735-7044.108.4.724. [DOI] [PubMed] [Google Scholar]
- Gao B, Moore RY. The sexually dimorphic nucleus of the hypothalamus contains GABA neurons in rat and man. Brain research. 1996;742(1–2):163–171. doi: 10.1016/s0006-8993(96)01005-0. [DOI] [PubMed] [Google Scholar]
- Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci U S A. 1990;87(17):6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarraci FA, Clark AS. Ibotenic acid lesions of the medial preoptic area disrupt the expression of partner preference in sexually receptive female rats. Brain research. 2006;1076(1):163–170. doi: 10.1016/j.brainres.2005.12.120. [DOI] [PubMed] [Google Scholar]
- Hagihara K, Hirata S, Osada T, Hirai M, Kato J. Distribution of cells containing progesterone receptor mRNA in the female rat di- and telencephalon: an in situ hybridization study. Brain Res Mol Brain Res. 1992;14(3):239–249. doi: 10.1016/0169-328x(92)90179-f. [DOI] [PubMed] [Google Scholar]
- Hansen S. Maternal behavior of female rats with 6-OHDA lesions in the ventral striatum: characterization of the pup retrieval deficit. Physiol Behav. 1994;55(4):615–620. doi: 10.1016/0031-9384(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Hansen S, Bergvall AH, Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav. 1993;45(3):673–676. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- 20.Hoebel BG, Monaco AP, Hernandez L, Aulisi EF, Stanley BG, Lenard L. Self-injection of amphetamine directly into the brain. Psychopharmacology (Berl) 1983;81(2):158–163. doi: 10.1007/BF00429012. [DOI] [PubMed] [Google Scholar]
- Hull EM, Dominguez JM. Sexual behavior in male rodents. Horm Behav. 2007;52(1):45–55. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Dominguez JM, Muschamp JW. Neurochemical mediators of male sexual behavior. In: Lajtha A, Blaustein J, editors. Handbook of Neurochemistry and Molecular Biology. 3rd Ed. Vol. 3rd. New York: Springer; 2007. pp. 37–94. [Google Scholar]
- Hull EM, Lorrain DS, Du J, Matuszewich L, Lumley LA, Putnam SK, Moses J. Hormone-neurotransmitter interactions in the control of sexual behavior. Behav Brain Res. 1999;105(1):105–116. doi: 10.1016/s0166-4328(99)00086-8. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci. 1997;17(21):8580–8587. doi: 10.1523/JNEUROSCI.17-21-08580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Kohl RR, McBride WJ. GABA(A) receptor blockade in the anterior ventral tegmental area increases extracellular levels of dopamine in the nucleus accumbens of rats. J Neurochem. 1997;69(1):137–143. doi: 10.1046/j.1471-4159.1997.69010137.x. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Murphy JM, McBride WJ. Self-infusion of GABA(A) antagonists directly into the ventral tegmental area and adjacent regions. Behav Neurosci. 1997;111(2):369–380. doi: 10.1037//0735-7044.111.2.369. [DOI] [PubMed] [Google Scholar]
- Jenkins WJ, Becker JB. Dynamic increases in dopamine during paced copulation in the female rat. Eur J Neurosci. 2003;18(7):1997–2001. doi: 10.1046/j.1460-9568.2003.02923.x. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61(5):786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse. 1990;5(1):48–58. doi: 10.1002/syn.890050104. [DOI] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. gamma-Aminobutyric acid cells with cocaine-induced DeltaFosB in the ventral tegmental area innervate mesolimbic neurons. Biol Psychiatry. 2010;67(1):88–92. doi: 10.1016/j.biopsych.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Keer SE, Stern JM. Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol Behav. 1999;67(5):659–669. doi: 10.1016/s0031-9384(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21(3):467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol. 2007;15(5):461–471. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. GABA(A) receptors in the ventral tegmental area control bidirectional reward signalling between dopaminergic and non-dopaminergic neural motivational systems. The European journal of neuroscience. 2001;13(5):1009–1015. doi: 10.1046/j.1460-9568.2001.01458.x. [DOI] [PubMed] [Google Scholar]
- Lecca S, Melis M, Luchicchi A, Muntoni AL, Pistis M. Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse. Neuropsychopharmacology. 2012;37(5):1164–1176. doi: 10.1038/npp.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 2000;108(2):215–231. doi: 10.1016/s0166-4328(99)00170-9. [DOI] [PubMed] [Google Scholar]
- Lonstein JS. Frank A. Beach Award: Parenting--the other reproductive behavior. Horm Behav. 2002;42(3):258–262. doi: 10.1006/hbeh.2002.1818. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Morrell JI. Neuroendocrinology and Neurochemistry of Maternal Motivation and Behavior. In: Blaustein JD, editor. Handbook of Neurochemistry and Molecular Neurobiology. New York, NY: Springer; 2007. pp. 195–246. [Google Scholar]
- Lynch WJ. Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol. 2006;14(1):34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164(2):121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Lyness WH, Friedle NM, Moore KE. Destruction of dopaminergic nerve terminals in nucleus accumbens: effect on d-amphetamine self-administration. Pharmacol Biochem Behav. 1979;11(5):553–556. doi: 10.1016/0091-3057(79)90040-6. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577(Pt 3):907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152(4):1024–1031. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nature neuroscience. 2005;8(11):1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The Neurobiology of Parental Behavior. New York: Springer-Verlag; 2003. [Google Scholar]
- Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol. 2009;30(1):46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl) 1984;84(2):167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- Pfaus JG. Neurobiology of sexual behavior. Current opinion in neurobiology. 1999;9(6):751–758. doi: 10.1016/s0959-4388(99)00034-3. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Heeb MM. Implications of immediate-early gene induction in the brain following sexual stimulation of female and male rodents. Brain research bulletin. 1997;44(4):397–407. doi: 10.1016/s0361-9230(97)00219-0. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Phillips AG. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav Neurosci. 1991;105(5):727–743. doi: 10.1037//0735-7044.105.5.727. [DOI] [PubMed] [Google Scholar]
- Phillips GD, Robbins TW, Everitt BJ. Bilateral intra-accumbens self-administration of d-amphetamine: antagonism with intra-accumbens SCH-23390 and sulpiride. Psychopharmacology (Berl) 1994;114(3):477–485. doi: 10.1007/BF02249339. [DOI] [PubMed] [Google Scholar]
- Phillipson OT, Griffiths AC. The topographic order of inputs to nucleus accumbens in the rat. Neuroscience. 1985;16(2):275–296. doi: 10.1016/0306-4522(85)90002-8. [DOI] [PubMed] [Google Scholar]
- Quadros PS, Pfau JL, Wagner CK. Distribution of progesterone receptor immunoreactivity in the fetal and neonatal rat forebrain. J Comp Neurol. 2007;504(1):42–56. doi: 10.1002/cne.21427. [DOI] [PubMed] [Google Scholar]
- Raisman G, Field PM. Sexual dimorphism in the preoptic area of the rat. Science. 1971;173:731–733. doi: 10.1126/science.173.3998.731. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, Jenab S, Quinones-Jenab V. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003;120(2):523–533. doi: 10.1016/s0306-4522(03)00317-8. [DOI] [PubMed] [Google Scholar]
- Shank EJ, Seitz PK, Bubar MJ, Stutz SJ, Cunningham KA. Selective ablation of GABA neurons in the ventral tegmental area increases spontaneous locomotor activity. Behav Neurosci. 2007;121(6):1224–1233. doi: 10.1037/0735-7044.121.6.1224. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388(4):507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270(2):209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Stobbs SH, Ohran AJ, Lassen MB, Allison DW, Brown JE, Steffensen SC. Ethanol suppression of ventral tegmental area GABA neuron electrical transmission involves N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 2004;311(1):282–289. doi: 10.1124/jpet.104.071860. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Numan M. Hypothalamic interaction with the mesolimbic DA system in the control of the maternal and sexual behaviors in rats. Neurosci Biobehav Rev. 2011;35(3):826–847. doi: 10.1016/j.neubiorev.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Chung WC, Kruijver FP, Hofman MA, Ishunina TA. Structural and functional sex differences in the human hypothalamus. Horm Behav. 2001;40:93–98. doi: 10.1006/hbeh.2001.1682. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain research bulletin. 1982;9(1–6):321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Sweet WH, Mark VH. Unipolar anodal electrolytic lesions in the brain of man and cat; report of five human cases with electrically produced bulbar or mesencephalic tractotomies. AMA Arch Neurol Psychiatry. 1953;70(2):224–234. doi: 10.1001/archneurpsyc.1953.02320320090007. [DOI] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73(6):1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM, Packard MG, Hiroi N. Place conditioning with dopamine D1 and D2 agonists injected peripherally or into nucleus accumbens. Psychopharmacology (Berl) 1991;103(2):271–276. doi: 10.1007/BF02244216. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36(2):229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Yang LY, Clements LG. MPOA lesions affect female pacing of copulation in rats. Behav Neurosci. 2000;114(6):1191–1202. [PubMed] [Google Scholar]
- Young ST, Porrino LJ, Iadarola MJ. Cocaine induces striatal c-fos-immunoreactive proteins via dopaminergic D1 receptors. Proc Natl Acad Sci U S A. 1991;88(4):1291–1295. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Becker ML, Freiman AJ, Strauch S, Degarmo B, Geisler S, Meredith GE, Marinelli M. Fos after single and repeated self-administration of cocaine and saline in the rat: emphasis on the Basal forebrain and recalibration of expression. Neuropsychopharmacology. 2010;35(2):445–463. doi: 10.1038/npp.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Cheng AY, Lee TJ, Ghobadi CW, Schwartz ZM, Geisler S, Parsely KP, Gruber C, Veh RW. Inputs to the midbrain dopaminergic complex in the rat, with emphasis on extended amygdala-recipient sectors. J Comp Neurol. 2011;519(16):3159–3188. doi: 10.1002/cne.22670. [DOI] [PMC free article] [PubMed] [Google Scholar]