Abstract
The abilities of docosahexaenoic acid (DHA) and exercise to counteract cognitive decay after TBI is getting increasing recognition; however, the possibility that these actions can be complementary remains just as an intriguing possibility. Here we have examined the likelihood that the combination of diet and exercise has the added potential to facilitate functional recovery following TBI. Rats received mild fluid percussion injury (mFPI) or sham injury and then were maintained on a diet high in DHA (1.2% DHA) with or without voluntary exercise for 12 days. We found that FPI reduced DHA content in the brain, which was accompanied by increased levels of lipid peroxidation assessed using 4-HHE. FPI reduced the enzymes Acox1 and 17 -HSD4, and the calcium-independent phospholipases A2 (iPLA2), which are involved in metabolism of membrane phospholipids. FPI reduced levels of syntaxin-3 (STX-3), involved in the action of membrane DHA on synaptic membrane expansion, and also reduced BDNF signaling through its TrkB receptor. These effects of FPI were optimally counteracted by the combination of DHA and exercise. Our results support the possibility that the complementary action of exercise is exerted on restoring membrane homeostasis after TBI, which is necessary for supporting synaptic plasticity and cognition. It is our contention that strategies that take advantage of the combined applications of diet and exercise may have additional effects to the injured brain.
Keywords: DHA, exercise, BDNF, omega-3 fatty acids, cognition
1. Introduction
Traumatic brain injury (TBI) is a devastating condition that has long-lasting consequences for cognitive ability of patients (Amen et al., 2011). Growing evidence indicates that diet (Wu et al., 2004) and exercise (Griesbach et al., 2007) have the ability to counteract several parameters of brain plasticity and cognitive function in animal models of TBI. Feeding and exercise are routine aspects of the daily living, which implies that their mechanisms may have evolved in concert. Therefore, it is important to understand the mechanisms by which the combined actions of diet and exercise can have enhanced therapeutic value for the treatment of TBI.
Docosahexaenoic acid (C22:6n-3; DHA) is abundant in the phospholipid composition of plasma membranes in the brain and its action is important for brain development and adult plasticity. DHA is a major modulator of synaptic membrane fluidity and function (Lukiw and Bazan, 2008), which is fundamental for supporting cell signaling (Stillwell et al., 2005; Calder and Yaqoob, 2009), and synaptic plasticity (Wu et al., 2008; Chytrova et al., 2010). An increasing number of reports indicate that TBI disrupts plasma membrane function (Bigford et al., 2009; Sharma et al., 2010), and synaptic plasticity (Chytrova et al., 2010; Wu et al., 2011), which may be responsible for deficits in neuronal function and cognition, even without major cell death in the brain. The plasma membrane is the site of regulation for many basic neuronal functions that are disrupted in TBI (Merenda et al., 2008) such as excitatory neurotransmitter efflux (Biegon, 2004), energy metabolism, and oxidative stress (Ansari et al., 2008). Molecules related to the metabolism of phospholipids in the plasma membrane, such as the calcium-independent PLA2 (iPLA2) and synatxin-3 are emerging as important regulators of plasticity and repair. In particular, iPLA2 seems to play an important role in synaptic stability (Allyson et al., 2012) and cognitive function (Schaeffer et al., 2009). Syntaxin 3 (STX-3), which is a structural component of synaptic membrane, is under the regulation of DHA (Cansev and Wurtman, 2007), and can contribute to neurite outgrowth, and membrane expansion (Darios and Davletov, 2006).
The enzymes acyl-CoA oxidase 1 (Acox1) and 17 -hydroxysteroid dehydrogenase type 4 (17 -HSD4) are localized in the peroxisome, and regulate alternative metabolic pathways involved in the DHA synthesis. 17 -HSD4 belongs to a family of related proteins which are essential for the lipid homeostasis in mice (Huyghe et al, 2006). Both Acox1 and 17 -HSD4 are involved in peroxisomal fatty acid -oxidation during the biosynthesis of DHA. In particular, It has been proposed that during the process of DHA synthesis, the intermediate C22:5n-3 is converted to C24:6n-3, which is then retroconverted to C22:6n-3 (DHA) by peroxisomal -oxidation. Peroxisomal enzymes straight-chain Acox1 and 17 -HSD4 were reported to participate in this process in human skin fibroblasts (Su et al., 2001; Ferdinandusse et al., 2001).
In turn, the action of exercise on brain plasticity and function has also been related to support neuronal signaling and associated to metabolic pathways related to fatty acids (Burdge and Calder, 2005; Kim, 2007; Calder, 2012). The current study aims to provide important information to better understand how exercise can support DHA metabolism important for membrane homeostasis in the pathobiology of TBI.
2. Materials and Methods
2.1. Experimental Designs and Tissue Preparation
Sprague-Dawley rats (Charles River Laboratories, Inc., Wilmington, MA, USA) weighing between 200 and 240g were housed in cages and maintained in environmentally controlled rooms (22–24 °C) with a 12h light/dark cycle. After acclimatization for 1 week on standard rat chow, one set of rats was exposed to a DHA-enriched diet (1.2% DHA), while another set were exposed to regular diet after fluid percussion injury was performed. The rats were then maintained on the diets with or without voluntary exercise for 12 days. The rats were divided into 5 groups: (1) Sham-RGD (regular diet)-Sed (sedentary) as control (CTL, dashed line in bar figures), (2) FPI-RGD-Sed (fed regular diet, sedentary), (3) FPI-RGD-Exc (fed regular diet, exercise), (4) FPI-DHA-Sed (fed DHA, sedentary), and (5) FPI-DHA-Exc (fed DHA, exercise). After one week, the rats (n=5–6 within each group) were tested in the Morris Water Maze (MWZ) for learning ability. The rats, still on the diet and exercise during the MWZ test, were then killed by decapitation; the fresh tissues including hippocampus were dissected, frozen in dry ice and stored at −70°C until use for biochemical analyses. The diets, fed ad libitum, were provided in powder. All experiments were performed in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals, and animal suffering was minimized.
2.2. Fluid percussion injury
The injury was performed as previously described (Wu et al., 2003). In brief, with the aid of a microscope (Wild, Heerburg, Switzerland) a 3.0-mm diameter craniotomy was made 3.0 mm posterior to bregma and 6.0 mm lateral (left) to the midline with a high-speed drill (Dremel, Racine, WI). A plastic injury cap was placed over the craniotomy with silicone adhesive and dental cement. When the dental cement hardened, the cap was filled with 0.9% saline solution. Anesthesia was discontinued and the injury cap was attached to the fluid percussion device. At the first sign of hind-limb withdrawal to a paw pinch, a mild fluid percussion pulse (1.5 atm) was administered. Sham animals underwent an identical preparation with the exception of the lesion. Immediately upon responding to a paw pinch, anesthesia was restored and the skull was sutured. Neomycin was applied on the suture and the rats were placed in a heated recovery chamber for approximately an hour before being returned to their cages.
2.3. Morris Water Maze
Briefly, the rats were trained in the water maze with 2 consecutive trials per day for 5 days. The rats were placed into the tank facing the wall from one of the equally spaced start locations that were randomly changed every trial. Each trial lasted until the rat found the platform or for a max of 1 min. If the rat failed to find the platform in the allocated time, it was gently placed on the platform. At the end of each trial, the rats were allowed to rest on the platform for 1 min, and the escape latencies were recorded.
2.4. Western blot
The left hippocampal tissue was homogenized in a lysis buffer containing 137 mM NaCl, 20 mM Tris–HCl pH 8.0, 1% NP40, 10% glycerol, 1 mM PMSF, 10 µg/ml aprotinin, 0.1 mM benzethonium chloride, 0.5 mM sodium vanadate. The homogenates were then centrifuged; the supernatants collected and total protein concentration was determined according to MicroBCA procedure (Pierce, Rockford, IL), using bovine serum albumin as standard. Briefly, protein samples were separated by electrophoresis on an 8% polyacrylamide gel and electrotransferred to a nitrocellulose membrane. Nonspecific binding sites were blocked in TBS, overnight at 4°C, with 2% BSA and 0.1% Tween-20. Membranes were rinsed for 10 min in buffer (0.1% Tween-20 in TBS) and then incubated with anti-actin or anti-BDNF, p-TrkB, iPLA2, STX-3, Acox1, 17 -HSD4 (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA); Sir2 (1:1000, Upstate, Billerica, MA), anti-4-HHE (1:1000, a gift from Dr. Picklo (Long et al., 2008)), followed by anti-rabbit IgG horseradish peroxidase-conjugate (Santa Cruz Biotechnology). After rinsing with buffer, the immunocomplexes were visualized by chemiluminescence using the ECL kit (Amersham Pharmacia Biotech Inc., Piscataway, NJ) according to the manufacturer's instructions. The film signals were digitally scanned and then quantified using NIH Image software. Actin was used as an internal control for western blot such that data were standardized according to actin values.
2.5. Lipids analysis
Total lipids were extracted from cerebral cortical tissues with lysis solution of chloroform-methanol (2:1, vol:vol) containing 0.005% Butylated hydroxytoluene. The ratio of sample to lysis solution is 1:20 (i.e. 100 mg tissue + 2ml lysis solution). After centrifugation at 9000 rpm for 10min, the chloroform layer was transferred to a 15 ml tube, and then mixed with 0.9% saline. After centrifugation again, the chloroform layer was transferred to another 15 ml tube and dried under nitrogen. The dried sample of total lipids was dissolved in hexane and methylated by methanol in the presence of BF3 at 90°C for lipids analysis. Fatty acid profiles were determined by using gas chromatography (GC). The system consisted of model Clarus 500 gas chromatograph (PerkinElmer) with a built-in Autosampler. An Elite-WAX column (60m, 0.32-mm internal diameter, PerkinElmer) was used, with hydrogen as the carrier gas. GC oven temperature was initially held at 140•°C for 2 min and raised with a gradient of 5•°C•min−1 until 250•°C and held for 10 min. The total run time is 34 min. The injector and detector were maintained at 250 and 300•°C, respectively. A 1 μl sample of fatty acid methyl esters (FAME) was injected in split injection mode with a 100:1 split ratio. Peaks of resolved fatty acid methyl esters were identified and quantified by comparison with standards (Supelco 37-component FAME Mix).
2.6. Statistical analysis
For Western blot, the values were expressed as a ratio of actin value and then converted to percent of Sham-Sed as presented in bar figures and represented the mean ± SEM. All statistical analyses were performed by SPSS 16.0 software. The data were analyzed by ANOVA followed by Bonferroni’s post hoc test. Statistical differences were considered significant at P < 0.05.
3. Results
3.1. Exercise regulates the content of DHA in the brain
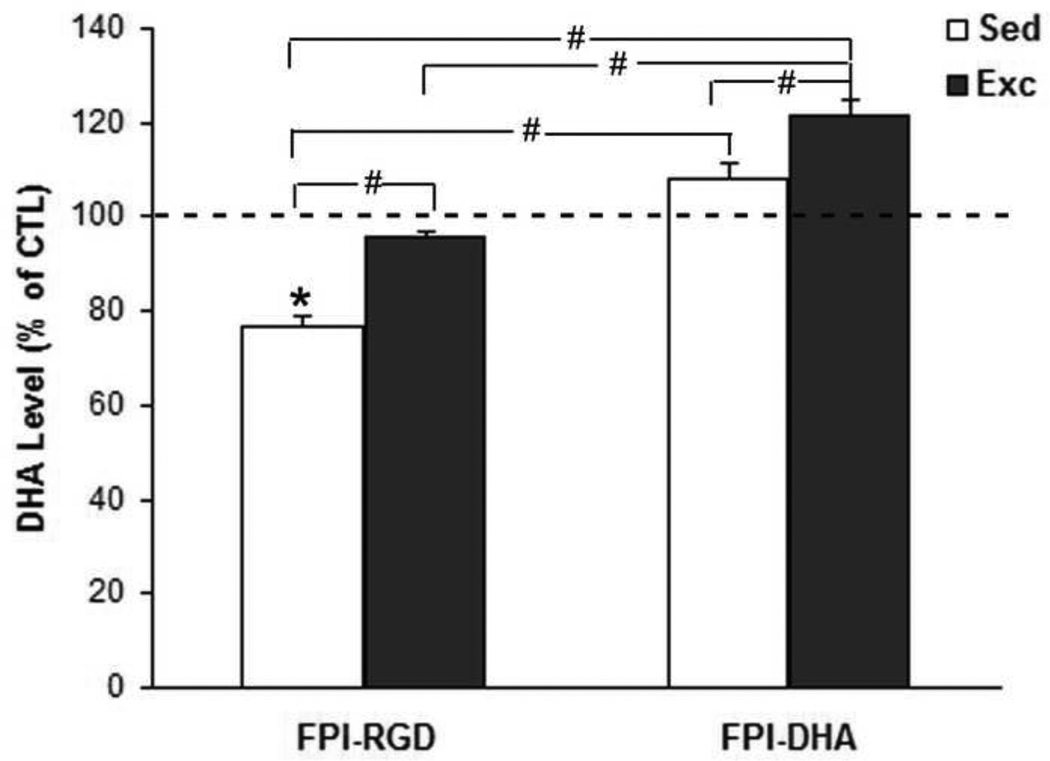
The DHA levels were measured by gas chromatography as described in Methods. The Sham-Sed rats fed regular diet (RGD) was used as control (CTL) group. The level of DHA were converted to percent of CTL group and presented in bar figure (Fig. 1). The results showed that there was a significant group effect for DHA content in the brain by ANOVA (F4,25=21.27; p<0.01). The DHA levels were higher in FPI-DHA-Exc (122% of CTL) than that in FPI-RGD-Sed (77% of CTL, p<0.05; Fig. 1), FPI-RGD-Exc (96% of CTL, p<0.05; Fig. 1), or FPI-DHA-Sed group (108% of CTL, p<0.05; Fig. 1). In addition, DHA levels were higher in FPI-RGD-Exc (96% of CTL, p<0.05; Fig. 1) and FPI-DHA-Sed (108% of CTL, p<0.05; Fig. 1) than that in FPI-RGD-Sed (77% of CTL; Fig. 1). These results suggest that exercise and dietary DHA have additive effects on the DHA levels in the brain after TBI.
Figure 1.
Exercise provides protection against FPI-reduced brain DHA. The DHA content in the brain was measured by gas chromatography (GC). The value was converted to percent of CTL and presented in bar figure. FPI resulted in reduction of DHA, which was reversed by both exercise and DHA supplementation. A greater increase of DHA was observed when exercise was combined with DHA. *, p<0.05 vs CTL. #, p< 0.05. CTL, Sham-RGD-Sed. FPI, fluid percussion injury. Sed, Sedentary. Exc, Exercise.
3.2. Exercise supports DHA stability by impacting enzymes (Acox1 and 17 -HSD4) associated with metabolism of PUFAs
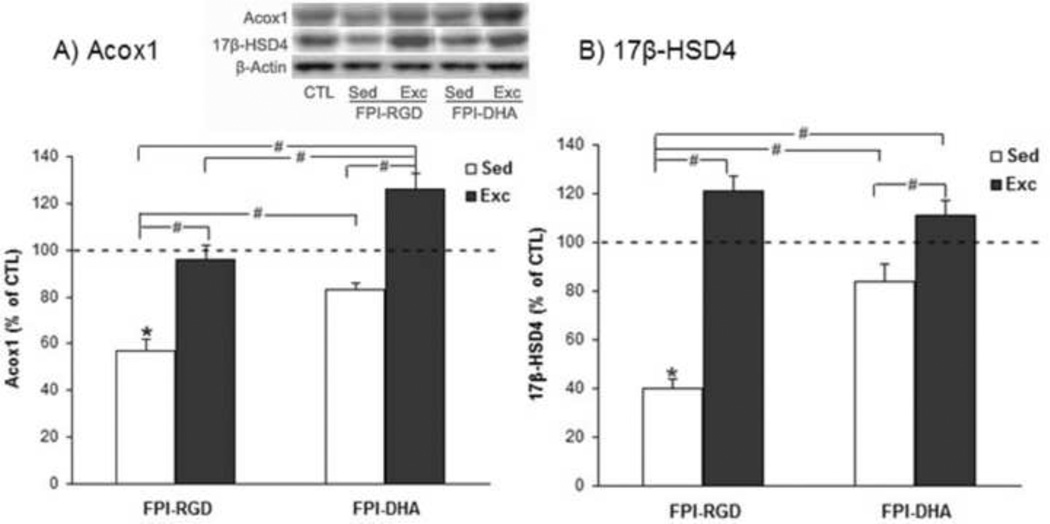
Acox1 and 17 -HSD4 are important enzymes involved in the metabolism of DHA from its precursor. The results showed that there was a significant group effect on Acox1 level (F4,25=4.07, p<0.05 by ANOVA) in the hippocampus. The Acox1 levels were higher in FPI-DHA-Exc (126% of CTL) than that in FPI-RGD-Sed (57% of CTL, p<0.05; Fig. 2A), FPI-RGD-Exc (95% of CTL, p<0.05; Fig. 2A), or FPI-DHA-Sed group (83% of CTL, p<0.05; Fig. 2A). In addition, Acox1 levels were higher in FPI-RGD-Exc (95% of CTL, p<0.05; Fig. 2A) and FPI-DHA-Sed (83% of CTL, p<0.05; Fig. 2A) than that in FPI-RGD-Sed (57% of CTL; Fig. 2A). These results suggest that exercise and dietary DHA have complimentary effects on the Acox1 levels in the hippocampus after TBI. There was a significant positive correlation between Acox1 and the amount of wheel running in FPI rats with exercise (r=0.959, p<0.05).
Figure 2.
Exercise and DHA supplementation regulate Acox1 and 17 -HSD4 levels in the hippocampus after FPI. (A) FPI resulted in reduction of Acox1, which was counteracted by DHA or exercise. A greater increase of Acox1 was observed when exercise and DHA was combined. (B) FPI resulted in significant reduction of 17 -HSD4, which was significantly reversed by exercise, DHA or combination of both. *, p<0.05 vs CTL. #, p< 0.05. CTL, Sham-RGD-Sed. FPI, fluid percussion injury. Sed, Sedentary. Exc, Exercise.
The results showed that there was a significant group effect on 17 -HSD4 level (F4,25=5.38, p<0.01 by ANOVA). The 17 -HSD4 levels were higher in FPI-DHA-Exc (111% of CTL) than that in FPI-RGD-Sed (40% of CTL, p<0.05; Fig. 2B), or FPI-DHA-Sed group (84% of CTL, p<0.05; Fig. 2B). In addition, 17 -HSD4 levels were higher in FPI-RGD-Exc (121% of CTL, p<0.05; Fig. 2B) and FPI-DHA-Sed (84% of CTL, p<0.05; Fig. 2B) than that in FPI-RGD-Sed (40% of CTL; Fig. 2B).
3.3. DHA and exercise reduce oxidative stress after TBI
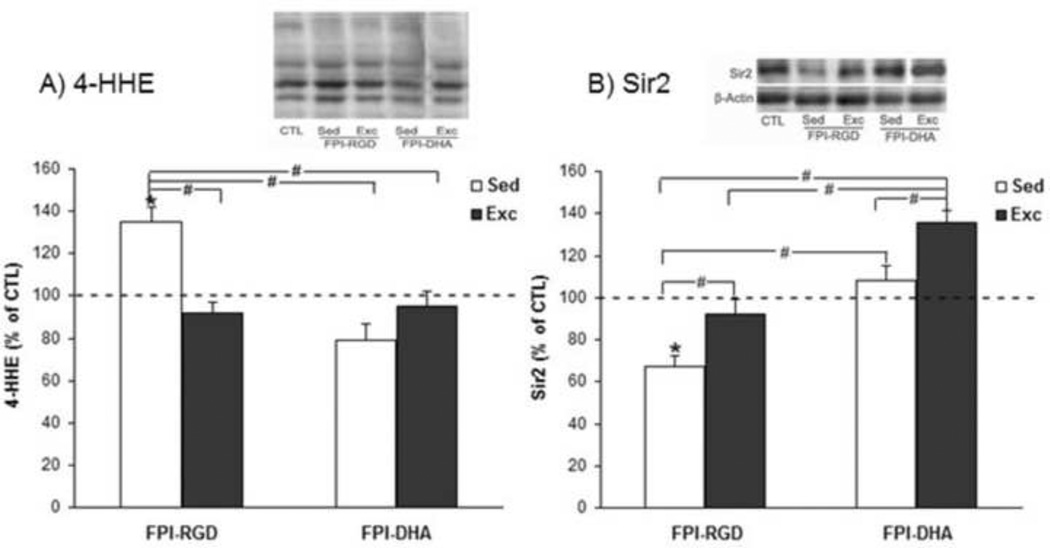
We measured hippocampal levels of 4-HHE, a maker of lipid peroxidation, which is derived from oxidative damage to n-3 polyunsaturated fatty acids. The results showed that there was a significant group effect on 4-HHE level (F4,25=11.69, p<0.01 by ANOVA). The 4-HHE levels were lower in FPI-DHA-Exc (95% of CTL) than that in FPI-RGD-Sed (135% of CTL, p<0.05; Fig. 3A). In addition, 4-HHE levels were lower in FPI-RGD-Exc (92% of CTL, p<0.05; Fig. 3A) and FPI-DHA-Sed (79% of CTL, p<0.05; Fig. 3A) than that in FPI-RGD-Sed (135% of CTL; Fig. 3A).
Figure 3.
Exercise and DHA supplementation increase resistance to oxidative stress after FPI. (A) FPI resulted in increased 4-HHE, which was counteracted by exercise, DHA, or combination of both. (B) FPI resulted in significant reduction of Sir2, which was significantly reversed by exercise or DHA. A greater elevation of Sir2 was observed when exercise was combined with DHA supplementation. *, p<0.05 vs CTL. #, p< 0.05. CTL, Sham-RGD-Sed. FPI, fluid percussion injury. Sed, Sedentary. Exc, Exercise.
We measured the levels of Sir2 in the hippocampus. The results showed that there was a significant group effect on Sir2 level (F4,25=18.42, p<0.01 by ANOVA). The Sir2 levels were higher in FPI-DHA-Exc (136% of CTL) than that in FPI-RGD-Sed (67% of CTL, p<0.05; Fig. 3B), FPI-RGD-Exc (92% of CTL, p<0.05; Fig. 3B), or FPI-DHA-Sed group (108% of CTL, p<0.05; Fig. 3B). In addition, Sir2 levels were higher in FPI-RGD-Exc (92% of CTL, p<0.05; Fig. 3B) and FPI-DHA-Sed (108% of CTL, p<0.05; Fig. 3B) than that in FPI-RGD-Sed (67% of CTL; Fig. 3B). These results suggest that exercise and dietary DHA have additive effects on the Sir2 levels in the hippocampus after TBI.
3.4. DHA and exercise may enhance membrane homeostasis by increasing iPLA2 and STX-3 after TBI
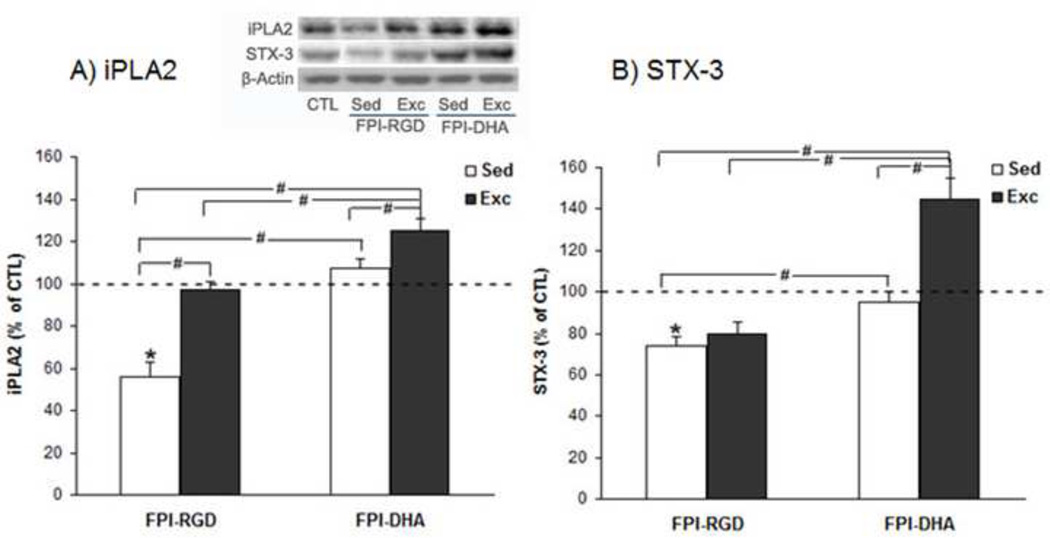
We measured iPLA2 levels based on its involvement in membrane homeostasis, which may affect neuronal signaling and learning and memory. The results showed that there was a significant group effect on iPLA2 level (F4,25=5.79, p<0.01 by ANOVA). The iPLA2 levels were higher in FPI-DHA-Exc (125% of CTL) than that in FPI-RGD-Sed (56% of CTL, p<0.05; Fig. 4A), FPI-RGD-Exc (97% of CTL, p<0.05; Fig. 4A), or FPI-DHA-Sed group (107% of CTL, p<0.05; Fig. 4A). In addition, iPLA2 levels were higher in FPI-RGD-Exc (97% of CTL, p<0.05; Fig. 3B) and FPI-DHA-Sed (107% of CTL, p<0.05; Fig. 4A) than that in FPI-RGD-Sed (56% of CTL; Fig. 4A). These results suggest that exercise and dietary DHA have enhanced effects on the iPLA2 levels in the hippocampus after TBI.
Figure 4.
Exercise and DHA supplementation regulate iPLA2 and STX-3 levels in the hippocampus after FPI. (A) FPI resulted in reduction of iPLA2, which was counteracted by DHA or exercise. A greater increase of iPLA2 was observed when exercise and DHA was combined. (B) FPI resulted in reduction of STX-3 which was significantly reversed by DHA. However, a greater elevation of STX-3 was observed when exercise and DHA was combined. *, p<0.05 vs CTL. #, p< 0.05. CTL, Sham-RGD-Sed. FPI, fluid percussion injury. Sed, Sedentary. Exc, Exercise.
We also measured STX-3 levels based on its involvement in membrane expansion and synaptic plasticity. The results showed that there was a significant group effect on STX-3 level (F4,25=12.77, p<0.01 by ANOVA). The STX-3 levels were higher in FPI-DHA-Exc (145% of CTL) than that in FPI-RGD-Sed (74% of CTL, p<0.05; Fig. 4B), FPI-RGD-Exc (80% of CTL, p<0.05; Fig. 4B), or FPI-DHA-Sed group (95% of CTL, p<0.05; Fig. 4B). In addition, STX-3 levels were higher in FPI-DHA-Sed (95% of CTL, p<0.05; Fig. 4B) than that in FPI-RGD-Sed (74% of CTL; Fig. 4B). These results suggest that exercise and dietary DHA have additive effects on the STX-3 levels in the hippocampus after TBI.
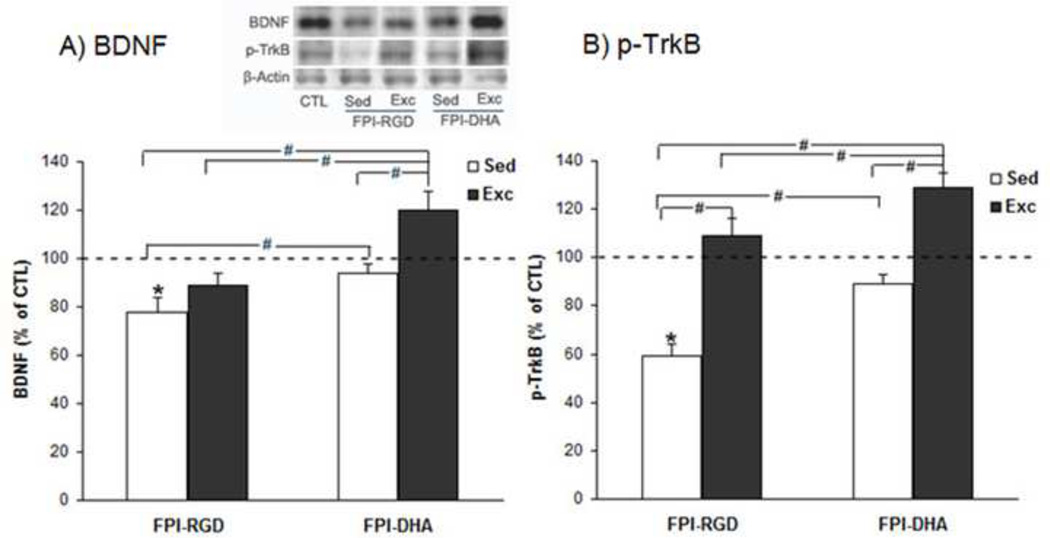
3.5. DHA alone or combination with exercise upregulate BDNF and p-TrkB after TBI
The results showed that there was a significant group effect on BDNF level (F4,25=10.83, p<0.01 by ANOVA). The BDNF levels were higher in FPI-DHA-Exc (120% of CTL) than that in FPI-RGD-Sed (78% of CTL, p<0.05; Fig. 5A), FPI-RGD-Exc (89% of CTL, p<0.05; Fig. 5A), or FPI-DHA-Sed group (94% of CTL, p<0.05; Fig. 5A). In addition, BDNF levels were higher in FPI-DHA-Sed (94% of CTL, p<0.05; Fig. 5A) than that in FPI-RGD-Sed (78% of CTL; Fig. 5A). These results suggest that exercise and dietary DHA have complimentary effects on the BDNF levels in the hippocampus after TBI.
Figure 5.
Exercise and DHA supplementation provide protection against BDNF and p-TrkB reduction after FPI. (A) FPI reduced BDNF levels, which were counteracted by DHA, exercise, or combination of both. A greater increase was observed when exercise was combined with DHA. (B) FPI reduced p-TrkB levels, which were counteracted by DHA or exercise. A greater effect was observed when exercise was combined with DHA. *, p<0.05 vs CTL. #, p< 0.05. CTL, Sham-RGD-Sed. FPI, fluid percussion injury. Sed, Sedentary. Exc, Exercise.
We measured the p-TrkB levels in the hippocampus. The results showed that there was a significant group effect on p-TrkB level (F4,25=12.85, p<0.01 by ANOVA). The p-TrkB levels were higher in FPI-DHA-Exc (129% of CTL) than that in FPI-RGD-Sed (59% of CTL, p<0.05; Fig. 5B), FPI-RGD-Exc (109% of CTL, p<0.05; Fig. 5B), or FPI-DHA-Sed group (89% of CTL, p<0.05; Fig. 5B). In addition, p-TrkB levels were higher in FPI-DHA-Sed (89% of CTL, p<0.05; Fig. 5B) and FPI-RGD-Exc (109% of CTL, p<0.05; Fig. 5B) than that in FPI-RGD-Sed (59% of CTL; Fig. 5B). These results suggest that exercise and dietary DHA have additive effects on the p-TrkB levels in the hippocampus after TBI.
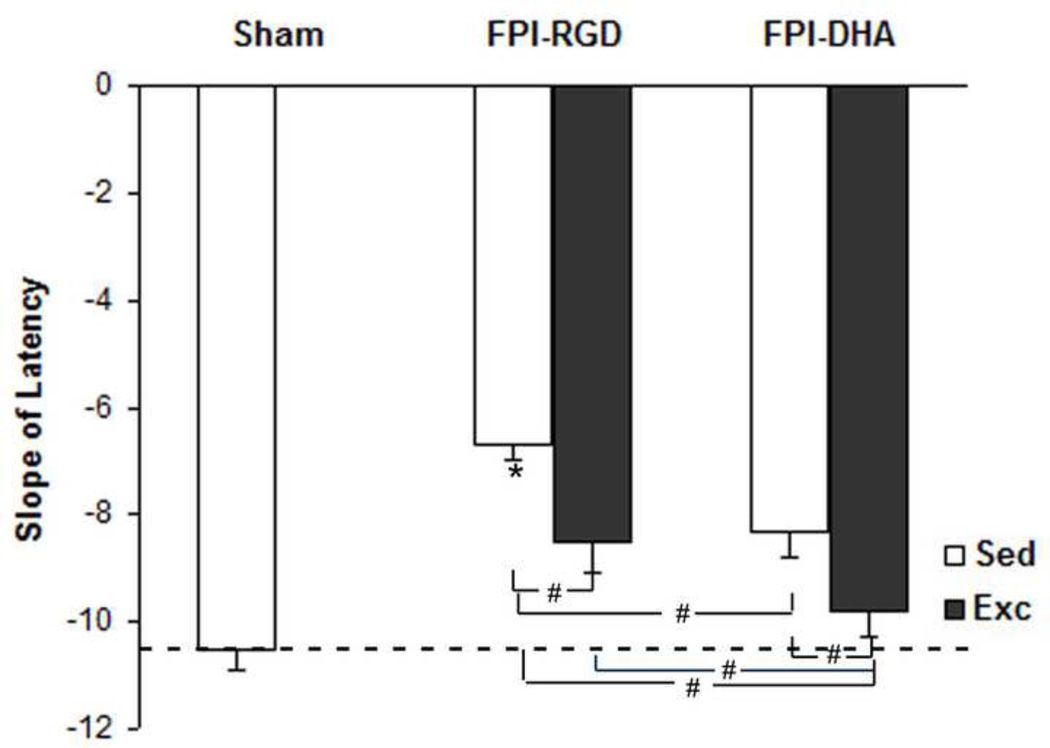
3.6. DHA and exercise combination enhance cognitive improvement after TBI
To determine whether exercise and DHA can provide neuroprotection against learning impairment after TBI, we evaluated the effects of exercise and dietary DHA supplementation on rats subjected to TBI. We calculated the slope of escape latency, which reflects the speed of learning during the testing: the steeper the slope, the faster the learning (Chytrova et al., 2010; Sharma et al., 2010). There was a significant group effect on learning slope (F4,25=2.98, p<0.05 by ANOVA). The learning slope was lower in FPI-DHA-Exc group than that in FPI-RGD-Sed group (p<0.05; Fig. 6), FPI-RGD-Exc group (p<0.05; Fig. 6, or FPI-DHA-Sed group (p<0.05; Fig. 6). In addition, learning slope was lower in FPI-DHA-Sed (p<0.05; Fig. 6) and FPI-RGD-Exc (p<0.05; Fig. 6) than that in FPI-RGD-Sed group. These results suggest that exercise and dietary DHA have additive effects on learning performance after TBI.
Figure 6.
Exercise and DHA supplementation provide protection against learning deficit after FPI. The learning speed (slope of escape latency to find the platform during Barnes Maze test) was shown. FPI resulted in reduced learning speed in rats fed control diet, which was counteracted by DHA or exercise. A greater effect in learning speed was observed when DHA and exercise was combined. *, p<0.05 vs CTL. #, p< 0.05. CTL, Sham-RGD-Sed. FPI, fluid percussion injury. Sed, Sedentary. Exc, Exercise.
4. Discussion
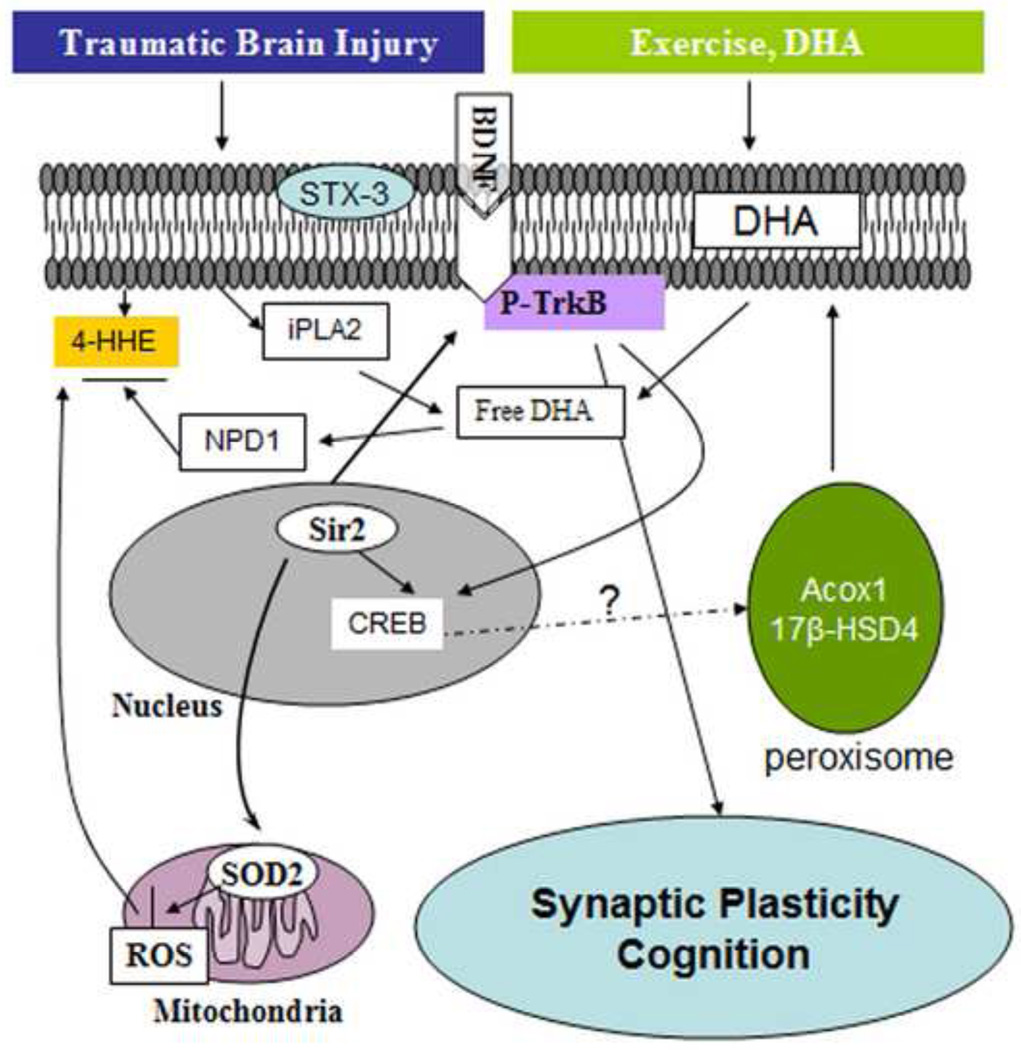
We found that the combination of dietary DHA and voluntary exercise was particularly effective to counteract the effects of TBI on cognitive function and several parameters of synaptic plasticity, and plasma membrane homeostasis. From a mechanistic point of view, we found that exercise influences DHA function by normalizing DHA content in the brain, which process seems mediated through enzymes that control the metabolism of DHA in the membrane. The collaborative action of exercise and diet was instrumental to compensate for the reduction in the levels of BDNF and the activation of the BDNF receptor p-TrkB. The interactive action between exercise and DHA was also instrumental for protecting against a decay in the spatial learning ability of the animals tested in the Morris water maze. These findings indicate that the combined actions of exercise and DHA supplementation have strong therapeutic potential for reducing the deleterious effects of TBI on membrane homeostasis, synaptic plasticity and cognition (Fig. 7).
Figure 7.
Possible mechanisms underlying the beneficial effects of exercise and DHA supplementation on cognitive improvement after TBI. Exercise can complement the action of DHA supplementation to maintain DHA levels in the plasma membrane by acting on the enzymes Acox1 and 17 -HSD4, which regulate DHA synthesis. The action of diet and exercise can also be exerted on maintaining membrane homeostasis by reversing the action of TBI on reducing iPLA2 and STX-3. Exercise and DHA can increase resistance to oxidative damage (i.e. reduction of 4-HHE and increase of Sir2). These beneficial effects may help activate BDNF-TrkB signaling, subsequently improving cognitive function after train trauma.
4.1. Membrane homeostasis after TBI
It is known that DHA is an essential component of nerve cell membranes (Marszalek and Lodish, 2005; Crawford et al., 2009). Current evidence indicates that the synthesis of DHA is very limited in the brain (Kim, 2007; Igarashi et al., 2007; Astarita et al., 2010), and our current results provides new evidence indicating that TBI reduced the enzymes Acox1 and 17 -HSD4 that regulate the synthesis of DHA in the brain. Our gas chromatography assays showing that TBI reduced the levels of DHA in the brain also support these results. Both of the studied enzymes are localized in the peroxisome and are involved in DHA biosynthesis from its precursor tetracosahexaenoic acid (Su et al., 2001). Acox1 and 17 -HSD4 participate in the process of DHA synthesis, in which the intermediate C22:5n-3 is converted to C24:6n-3, which is then retroconverted to C22:6n-3 (DHA) (Su et al., 2001; Ferdinandusse et al., 2001).
Although the separate applications of exercise or DHA supplementation were sufficient to counteract the TBI-related reductions of Acox1 and 17 -HSD4, exercise boosted the effects of DHA on Acox1. A potential effect of exercise on Acox1 was supported by results showing that levels of Acox1 were increased according to the amount of wheel running. Based on the roles of Acox1 and 17 -HSD4 supporting synthesis of DHA from precursors, it is reasonable to assume that exercise can impact the metabolism of DHA (Fig. 7). It is noteworthy that the actions of DHA supplementation and exercise were also expressed on higher levels of DHA. The enzyme iPLA2 also plays a role on phospholipid metabolism and it has been involved on maintaining membrane homeostasis after TBI. The current results showed that the effects of exercise or diet were sufficient to counteract the reduction of iPLA2 after TBI while the combined actions of DHA and exercise produced a greater elevation of iPLA2. It is known that iPLA2 plays an important role in stimulating phospholipids turnover in the membrane, reduction of iPLA2 may affect the mechanism that preserve membrane phospholipids, consequently impairing cognitive function and/or plasticity (Schaeffer et al., 2009; Allyson et al., 2012). Furthermore, DHA treatment combined with exercise counteracted the reduction of STX-3 after TBI. STX-3 is a plasma membrane-bound protein, which is found in neuronal growth cones and is susceptible to the influence of DHA on membrane expansion (Darios and Davletov, 2006). This implies that homeostatic regulation of membrane-related iPLA2 and STX-3 may be part of the mechanism by which diet and exercise preserve cognitive function after TBI. It is important to consider that the enzyme iPLA2 also has the capacity to produce free DHA that can be converted to the neuroprotective mediators NPD1 (Lukiw et al., 2005), suggesting a role of NPD1 on the protective effects of exercise and DHA in our paradigm. We measured the levels of the lipid peroxidation marker 4-HHE to have an estimate of the impact of the interventions on plasma membrane integrity. We found that TBI increased 4-HHE levels while DHA, exercise, and the combination of both significantly counteracted the effects of TBI on 4-HHE levels. Further, the reduction of Sir2 by TBI was significantly counteracted by DHA, exercise, and combination of both. Sir2 plays an important role in mitochondria function, energy homestasis, and stress resistance (Menzies et al., 2013). These results indicate that the membrane damage caused by TBI can be optimally counteracted by the combination of DHA and exercise. It is also noteworthy that alterations in the status of membrane damage appeared coordinated with molecular events underlying DHA metabolism.
4.2. Involvement of the BDNF system on the effects of the DHA diet and exercise
We used the Morris Water maze to assess how the diet and exercise interventions could influence the spatial learning performance, and the results showed a similar group pattern to that observed in the molecular assessment. Specifically, the combined applications of the DHA diet and exercise was more effective than the separate applications of DHA and exercise on the spatial learning ability after TBI. As discussed above, the plasma membrane plays a crucial role on the regulation of neuronal signaling through many receptors embedded in the membrane, thereby influencing behavioral performance. In particular, the receptors for BDNF are transmembrane receptors, and TrkB signaling is critical for learning and memory ability, which has been shown to be susceptible to the action of exercise (Vaynman et al., 2007). Our results showed that exercise greatly complements the action of DHA supplementation on levels of BDNF and TrkB activation. BDNF is an effective synaptic modulator (Tanaka et al., 2008; Adasme et al., 2011; Sajikumar and Korte, 2011), and a necessary element for various types of learning (Minichiello, 2009; Nagahara and Tuszynski, 2011). The overall evidence suggests that increased levels of BDNF under the actions of DHA and exercise may serve to support cognition and synaptic plasticity. Our previous study demonstrated that a combination of DHA and exercise can potentiate the cognition and BDNF-related plasticity in intact rats (Wu et al., 2008). A recent study from our group indicated that a combination of diet (DHA+curcumin) and exercise resulted in enhanced improvement in spinal cord learning via a BDNF-related mechanism (Joseph et al., 2012). These findings showing an interaction between diet and exercise are novel to demonstrate a potential mechanism by which two aspects of lifestyle can interact at the molecular level in both brain and spinal cord with subsequent effects on the modulation of learning under normal and challenging conditions.
5. Conclusions and Therapeutic Implications
Diet and exercise are integral aspects of the daily living and our results indicate that their combined application has the added capacity to benefit recovery events after TBI. The main novel aspect of these results is that exercise appears to act by providing support to metabolic pathways that preserve DHA in the plasma membrane. It is also significant that these actions of diet and exercise may be achieved through elevations of BDNF and p-TrkB involved in synaptic plasticity and cognitive function. These results suggest that the combined management of dietary DHA and/or exercise has the added therapeutic potential to reduce the deleterious effects of TBI on neuronal plasticity and cognitive function.
Exercise facilitates the action of DHA in preserving DHA homeostasis after TBI
Exercise enhances the effects of DHA on counteracting the outcome of TBI
These findings may have important therapeutic potential for the treatment of TBI
Acknowledgements
This study was supported by NIH award NS50465.
Abbreviations
- Acox1
acyl-CoA oxidase 1
- CTL
control
- DHA
Docosahexaenoic acid
- 17 -HSD4
17 -hydroxysteroid dehydrogenase type 4
- Exc
exercise
- FPI
fluid percussion injury
- iPLA2
calcium-independent phospholipase A2
- MWZ
Morris Water Maze
- RGD
regular diet
- Sed
sedentary
- STX-3
syntaxin 3
- TBI
Traumatic brain injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adasme T, Haeger P, Paula-Lima AC, Espinoza I, Casas-Alarcón MM, Carrasco MA, Hidalgo C. Involvement of ryanodine receptors in neurotrophin-induced hippocampal synaptic plasticity and spatial memory formation. Proc Natl Acad Sci USA. 2011;108:3029–3034. doi: 10.1073/pnas.1013580108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allyson J, Bi X, Baudry M, Massicotte G. Maintenance of synaptic stability requires calcium-independent phospholipase A2 activity. Neural Plast. 2012;2012:569149. doi: 10.1155/2012/569149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amen DG, Wu JC, Taylor D, Willeumier K. Reversing brain damage in former NFL players: implications for traumatic brain injury and substance abuse rehabilitation. J Psychoactive Drugs. 2011;43:1–5. doi: 10.1080/02791072.2011.566489. [DOI] [PubMed] [Google Scholar]
- Ansari MA, Roberts KN, Scheff SW. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic Biol Med. 2008;45:443–452. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astarita G, Jung KM, Berchtold NC, Nguyen VQ, Gillen DL, Head E, Cotman CW, Piomelli D. Deficient liver biosynthesis of docosahexaenoic acid correlates with cognitive impairment in Alzheimer's disease. PLoS OneM. 2010;5:e12538. doi: 10.1371/journal.pone.0012538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegon A. Cannabinoids as neuroprotective agents in traumatic brain injury. Curr Pharm Des. 2004;10:2177–2183. doi: 10.2174/1381612043384196. [DOI] [PubMed] [Google Scholar]
- Bigford GE, Alonso OF, Dietrich D, Keane RW. A novel protein complex in membrane rafts linking the NR2B glutamate receptor and autophagy is disrupted following traumatic brain injury. J Neurotrauma. 2009;26:703–720. doi: 10.1089/neu.2008.0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdge GC, Calder PC. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod Nutr Dev. 2005;45:581–597. doi: 10.1051/rnd:2005047. [DOI] [PubMed] [Google Scholar]
- Calder PC, Yaqoob P. Understanding omega-3 polyunsaturated fatty acids. Postgrad Med. 2009;121:148–157. doi: 10.3810/pgm.2009.11.2083. [DOI] [PubMed] [Google Scholar]
- Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr. 2012;142:592S–599S. doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
- Cansev M, Wurtman RJ. Chronic administration of docosahexaenoic acid or eicosapentaenoic acid, but not arachidonic acid, alone or in combination with uridine, increases brain phosphatide and synaptic protein levels in gerbils. Neuroscience. 2007;148:421–431. doi: 10.1016/j.neuroscience.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chytrova G, Ying Z, Gomez-Pinilla F. Exercise contributes to the effects of DHA dietary supplementation by acting on membrane-related synaptic systems. Brain Res. 2010;1341:32–40. doi: 10.1016/j.brainres.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford MA, Bazinet RP, Sinclair AJ. Fat intake and CNS functioning: ageing and disease. Ann Nutr Metab. 2009;55:202–228. doi: 10.1159/000229003. [DOI] [PubMed] [Google Scholar]
- Darios F, Davletov B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature. 2006;440:813–817. doi: 10.1038/nature04598. [DOI] [PubMed] [Google Scholar]
- Ferdinandusse S, Denis S, Mooijer PA, Zhang Z, Reddy JK, Spector AA, Wanders RJ. Identification of the peroxisomal beta-oxidation enzymes involved in the biosynthesis of docosahexaenoic acid. J Lipid Res. 2001;42:1987–1995. [PubMed] [Google Scholar]
- Griesbach GS, Gómez-Pinilla F, Hovda DA. Time window for voluntary exerciseinduced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. J Neurotrauma. 2007;24:1161–1171. doi: 10.1089/neu.2006.0255. [DOI] [PubMed] [Google Scholar]
- Huyghe S, Schmalbruch H, De Gendt K, Verhoeven G, Guillou F, Van Veldhoven PP, Baes M. Peroxisomal multifunctional protein 2 is essential for lipid homeostasis in Sertoli cells and male fertility in mice. Endocrinology. 2006;147:2228–2236. doi: 10.1210/en.2005-1571. [DOI] [PubMed] [Google Scholar]
- Igarashi M, DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. Docosahexaenoic acid synthesis from alpha-linolenic acid by rat brain is unaffected by dietary n-3 PUFA deprivation. J Lipid Res. 2007;48:1150–1158. doi: 10.1194/jlr.M600549-JLR200. [DOI] [PubMed] [Google Scholar]
- Joseph MS, Ying Z, Zhuang Y, Zhong H, Wu A, Bhatia HS, Cruz R, Tillakaratne NJ, Roy RR, Edgerton VR, Gomez-Pinilla F. Effects of diet and/or exercise in enhancing spinal cord sensorimotor learning. PLoS One. 2012;7:e41288. doi: 10.1371/journal.pone.0041288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY. Novel metabolism of docosahexaenoic acid in neural cells. J Biol Chem. 2007;282:18661–18665. doi: 10.1074/jbc.R700015200. [DOI] [PubMed] [Google Scholar]
- Long EK, Murphy TC, Leiphon LJ, Watt J, Morrow JD, Milne GL, Howard JR, Picklo MJ Sr. Trans-4-hydroxy-2-hexenal is a neurotoxic product of docosahexaenoic (22: 6; n-3) acid oxidation. J Neurochem. 2008;105:714–724. doi: 10.1111/j.1471-4159.2007.05175.x. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ, Bazan NG. Docosahexaenoic acid and the aging brain. J Nutr. 2008;138:2510–2514. doi: 10.3945/jn.108.096016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek JR, Lodish HF. Docosahexaenoic acid, fatty acid-interacting proteins, and neuronal function: breastmilk and fish are good for you. Annu Rev Cell Dev Biol. 2005;21:633–657. doi: 10.1146/annurev.cellbio.21.122303.120624. [DOI] [PubMed] [Google Scholar]
- Menzies KJ, Singh K, Saleem A, Hood DA. Sirtuin 1-mediated effects of exercise and resveratrol on mitochondrial biogenesis. J Biol Chem. 2013;288:6968–6979. doi: 10.1074/jbc.M112.431155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merenda A, Gugliotta M, Holloway R, Levasseur JE, Alessandri B, Sun D, Bullock MR. Validation of brain extracellular glycerol as an indicator of cellular membrane damage due to free radical activity after traumatic brain injury. J Neurotrauma. 2008;25:527–537. doi: 10.1089/neu.2007.0359. [DOI] [PubMed] [Google Scholar]
- Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci. 2009;10:850–860. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10:209–219. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Korte M. Metaplasticity governs compartmentalization of synaptic tagging and capture through brain-derived neurotrophic factor (BDNF) and protein kinase Mzeta (PKMzeta). Proc Natl Acad Sci USA. 2011;108:2551–2556. doi: 10.1073/pnas.1016849108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer EL, Forlenza OV, Gattaz WF. Phospholipase A2 activation as a therapeutic approach for cognitive enhancement in early-stage Alzheimer disease. Psychopharmacology (Berl) 2009;202:37–51. doi: 10.1007/s00213-008-1351-0. [DOI] [PubMed] [Google Scholar]
- Sharma S, Ying Z, Gomez-Pinilla F. A pyrazole curcumin derivative restores membrane homeostasis disrupted after brain trauma. Exp Neurol. 2010;226:191–199. doi: 10.1016/j.expneurol.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillwell W, Shaikh SR, Zerouga M, Siddiqui R, Wassall SR. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod Nutr Dev. 2005;45:559–579. doi: 10.1051/rnd:2005046. [DOI] [PubMed] [Google Scholar]
- Su HM, Moser AB, Moser HW, Watkins PA. Peroxisomal straight-chain Acyl-CoA oxidase and D-bifunctional protein are essential for the retroconversion step in docosahexaenoic acid synthesis. J Biol Chem. 2001;276:38115–38120. doi: 10.1074/jbc.M106326200. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GC, Kasai H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319:1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. The select action of hippocampal calcium calmodulin protein kinase II in mediating exercise-enhanced cognitive function. Neuroscience. 2007;144:825–833. doi: 10.1016/j.neuroscience.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Molteni R, Ying Z, Gomez-Pinilla F. A saturated-fat diet aggravates the outcome of traumatic brain injury on hippocampal plasticity and cognitive function by reducing brain-derived neurotrophic factor. Neuroscience. 2003;119:365–375. doi: 10.1016/s0306-4522(03)00154-4. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J. Neurotrauma. 2004;21:1457–1467. doi: 10.1089/neu.2004.21.1457. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. 2008;155:751–759. doi: 10.1016/j.neuroscience.2008.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. The salutary effects of DHA dietary supplementation on cognition, neuroplasticity, and membrane homeostasis after brain trauma. J Neurotrauma. 2011;28:2113–2122. doi: 10.1089/neu.2011.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]