Abstract
Modern pupillometry has expanded the study and utility of pupil responses in many new domains including psychiatry, particularly for understanding aspects of cognitive and emotional information processing. Here, we review the applications of pupillometry in psychiatry for understanding patients’ information processing styles, predicting treatment, and augmenting function. In the past year pupillometry has been shown to be useful in specifying cognitive/affective occurrences during experimental tasks and informing clinical diagnoses. Such studies demonstrate the potential of pupillary motility to be used in clinical psychiatry much as it has been in neurology for the past century.
Keywords: pupil, pupillometry, cognition, emotion, psychopathology, depression, anxiety, pupil dilation, psychiatry, cognitive processing, emotional processing, psychotherapy, limbic system, executive control, autism, Parkinson's Disease, Autonomic Dysfunction, brain computer interaction, vulnerability
Introduction
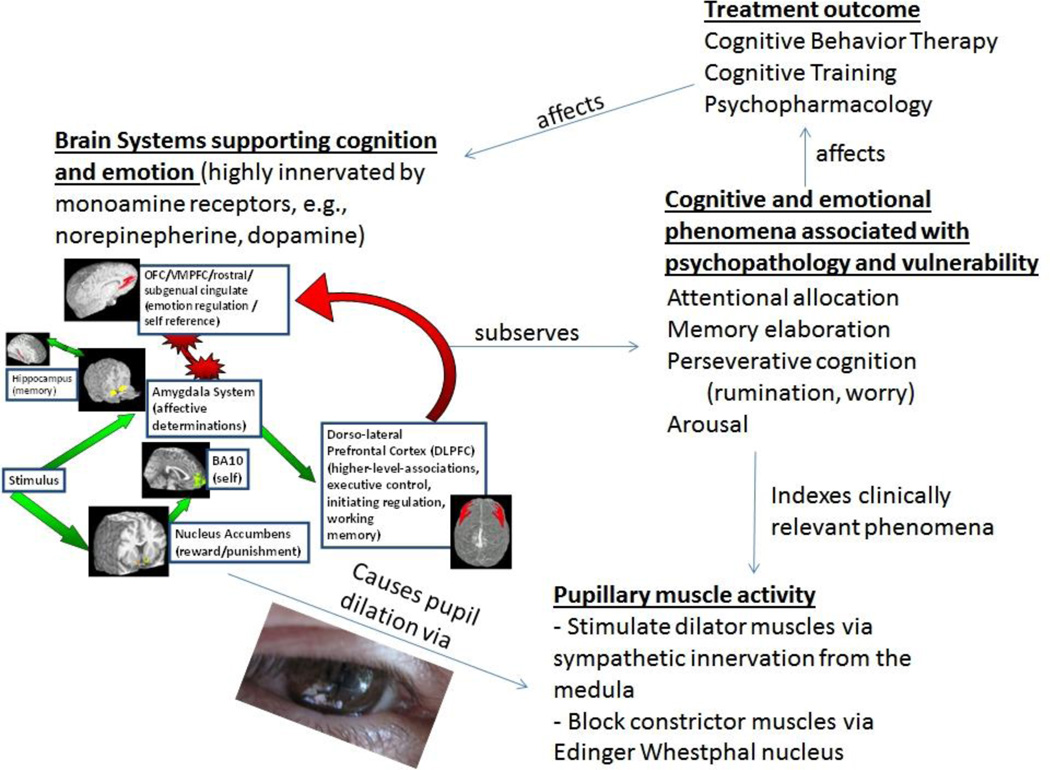
The pupil has a venerable history in medicine, from the diagnosis of neurological syndromes such as Addison disease and Horner syndrome to monitoring the depth of anesthesia [1]. As pupillometry is a quick, painless, and noninvasive process, assessment of the pupil response to light stimulation and its re-dilation is used in the clinical setting for detecting optic nerve dysfunction, diagnosing certain drug effects, assessing brainstem integrity and autonomic functions. [2]. The use of pupillometry in psychology and psychiatry has had a lower profile stemming from initial forays in the 1960’s as a measure of affect, cognition [3, 4], and specifically, the observation that the pupil dilates with cognitive load [5]. In the 1980s the application of pupillometry was extended to reflect individual differences in cognitive and affective processing [6] yielding the potential for use in understanding psychiatric disorders which are frequently characterized by cognitive and affective disturbances [7]. Here, we summarize specific recent advances in areas applicable to psychiatry from the past two years. Psychiatric uses have stemmed from recent re-recognition of the correspondence of pupil dilation to aspects of information processing including cognition and emotion [8]. We thus concentrate on associations of altered pupillary motility in psychiatric disorders when patients are performing cognitive and emotional tasks, along with specific alterations in developmental populations, particularly autism spectrum disorders. Figure 1 portrays a theoretical model of how the pupil may be used to index relevant phenomena in the psychiatric clinic.
Figure 1.
Theoretical model of how pupil dilation indexes phenomena relevant to cognitive and emotional information processing relevant to the psychiatry clinic.
1. Brain Influences on the Pupil
Changes in pupillary dilation in response to cognitive and affective load are due to direct innervation of the sympathetically mediated dilator muscle of the iris and the parasympathetically mediated sphincter muscle by brain regions associated with cognitive and emotional processing [2, 7]. For example, stimulation of limbic regions such as the amygdala (a brain area used in emotion and memory) increases pupil dilation, likely through a variety of mechanisms involving pathways to the medulla [9] such as direct excitatory inputs from the amygdala to the descending sympathetic pathway in the medulla, and mediation through pathways from the amygdala to the posterior hypothalamus.. Pupillary dilation has been linked to executive control with dorso-lateral prefrontal activity observed during a working memory task in a dual pupillometry and neuro-imaging study [10], likely due to cortical inhibition of the Edinger Westphal nucleus. Pupillary responses have also been associated with the anterior cingulate cortex (as evidenced by dual anterior cingulate cortex activity and autonomic dilatory pupil response in an error processing task) [11] and to the activation of the locus coeruleus (LC) and the noradrenergic (NE) system [12] The LC communicates with the prefrontal cortex, pulvinar nucleaus, superior colliculus and other areas of the brain in involved in selective attention. Though increasing data suggests the importance of measuring brain function associated with cognition and emotion in the psychiatry clinic [13–15], conventional neuroimaging is too expensive to use on a regular basis. Physiological proxies such as pupil dilation are emerging as promising measures for the psychiatric clinical setting. The remainder of this article thus reviews recent findings relevant to this topic.
2. Pupil Dilation, Cognition, and Emotion
The current state-of-the-art in pupillary applications to psychology and psychiatry stems from assessment of pupil dilation in language, arithmetic, perception tasks, and short term memory tasks [6]. Many studies support the conclusion that pupil diameterincreases with cognitive load and that pupil diameter decreases when an individual attempts to process more than he or she can handle [16–19]. Specifically, the pupil dilates when an individual is processing positive and negative information [20] including pictures [21, 22], words, [23], and sounds [24]. While initial research has suggested that the pupil dilates only in response to a positive stimulus, ([25]; [26]; [21], it is now believed changes in pupil size occur when emotionally arousing stimuli are present, regardless of positive or negative valence ([22]; [21]; [24]; [27]).
3:Pupillometry to Assess Mechanisms of Psychiatric Disorder and Vulnerability
Initial studies of abnormal pupil dilation in depression [28–30] were augmented by a recent study showing that pupil dilation indexes one possible neural mechanism for cognitive deficiencies in depression [31]. Depressed participants not only made more mistakes on a cognitive task, but were more likely to display non-task related pupil dilation signifying cognitive load, during a task not due to the task itself – potentially reflecting other processes such as rumination. Indeed, task-related dilation occurred immediately following the cognitive prompt, suggesting task-related cognition. Depressed participants were more likely to exhibit pupillary dilation not in relation to the cognitive prompt time frame. These participants also scored higher on rumination traits suggesting that depressed individuals engage in increased intrinsic processing that depletes their ability to process information. This application is potentially important as there are assessments of cognitive problems such as attention deficits, but no clinical assessments for why they occur. Pupillar dilation dynamics thus provides an objective index of non-task-related processing that could be addressed in behavioral treatments.
Of note, the pupil has continued to be associated with other features common to psychiatric disorder such as reward and risk processing [32, 33] which are decreased in disorders such as depression. The pupil is also associated with facilities such as arousal [34], working memory [35] and attention [36] which are impaired in many psychiatric conditions and decision making [37] which is particularly disrupted in externalizing disorders. A large literature on sleep disturbance and the pupil has similarly been augmented to the place where clinical measures are possible [38, 39]; it has thus seen initial uses in clinical trials, e.g., of modafinil (an awake-promoting agent) [40]. Thus, pupillary responses have been increasingly considered across the psychiatric spectrum, e.g., to high calorie foods in individuals with high BMI [41], and in a demonstration of particularly low pupillary motility in association with worry [42], potentially reflecting avoidance processes.
The pupil has also emerged as a potential indicator of vulnerability in individuals who are not yet depressed [43]. Current studies have demonstrated that non-depressed individuals with a previous depressive episode (suggesting depression vulnerability) haveincreased responses to personally relevant negative words compared to never-depressed controls. However, during a negative mood-state task in which participants were instructed to think of a sad event while listening to sad music, the depression-vulnerable individuals exhibited less pupil dilation than non-vulnerable individuals. One proposed explanation for these findings is that depression-vulnerable individuals are at a heightened reactivity for emotional information and highly salient emotional information overloads their ability to process it. Much like when the pupil ceases to dilate when an individual is cognitively overworked [17], perhaps a similar mechanism is working with emotional information for those at risk for depression. In addition depression-vulnerable individuals did not report an increase in negative mood during the mood-state task, suggesting those at risk for depression engage in emotional and cognitive blunting when processing highly salient negative information. From a practical perspective, with replication this result could suggest a clinically applicable assessment of vulnerability to depression as well as relevant mechanisms for pre-emptive targeting, e.g., via cognitive neurorehabilitative exercises.
Pupil abnormalities can also be seen in those suffering from anxiety. In one study, individuals scoring high on worry and rumination trait tendencies h smaller pupil dilation following personally relevant negative emotional stimuli than those scoring low on these traits [42]. These “worriers” performed behavioral tasks exceptionally well, suggesting that their relatively poor pupil dilation was not a result of demands exceeding cognitive capacity [44], but of a pattern of emotional avoidance in chronic worriers.
Pupil dilation differences are also present for individuals with Parkinson’s Disease (PD) [45], a neurodegenerative disorder which eventually leads to cognitive problems and dementia in its advanced stages. Pupillary unrest, the spontaneous changes of pupil diameter in darkness is associated with alternating sympathetic and parasympathetic influences, is increasingly associated with fatigue/arousal [46] and autonomic instability [47]. Jain et al. [48] measured pupillary unrest in individuals with PD and controls and found that arousal symptoms in PD were associated with increased pupillary unrest, suggesting pupillary unrest marks features of disorders affecting arousal such as PD and pupillary unrest is a marker of disordered arousal in PD. Furthermore, cardiovascular autonomic dysfunction (as measured by resting heart rate variability) reflecting lower parasympathetic function, was negatively correlated with pupillary unrest in PD [49]. Additional studies are needed to relate autonomic changes with motor symptoms in PD, but perhaps autonomic physiology (including the use of pupillary measures) can be used as a marker of PD progression.
4.. Using Pupillometry to Inform Treatment
The past two years have also seen increasing use of the pupil to directly inform treatment decisions and progress outcome in the psychiatric clinic. Pupillary reactivity is already used in a predictive capacity for treatment outcome in other disciplines, e.g., as an early index of intracranial pressure [50] as a predictor for success following surgical intervention in traumatic brain injury [51], and as an indicator of drug efficiency, e.g., for tramadol pharmacokinetics [52]; here we focus on its potential for similar uses in the psychiatry clinic. For example, while Cognitive Therapy (CT) is one of the most efficient treatments for depression [53], only 40% to 60% of patients experience symptom remission using this therapy [54]. Currently, there is no efficient mode of determining which patients would benefit from CT, a therapy that targets deficient regulatory control and fosters the depressed individual’s ability to recruit executive control in response to negative stimuli or thoughts. Functional magnetic resonance imaging studies have shown that successful CT treatments alter limbic activity and prefrontal function ([55]; [56]; [57]. Pre-treatment neuroimaging has been useful in identifying which patients would benefit from CT, particularly those with decreased pretreatment reactivity in the subgenual cingulate cortex (suggesting capitalization on unaffected limbic monitoring) [55, 58]. However the feasibility of neuroimaging to assess clinical patients is limited as such tests are time-consuming and expensive. Pupil dilation measures, however, could provide an excellent alternative in this setting.
Siegle, et al, [59] found that low levels of pupil dilation during a task involving alternation between naming the emotional valence of words and putting digits in order predicted remission in CT in participants with depression. Specifically, remission was associated with either low initial depressive severity or the combination of higher initial depressive severity and low sustained pupillary responses in the period following presentation of negative words lasting into the period in which digit sorting was to begin (i.e., during the operation of switching between cognitive tasks). Brain imaging studies showed – as in healthy individuals –increased pupillary responses were associated with increased activity in dorsolateral prefrontal regions associated with executive control and emotion regulation. If sustained pupillary dilation reflects prefrontally-mediated executive control, more severely depressed patients who do not have this capability may benefit from CT. Conversely, those who already have this capability may benefit from other treatments that do not act by teaching executive control (such as antidepressant medications). That said, there may be a critical level of recruitment of cognitive and emotional resources to the task, also indexed by the pupil necessary for response. For example, initial data suggest that periodic pupillary dilation indicating trial-related responsivity is associated with better response to cognitive exercises designed specifically to target prefrontally mediated executive control in unipolar depressed individuals [60].
These results suggest that a quick (less than ten minute) assessment of the pupil may be able to help clinicians decide when to allocate time and resources of CT therapy to a selected group of severely depressed patients who would be expected to respond favorably to this mode of treatment. Theoretically, patients with decreased sustained pupillary responses may respond well to targeted intervention specifically designed to increase executive control. Currently, there is no method of determining the optimal treatment for patients with depression and depressed individuals are often administered treatments that are inefficient. Our current unsystematic prescription of depression treatments yields a severe cost not only to individuals, but to society at large. The cost of depression, in terms of lost productivity and increased medical expenses, is $83 billion each year [61]. Clinicians and patients must begin making better informed decisions about psychiatric treatment, a decision pupillometry can easily, quickly, and cheaply aid.
The primary alternative to psychotherapy for psychiatric disorders is medication. As medications such as antidepressants are increasingly thought to act on cognitive features of disorder indexed by the pupil such as attentional allocation [62, 63], it is reasonable that pupillary motility would also index their utility. Initial data indeed suggests that pupillary motility has shown progress in understanding drug effects. There is a strong history of exploring pupillary dilation as a measure of drug occupancy and action [64]. In the past two years, this literature has specifically expanded to include ketamine which has recently been explored as an acute intervention for suicidal depression [65]. Pupil dilation was shown to increase with ketamine administration in dogs [66] potentially yielding a measure of drug occupancy. Effects on pupillary motility have been explored as well for other psychoactive agents including antipsychotics [67] and buprenorphine [68]. Relationships of pupillary motility to release of neurotransmitters such as norepinephrine have also been considered [69–72] potentially yielding useful clinical predictors or measures in association with prescriptions, e.g., for common antidepressants such as selective norepinephrine reuptake inhibitors (SNRI’s). Together, it is hoped that such literature will eventually yield predictive algorithms such that by observing features such as pupil dilation, an individual can understand their likelihood of response to, and optimal dose of, a variety of possible interventions.
5. Pupillometry in Developmental Populations
Differences in pupillary dilation for various experimental tasks have also been found in younger populations. For example, depressed youths who are presented with negative words show decreased pupil dilation 9 to 12 seconds after the word presentation, compared to non-depressed youths [30]. These participants had a greater severity of depression; thus, decreased pupil dilation suggested an impaired ability to recruit regulatory mechanisms. This led to the question of whether pupil dilation might also index vulnerability in developmental populations. Anxious youths frequently become depressed in their late teens and early adulthood [73–75], and are thus a model vulnerable population. A task involving attention to emotional faces used in conjunction with pupillometry revealed that unlike controls and like vulnerable adults [43], anxious youths displayed pupil dilation long after an emotional or threatening stimulus had been presented [76]. These results suggest anxious youths have increased and sustained reactions to emotional faces, a response that is possibly rooted in an enhanced recall or elaboration of threat-relevant information and/or an increased effort to emotionally regulate emotional stimulus long after it is gone. These responses to threat may lead to the development of other maladaptive patterns; thus, early intervention specifically targeting these responses may be useful in treatment.
To create a tool for examining the extent to which these biases generalized to a more “real-world” context involving social rejection, Silk et al. [77] measured adolescent pupillary responses to rejection and acceptance using a simulated internet chatroom task. Participants were put in a scenario where an alleged online peer had to pick between the participant and another alleged peer to talk about common interests (in truth, all decisions were pre-determined and there was no true interaction). Pupil responses were obtained to differentiate pupil responses during acceptance (being picked by their online peer) and rejection (not being picked). Youths (aged 9–17) showed heightened peak pupil dilation in response to peer rejection. The findings suggest increased prefrontal activity in response to rejection, consistent with previous fMRI research showing greater activity in ventral prefrontal regions in response to exclusion [78]. Potentially, youths engage prefrontal areas of the brain in order to regulate emotions in times of social digress. Furthermore, and relevant to the task’s utility in assessing vulnerability, measuring participant social and emotional behavior in the real world via brief telephone interviews showed that adolescents with greater pupil dilation during rejection also had lower feelings of connectedness in their daily peer interactions. These participants also had increased pupil dilation prior to rejection, suggesting a particularly sensitive anticipatory response to rejection. These youths are also those that feel less connected to their peers. Such results suggest that pupillometry might help to identify youths who might benefit from interventions that seek to increase social connectedness.
Pupillometry has also been used recently to better understand emotional information processing in adolescents with inflammatory bowel disease (IBD), a disease which is associated with higher rates of depression than other physical diseases. Youths with IBD had increased initial pupil dilation to negative words, regardless of depression status, suggesting an increased sensitivity to negative emotional stimuli [79]. While further research is needed to tease out why this sensitivity exists in this population (high-dose steroid treatment, effect of IBD-related inflammatory cytokines, psychosocial stress related to the disease all might affect neural processing), pupillometry suggests that increased reactivity to negative emotional information is an integral part of the expression of this disorder.
6. Autism Research
In the psychiatric clinic of the future it will be important to assess not only verbal but non-verbal nonverbal populations. A specific, often non-verbal group that may benefit from insights and use of pupillometry are individuals with Autism Spectrum Disorders (ASD). Indeed, recent years have observed features such as increased pupil diameter along with other indicators of autonomic dysfunction in autism spectrum disorders [80]. The pupil has also been associated with key features of autism such as empathy in adults [81] and children as young as two years old [82], and has specifically been shown to be decreased in response to social reward in autistic children [83] and positive information in Asperger’s disorder [84]. Pupillary measures have, indeed proved useful clinically with the potential for charting the efficacy of behavioral treatments for autism spectrum disorders such as Fragile X syndrome [85].
Of particular note, while it is known that individuals with ASD attend abnormally to the eyes, different theories exist on why this is so. Previous research has suggested that unusual eye fixations of individuals with ASD might be due to eyes being aversive ([86]; [87]; [88]). An alternative theory is the social motivation hypothesis which states that individuals with ASD do not attend to social stimuli because they fail to adequately form representations of the reward value of such stimuli ([89]; [90]), while typically developing individuals find social stimuli rewarding ([91]; [92]).
Pupil dilation has been used recently to help discern between these two differing hypotheses [83]. The researchers looked at the pupil dilations of children with ASD viewing static images of emotional faces. They found that while the responses of ASD children were similar to controls for most emotions, they lacked pupillary dilation to happy faces with direct gaze and concluded smiling faces were neither emotionally arousing nor particularly interesting to ASD children, i.e., that children with autism do not find eyes aversive. In contrast, happy faces have been shown to activate reward circuitry in typically developing individuals [91], [93]. In fact, attractive faces with direct gaze elicits activity in the ventral striatum, an area associated with reward processing [94], and pupillary response has also been linked to reward processing [95]; [92]; [96]. Thus, typically developing individuals show a pupillary response to smiling faces due to the reward value of a smiling face. The lack of this phenomenon occurring for those with ASD, might be due to impaired social reward processing system [90], not an aversion to eyes. These studies demonstrate the utility of pupillometry as one of multiple measures which can improve information-gathering about populations that cannot speak for themselves.
7. Brain Computer Interactions
The burgeoning area of Brain Computer Interactions (BCI) has been a hotbed of activity for assisting individuals with movement difficulties to interact with computers. Similarly for individuals with trouble expressing or recognizing their own emotions, pupil dilation could help to fill in what is for them, a critical missing piece. This area sits at the intersection between cognitive neuroscience and neurology, in that the problems are frequently neurological but techniques from cognitive neuroscience are being brought to the table as solutions. In particular, if personal computers could perceive a user’s emotion via the use of physiological measures such as heart rate, galvanic skin response, EEG, pupil diameter etc. [97], then it could intelligently alter a user’s experience. For patients with disorders that impair muscle movement (such as amyotrophic lateral sclerosis), computers and devices that could detect one’s underlying state of mind, would be a highly effective communication tool. This was the premise used to guide the recent development of an automatic filtering system to classify individual unpleasant emotions based on pupil size changes and thus remove perceived unpleasant images from a database [98]. More research must be done in this area to determine how various stimuli affect different individuals. For example previous work found that women showed significantly larger pupil responses than males to neutral auditory stimuli [24]. However, it would certainly be possible (and practical) to create a baseline assessment of pupillary responses in individuals and use one’s personal data to inform pupillary input systems.
Conclusion
Together, the current body of literature indicates that pupillometry has recently moved from a basic research tool toward a clinical use in the domain of psychiatry. The route is not direct, but rather depends on the pupil’s ability to assess emotional information processing which is at the core of both the expression of many psychopathologies as well as their treatments, as described in Figure 1. As an assessment tool it can be used to reveal otherwise hidden dimensions of patient functioning (e.g., resource limitations) and has applications in helping to guide treatment selection. It is appropriate for use throughout the lifespan of the patient in these capacities. Moreover, initial data suggests that as a BCI-based extension of consciousness, the pupil may be an even more potent tool, particularly for those who are not strongly verbal.
Future Directions
For pupillometry to be routinely employed in clinics, critical research gaps will need to be addressed. As with any potential psycho-physiological assessment, measurements must be reliable for single subjects. While single subject research has helped make neuroimaging reliable [99], similar research needs to be done in pupillometry. Results that are understandable to researchers are not necessarily understandable to those in other professions. Developing software programs for proper analysis would help make pupil data understandable to clinicians. Furthermore pupil data must be meaningful to those that use it; thus, developing pupil norms tables for various pathologies (as well as differences in gender, age, ethnicity, etc.) would be crucial in making pupil data interpretable. Initial data in these regards has begun to emerge concerning one of the most popular measures – pupillary unrest [38]. Adding such data for task-related changes will be critical. With the strongly established base of research in the past few years, the field is now poised to begin such translational efforts. We thus look forward to seeing pupillometry in the psychiatry clinic rival its well-established uses in neurology in the coming years.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Simona Graur declares no potential conflicts of interest.
Greg Siegle declares no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Simona Graur, University of Pittsburgh School of Medicine, 121 Meyran St, Pittsburgh, PA 15213
Greg Siegle, University of Pittsburgh School of Medicine, Western Psychiatric Institute and Clinic, 3811 O’Hara St, Pittsburgh, PA 15213
References
- 1.Wilhelm H. Disorders of the pupil. Handb Clin Neurol. 2011;102:427–466. doi: 10.1016/B978-0-444-52903-9.00022-4. [DOI] [PubMed] [Google Scholar]
- 2.Lowenfeld IE. The pupil: Anatomy, physiology, and clinical applications. Boston: Butterworth Heinemann; 1999. [Google Scholar]
- 3.Hess EH, Polt JH. Pupil size in relation to mental activity during simple problem solving. Science. 1964;143:1190–1192. doi: 10.1126/science.143.3611.1190. [DOI] [PubMed] [Google Scholar]
- 4.Hess EH. Pupillometrics: A method of studying mental emotional and sensory processes. In: Greenfield NS, Sternbaum RA, editors. Handbook of psychophysiology. New York, N.Y: Rineholt, Holt, Winston; 1972. pp. 491–531. [Google Scholar]
- 5.Kahneman D, Beatty J. Pupil diameter and load on memory. Science. 1966;154(3756):1583–1885. doi: 10.1126/science.154.3756.1583. [DOI] [PubMed] [Google Scholar]
- 6.Beatty J. Task-evoked pupillary responses processing load and the structure of processing resources. Psychological Bulletin. 1982;91:276–292. [PubMed] [Google Scholar]
- 7.Steinhauer SR, Hakerem G. The pupillary response in cognitive psychophysiology and schizophrenia. Annals of the New York Academy of Sciences. 1992;658:182–204. doi: 10.1111/j.1749-6632.1992.tb22845.x. [DOI] [PubMed] [Google Scholar]
- 8.Beatty J, Lucero-Wagoner B. The pupillary system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. New York, NY: Cambridge University Press; 2000. pp. 142–162. [Google Scholar]
- 9.Koikegami H, Yoshida K. Pupillary dilation induced by stimulation of amygdaloid nuclei. Folia Pychiatrica Neurologica Japonica. 1953;7:109–125. doi: 10.1111/j.1440-1819.1953.tb00600.x. [DOI] [PubMed] [Google Scholar]
- 10.Siegle GJ, et al. Use of concurrent pupil dilation assessment to inform interpretation and analysis of fMRI data. Neuroimage. 2003;20(1):114–124. doi: 10.1016/s1053-8119(03)00298-2. [DOI] [PubMed] [Google Scholar]
- 11.Critchley HD, et al. Anterior cingulate activity during error and autonomic response. Neuroimage. 2005;27(4):885–895. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 12.Koss MC. Pupillary dilation as an index of central nervous system alpha 2- adrenoceptor activation. J Pharmacol Methods. 1986;15(1):1–19. doi: 10.1016/0160-5402(86)90002-1. [DOI] [PubMed] [Google Scholar]
- 13.Siegle GJ. Beyond depression commentary: Wherefore art thou, Depression Clinic of Tomorrow? Clinical Psychology Science and Practice. 2011;18:305–310. doi: 10.1111/j.1468-2850.2011.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forgeard MJ, et al. Beyond Depression: Towards a Process-Based Approach to Research, Diagnosis, and Treatment. Clin Psychol (New York) 2011;18(4):275–299. doi: 10.1111/j.1468-2850.2011.01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Illes J, et al. In the mind's eye: provider and patient attitudes on functional brain imaging. J Psychiatr Res. 2008;43(2):107–14. doi: 10.1016/j.jpsychires.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granholm E, et al. Pupillary responses index cognitive resource limitations. Psychophysiology. 1996;33:457–461. doi: 10.1111/j.1469-8986.1996.tb01071.x. [DOI] [PubMed] [Google Scholar]
- 17.Granholm E, et al. Pupillary responses index overload of working memory resources in schizophrenia. J Abnorm Psychol. 1997;106(3):458–467. doi: 10.1037//0021-843x.106.3.458. [DOI] [PubMed] [Google Scholar]
- 18.Granholm E, Verney SP. Pupillary responses and attentional allocation problems on the backward masking task in schizophrenia. Int J Psychophysiol. 2004;52(1):37–51. doi: 10.1016/j.ijpsycho.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Poock GK. Information processing vs pupil diameter. Percept Mot Skills. 1973;37(3):1000–1002. doi: 10.1177/003151257303700363. [DOI] [PubMed] [Google Scholar]
- 20.Janisse MP. Pupil size and affect: A critical review of the literature since 1960. The Canadian Psychologist. 1973;14(4):311–329. [Google Scholar]
- 21.Janisse MP. Pupillary response today: Emotion in the eye. Psychology Today. 1974;7:60–63. [Google Scholar]
- 22.Bradley MM, et al. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45(4):602–607. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegle GJ, et al. Use of concurrent pupil dilation assessment to inform interpretation and analysis of fMRI data. Neuroimage. 2003;20(1):114–124. doi: 10.1016/s1053-8119(03)00298-2. [DOI] [PubMed] [Google Scholar]
- 24.Partala T, Surakka V. Pupil size variation as an indication of affective processing. International Journal of Human-Computer Studies. 2003;59(1–2):185–198. [Google Scholar]
- 25.Mudd S, Conway CG, Schindler DE. The eye as music critic: Pupil response and verbal preferences. Studia Psychologica. 1990;32(1–2):23–30. [Google Scholar]
- 26.Hess EH, Polt JM. Pupil size as related to interest value of visual stimuli. Science. 1960;132(3423):349–350. doi: 10.1126/science.132.3423.349. [DOI] [PubMed] [Google Scholar]
- 27.Steinhauer SR, et al. Pupillary dilation to emotional visual stimuli revisited. Psychophysiology. 1983;20:472–472. [Google Scholar]
- 28.Siegle GJ, et al. Pupillary and reaction time measures of sustained processing of negative information in depression. Biol Psychiatry. 2001;49(7):624–636. doi: 10.1016/s0006-3223(00)01024-6. [DOI] [PubMed] [Google Scholar]
- 29.Siegle GJ, et al. Do the seconds turn into hours? Relationships between sustained pupil dilation in response to emotional information and self-reported rumination. Cognitive Therapy and Research. 2003;27(3):365–382. [Google Scholar]
- 30.Silk JS, et al. Pupillary reactivity to emotional information in child and adolescent depression: links to clinical and ecological measures. Am J Psychiatry. 2007;164(12):1873–1880. doi: 10.1176/appi.ajp.2007.06111816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones NP, et al. Poor performance on cognitive tasks in depression: Doing too much or not enough? Cogn Affect Behav Neurosci. 2010;10(1):129–140. doi: 10.3758/CABN.10.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson BA, Yantis S. Value-driven attentional and oculomotor capture during goal-directed, unconstrained viewing. Atten Percept Psychophys. 2012;74(8):1644–1653. doi: 10.3758/s13414-012-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yechiam E, Telpaz A. To Take Risk is to Face Loss: A Tonic Pupillometry Study. Front Psychol. 2011;2:344. doi: 10.3389/fpsyg.2011.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bayer M, Sommer W, Schacht A. Emotional words impact the mind but not the body: evidence from pupillary responses. Psychophysiology. 2011;48(11):1554–62. doi: 10.1111/j.1469-8986.2011.01219.x. [DOI] [PubMed] [Google Scholar]
- 35.van Rijn H, et al. Pupil dilation co-varies with memory strength of individual traces in a delayed response paired-associate task. PLoS ONE. 2012;7(12):e51134. doi: 10.1371/journal.pone.0051134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zylberberg A, Oliva M, Sigman M. Pupil dilation: a fingerprint of temporal selection during the "attentional blink". Front Psychol. 2012;3:316. doi: 10.3389/fpsyg.2012.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiedler S, Glockner A. The dynamics of decision making in risky choice: an eye-tracking analysis. Front Psychol. 2012;3:335. doi: 10.3389/fpsyg.2012.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eggert T, et al. The Pupillographic SleepinessTest in adults: effect of, age, gender, and time of day on pupillometric variables. Am J Hum Biol. 2012;24(6):820–828. doi: 10.1002/ajhb.22326. This publication helps to move a vererated measure, the Pupillary Unrest Index, observed to be associated with sleepiness in the 1970’s and strongly quantified in the 1990’s, into the clinic. This measure has been associated with a variety of psychiatric conditions from those characterized by sleepiness to others characterized by hypo- and hyper-arousal such as Parkinson’s disease. The article formally notes effects of demographic and measurement variables on the assessment of pupillary unrest increasing the chances that clinical will be valid.
- 39.Nakayama M, Yamamoto K, Kobayashi F. Estimation of sleepiness using pupillary response and its frequency components. Int J Bioinform Res Appl. 2012;8(5–6):342–365. doi: 10.1504/IJBRA.2012.049621. [DOI] [PubMed] [Google Scholar]
- 40.Niepel G, et al. Association of a deficit of arousal with fatigue in multiple sclerosis: effect of modafinil. Neuropharmacology. 2013;64:380–388. doi: 10.1016/j.neuropharm.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 41.Graham R, et al. Body mass index moderates gaze orienting biases and pupil diameter to high and low calorie food images. Appetite. 2011;56(3):577–586. doi: 10.1016/j.appet.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 42.Oathes DJ, Siegle GJ, Ray WJ. Chronic worry and the temporal dynamics of emotional processing. Emotion. 2011;11(1):101–114. doi: 10.1037/a0021781. [DOI] [PubMed] [Google Scholar]
- 43.Steidtmann D, Ingram RE, Siegle GJ. Pupil response to negative emotional information in individuals at-risk for depression. Cognition and Emotion. 2010;24:480–496. [Google Scholar]
- 44.Granholm E, et al. Pupillary responses index cognitive resource limitations. Psychophysiology. 1996;33(4):457–461. doi: 10.1111/j.1469-8986.1996.tb01071.x. [DOI] [PubMed] [Google Scholar]
- 45.Giza E, et al. Pupil light reflex in Parkinson's disease: evaluation with pupillometry. Int J Neurosci. 2011;121(1):37–43. doi: 10.3109/00207454.2010.526730. [DOI] [PubMed] [Google Scholar]
- 46.Ludtke H, et al. Mathematical procedures in data recording and processing of pupillary fatigue waves. Vision Res. 1998;38(19):2889–2896. doi: 10.1016/s0042-6989(98)00081-9. [DOI] [PubMed] [Google Scholar]
- 47.Egg R, et al. Autonomic instability, as measured by pupillary unrest, is not associated with multiple sclerosis fatigue severity. Mult Scler. 2002;8(3):256–260. doi: 10.1191/1352458502ms793oa. [DOI] [PubMed] [Google Scholar]
- 48.Jain S, et al. Pupillary unrest correlates with arousal symptoms and motor signs in Parkinson disease. Mov Disord. 2011;26(7):1344–1347. doi: 10.1002/mds.23628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jain S, et al. Autonomic insufficiency in pupillary and cardiovascular systems in Parkinson's disease. Parkinsonism Relat Disord. 2011;17(2):119–122. doi: 10.1016/j.parkreldis.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen JW, et al. Pupillary reactivity as an early indicator of increased intracranial pressure: The introduction of the Neurological Pupil index. Surg Neurol Int. 2011;2:82. doi: 10.4103/2152-7806.82248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuo JR, et al. Prognostic predictors of outcome in an operative series in traumatic brain injury patients. J Formos Med Assoc. 2011;110(4):258–264. doi: 10.1016/S0929-6646(11)60038-7. [DOI] [PubMed] [Google Scholar]
- 52.Matouskova O, et al. Pupillometry in healthy volunteers as a biomarker of tramadol efficacy. J Clin Pharm Ther. 2011;36(4):513–517. doi: 10.1111/j.1365-2710.2010.01203.x. [DOI] [PubMed] [Google Scholar]
- 53.Beck AT. Guilford clinical psychology and psychotherapy series. New York: Guilford Press; 1979. Cognitive therapy of depression; p. 425. [Google Scholar]
- 54.Hollon SD, Thase ME, Markowitz JC. Treatment and Prevention of Depression. Psychological Science in the Public Interest. 2002;3(2):39–77. doi: 10.1111/1529-1006.00008. [DOI] [PubMed] [Google Scholar]
- 55.Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry. 2006;163(4):735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- 56.McClure EB, et al. fMRI predictors of treatment outcome in pediatric anxiety disorders. Psychopharmacology (Berl) 2007;191(1):97–105. doi: 10.1007/s00213-006-0542-9. [DOI] [PubMed] [Google Scholar]
- 57.Fu CH, et al. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biol Psychiatry. 2008;64(6):505–512. doi: 10.1016/j.biopsych.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 58.Siegle GJ, et al. Toward clinically useful neuroimaging in depression treatment: prognostic utility of subgenual cingulate activity for determining depression outcome in cognitive therapy across studies, scanners, and patient characteristics. Arch Gen Psychiatry. 2012;69(9):913–924. doi: 10.1001/archgenpsychiatry.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Siegle GJ, et al. Remission prognosis for cognitive therapy for recurrent depression using the pupil: utility and neural correlates. Biol Psychiatry. 2011;69(8):726–33. doi: 10.1016/j.biopsych.2010.12.041. Here we advance the idea that pupillometry can play a significant routine role in the psychiatry clinic. This publication is prototypical of what we believe could be a move towards adoption of inexpensive biomarkers of probable treatment response, with assessments of pupil dilation leading the charge.
- 60.Siegle GJ, et al. You gotta work at it: Pupillary indices of task focus are prognostic for response to a neurocognitive intervention for depression. Clinical Psychological Science. in press. [Google Scholar]
- 61.Greenberg PE, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64(12):1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- 62.Godlewska BR, et al. Short-term SSRI treatment normalises amygdala hyperactivity in depressed patients. Psychol Med. 2012;42(12):2609–2617. doi: 10.1017/S0033291712000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Simplicio M, Norbury R, Harmer CJ. Short-term antidepressant administration reduces negative self-referential processing in the medial prefrontal cortex in subjects at risk for depression. Mol Psychiatry. 2012;17(5):503–510. doi: 10.1038/mp.2011.16. [DOI] [PubMed] [Google Scholar]
- 64.Siepmann T, et al. The effects of venlafaxine on autonomic functions in healthy volunteers. J Clin Psychopharmacol. 2007;27(6):687–691. doi: 10.1097/jcp.0b013e31815a255b. [DOI] [PubMed] [Google Scholar]
- 65.Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14(8):1127–1131. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- 66.Kovalcuka L, et al. The effects of ketamine hydrochloride and diazepam on the intraocular pressure and pupil diameter of the dog's eye. Vet Ophthalmol. 2013;16(1):29–34. doi: 10.1111/j.1463-5224.2012.01015.x. [DOI] [PubMed] [Google Scholar]
- 67.Kleinloog D, et al. Does olanzapine inhibit the psychomimetic effects of Delta(9)-tetrahydrocannabinol? J Psychopharmacol. 2012;26(10):1307–1316. doi: 10.1177/0269881112446534. [DOI] [PubMed] [Google Scholar]
- 68.Megarbane B, Alhaddad H, Decleves X. Reduced pupil diameter in volunteers on stable buprenorphine maintenance therapy with telaprevir: a drugdrug interaction involving p-glycoprotein at the blood-brain barrier? Antimicrob Agents Chemother. 2012;56(11):6070. doi: 10.1128/AAC.01295-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gabay S, Pertzov Y, Henik A. Orienting of attention, pupil size, and the norepinephrine system. Atten Percept Psychophys. 2011;73(1):123–129. doi: 10.3758/s13414-010-0015-4. [DOI] [PubMed] [Google Scholar]
- 70.Jepma M, Nieuwenhuis S. Pupil diameter predicts changes in the exploration-exploitation trade-off: evidence for the adaptive gain theory. J Cogn Neurosci. 2011;23(7):1587–1596. doi: 10.1162/jocn.2010.21548. [DOI] [PubMed] [Google Scholar]
- 71.Kuipers JR, Thierry G. N400 amplitude reduction correlates with an increase in pupil size. Front Hum Neurosci. 2011;5:61. doi: 10.3389/fnhum.2011.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Preuschoff K, t Hart BM, Einhauser W. Pupil Dilation Signals Surprise: Evidence for Noradrenaline's Role in Decision Making. Front Neurosci. 2011;5:115. doi: 10.3389/fnins.2011.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brady EU, Kendall PC. Comorbidity of anxiety and depression in children and adolescents. Psychological Bulletin. 1992;111(2):244–255. doi: 10.1037/0033-2909.111.2.244. [DOI] [PubMed] [Google Scholar]
- 74.Kovacs M, et al. Depressive disorders in childhood. IV. A longitudinal study of comorbidity with and risk for anxiety disorders. Arch Gen Psychiatry. 1989;46(9):776–782. doi: 10.1001/archpsyc.1989.01810090018003. [DOI] [PubMed] [Google Scholar]
- 75.Pine DS, et al. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry. 1998;55(1):56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- 76. Price RB, et al. Sustained neural alterations in anxious youth performing an attentional bias task: a pupilometry study. Depress Anxiety. 2013;30(1):22–30. doi: 10.1002/da.21966. Here we used pupillometry to show that anxious youth potentially have inflexible or insufficient cognitive control. Anxious youth had sustained pupil dilation (thus hypothesized sustained cognitive-affective load) following emotional face viewing. These results might help inform clinicians which maladaptive patterns to target in treatment
- 77. Silk JS, et al. Peer acceptance and rejection through the eyes of youth: pupillary, eyetracking and ecological data from the Chatroom Interact task. Soc Cogn Affect Neurosci. 2012;7(1):93–105. doi: 10.1093/scan/nsr044. Here we found that adolescents with increased pupillary reactivity to rejection also felt less socially connected to their peers, suggesting pupillometry as a mode to identify youth who might benefit from interventions that seek to increase social connectedness
- 78.Masten CL, et al. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4(2):143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jones NP, et al. Impact of inflammatory bowel disease and high-dose steroid exposure on pupillary responses to negative information in pediatric depression. Psychosom Med. 2011;73(2):151–157. doi: 10.1097/PSY.0b013e318207ffea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anderson CJ, Colombo J, Unruh KE. Pupil and salivary indicators of autonomic dysfunction in autism spectrum disorder. Dev Psychobiol. 2012 doi: 10.1002/dev.21051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Azevedo RT, et al. Their pain is not our pain: Brain and autonomic correlates of empathic resonance with the pain of same and different race individuals. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hepach R, Vaish A, Tomasello M. Young children are intrinsically motivated to see others helped. Psychol Sci. 2012;23(9):967–972. doi: 10.1177/0956797612440571. [DOI] [PubMed] [Google Scholar]
- 83. Sepeta L, et al. Abnormal social reward processing in autism as indexed by pupillary responses to happy faces. Journal of neurodevelopmental disorders. 2012;4(1):17. doi: 10.1186/1866-1955-4-17. This publication is significant in that pupil dilation is used to augment understanding of eye-tracking data in autism. Pupil dilation is acquired in almost every eye-tracking study but is rarely analyzed. The authors take advantage of the additional provided data to make novel insights about autism. In addition, this article rekindles consideration regarding valence-specific responses in the pupil which was a hot topic in understanding the utility of pupillary responses in cognition in the 1960’s and 1970’s. Here, the authors suggest such response patterns may reflect individual differences in cognitive and affective information processing rather than differential valence-dependent mechanisms.
- 84.Kuchinke L, et al. Spontaneous but not explicit processing of positive sentences impaired in Asperger's syndrome: pupillometric evidence. Neuropsychologia. 2011;49(3):331–338. doi: 10.1016/j.neuropsychologia.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 85.Farzin F, et al. Reliability of eye tracking and pupillometry measures in individuals with fragile X syndrome. J Autism Dev Disord. 2011;41(11):1515–1522. doi: 10.1007/s10803-011-1176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dalton KM, et al. Gaze fixation and the neural circuitry of face processing in autism. Nature neuroscience. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kylliäinen A, et al. Face-and gaze-sensitive neural responses in children with autism: a magnetoencephalographic study. European Journal of Neuroscience. 2006;24(9):2679–2690. doi: 10.1111/j.1460-9568.2006.05132.x. [DOI] [PubMed] [Google Scholar]
- 88.Joseph RM, et al. Affective response to eye contact and face recognition ability in children with ASD. Journal of the International Neuropsychological Society. 2008;14(6):947. doi: 10.1017/S1355617708081344. [DOI] [PubMed] [Google Scholar]
- 89.Dawson G, et al. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73(3):700–717. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Developmental neuropsychology. 2005;27(3):403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- 91.Phillips ML, et al. Investigation of facial recognition memory and happy and sad facial expression perception: an fMRI study. Psychiatry Research: Neuroimaging. 1998;83(3):127–138. doi: 10.1016/s0925-4927(98)00036-5. [DOI] [PubMed] [Google Scholar]
- 92.O'Doherty JP, et al. Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron. 2006;49(1):157. doi: 10.1016/j.neuron.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 93.O’Doherty J, et al. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41(2):147–155. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- 94.Kampe K, et al. Reward value of attractiveness and gaze. Nature. 2001;413M(6856):589. doi: 10.1038/35098149. [DOI] [PubMed] [Google Scholar]
- 95.Steinhauer SR, Hakerem G. The Pupillary Response in Cognitive Psychophysiology and Schizophreniaa. Annals of the New York Academy of Sciences. 1992;658(1):182–204. doi: 10.1111/j.1749-6632.1992.tb22845.x. [DOI] [PubMed] [Google Scholar]
- 96.O’Doherty JP, et al. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38(2):329. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- 97.Jacob RJK. Eye tracking in advanced interface design. In: Woodrow B, Furness Thomas A III, editors. Virtual environments and advanced interface design. Oxford University Press, Inc.; 1995. pp. 258–288. [Google Scholar]
- 98. Kashihara K, Ito M, Fukumi M. Automatic system to remove unpleasant images detected by pupil-size changes. International Journal of Computer Science Issues. 2012;9(1):68–73. This publication uses pupil dilation to automatically remove negatively-perceived images for users in a computer-interface program. The results suggest that pupillometry could be used as a communication tool for individuals with movement or emotion difficulties.
- 99.Gorgolewski KJ, et al. Single subject fMRI test-retest reliability metrics and confounding factors. Neuroimage. 2013;69:231–243. doi: 10.1016/j.neuroimage.2012.10.085. [DOI] [PubMed] [Google Scholar]