Abstract
Introduction
Sustained and elaborative emotional information processing in depression and decreased affective elaboration in schizophrenia are considered hallmarks of these disorders but have not been directly measured. Gamma-band (35–45 Hz) EEG, has been associated with semantic functions such as feature binding and may index these elaborative processing. This study examined whether there were group differences in baseline and sustained gamma-band EEG following emotional stimuli in healthy adults as well as adults with depression and schizophrenia.
Methods
24 never-depressed healthy controls, 14 patients with DSM-IV unipolar major depressive disorder, and 15 patients with DSM-IV schizophrenia completed a lexical emotion identification task during EEG assessment. Gamma band EEG (35–45 Hz) in response to negative words was the primary dependent measure.
Results
As predicted, depressed individuals displayed sustained and increased gamma-band EEG throughout the task, and particularly in the seconds following negative words. Individuals with schizophrenia displayed decreased gamma-band activity throughout the task.
Conclusions
These data suggest that gamma-band EEG, measured over several seconds, may serve as a useful index of sustained semantic information processing. Depressed individuals appear to engage in sustained elaboration following emotional stimuli, whereas individuals with schizophrenia are not as prone to this type of elaborative processing.
Introduction
This study contrasts neural indices of elaboration of emotional information in healthy individuals with that of individuals with depression and schizophrenia. Prolonged involuntary elaboration (MacLeod and Mathews 1991) or rumination (Nolen-Hoeksema et al 1993) on negative aspects of information is a debilitating aspect of depression which has been linked to its persistence (Beck 1967; Ingram 1984; Ingram 1990; Ingram et al 1998; MacLeod and Mathews 1991; Teasdale 1988). Sustained elaboration has been inferred from a variety of indirect behavioral measures including enhanced memory for negative information (Matt et al 1992) and a tendency to interpret events as negative (Norman et al 1988) in depression. A parallel literature demonstrates that depressed individuals display sustained physiological reactivity in the seconds following the presentation of emotional information, including increased slow-wave ERP activity up to 13 seconds following presentation of negative material (Deldin et al 2001), sustained pupil dilation for up to 30 seconds following briefly presented emotional words (Siegle et al 2001; Siegle et al 2003b), and sustained fMRI-derived amygdala reactivity in depressed individuals for up to 30 seconds following briefly presented emotional words (Siegle et al 2002; 2007). These physiological indices are correlated with self-reported rumination (e.g., Siegle et al 2006; 2003b; 2002).
Different affective information processing disruptions are apparent in other psychopathologies. Individuals with schizophrenia display increased communication or speech disturbance (e.g., vague references, confused references, and ambiguous word meanings coded from speech transcripts) in the context of negative affect (Cohen and Docherty 2004; Docherty et al 1998; Docherty et al 2001; Rhinewine and Docherty 2002). In addition, they appear to display decreased limbic activity during affect labeling (Hempel et al 2003). Individuals with schizophrenia whose speech was characterized by affective reactivity exhibited enhanced initial startle reactivity but also greater habituation to repeated stimuli suggestive of reduced affective modulation. (Docherty et al 2001). These data contrast with depressed individuals, who display decreased sustained startle reactivity following emotional stimuli, presumably due to sustained affective modulation (Larson and Davidson 2001).
An intuitive explanation is that sustained physiological reactivity in depression reflects sustained elaborative emotional processing, and that individuals with schizophrenia display decreased elaborative emotional processing. Yet, traditionally employed physiological indices are not uniquely associated with semantic or elaborative processing. For example, the amygdala has been shown to respond to subliminally presented information (e.g., Whalen et al 2001), and sustained amygdala reactivity to foot-shock has been demonstrated in rats, who presumably do not semantically elaborate upon their experiences (2002). Similarly, individuals with schizophrenia appear to display increased physiological disturbances (e.g., corrugator EMG) following guided imagery, and do not report decreased affective intensity (Sison et al 1996), possibly suggesting a disconnection between traditional physiological measures and semantic affective processing.
Thus, to test whether elaborative processes are engaged in the seconds following presentation of emotional information in depressed individuals and individuals with schizophrenia more directly, it is important to examine an index of neural activity specific to associative processing in these populations. Stimulus-induced gamma-band (~40Hz) EEG activity has been suggested to specifically reflect feature binding processes (Tallon-Baudry and Bertrand 1999). While this high frequency activity is typically assessed within one second following stimuli, such research has generally targeted binding of visual or low-level (e.g., orthographic) features. If gamma activity also indexes higher-order feature binding, as occurs for semantic association (Braeutigam et al 2001; Pulvermuller et al 1996; Pulvermuller et al 1999; Pulvermuller et al 1995) or working memory processes as are involved in semantic elaboration, it could continue for a longer period on the order of many seconds. In support of this idea, stimulus related gamma EEG has been observed during semantic decision tasks (Lachaux et al 2007) and in comprehending grammatical, but not agrammatical sentences (Hald et al 2006).
As such, measuring gamma-band EEG over a longer time-scale may help to understand the time course specifically of semantic elaboration of emotional information in psychopathology. This study therefore examined sustained gamma-band EEG in response to briefly presented emotional information in healthy individuals and individuals with unipolar depression and schizophrenia. Semantic elaboration is often considered to reflect successive association of features of emotional stimuli with other related emotional stimuli (Bower 1981; Ingram 1984; Siegle and Hasselmo 2002). We therefore hypothesized that depressed individuals would display sustained semantic elaboration, indexed by sustained gamma-band EEG following exposure to negative information. We further hypothesized that individuals with schizophrenia would display decreased sustained gamma-band EEG in response to negative information as an index of decreased semantic elaboration. Because observed effects could be a function of either task-set (the entire task-related attention to emotional features) or responses to specific stimuli, we examined both stimulus-related changes in gamma, and overall levels of gamma throughout the task. As our hypotheses regard how participants process negative information, primary planned analyses regarded negative word stimuli.
To understand the generality of these observations we also examined the specificity of observed findings to negative versus neutral stimuli. This comparison could aid in assessing whether observed findings regard general hyper-reactivity or emotionally-biased information processing. Sensitivity analyses examined continuous associations of sustained gamma-power with potentially relevant trait variables (rumination, depressive severity), age, and potential medication effects.
Method
Participants
Participants included 14 patients diagnosed with unipolar major depression (i.e., in the midst of a current major depressive episode) and 15 individuals diagnosed with schizophrenia but not a significant secondary depressive syndrome using a structured clinical interview (SCID I, First et al 1996), and 24 healthy, never-depressed controls. Demographics are reported in Table 1. Participants stated they had no significant eye problems; a subset who were assessed with a hand-held eye chart had normal corrected vision (20/30) with both eyes open; the remainder reported no difficulty reading text on a computer screen much smaller than the text used in the experiment. Participants described no health problems thought to interfere with performance or psychoactive drug or alcohol abuse within the past six months. Participants were excluded if they had a previous history of manic episodes. Depressed and control participants with a previous history of psychosis were also excluded. Most depressed participants were taking antidepressants (n=9), particularly selective serotonin reuptake inhibitors (SSRI’s; n=6) as well as sleep medication (n=1) and pain medication (n=1). Most participants with schizophrenia were taking atypical antipsychotic medications (n=14) along with antidepressants (n=7) particularly SSRI’s (n=6), anticonvulsants (n=3), benzodiazepines (n=4), and beta-blockers (n=1). All participants scored in the normal range on a cognitive screen (VIQ equivalent (computed based either on subscales of the WAIS (Wechsler 1997) or the NAART (Nelson and Willison 1991)) >80). ANOVAs followed by relevant simple effects tests on all demographic variables revealed that control participants were significantly younger than both depressed participants, t(36)=−3.1, p=.001, and participants with schizophrenia, t(37)=3.6, p=.001. The groups did not otherwise differ on demographic characteristics including gender and ethnic composition (p>.9).
Table 1.
Subject demographics and behavioral data
| Measure | Depressed | Schizophrenia | Control | Significant Difference (ns=not significant, blank = not tested) |
|---|---|---|---|---|
| N | 14 | 15 | 24 | |
| # male | 8 | 8 | 12 | ns |
| # Caucasian | 11 | 12 | 20 | ns |
| Age range | 21–58 | 33–50 | 18–53 | |
| Age M (SD) | 43.1 (14.2) | 41.5 (5.6) | 30.2 (11.4) | F(2,50)=8.00, p=.001, η2=.24 |
| Age M (SD)-Age-matched Subsample | 41.9 (14.0) | 40.2 (5.0) | 38.1 (9.1) | F(2,39)<1, p=.64 |
| BDI M (SD) | 22.8 (8.0) | 13.2 (8.1) | 2.8 (2.9) | F(2,49)=47.1, p<.0005, η2=.66 |
| Post-scan Emotion ratings of employed words (1= very negative and 7 = very positive) | ||||
| Positive words M (SD) | 5.39(.84) | 5.76(.56) | 5.73(.62) | |
| Negative words M (SD) | 2.24(1.06) | 1.75(.43) | 2.17(1.00) | |
| Neutral words M (SD) | 4.18(.33) | 4.32(.22) | 4.14(.45) | |
| Reaction times in seconds | ||||
| Positive words M (SD) | 1.31(.41) | 1.49(.26) | 1.06(.32) | F(2,51)=7.79, p=.001, η2=.23 |
| Negative words M (SD) | 1.29(.52) | 1.41(.11) | 1.09(.37) | F(2,51)=3.49, p=.038, η2=.12 |
| Neutral words M (SD) | 1.38(.41) | 1.60(.30) | 1.30(.42) | F(2,51)=2.87, p=.066, η2=.10 |
Measures of Mood and Rumination
To assess depressive severity at the time of testing, the Beck Depression Inventory (Beck 1967) was administered. The Response Styles Questionnaire (RSQ, Nolen-Hoeksema et al 1993) was given after the information processing measures to assess a trait-like disposition to sustain attention to negative information. The RSQ is a 71 item self-report measure containing rumination and distraction subscales. The 22 item rumination subscale assesses the frequency with which individuals think about their symptoms of depression, when they feel sad or depressed, on a four point scale from “almost never” to “almost always.” It contains items such as “think about how alone you feel” and “think about how passive and unmotivated you feel.” Previous studies have shown the RSQ rumination scale to be internally consistent and factor-analytically derivable. Higher scores are related to more severe and longer episodes of depression (Nolen-Hoeksema et al 1993) as well as distorted interpretations of hypothetical life events (Lyubomirsky et al 1998).
Apparatus
Stimuli were displayed in white on a black computer screen. Participants sat approximately 77 cm from the stimuli. Stimuli were lowercase letters approximately 1.59 cm high, subtending 1.18 degrees of visual angle. Reaction times were recorded using button presses on a game pad capable of reading reaction times with millisecond resolution. It was modified to contain three buttons, arranged in a triangle, so that respondents’ fingers were nearly equidistant from each possible response. To account for differential response latencies to different buttons, the mapping of game-pad buttons to responses was counterbalanced across participants.
EEG data were recorded using methods previously described and tested (Condray et al 2003). In brief, 20 channel EEG data collection was accomplished using a preconfigured Physiometrix cap referenced to nose with forehead as ground. Electrodes medial to the left eye (above, below) and at the outer canthi of both eyes measured eye movements. Data were recorded at 250 Hz (1samp/4ms), using a .02–100 Hz band-pass filter, via Sensorium amplifiers. Impedances were below 30 kΩ (standard recommended impedance threshold for the employed amplifiers). Data were collected using the EEGSYS software data acquisition platform (Hartwell 1995).
Target Stimulus Materials
Stimuli included 80 negative words in addition to 80 positive and 80 neutral words used as controls, from the ANEW corpus (Bradley and Lang 1997). Selected words within each normed affective valence category (valence ratings: <3 = negative, 4–6 = neutral, >7 = positive) were balanced for word length, frequency, and arousal, using a computer program designed to create balanced affective word lists (Siegle 1994).
Procedure
At a first appointment, participants were told about the experiment and signed a University of Pittsburgh IRB approved consent form. A subset of participants received a brief vision test (implemented late in our protocol); all received a cognitive screen. At a second appointment, usually within two weeks, participants completed the battery of information processing and self-report measures. Testing occurred in a moderately lit room in which the experimenter was not present. Time of day was not controlled. The order of administration of a lexical decision (not described or analyzed in this manuscript) and valence identification task was counterbalanced across participants. The tasks were the same as our previous study (Siegle et al 2001) with the exception that more words were used, and all words were from a normed corpus.
Emotional Valence Identification Task
Each word was presented in the following manner. A fixation square appeared and remained on the screen for 200 ms. The fixation square was then replaced by a row of X’s (forward mask) for 2000 ms. The X’s in the middle of the string were next replaced by letters spelling a word (the target stimulus). After a target-stimulus duration of 150 ms, the letters were masked by a row of X’s again, and the participant was allowed to respond. EEG data were recorded for eight seconds after the initial onset of the word stimuli, regardless of when the participant responded. Thus, the total inter-trial interval was 10.35 seconds. All masks and stimuli were drawn in white on a black background. Research participants were instructed to judge the emotionality of stimuli by pushing buttons for “Positive”, “Negative”, or “Neutral” as quickly and accurately as they could using a three-button game-pad under their right hand, in response to each stimulus. Labels for these responses were on a card attached to the upper-right of the computer screen. The research participant’s EEG throughout the trial, as well as reaction time and response were recorded on the computer for each stimulus.
Data Selection, Cleaning, and Reduction
Selection of Stimuli for Analysis
Trials with reaction times below 150 ms were discarded as outliers, because previous results suggest that reaction times in this range indicate that a response was made without regard for the stimulus (Matthews and Southall 1991). Trials for which the normed valence did not match the participant’s response were not eliminated from participant averages because it was assumed that the decision processes were the same, and also because there is truly no “correct” response for the emotionality of words.
Calculation of Reaction Times
Harmonic means of reaction times were used to reliably index the central tendency of an individual’s reaction times within a condition (as recommended by Ratcliff 1993). To eliminate spurious skew due to outliers while preserving rank-ordering of data, outliers more than 1.5 times the interquartile range from the median harmonic mean on any variable were scaled to the closest obtained value below this cutoff plus the difference between this value and the next closest value. Analyses of mean harmonic reaction times per subject were done within group x valence multivariate ANOVAs. Family-wise alpha for all planned pairwise contrasts was controlled at 0.05 using a Bonferroni correction, unless all pairwise contrasts were tested, in which case a Tukey correction was performed (as suggested by (Maxwell and Delaney 2000))
Calculation of EEG Indices
Data were pre-processed using methodology similar to that which our group has used in the past (e.g., Condray et al 2003). Blinks, eye movements, and other artifacts (voltage exceeding ± 150 μV) were corrected by applying the Gratton et al (1983) procedure (code by G. Miller) using the EOG data. Remaining regions of sustained blinks were corrected using linear interpolation (effectively removing frequency domain information associated with large blinks). Trials comprised of over 50% blinks were removed from consideration.
For time-frequency analyses, each trial for each subject was subjected to wavelet decomposition (Morlet mother wavelet as in Tallon-Baudry and Bertrand 1999) yielding a trial x time x frequency matrix for each subject. Power at each frequency during a 200ms baseline was subtracted for each trial. Average matrices for each valence (positive, negative, neutral) were computed for each subject of “induced” (i.e., non-phase-locked) activity and averaged across subjects. Mean power in the 35–45 Hz range was extracted for analyses of “gamma” band activity.
Analytic Strategy for Between-Groups Comparisons
The one-second window after the stimulus in which gamma-band activity to negative words was maximal was chosen for primary group comparisons as we reasoned that the interval with the largest signal would produce the greatest signal-to-noise ratio for group comparisons. As described previously, primary hypotheses regarded only responses to negative words. Main effects of group in response to negative words during the empirically identified time-window were computed using ANOVA’s. For sensitivity analyses, main effects of condition (e.g., differential response to negative vs. neutral words) were examined using paired t-tests. Group by condition interactions were evaluated with independent samples t-tests between condition-related difference waveforms from each group.
Group differences over time are illustrated in figures depicting waveforms by showing significant statistical tests at each sample along relevant waveforms; because that technique does not control for type I error across the large number of tests these figures should be interpreted descriptively only.
Analytic Strategy for Scalp Maps
Tests of hypotheses in specific time-windows were examined at each of the 20 electrodes across the scalp using the same ANOVAs and simple effects decompositions as described previously. To control type I error for tests of whether there were differences at any specific electrode, a Bonferroni correction was employed in which electrodes significant at p<.05/20 or p<.0025 were considered to represent locations of significant group differences. Since mechanisms underlying sustained elaborative processing in depression could have reflected deep subcortical generators and because gamma-band EEG is often associated with subcortical and thalamic circuits (Kraut et al 2003; Skinner et al 2000), particularly including disruptions of gamma-band EEG in schizophrenia (Behrendt 2003) which are expected to lead to wide-spread surface-EEG effects, relevant tests were not best considered by examining single electrodes but, rather, clusters of significant electrodes. Our primary question was, specifically, whether there were reliable differences anywhere across the scalp, without regard to specific locations. To examine this question, the 3-group contrast of baseline-corrected gamma to negative words was subjected to 1000 Monte-Carlo simulations in which group membership was shuffled randomly, and for each simulation, the number of electrodes significant at p<.1 was recorded. Across 2 replications of this exercise it was determined that 4 electrodes were significant at p<.1 5% of the time. Thus, differences between groups were considered reliable if >4 electrodes were significant for relevant contrasts.
Analytic Strategy for Tests of Rumination
A more exploratory aim was to relate continuous scores on a measure of rumination or perseverative thought to EEG data. For this analysis we did not restrict investigations to the window of peak gamma activity because we reasoned that sustained thought processes might be associated with sustained gamma-band activity. Thus, we examined the 0.25 second-long time-window in which rumination scores were maximally correlated with gamma-band EEG responses. Because of the potential for type I error in this analysis we examined other contrasts such as associations of rumination with gamma band activity to neutral words to suggest whether results were unique to negative words.
Results
Behavioral Data
Post-hoc emotion ratings and harmonic mean reaction times to all conditions are shown in Table 1. Multivariate split-plot group (depressed, schizophrenia, control) x valence (positive, negative, neutral) ANOVAs on valence ratings suggested that words were reliably rated as belonging to different categories, valence main effect Wilks’ Λ=.11, F(2,50)=209.4, p<.0005, η2 (% variance accounted for) =.89. The group x valence effect was not significant, Wilks’ Λ=.87, F(4,100)=1.8, p=.13, η2=.07, and there was no main effect of group, p=.70.
A similar analysis on reaction times also revealed significant main effects of valence, Wilks’ Λ=.73, F(2,50)=8.96, p<.0005, η2=.26 (negative words were responded to most quickly) and group, F(2,51)=5.1, p=.01, η2=.17 (controls were fastest). While the a priori chosen multivariate test did not reveal a valence x group interaction, Wilks’ Λ=.87, F(4,100)=1.85, p=.13, η2=.07, the less conservative Roy’s Largest Root test of the same effect was significant, Roy’s Largest Root = .13, F(2,51)=3.43, p=.04, η2=.11. As we had an a priori hypothesis regarding this interaction it was examined in the context of simple effects analyses. Control participants responded more slowly to neutral words than to both positive words, F(1,22)=36.3, p<.0005, η2=.62 and negative words, F(1,22)=14.1, p=.001, η2=.39. Depressed participants did not respond differently to the different valences (p>.4). Individuals with schizophrenia responded more quickly to negative than neutral words F(1,14)=6.7, p=.02, η2=.32, and nonsignificantly faster to positive than neutral words F(1,14)=3.4, p=.09, η2=.19.
Primary Gamma EEG analyses
Sustained gamma-band reactivity negative words in depression
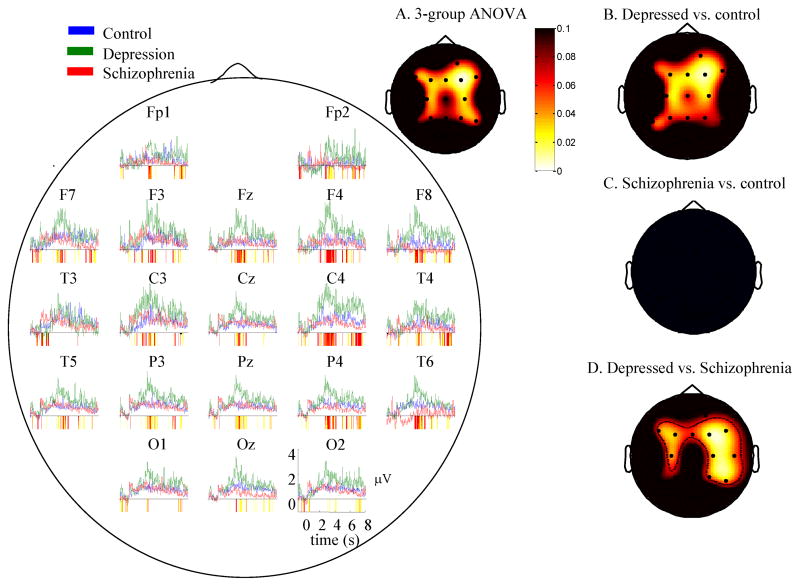
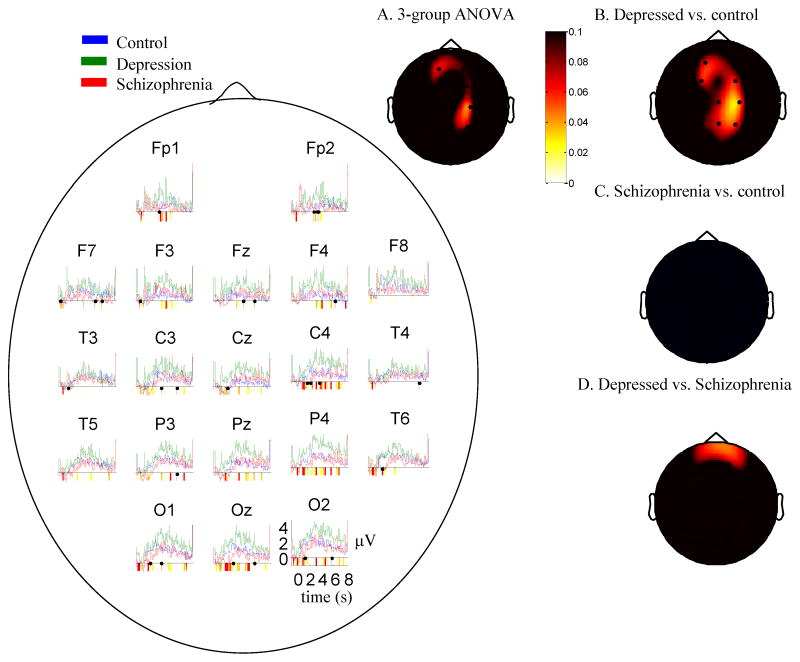
The time-series in Figure 1 depict the mean trial-related gamma power (35–45Hz) for all electrodes to negative words throughout trials as an index of stimulus-related reactivity. Tests of deviation suggested that for all groups, at all electrodes, gamma activity increased in the 3–5 seconds following presentation of the stimuli. Regions of significant differences between groups are highlighted in the colored bars below the x-axis of Figure 1. As shown in the figure, depressed individuals displayed increased gamma-band EEG across most electrodes throughout trials relative to healthy individuals and individuals with schizophrenia. Figure 1A displays the scalp distribution of p-values from a group ANOVA on gamma power in the window of maximal gamma (3–4 seconds following the offset of stimuli). These values are reported in Table 2, column 1. As shown in the figure and table, this analysis was significant for the central electrodes (>4 electrodes with significant differences, suggesting a group difference across electrodes). To decompose these effects, The other scalp plots show simple effects scalp maps of t-tests for depressed versus control (Figure 1B), schizophrenia versus control participants (1C), and depressed versus schizophrenia (1D). As shown in the figure group differences were driven by the fact that depressed individuals displayed increased gamma EEG power relative to the other groups; healthy individuals and individuals with schizophrenia displayed few differences. Table 2, column 2 reports test magnitudes for the depressed v. control comparison. For each map, >4 electrodes were significant suggesting that group differences were reliable.
Figure 1.
Time series graphs: Mean EEG gamma power (35–45 Hz) over time for each group in response to negative words. Periods of significant differences between groups are highlighted below the x-axis (yellow in color – light gray in black/white: p<0.1; red in color, dark gray in black/white: p<0.05). A. Scalp map for three group ANOVA (control, depression, schizophrenia) 3–4 seconds after stimulus offset minus a pre-stimulus baseline. Whiter values represent lower p-values. Groups differed on sustained gamma power throughout the scalp. B–D. p values for t-tests of group difference contrasts in mean Gamma Power (35–45 Hz) 3–4s following the offset of the stimulus minus a pre-stimulus baseline, all groups, all electrodes, negative words. Whiter values represent lower p-values. Values in the red/yellow (gray in black/white) spectrum represent positively-valued t-tests. Individuals with depression displayed increased sustained gamma power compared to controls.
Table 2.
p-values for tests of group differences across electrodes for the period 3–4 seconds post-stimulus onset (baseline-corrected by subtracting out the mean gamma power during the 1 second baseline period) and for the 1s baseline period (not baseline-corrected).
| 1. 3-group ANOVA negative words — 3-4s | 2. Depressed vs control on negative words – 3-4s | 3. Schizophrenia vs control – 1s baseline period | 4. 3-group ANOVA negative vs. neutral words – 3-4s | 5. Schizophrenia sample bdi>15 vs. bdi<9 negative words – 3-4s | 6. Schizophrenia sample bdi>15 vs. bdi<9 1s baseline period only | |
|---|---|---|---|---|---|---|
| Fz | 0.03 | 0.03 | 0.06 | 0.24 | 0.55 | 0.91 |
| Cz | 0.10 | 0.06 | 0.06 | 0.26 | 0.69 | 0.55 |
| Pz | 0.09 | 0.06 | 0.16 | 0.04 | 0.81 | 0.36 |
| Oz | 0.17 | 0.25 | 0.31 | 0.57 | 0.54 | 0.92 |
| Fp1 | 0.15 | 0.14 | 0.38 | 0.53 | 0.04 | 0.79 |
| F3 | 0.03 | 0.04 | 0.03 | 0.22 | 0.31 | 0.75 |
| F7 | 0.07 | 0.11 | 0.02 | 0.27 | 0.97 | 1.00 |
| C3 | 0.06 | 0.07 | 0.06 | 0.17 | 0.35 | 0.44 |
| P3 | 0.07 | 0.06 | 0.14 | 0.10 | 0.75 | 0.68 |
| T3 | 0.60 | 0.72 | 0.37 | 0.18 | 0.70 | 0.10 |
| T5 | 0.11 | 0.08 | 0.15 | 0.54 | 0.95 | 0.66 |
| O1 | 0.31 | 0.21 | 0.61 | 0.11 | 0.89 | 0.73 |
| Fp2 | 0.06 | 0.05 | 0.08 | 0.88 | 0.13 | 0.32 |
| F4 | 0.002 | 0.01 | 0.07 | 0.19 | 0.51 | 0.83 |
| F8 | 0.04 | 0.05 | 0.33 | 0.83 | 0.03 | 0.77 |
| C4 | 0.03 | 0.03 | 0.09 | 0.55 | 0.44 | 0.90 |
| P4 | 0.06 | 0.07 | 0.23 | 0.04 | 0.57 | 0.89 |
| T4 | 0.18 | 0.22 | 0.52 | 0.08 | 0.32 | 0.35 |
| T6 | 0.05 | 0.09 | 0.52 | 0.10 | 0.46 | 0.46 |
| O2 | 0.23 | 0.17 | 0.43 | 0.62 | 0.92 | 0.46 |
| # electro-des p<.1 | 13 | 13 | 8 | 5 | 2 | 1 |
Bold = values significant at p<.0025, which represents a significant result at that electrode at p<.05 after accounting for multiple tests across electrodes
Italic = values significant at p<.1. Monte Carlo simulations suggested that for these data, 4 electrodes significant at p<.1 were needed to suggest there were reliable differences between groups at a level of p<.05.
Group differences in overall levels of gamma during the task
Gamma-band EEG during the 1 second pre-stimulus baseline period was examined to quantify processing not specifically associated with stimulus characteristics. Figure 2 displays the scalp distributions for group contrasts during this period. Table 2, column 3 reports test magnitudes for the schizophrenia vs. control comparison in which >4 electrodes were significantly different. As shown in the figure and table, depressed individuals displayed increased task-related gamma-band activity compared to the other groups, whereas individuals with schizophrenia displayed decreased task-related gamma-band activity, across the scalp.
Figure 2.
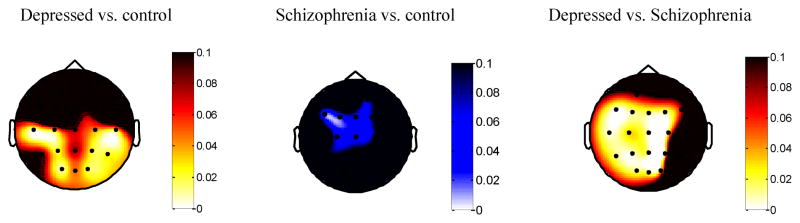
p values for t-tests of group differences in mean Gamma Power (35–45 Hz) in the 1s pre-stimulus mask period, all groups, all electrodes, negative words. Whiter values represent lower p-values. Values in the red/yellow (white/gray in black and white) spectrum represent positive t-tests; values in the blue spectrum (white gray in black and white – all non-black values in the Schizophrenia vs. Control subfigure apply, and no others apply) represent negative t-tests. Individuals with depression display increased gamma power throughout the task compared to controls. Individuals with schizophrenia displayed decreased gamma power throughout the task compared to controls.
Uniqueness of group differences to negative words
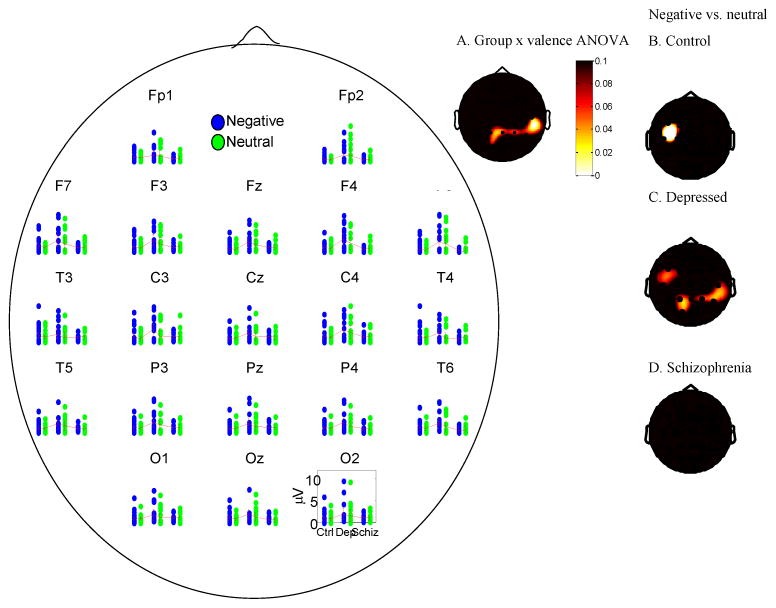
To understand whether observed group differences in sustained gamma band responses were unique to negative words all comparisons for negative words were repeated in the context of a group x valence (negative, neutral) analysis. Thus, Table 2 column 4 and figure 3 show the scalp distribution of p-values from a group (depressed, control, schizophrenia) ANOVA in which trial-related gamma power averaged over the period from 3–4 seconds following the offset of the stimulus across all trials was computed on the baseline-corrected data for negative compared to neutral words. As shown in the table and figure, group differences in sustained gamma following negative vs. neutral words were apparent throughout (>4) primarily parietal electrodes. To decompose this interaction, Table 3 shows the result of t-tests of differences between negative and neutral words at each electrode within each group. As shown in the table, sustained gamma band responses were significantly larger for negative than neutral words throughout the head for depressed participants, but not for control participants or participants with schizophrenia. To put the magnitude of valence-related versus group-related differences in context, the time-series in figure 3 show the raw data underlying the scalpmaps in Figure 1, Table 2, columns 1, 2, 4, and Table 3 are based. The scalpmap shows regions of significant differences. Figure 3 highlights that the primary pattern of group differences regards increased gamma band responses in depressed individuals, with valence-related differences being visible but of a qualitatively smaller magnitude.
Figure 3.
Scatterplot and 95% confidence intervals showing gamma power for all participants in response to negative and neutral words 3–4 seconds following stimulus onset minus a pre-stimulus baseline. A. Scalp map of p-values from a group 3-way ANOVA (depressed, control, schizophrenia) on the contrast of negative minus neutral words in which trial-related gamma power 3–4 seconds following the offset of the stimulus minus a pre-stimulus baseline. The groups differed in parietal regions as a function of valence. B–D. Scalp maps of within-group differences in gamma power to negative and neutral words 3–4 seconds following stimulus offset minus a pre-stimulus baseline, showing that the primary ANOVA was driven by differences present in depressed participants compared to the other groups.
Table 3.
t-tests of differences in gamma power in response to negative versus neutral words across electrodes for the period 3–4 seconds post-stimulus onset.
| Electrode | Control | Depressed | Schizophrenia | ||||||
|---|---|---|---|---|---|---|---|---|---|
| t(23) | p | d | t(15) | p | d | t(14) | p | d | |
| Fz | 0.78 | 0.44 | 0.16 | 1.41 | 0.18 | 0.35 | −0.77 | 0.45 | −0.2 |
| Cz | −0.03 | 0.98 | −0 | 1.25 | 0.23 | 0.31 | −0.37 | 0.72 | −0.1 |
| Pz | −0.17 | 0.86 | −0 | 1.92 | 0.07 | 0.48 | −0.21 | 0.84 | −0.05 |
| Oz | 1.35 | 0.19 | 0.28 | 1.41 | 0.18 | 0.35 | 0.75 | 0.47 | 0.19 |
| Fp1 | 0.14 | 0.89 | 0.03 | 0.79 | 0.44 | 0.2 | −0.5 | 0.62 | −0.13 |
| F3 | 1.53 | 0.14 | 0.31 | 1.76 | 0.1 | 0.44 | −0.26 | 0.8 | −0.07 |
| F7 | 1.51 | 0.14 | 0.31 | 1.84 | 0.09 | 0.46 | −0.17 | 0.87 | −0.04 |
| C3 | 1.86 | 0.08 | 0.38 | 1.54 | 0.14 | 0.39 | −0.56 | 0.58 | −0.15 |
| P3 | 0.69 | 0.5 | 0.14 | 2.07 | 0.06 | 0.52 | −0.33 | 0.75 | −0.09 |
| T3 | 1.87 | 0.07 | 0.38 | 1.85 | 0.08 | 0.46 | −0.27 | 0.79 | −0.07 |
| T5 | 0.67 | 0.51 | 0.14 | 1.12 | 0.28 | 0.28 | 0.37 | 0.72 | 0.09 |
| O1 | 0.27 | 0.79 | 0.05 | 1.93 | 0.07 | 0.48 | −0.89 | 0.39 | −0.23 |
| Fp2 | 0.37 | 0.72 | 0.07 | 0.19 | 0.85 | 0.05 | −0.58 | 0.57 | −0.15 |
| F4 | 0.74 | 0.47 | 0.15 | 1.71 | 0.11 | 0.43 | 0.01 | 0.99 | 0 |
| F8 | 0.8 | 0.43 | 0.16 | 1.07 | 0.3 | 0.27 | 0.52 | 0.61 | 0.14 |
| C4 | 0.79 | 0.44 | 0.16 | 1.05 | 0.31 | 0.26 | 0.22 | 0.83 | 0.06 |
| P4 | 1.02 | 0.32 | 0.21 | 2.17 | 0.05 | 0.54 | −0.7 | 0.49 | −0.18 |
| T4 | 0.35 | 0.73 | 0.07 | 1.96 | 0.07 | 0.49 | −1.01 | 0.33 | −0.26 |
| T6 | 0.65 | 0.52 | 0.13 | 2.05 | 0.06 | 0.51 | −0.13 | 0.9 | −0.03 |
| O2 | 0.28 | 0.78 | 0.06 | 0.84 | 0.42 | 0.21 | −0.14 | 0.89 | −0.04 |
| # electro-des p<.1 | 2 | 8 | 0 | ||||||
Columns labeled d represent Cohen’s d, calculated as the difference in means divided by the pooled standard deviation.
Bold = values significant at p<.1. Monte Carlo simulations suggested that for these data, 4 electrodes significant at p<.1 were needed to suggest there were reliable differences between conditions at a level of p<.05.
Sensitivity analyses
Associations of sustained high-frequency EEG responses with self-reported rumination and depressive symptomatology
To examine correlations of RSQ rumination scores with gamma band activity, correlations of RSQ rumination at each electrode at each sample were computed. The 0.25 second window with the strongest correlations lasted from 3.14–3.39 seconds post stimulus onset. In that window, correlations with rumination scores were significant (p<.1) along the entire midline, Fz, R2 = 0.10, F(1,54)=5.82, p=0.02, Cz,. R2 = 0.13, F(1,54)=8.23, p=0.006, Pz, R2 = 0.08, F(1,54)=4.72, p=0.03 as well as F3, F7, and P3. Inspection of scatter plots showed that these relationships reflected the full range of RSQ scores and were not driven by just one group or outliers (available from the authors upon request). In contrast, using the same procedure there were no 0.25 second intervals in which more than 1 electrode was significant for neutral words or more than two electrodes for positive words, suggesting that associations of gamma band activity with rumination were unique to responses to negative words.
Using analogous tests, there were no 0.25 second intervals in which gamma band activity to negative words at more than 3 electrodes were significantly (p<.1) correlated with BDI scores in the entire sample or the sample restricted to just depressed participants. Along similar lines, some individuals with schizophrenia scored in the median range for depressed individuals on the BDI. To understand whether these individuals also had gamma patterns associated with the depressed group, we contrasted the schizophrenia groups separated by having BDI scores >=15 (n=7) and those having BDI scores <9 (n=6). Table 2, columns 5 and 6 also show the analogous differences in the 3–4 second and baseline periods for comparison with our other reported tests – no regions in which >4 electrodes were significantly different were apparent. To test whether this was a function of the chosen interval, testing for any 0.25 second windows in which the groups differed most reliably on 4 electrodes revealed no windows with significant differences (p<.1) at any electrode. In the window of maximal differences (2.66–2.92 seconds after stimulus onset); effects were uniformly small and thus not likely to be due to low power compared to the other effects examined in this sample. Thus dysphoric individuals with schizophrenia appear to have gamma patterns more characteristic of schizophrenia than depression.
Examination of better-age-matched subsamples
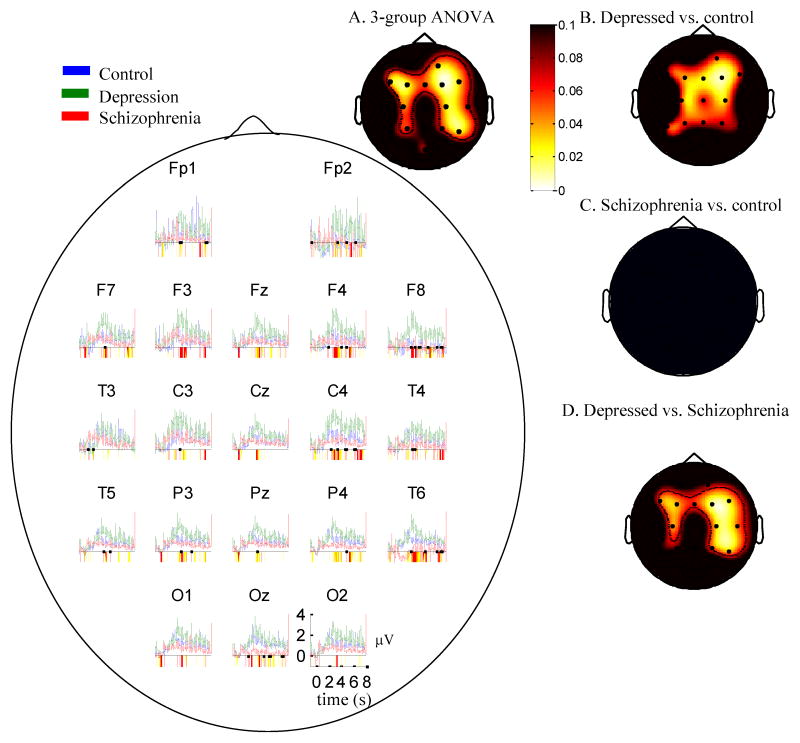
Because the groups differed in age it was important to examine whether results were a function of age differences. A better-age-matched subset of participants were constructed by eliminating the 9 youngest controls and the 2 oldest participants with schizophrenia and the oldest participant with depression yielding 13 subjects per group. In the new groups the mean age differences between any two groups were less than 2 years (controls: 38.1, depression: 41.9, schizophrenia: 40.2), and a test of group differences in age was not significant (F(2,39)=.5, p=.64). Figure 4, which parallels Figure 1, shows that the overall character of results in these groups is the same as for the original data. Depressed participants had the greatest stimulus related, and greatest baseline gamma values throughout the majority of the head. The scalp-plot on the top right further illustrates that there were significant group differences in the baseline-corrected gamma in the 4–5s window through the same topographic regions as in the plot for the full sample. As such, it is unlikely that results based on the full sample were due to age.
Figure 4.
Mean EEG gamma power (35–45 Hz) over time for age-matched subsamples in each group in response to negative words. Periods of significant differences between groups are highlighted below the x-axis (yellow (light gray in black/white): p<0.1; red (dark gray in black/white): p<0.05). a. Scalp map for three group ANOVA (control, depression, schizophrenia) 3–4 seconds after stimulus offset minus a pre-stimulus baseline. B–D. Two-group contrast maps analogous to those in Figure 1. Whiter values represent lower p-values. Depressed individuals displayed increased high-frequency activity compared to the other groups throughout the scalp.
Medication effects
To understand whether medication effects likely affected observed results we examined our primary contrast (gamma to negative words 3–4 seconds post-stimulus onset) as a function of medications within the patient groups. The only medications with enough variation for which to examine group differences were SSRIs. Among participants with depression and schizophrenia 17 were not taking SSRI’s and 12 were. At no electrode were differences associated with SSRIs apparent (p>.22 for all electrodes; generally p>.4). Within the depressed participants 8 were medicated with SSRIs and 6 were not. In no case did results differ by SSRI status (p>.4 for all electrodes but one for which p>.25). Within participants with schizophrenia 6 participants were medicated with SSRI’s and 9 were not. In no case did results differ by SSRI status (all p’s>.28). Within participants with schizophrenia, all but one participant received anti-psychotic medications, so the role of these medications could not be examined. Similarly no more than four participants were taking any other drug class, prohibiting analysis of drug effects, but also making them unlikely to have confounded results.
Invoked gamma band differences independent of baseline differences
The “industry standard” in physiological research involves subtracting a pre-stimulus baseline signal from a stimulus-related response. This technique assumes that the magnitude of the stimulus-related response was unrelated to the baseline. Rather, we found that the stimulus-related response was correlated with the pre-stimulus gamma-band activity at 17/20 electrodes across all participants. We therefore covaried baseline activity from the stimulus-related activity 3–4 seconds following stimulus onset. This analysis revealed that even after this technique, depressed individuals displayed increased stimulus-related gamma-band responses to negative words compared to controls at all electrodes, with significant differences at six electrodes (Fz, F3. Fp2, F4, F8, A1), consistent with the observed right-frontal differences observed in our other analyses. Individuals with schizophrenia did not differ from controls at any electrode. Valence-related differences were not present in any group when baseline data were covaried rather than subtracted. Thus, this more conservative type of analysis revealed data which were largely but not completely consistent with our primary analyses. (Figures and formal statistics for these analyses are available from the authors upon request.)
EEG differences in other frequency bands
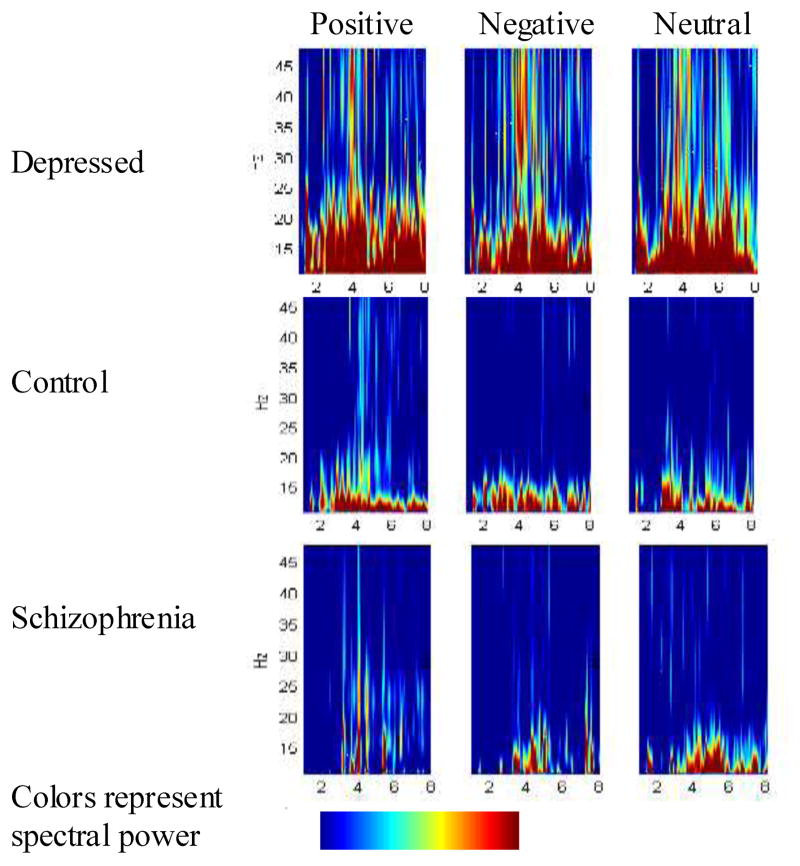
We examined whether the observed event-related EEG responses were unique to gamma-band activity using group contrasts of activity throughout the frequency spectrum at each sampled point. Figure 5 depicts data for one representative electrode; data was similar at other relevant sites. These data revealed that depressed individuals displayed increased activity throughout a wide range of the frequency spectrum throughout the scalp. This pattern may indicate that multiple cognitive processes indexed by different frequencies of oscillation are sustained in depression.
Figure 5.
Wavelet decomposition for each group, all valences, all frequencies at Fz. Spectra represent power in response to the stimulus minus the power during a prestimulus baseline period. Depressed individuals displayed increased high-frequency activity compared to the other groups, particularly for negative words.
Based on these graphs, to examine formally whether group differences were unique to gamma-band related activity, differences in the 15–25 hz band were compared. Again, as shown in Figure 6, depressed individuals displayed increased high-frequency activity compared to the controls at midline electrodes but other differences were less reliably pronounced.
Figure 6.
Mean EEG power in the 15–25 Hz range over time for each group in response to negative words. Periods of significant differences between groups are highlighted below the x-axis (yellow (light gray in black/white): p<0.1; red (dark gray in black/white): p<0.05). Scalp map for three group ANOVA (control, depression, schizophrenia) 3–4 seconds after stimulus offset minus a pre-stimulus baseline. Whiter values represent lower p-values. Depressed individuals displayed increased high-frequency activity compared to the other groups throughout the scalp.
Discussion
Together, these data suggest that though gamma-band EEG responses are usually considered during just early information processing, gamma-band activity is sustained in the eight seconds following presentation of emotional information. This result is consistent with the idea that gamma could represent an index of sustained feature-binding/semantic association/working-memory processing such as elaboration. The frontal distribution of sustained gamma increases in depressed participants is consistent with sustained working memory processes as might occur in semantic elaboration. The correlation of sustained gamma band activity seconds after a stimulus with self-reported rumination further supports the notion that this phenomenon represents sustained elaborative processing.
Depressed individuals displayed sustained and increased gamma-band EEG throughout the task, and particularly increased stimulus-related gamma-band EEG to negative versus neutral words, compared to control participants and individuals with schizophrenia. These data support the idea that depressed individuals tend to engage in sustained elaborative processing of negative information for seconds after it is presented. Potentially such processes occurring in the seconds following emotional stimuli are linked to more debilitating phenomena such as rumination which are usually examined on a longer time-scale (Siegle et al 2003a). Assessment of gamma-band activity may more uniquely support this observation than other measures such as those derived from pupil dilation or fMRI as gamma band activity is thought to more uniquely index semantic and associative processes.
In contrast, consistent with other data (Spencer et al 2004), individuals with schizophrenia displayed decreased pre-stimulus gamma-band activity compared to both healthy controls and depressed individuals. While increased baseline gamma has been observed for patients with both depression and schizophrenia during sleep (Tekell et al 2005), the current data suggest that at least for schizophrenia, waking states may be characterized by different mechanisms. Additionally, in contrast to control participants, individuals with schizophrenia displayed no differences in processing of negative and neutral stimuli, suggesting a specific lack of emotional elaboration in this group.
Of course, the observation of sustained gamma band activity could represent other sustained cognitive processes such as sustained attentional allocation or vigilance to the task. We believe this is unlikely as 1) sustained gamma activity in depression was specific to negative words, 2) the only visual information to attend to following the initial 250ms word presentation was a mask consisting of X’s, and 3) our previous work suggests that sustained task vigilance is decreased in depressed individuals on more cognitive tasks with similarly long stimulus durations (Siegle et al 2004). Similarly, the observation of decreased gamma-band activity in individuals with schizophrenia could simply represent other processes such as a lack of semantic processing due to poor memory or working memory. Yet, as the decreased gamma-band activity was present even at baseline measurements, such an explanation may be less likely.
Increased sustained gamma-band responses to negative, compared to neutral words were only apparent for depressed individuals. This is consistent with the notion that depressed individuals may elaborate or ruminate specifically on negative information, and is consistent with our observations of increased sustained physiological reactivity to negative information (Siegle et al 2003a) as well as sustained amygdala activity (Siegle et al 2002) on valence identification tasks.
Results were a function of group and were not associated with continuous measures of depressive symptomatology. This could be because sustained gamma and symptom reporting are associated with different brain processes, e.g., gamma is due to sustained subcortical processes and self-reported negative affect is due to more cortical processing. Similarly, individuals with schizophrenia were not differentiated on relevant measures by their level of dysphoria. Also, results were not, as originally hypothesized, specific to the 35–45 Hz range. Rather, a wider range of high frequency data encompassing the frequencies sometimes considered “low gamma” (Shibata et al 1999) or “beta” activity (Crone et al 1998) displayed similar patterns to that observed for the “high gamma” range. This suggests that sustained gamma activity in the depressed group and decreased overall gamma in the group with schizophrenia may be representative of a broader set of processes than just semantic binding, association, and elaboration. Support for this possibility is provided by recent work showing schizophrenia patients to be characterized by reduced EEG power to gamma-range auditory stimulation (Kwon et al 1999), reduced EEG gamma-band response to the auditory oddball paradigm (Gallinat et al 2004; Symond et al 2005), and lower event-related EEG coherence between frontal and temporal regions following presentation of acoustic stimuli (Ford et al 2002). Yet, increased gamma-power has not been observed in depression on cognitive tasks (Pizzagalli et al in press), suggesting the result for depressed participants may be specific to semantic elaborative processing in the context of attention to emotional aspects of information.
The present study was characterized by a number of limitations. Most notably, the subject populations were relatively heterogeneous; comorbidities and medications were not controlled for, and depressive severity varied in a wide range, though analyses did not suggest that results were a function of depressive severity. Thus, effects could be strongest in specific sub-populations. The groups also differed in age, although additional analyses based on a subsample of better-age-matched subgroups yielded comparable effects. Given that trials were very long, many trials had blinks; while these were largely corrected by the Gratton algorithm, windows that were not corrected were replaced using linear interpolation which could have introduced frequency domain artifacts. Inspections suggested this phenomenon was unlikely but further studies examining different types of blink correction may be useful to clarify this data. In addition, the task was quite long – over an hour. Sometimes the described task was administered after an hour of testing. Thus, differential fatigue effects could have contributed to observed group differences. Finally, the observed group differences were in a wide bandwidth in which differences could be due to processes other than brain function, e.g., muscle artifacts (e.g., if depressed individuals became more tense over eight seconds following a stimulus). We did not have concurrent EMG data to rule this out, but the correlations with rumination, particularly in the depressed group speak against the probability of such artifacts. Similarly, the observed differences in baseline activity may not be associated with the task at all, but rather with a general predisposition – future research examining non-emotional tasks will be necessary to evaluate this potential alternative explanation.
These limitations notwithstanding, the presented data represent the first examination of sustained gamma band activity up to eight seconds after an emotional stimulus is presented. These data provide direct support for the idea that depressed and ruminating individuals engage in sustained elaborative semantic processing of emotional information relative to healthy individuals, and individuals with other psychopathologies. In contrast, individuals with schizophrenia did not display this pattern, but rather, appear to be characterized more by blunted elaborative processing. Together, these data potentially suggest that whereas depression involves increased and sustained elaborative processing of emotional information, schizophrenia is characterized by decreased preparation for and engagement in elaborative processing, particularly of emotional information.
Acknowledgments
We gratefully acknowledge contributions of the volunteers who participated in this study as well as Lisa Ledonne, Carole Turocy, Jean Turocy, Roma Konecky, Lisa Farace, and Vince Penkrot for contributions to data collection and analysis, the staff of the WPIC Mood Disorders Treatment and Research Program for assistance in recruitment, and to anonymous reviewers for insightful comments. Data was collected and analyzed in the Psycholinguistics Laboratory and Program in Cognitive Affective Neuroscience at the Western Psychiatric Institute and Clinic. This research was supported by MH50631, MH64159, MH60473, MH55762, MH-58397 and MH-58356, NARSAD, and the Veteran’s Research Foundation. This material is the result of work supported with resources and the use of facilities at the Pittsburgh VA Healthcare System, Highland Drive Division.
References
- Beck AT. Depression Clinical experimental and theoretical aspects. New York: Hoeber; 1967. [Google Scholar]
- Behrendt RP. Hallucinations: synchronisation of thalamocortical gamma oscillations underconstrained by sensory input. Conscious Cogn. 2003;12:413–451. doi: 10.1016/s1053-8100(03)00017-5. [DOI] [PubMed] [Google Scholar]
- Bower G. Mood and memory. American Psychologist. 1981;36:129–148. doi: 10.1037//0003-066x.36.2.129. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective Norms for English Words ANEW Technical Manual and Affective Ratings. Gainsville FL: The Center for Research in Psychophysiology University of Florida; 1997. [Google Scholar]
- Braeutigam S, Bailey AJ, Swithenby SJ. Phase-locked gamma band responses to semantic violation stimuli. Brain Res Cogn Brain Res. 2001;10:365–377. doi: 10.1016/s0926-6410(00)00055-0. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Docherty NM. Affective reactivity of speech and emotional experience in patients with schizophrenia. Schizophr Res. 2004;69:7–14. doi: 10.1016/S0920-9964(03)00069-0. [DOI] [PubMed] [Google Scholar]
- Condray R, Siegle GJ, Cohen JD, van Kammen DP, Steinhauer SR. Automatic activation of the semantic network in schizophrenia: evidence from event-related brain potentials. Biological Psychiatry. 2003;54:1134–1148. doi: 10.1016/s0006-3223(03)00699-1. [DOI] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;121:2301–2315. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- Deldin P, Deveney C, Kim A, Casas BR. A slow wave investigation of working memory biases in mood disorders. Journal of Abnormal Psychology. 2001;110:267–281. doi: 10.1037//0021-843x.110.2.267. [DOI] [PubMed] [Google Scholar]
- Docherty NM, Hall MJ, Gordinier SW. Affective reactivity of speech in schizophrenia patients and their nonschizophrenic relatives. Journal of Abnormal Psychology. 1998;107:461–467. doi: 10.1037//0021-843x.107.3.461. [DOI] [PubMed] [Google Scholar]
- Docherty NM, Rhinewine JP, Nienow TM, Cohen AS. Affective reactivity of language symptoms, startle responding, and inhibition in schizophrenia. Journal of Abnormal Psychology. 2001;110:194–198. doi: 10.1037//0021-843x.110.1.194. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM IV Axis I Disorders Patient Edition. Vol. 20. New York: Biometrics Research Department New York State Psychiatric Institute; 1996. [Google Scholar]
- Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry. 2002;51:485–492. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Winterer G, Herrmann CS, Senkowski D. Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin Neurophysiol. 2004;115:1863–1874. doi: 10.1016/j.clinph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hald LA, Bastiaansen MC, Hagoort P. EEG theta and gamma responses to semantic violations in online sentence processing. Brain Lang. 2006;96:90–105. doi: 10.1016/j.bandl.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Hartwell JW. EEGSYS Version 5.7 User’s Guide. Baltimore, MD: Friends Medical Science Research Center, Inc; 1995. [Google Scholar]
- Hempel A, Hempel E, Schonknecht P, Stippich C, Schroder J. Impairment in basal limbic function in schizophrenia during affect recognition. Psychiatry Research. 2003;122:115–124. doi: 10.1016/s0925-4927(02)00126-9. [DOI] [PubMed] [Google Scholar]
- Ingram RE. Toward an information processing analysis of depression. Cognitive Therapy and Research. 1984;8:443–478. [Google Scholar]
- Ingram RE. Self focused attention in clinical disorders Review and a conceptual model. Psychological Bulletin. 1990;107:156–176. doi: 10.1037/0033-2909.107.2.156. [DOI] [PubMed] [Google Scholar]
- Ingram RE, Miranda J, Segal ZV. Cognitive vulnerability to depression. New York NY: Guilford; 1998. [Google Scholar]
- Kraut MA, Calhoun V, Pitcock JA, Cusick C, Hart J., Jr Neural hybrid model of semantic object memory: implications from event-related timing using fMRI. J Int Neuropsychol Soc. 2003;9:1031–1040. [Google Scholar]
- Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, Fonlupt P, Kahane P, Minotti L, Hoffmann D, Bertrand O, Baciu M. Relationship between task-related gamma oscillations and BOLD signal: New insights from combined fMRI and intracranial EEG. Hum Brain Mapp. 2007 doi: 10.1002/hbm.20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson C, Davidson R. Prolonged Startle Blink Potentiation following Negative Stimuli among Individuals with Relative Right Frontal EEG Asymmetry. Psychophysiology. 2001:3. [Google Scholar]
- Lyubomirsky S, Caldwell ND, Nolen-Hoeksema S. Effects of ruminative and distracting responses to depressed mood on retrieval of autobiographical memories. Journal of Personality & Social Psychology. 1998;75:166–177. doi: 10.1037//0022-3514.75.1.166. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews AM. Cognitive experimental approaches to the emotional disorders. In: Martin PR, editor. Handbook of Behavior Therapy and Psychological Science An Integrative Approach. Vol. 16. 1991. pp. 116–150. [Google Scholar]
- Matt GE, Vazquez C, Campbell W. Mood congruent recall of affectively toned stimuli: A meta analytic review. Clinical Psychology Review. 1992;12:227–255. [Google Scholar]
- Matthews G, Southall A. Depression and the processing of emotional stimuli A study of semantic priming. Cognitive Therapy and Research. 1991;15:283–302. [Google Scholar]
- Maxwell SE, Delaney HD. Designing experiments and analyzing data: a model comparison perspective. Mahwah N.J: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- McIntyre CK, Hatfield T, McGaugh JL. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. European Journal of Neuroscience. 2002;16:1223–1226. doi: 10.1046/j.1460-9568.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- Nelson HE, Willison J. The National Adult Reading Test. Berkshire, England: Nfer-Nelson Publishing Co; 1991. [Google Scholar]
- Nolen-Hoeksema S, Morrow J, Fredrickson BL. Response styles and the duration of episodes of depressed mood. Journal of Abnormal Psychology. 1993;102:20–28. doi: 10.1037//0021-843x.102.1.20. [DOI] [PubMed] [Google Scholar]
- Norman W, Miller I, Dow M. Characteristics of depressed patients with elevated levels of dysfunctional cognitions. Cognitive Therapy and Research. 1988;12:39–52. [Google Scholar]
- Pizzagalli DA, Peccoralo LA, Davidson RJ, Cohen JD. Resting anterior cingulate activity and abnormal responses to errors in subjects with elevated depressive symptoms: A 128-channel EEG study. Human Brain Mapping. doi: 10.1002/hbm.20172. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermuller F, Eulitz C, Pantev C, Mohr B, Feige B, Lutzenberger W, et al. High-frequency cortical responses reflect lexical processing: an MEG study. Electroencephalogr Clin Neurophysiol. 1996;98:76–85. doi: 10.1016/0013-4694(95)00191-3. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F, Lutzenberger W, Preissl H. Nouns and verbs in the intact brain: evidence from event-related potentials and high-frequency cortical responses. Cereb Cortex. 1999;9:497–506. doi: 10.1093/cercor/9.5.497. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F, Lutzenberger W, Preissl H, Birbaumer N. Spectral responses in the gamma-band: physiological signs of higher cognitive processes? Neuroreport. 1995;6:2059–2064. doi: 10.1097/00001756-199510010-00025. [DOI] [PubMed] [Google Scholar]
- Ratcliff R. Methods for dealing with reaction time outliers. Psychological Bulletin. 1993;114:510–532. doi: 10.1037/0033-2909.114.3.510. [DOI] [PubMed] [Google Scholar]
- Rhinewine JP, Docherty NM. Affective reactivity of language and right-ear advantage in schizophrenia. Schizophrenia Research. 2002;53:181–186. doi: 10.1016/s0920-9964(01)00228-6. [DOI] [PubMed] [Google Scholar]
- Shibata T, Shimoyama I, Ito T, Abla D, Iwasa H, Koseki K, et al. Event-related dynamics of the gamma-band oscillation in the human brain: information processing during a GO/NOGO hand movement task. Neuroscience Research. 1999;33:215–222. doi: 10.1016/s0168-0102(99)00003-6. [DOI] [PubMed] [Google Scholar]
- Siegle GJ. The Balanced Affective Word List Creation Program. 1994 Available Web at http://www.sci.sdsu.edu/CAL/wordlist/
- Siegle GJ, Carter CS, Thase ME. Use of fMRI to predict recovery from unipolar depression with cognitive behavior therapy. American Journal of Psychiatry. 2006;163:735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Granholm E, Ingram RE, Matt GE. Pupillary response and reaction time measures of sustained processing of negative information in depression. Biological Psychiatry. 2001;49:624–636. doi: 10.1016/s0006-3223(00)01024-6. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Hasselmo ME. Using connectionist models to guide assessment of psychological disorder. Psychological Assessment. 2002;14:263–278. [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Carter CS, Ramel W, Thase ME. Do the seconds turn into hours? Relationships between sustained pupil dilation in response to emotional information and self-reported rumination. Cognitive Therapy and Research. 2003a;27:365–382. [Google Scholar]
- Siegle GJ, Steinhauer SR, Carter CS, Ramel W, Thase ME. Do the seconds turn into hours? Relationships between sustained pupil dilation in response to emotional information and self reported rumination. Cognitive Therapy and Research. 2003b;27:365–383. [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME. Pupillary assessment and computational modeling of the Stroop task in depression. International Journal Psychophysiology. 2004;52:63–76. doi: 10.1016/j.ijpsycho.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Sison CE, Alpert M, Fudge R, Stern RM. Constricted expressiveness and psychophysiological reactivity in schizophrenia. Journal of Nervous & Mental Disease. 1996;184:589–597. doi: 10.1097/00005053-199610000-00002. [DOI] [PubMed] [Google Scholar]
- Skinner JE, Molnar M, Kowalik ZJ. The role of the thalamic reticular neurons in alpha- and gamma-oscillations in neocortex: a mechanism for selective perception and stimulus binding. Acta Neurobiol Exp (Wars) 2000;60:123–142. doi: 10.55782/ane-2000-1330. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symond MP, Harris AW, Gordon E, Williams LM. “Gamma synchrony” in first-episode schizophrenia: a disorder of temporal connectivity? Am J Psychiatry. 2005;162:459–465. doi: 10.1176/appi.ajp.162.3.459. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Teasdale JD. Cognitive vulnerability to persistent depression. Cognition and Emotion. 1988;2:247–274. [Google Scholar]
- Tekell JL, Hoffmann R, Hendrickse W, Greene RW, Rush AJ, Armitage R. High frequency EEG activity during sleep: characteristics in schizophrenia and depression. Clin EEG Neurosci. 2005;36:25–35. doi: 10.1177/155005940503600107. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: Psychological Corporation; 1997. (WAIS-III) [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]