Abstract
Immunotherapeutic approaches to treating Alzheimer’s disease (AD) using vaccination strategies must overcome the obstacle of achieving adequate responses to vaccination in the elderly. Here we demonstrate for the first time that application of the E. coli heat-labile enterotoxin adjuvant-laden immunostimulatory patches (LT-IS) dramatically enhances the onset and magnitude of immune responses to DNA- and protein-based vaccines for Alzheimer’s disease following intradermal immunization via gene gun and conventional needles, respectively. Our studies suggest that the immune activation mediated by LT-IS offers improved potency for generating AD-specific vaccination responses that should be investigated as an adjuvant in the clinical arena.
Keywords: Alzheimer’s disease, epitope vaccine, immunostimulatory patch, immune responses
1. Introduction
Alzheimer’s disease (AD) is characterized by dementia that typically begins with subtle and poorly recognized failure of memory that gradually becomes severe and incapacitating. Other common findings include confusion, poor judgment, language disturbance, agitation, withdrawal, and hallucinations (Brookmeyer et al., 2007). The neuropathological features of the disease include neurofibrillary tangles (NFT), deposition of amyloid-β (Aβ) in senile plaques, and neuronal loss in affected brain regions (Price and Sisodia, 1994). Based on the amyloid cascade hypothesis (Hardy and Higgins, 1992, Hardy and Selkoe, 2002), many active and passive vaccination strategies directed toward reducing the Aβ deposits are currently being tested in clinical trials (Lemere and Masliah, 2010). In the first AN-1792 clinical trial the vaccination led to the development of meningoencephalitis in some of the patients, which was believed to be associated with anti-Aβ specific T cell autoreactivity (Nicoll et al., 2003, Masliah et al., 2005). Of note, only low titers of anti-Aβ antibodies were generated in the majority of AD patients, and there was a lack of measurable antibody responses in approximately 40% of the patients even after additional injections with modified formulations (Bayer et al., 2005)-(Fox et al., 2005). Hence, overcoming both safety issues and poor immunogenicity has evolved as being the primary barriers to successful immunotherapy for AD.
We have previously generated DNA-, peptide- and protein-based epitope vaccines composed of three copies of a self B-cell epitope of Aβ42 (Aβ11 or Aβ12) and the foreign T helper (Th) cell epitope or string of multiple Th epitopes, collectively designated as universal MultiTEP platform. These prototype AD vaccines were found to be safe based on lack of induction of T cell autoreactivity, and were also highly immunogenic in wild-type mice, APP/Tg mice, rabbits and monkeys (Petrushina et al., 2007, Movsesyan et al., 2008, Davtyan et al., 2010, Davtyan et al., 2013, Ghochikyan et al., 2013, Evans et al., 2013). However, induction of immune responses in humans is more challenging and requires a potent adjuvant (in case of protein vaccines) or delivery system (in case of DNA vaccines) to induce potent Th responses and subsequently high titers of antibodies. Of note, the only conventional adjuvant approved by FDA for protein vaccines are aluminum based adjuvants. On the other hand, the application of DNA immunization methods used in mice did not provide encouraging results in humans or large animals (Babiuk et al., 2003) although three types of veterinary DNA-based vaccines have been approved (Kutzler and Weiner, 2008).
In this study as an effort to improve the immunogenicity of the AD DNA vaccine, we have evaluated the addition of a topical adjuvant to the vaccine regimen. Transcutaneous immunization has been shown to be effective at inducing strong systemic and mucosal responses (Glenn et al., 2003, Guerena-Burgueno et al., 2002) and adjuvants such as heat-labile enterotoxin of Escherichia coli (LT) have very strong immune stimulatory activity when administered transcutaneously at sites of antigen injection (Glenn et al., 1999, Scharton-Kersten et al., 2000). In previous studies, immune stimulating patches containing LT that were applied at the injection site of influenza protein and DNA vaccines were found to dramatically enhance the virus-specific immune response in mice (Guebre-Xabier et al., 2004, Mkrtichyan et al., 2008). Here, we extended this approach to test the ability of LT-IS patches to enhance the efficacy of a DNA epitope vaccine, DepVac (Davtyan et al., 2012) and cGMP grade recombinant protein epitope vaccine, Lu AF20513 (Davtyan et al., 2013) for AD. This report demonstrates that LT-IS can dramatically enhance humoral and cellular immune responses to DNA and protein vaccines against AD.
2. Materials and methods
2.1 Mice
Female, 5–6 week-old C57BL/6 and B6SJL mice were obtained from The Jackson Laboratory (ME). 12–16 month-old 3xTg-AD and 4–6 month-old Tg2576 mice were provided by the UCI-Alzheimer’s Disease Research Center (ADRC). All animals were housed in a temperature and light-cycle controlled facility, and their care was under the guidelines of the National Institutes of Health and an approved IACUC protocol at University of California, Irvine.
2.2 Immunogens and immunization
DNA construct
The construction strategy of pCMVE/MDC-3Aβ11-PADRE (DepVac) has been previously described (Movsesyan et al., 2008). C57BL/6 (n=16) and 3xTg-AD mice (n=16) were immunized biweekly by gene gun for 6 weeks as described previously (Movsesyan et al., 2008, Davtyan et al., 2010).
Protein epitope vaccine
Lu AF20513 protein composed of three copies of B cell epitope from Aβ42, Aβ1–12, and two foreign Th cell epitopes from Tetanus Toxin (TT), P30 and P2, was purified as previously described (Davtyan et al., 2013). B6SJL (n=18) and Tg2576 mice (n=20) were immunized three and five times biweekly, respectively. Mice were immunized intradermally (i.d.) in the abdomen with 50 μg Lu AF20513 in 30 μl volume by conventional needle and immediately after injection, LT-IS or placebo patches were applied to the immunization site. One group of Tg2576 mice (n=7) was immunized s.c. with the same amount of Lu AF20513 formulated in aluminum based adjuvants, Alhydrogel® (Brenntag Biosector, Denmark). For analysis of the humoral responses, sera were collected on day 12 after first and second immunizations and 7 days after the third immunization.
2.3 Patch application
Patches were applied as described previously (Mkrtichyan et al., 2008). Briefly, mice were anesthetized and the skin was shaved at the site of immunization. The shaved skin was pretreated by hydration with saline and the stratum corneum was disrupted by mild abrasion with emery paper (GE Medical Systems, NJ). Wet patches containing phosphate buffered saline (placebo patch) or 10 μg LT (LT-IS patch) were applied on pretreated skin overnight.
2.4 Detection of anti-Aβ antibody concentration using ELISA
Concentrations of anti-amyloid β (Aβ) antibodies were measured in sera of immunized and control mice as we described previously (Ghochikyan et al., 2006, Davtyan et al., 2010). Antibody concentrations in sera collected from individual mice or in pooled sera were calculated using a calibration curve generated with the 6E10 (anti-Aβ) monoclonal antibody (Signet, MA). HRP-conjugated anti-IgG1, IgG2ab, IgG2b and IgM specific antibodies (Bethyl Laboratories, Inc., TX) were used to characterize the isotype profiles of antibodies in pooled sera from wild-type and transgenic mice at dilutions of 1:500 and 1:200, respectively.
2.5 T cell proliferation and detection of cytokine production
On day 7 after the third immunization mice were euthanized and cellular responses were evaluated in splenocytes. T cell proliferation was analyzed in splenocyte cultures using [3H] thymidine incorporation assays and stimulation indices were calculated as described previously (Agadjanyan et al., 1997, Cribbs et al., 2003, Davtyan et al., 2010). ELISPOT assay was used to determine the number of antigen-specific cells producing cytokines (IFN-γ and IL-4) in splenocyte cultures from individual mice as described previously (Davtyan et al., 2013). Cultured splenocytes from experimental and control mice were re-stimulated in vitro with PADRE, P30, P2 (all are from GenScript, NJ), Aβ40 (American Peptide, CA), Lu AF20513, or irrelevant peptides (10 μg/ml of each peptide).
2.6 Statistical Analysis
Statistical parameters [mean, standard deviations (SD), and p values] were calculated using Prism 3.03 software (GraphPad Software, Inc., CA). Statistically significant differences were examined using a t-test or analysis of variance (ANOVA) and Tukey’s multiple comparisons post-test (P < 0.05 was considered significant).
3. Results
3.1 Effects of LT-IS patches on the humoral and cellular immune responses in C57BL/6 and 3xTg-AD mice immunized with DNA vaccine
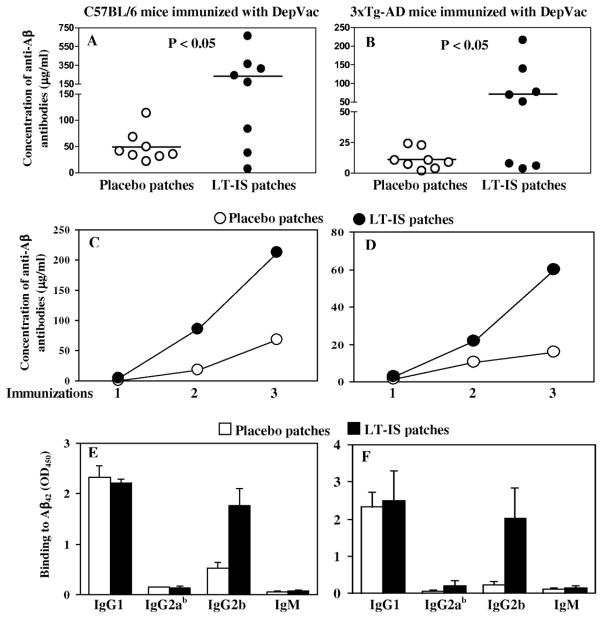
In this study, we examined the role of LT-IS patches in enhancing anti-Aβ immune responses in wild-type C57BL/6 and the 3xTg-AD strain (both has the same H2b immune haplotype) mice. The latter is a mouse model of AD, which mimics the Aβ and tau pathology found in human AD. The mice were immunized with DNA epitope vaccine, DepVac, delivered by i.d. route with gene gun and treated with either LT-IS (experiment) or placebo (control) patches that were applied at the site of immunization immediately after injection. Measurement of anti-Aβ titers after the last immunization showed that application of LT-IS patches significantly (P ≤ 0.05) enhanced anti-Aβ antibody response in both C57BL/6 and 3xTg-AD mice (Fig. 1A, B). Interestingly, in both cases 5 out of 8 animals generated very high concentrations of antibodies specific to amyloid, while LT-IS patches did not enhanced immune responses in 3 mice from each group. Analysis of the kinetics of anti-Aβ antibody responses showed that the first injection of DepVac induced only low levels of antibodies in all groups; however, after the third immunization, high antibody concentrations were attained in the LT-IS and control treatment groups (Fig. 1C, D). Despite the similar kinetic profile of antibody responses in the two groups, this vaccination regimen elicited higher overall antibody titers in the DepVac/LT-IS group vs. the DepVac/placebo group. As we expected from our previous studies where antibodies produced after DepVac immunizations were predominantly of the IgG1 isotype (Movsesyan et al., 2008, Davtyan et al., 2010), application of LT-IS patches did not change the profile of isotype of anti-Aβ antibodies (ratio of IgG1/IgG2ab equals 15 and 16 for LT-IS patch and placebo patch application, respectively), although there were increased concentrations of IgG2b antibodies in LT-IS vs. control treated mice (Fig. 1E, F).
Figure 1. LT-IS patches significantly increased humoral immune responses in C57BL/6 (A,C,E) and 12–16 month-old 3xTg-AD mice (B,D,F) immunized with DepVac administered intradermally via gene gun.
Concentrations of anti-Aβ antibodies were measured in individual mice after the 3rd immunization (A, B). Kinetics of anti-Aβ responses were analyzed in pooled sera (C, D). The isotypes of serum antibodies were analyzed in individual mice (E, F). Sera from wild-type and 3xTg-AD animals were used at dilutions of 1:500 and 1:200, respectively. Lines and error bars represent the average value ± SD.
To determine whether application of LT-IS patches also affects T helper cell activation, we analyzed cytokine profiles and proliferation of splenocytes from vaccinated mice that were re-stimulated in vitro with PADRE peptide, which is encoded by the DepVac construct (Fig. 2). As shown in Fig. 2A and B application of LT-IS patches to DepVac-vaccinated C57BL/6 and 3xTg-AD mice elicited increased frequencies of T cells producing IFN-γ and IL-4 compared with placebo patches (P ≤ 0.01 and P ≤ 0.05; respectively, for C57BL/6 mice; Fig. 2A and P ≤ 0.01 and P ≤ 0.001; respectively, for 3xTg-AD mice; Fig. 2B). When T cell proliferation was examined, splenocytes from DepVac-vaccinated C57BL/6 and 3xTg-AD mice that were treated with LT-IS patches also exhibited increased antigen-specific T cell proliferation compared to the groups that received placebo patches (P ≤ 0.05) (Fig. 2C, D). Of note, anti-Aβ T cell responses were not detected in cultures of immune splenocytes (data not shown). These data indicate that PADRE specific cellular immune responses are perfectly correlated with anti-Aβ antibody responses. Thus, LT-IS patches dramatically improved anti-PADRE cellular and anti-Aβ antibody responses to DepVac delivered i.d. by gene gun without drastically changing the isotype profile of antibodies generated in C57BL/6 and 3xTg-AD mice (Fig. 1 and 2).
Figure 2. LT-IS patches significantly increased cellular immune responses in C57BL/6 (A,C) and 12–16 month-old 3xTg-AD mice (B,D) immunized with DepVac administered intradermally via gene gun.
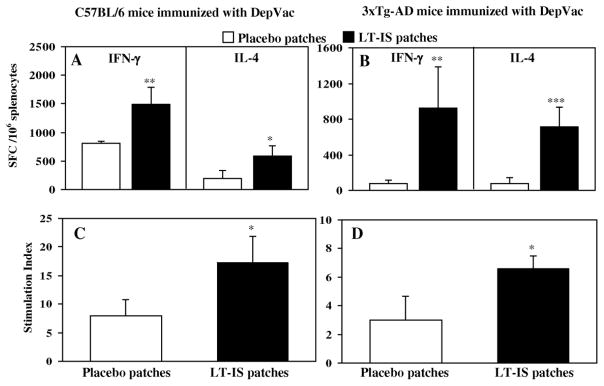
Cytokine-producing T cells (A, B) and proliferation of T cells (C,D) were detected in splenocyte cultures after three immunizations by ELISPOT and [3H] thymidine incorporation assays, respectively. Splenocytes from individual mice were re-stimulated in vitro with the PADRE peptide (10 μg/ml). Error bars represent the average value ± SD (n=4; *P≤0.05, **P≤0.01, ***P≤0.001).
3.2 Effects of LT-IS patches on the immune responses in B6SJL and Tg2576 mice vaccinated with Lu AF20513 recombinant protein vaccine
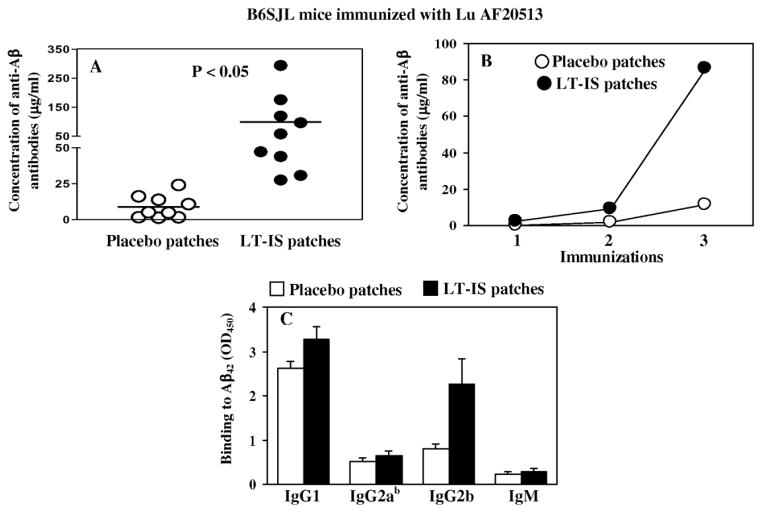
In this study we evaluated the effect of LT-IS patches on immune responses induced by a GMP grade recombinant protein-based AD epitope vaccine, Lu AF20513 (Davtyan et al., 2013) delivered to B6SJL mice of H2bxs haplotype intradermally using a conventional needle. Three immunizations of Lu AF20513 induced the production of an average of 8.9 μg/ml anti-Aβ antibodies whereas application of LT-IS patches to the site of immunization increased the antibody production more than 10 times, 99.21 ± 87.5μg/ml (Fig. 3A and B). Analysis of the production of IgG1, IgG2ab, IgG2b, and IgM isotypes of anti-Aβ antibodies in Lu AF20513-immunized mice showed a predominance of the IgG1 isotype, corresponding to Th2 type of immune responses, although there were increased concentrations of IgG2b antibodies in LT-IS vs. control treated mice (Fig. 3C).
Figure 3. LT-IS patches significantly increased humoral immune responses in B6SJL mice immunized with Lu AF20513 protein by intradermal administration with conventional syringe.
Concentrations of anti-Aβ antibodies were measured in individual mice after 3rd immunization (A). Kinetics of anti-Aβ responses were analyzed in pooled sera (B). The isotypes of serum antibodies were analyzed in individual mice (C). Sera from vaccinated mice were used at dilution 1:500. Lines and error bars represent the average value ± SD.
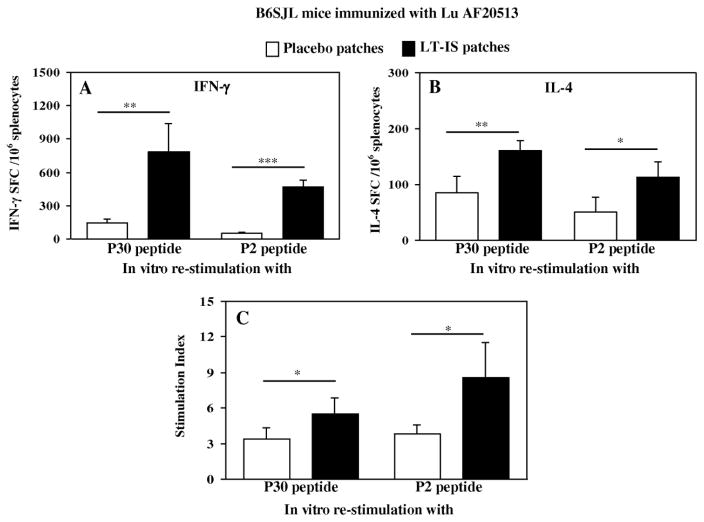
Analysis of T helper cell responses was also conducted by re-stimulating splenocytes from Lu AF20513-vaccinated B6SJL mice with appropriate Th epitopes, P2 and P30 peptides in vitro (Fig. 4). Application of LT-IS patches to mice led to significantly increased frequencies of cells producing IFN-γ and IL4 cytokines upon re-stimulation (Fig. 4A and B). These results were confirmed by proliferation assays where splenocytes from mice immunized with Lu AF20513 plus LT-IS patches induced significantly stronger T cell proliferation responses to the both Th peptides, P2 and P30, than those from mice immunized with Lu AF20513 plus placebo patches (Fig. 4C). As we anticipated from our studies with Tg2576 mice immunized with Lu AF20513 (Davtyan et al., 2013), wild-type mice of the same immune haplotype did not induce anti-Aβ specific Th cell responses to immunizations with Lu AF20513 as well (data not shown).
Figure 4. LT-IS patches significantly increased cellular immune responses in B6SJL mice immunized with Lu AF20513 protein by intradermal administration with conventional syringe.
IFN-γ (A) and IL-4 (B) producing T cells and proliferation of T cells (C) were detected in splenocyte cultures after 3 immunizations by ELISPOT and [3H] thymidine incorporation assays, respectively. Splenocytes from individual mice were re-stimulated in vitro with the P30 and P2 peptides (10 μg/ml). Error bars represent the average value ± SD (n=4; *P≤0.05, **P≤0.01, ***P≤0.001).
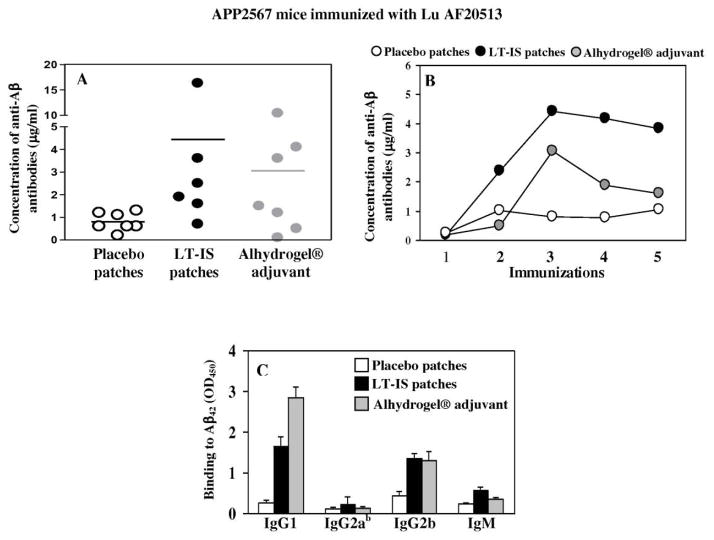
As mentioned above, Lu AF20513 formulated in strong Quil-A adjuvant is immunogenic in Tg2576 mice: anti-Aβ antibody generated by this vaccine reduce AD-like pathology in the brains of this mouse model of AD without inducing microglial activation and enhancing astrocytosis or cerebral amyloid angiopathy (Davtyan et al., 2013). Accordingly, in this study we decided to measure the effect of LT-IS patches in enhancing of immune responses to immunizations with Lu AF20513 in the same mouse model of AD. Intradermal immunization of Tg2576 mice with Lu AF20513 followed by application of LT-IS patch only slightly increased anti-Aβ antibody responses vs placebo patches (Fig. 5A). In one hand these data were unexpected, because of results presented above in Figure 3 and because immunization of Tg2576 mice with Lu AF20513 formulated in Quil-A induced strong antibody production (Davtyan et al., 2013). On the other hand, our previous studies indicated that fibrillar Aβ42 vaccine formulated in conventional adjuvant, Alhydrogel® was also weakly immunogenic in Tg2576 mice (Petrushina et al., 2003). Thus, we analyzed humoral immune responses in Tg2576 mice vaccinated with Lu-AF20513 formulated in Alhydrogel® and showed that similar to fibrillar Aβ42 immunizations, vaccinated mice induced only low titers of anti-Aβ antibodies. Although concentrations of anti-Aβ antibody responses was quite low in both groups of mice, analysis of the kinetics of humoral immune responses showed that LT-IS patches induced the highest antibody titers after three immunizations, and the antibody responses were more sustained following subsequent immunizations than in the Lu AF20513/Alhydrogel® immunized group (Fig. 5B). Of note, LT-IS and Alhydrogel® both induced in Tg2576 mice Th2 type of humoral immune responses: IgG1/IgG2ab ratio equals 7.8 and 22.2 in mice immunized with Lu AF20513/LT-IS and Lu AF20513/Alhydrogel®, respectively ((Fig. 5C). Collectively mice study is indicating that LT-IS patches are potent adjuvant that are enhancing the immunogenicity of Lu AF20513 vaccine.
Figure 5. Intradermal immunization with Lu AF20513 followed by application of LT-IS patch and s.c. injection of Lu AF20513 formulated in Alhydrogel® induced equal levels of anti-Aβ antibody responses in Tg2576 mice.
Concentrations of anti-Aβ antibodies were measured in individual mice after 3rd immunization (A). Tg2576 mice were immunized 5 times and kinetics of anti-Aβ responses were analyzed in pooled sera. (B). Antibody isotypes were analyzed in sera of individual mice after 3rd immunization (C). Sera from Tg2576 animals were used at dilution 1:200. Lines and error bars represent the average value ± SD.
4. Discussion
In the present study, we investigated a novel approach for bolstering immune responses to DNA and protein based AD epitope vaccines using transcutaneously applied heat-labile enterotoxin (LT) adjuvant. Data from preclinical studies (Petrushina et al., 2007) as well as from the first clinical trial AN1792 (Patton et al., 2006, Holmes et al., 2008) argue that high titers of long lasting anti-Aβ antibody could be beneficial in reduction of Aβ-deposits in the brain and in improvement of cognitive function. Currently several active immunotherapy strategies are being tested in clinical trials. The available data demonstrate that induction of high titers of anti-Aβ antibody in AD patients is difficult to achieve and potent adjuvants are required. Recently, it was shown that topical administration of LT-IS patches (Intercell USA, Inc.) at the site of protein vaccine injection resulted in 10- to 50-fold increases in the influenza virus-specific immune response (Guebre-Xabier et al., 2004). In response to this adjuvant, activated Langerhans cells migrate to the draining lymph nodes where they present the antigen to T cells. Importantly, transcutaneous delivery of LT to the skin can be achieved with a high degree of safety, overcoming the toxicities observed when the adjuvant is injected with vaccine (Glenn et al., 2003). Accordingly LT-IS patches currently have been evaluated in various clinical trials with the goal to enhance the efficacy of conventional vaccines (Frech et al., 2008, Glenn et al., 2009). Indeed, our group has also demonstrated that LT-IS patches augment anti-influenza IgG1 antibody responses induced by DNA vaccination of wild-type mice (Mkrtichyan et al., 2008).
DNA vaccines have many advantages over conventional protein/peptide based vaccines, being relatively safe, cost efficient, and capable of sustaining reasonable levels of antigen expression within cells. DNA vaccines can be easily manipulated in order to modify genes and to target the desired type of immune response. However, one caveat is that the immunogenicity of DNA vaccines is low in large animals and humans, perhaps owing to inefficient transfection of cells by naked DNA. It has been estimated that only 10% of injected DNA gets into the cytoplasm and less than 1% of the DNA within the cell enters the nucleus (Babiuk et al., 2003). Thus, numerous approaches have been tested for improving the in vivo uptake and expression of plasmid DNA, including gene gun (Fuller et al., 1997, Torres et al., 1997), electroporation (McCray et al., 2006, Luxembourg et al., 2007) and fluid-jet (Walther et al., 2004) devices; lipid based delivery systems (Gregoriadis et al., 1997), encapsulation of DNA in different microparticles (Jones et al., 1997, Hedley et al., 1998) etc.). Another approach to enhance the humoral and cellular immune responses is co-delivery of DNA immunogens and molecular adjuvant-gene encoding different cytokines or chemokines (Ertl and Xiang, 1996, Kim et al., 1998), or combinations of molecular and conventional adjuvants. In this report, we demonstrated that application of LT-IS patches significantly enhanced the onset and amplitude of anti-Aβ antibody responses to gene gun immunization with a DNA-based AD epitope vaccine as well as to a protein vaccine delivered intradermally. The enhancement of anti-Aβ antibody responses correlated with higher numbers of activated T cells specific to foreign T cell epitopes incorporated into the vaccine.
To confirm that the LT-IS patch may be as effective as clinically approved conventional adjuvants we compared antibody responses induced by Lu AF20513 vaccine formulated Alhydrogel® or in combination with LT-IS in Tg2576 mice, known as a poor responder to β-amyloid based vaccines (Petrushina et al., 2003, Movsesyan et al., 2010). We demonstrated that i.d. injection of Lu AF20513 and application of LT-IS patch induced the same low levels of anti-Aβ antibody response in Tg2576 mice as s.c. injection of Lu AF20513 formulated in Alhydrogel® (Fig. 5) as we reported with other epitope vaccine (Movsesyan et al., 2010). Of note, both vaccination protocols did not induce meningoencephalitis (no infiltration of CD3+ CD4+ or CD3+CD8+ T cells in the brains of injected animals was observed). Although Alhydrogel® remains the most commonly used adjuvant and currently approved for use in humans by the FDA, it sometimes demonstrates limited effects in clinical settings (Langley et al., 2009). Having in mind that the LT-IS patch proved to be well tolerated in clinical trials (Glenn et al., 2009) and considering the ease of storage and manipulations of patches, it could be a superior alternative to Alhydrogel® adjuvant. Importantly, we should mention here that the strong immunogenicity of the Lu AF20513 vaccine formulated in Alhydrogel® was shown in two animal species, guinea pigs and monkeys (Davtyan et al., 2013). Collectively, these results suggest that the Lu AF20513 formulated into the Alhydrogel® may induce different immune responses in different animal models and only future clinical trials may show the efficacy of this formulation in vaccinated subjects.
In conclusion, this report has uncovered an improved DNA and protein immunization protocol associated with adjuvant delivered by LT-IS patches for AD. Further development of these technologies may improve the utility of any plasmid DNA and protein vaccination method.
Highlights.
LT-IS patches enhances the onset and magnitude of immune responses to AD DNA vaccine
LT-IS patches enhances humoral immune responses to protein-based AD vaccine
LT-IS patches may improve the utility of any DNA and protein vaccination method
Acknowledgments
We would like to thank Dr. Annette Marleau for help with editing and valuable comments. This work was supported by funding from Alzheimer’s Association (IIRG-07-283140 and IIRG-12-239626) and from NIH (NS-50895; AG-20241 and NS-057395). HD was supported by NIA T32 training grant AG00096.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AGADJANYAN MG, TRIVEDI NN, KUDCHODKAR S, BENNETT M, LEVINE W, LIN A, BOYER J, LEVY D, UGEN KE, KIM JJ, WEINER DB. An HIV type 2 DNA vaccines induces cross-reactive immune responses against HIV type 2 and SIV. AIDS Res Hum Retroviruses. 1997;13:1561–1572. doi: 10.1089/aid.1997.13.1561. [DOI] [PubMed] [Google Scholar]
- BABIUK LA, PONTAROLLO R, BABIUK S, LOEHR B, VAN DRUNEN LITTEL-VAN DEN HURK S. Induction of immune responses by DNA vaccines in large animals. Vaccine. 2003;21:649–58. doi: 10.1016/s0264-410x(02)00574-1. [DOI] [PubMed] [Google Scholar]
- BAYER AJ, BULLOCK R, JONES RW, WILKINSON D, PATERSON KR, JENKINS L, MILLAIS SB, DONOGHUE S. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology. 2005;64:94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- BROOKMEYER R, JOHNSON E, ZIEGLER-GRAHAM K, ARRIGHI HM. Forecasting the global burden of Alzheimer’s disease. Journal of Alzheimer’s Association. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- CRIBBS DH, GHOCHIKYAN A, TRAN M, VASILEVKO V, PETRUSHINA I, SADZIKAVA N, KESSLAK P, KIEBER-EMMONS T, COTMAN CW, AGADJANYAN MG. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int Immunol. 2003;15:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVTYAN H, GHOCHIKYAN A, MOVSESYAN N, ELLEFSEN B, PETRUSHINA I, CRIBBS DH, HANNAMAN D, EVANS CF, AGADJANYAN MG. Delivery of a DNA Vaccine for Alzheimer’s Disease by Electroporation versus Gene Gun Generates Potent and Similar Immune Responses. Neurodegener Dis. 2012;10:261–4. doi: 10.1159/000333359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVTYAN H, GHOCHIKYAN A, PETRUSHINA I, HOVAKIMYAN A, DAVTYAN A, POGHOSYAN A, MARLEAU AM, MOVSESYAN N, KIYATKIN A, RASOOL S, LARSEN AK, MADSEN PJ, WEGENER KM, DITLEVSEN DK, CRIBBS DH, PEDERSEN LO, AGADJANYAN MG. Immunogenicity, Efficacy, Safety, and Mechanism of Action of Epitope Vaccine (Lu AF20513) for Alzheimer’s Disease: Prelude to a Clinical Trial. J Neurosci. 2013;33:4923–34. doi: 10.1523/JNEUROSCI.4672-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVTYAN H, MKRTICHYAN M, MOVSESYAN N, PETRUSHINA I, MAMIKONYAN G, CRIBBS DH, AGADJANYAN MG, GHOCHIKYAN A. DNA prime-protein boost increased the titer, avidity and persistence of anti-Abeta antibodies in wild-type mice. Gene Ther. 2010;17:261–71. doi: 10.1038/gt.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERTL HC, XIANG ZQ. Genetic immunization. Viral Immunol. 1996;9:1–9. doi: 10.1089/vim.1996.9.1. [DOI] [PubMed] [Google Scholar]
- EVANS CF, DAVTYAN H, PETRUSHINA I, HOVAKIMYAN A, DAVTYAN A, HANNAMAN D, CRIBBS DH, AGADJANYAN MG, GHOCHIKYAN A. Epitope-based DNA vaccine for Alzheimer’s disease: Translational study in macaques. Alzheimers Dement. 2013 doi: 10.1016/j.jalz.2013.04.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX NC, BLACK RS, GILMAN S, ROSSOR MN, GRIFFITH SG, JENKINS L, KOLLER M. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563–72. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- FRECH SA, DUPONT HL, BOURGEOIS AL, MCKENZIE R, BELKIND-GERSON J, FIGUEROA JF, OKHUYSEN PC, GUERRERO NH, MARTINEZ-SANDOVAL FG, MELENDEZ-ROMERO JH, JIANG ZD, ASTURIAS EJ, HALPERN J, TORRES OR, HOFFMAN AS, VILLAR CP, KASSEM RN, FLYER DC, ANDERSEN BH, KAZEMPOUR K, BREISCH SA, GLENN GM. Use of a patch containing heat-labile toxin from Escherichia coli against travellers’ diarrhoea: a phase II, randomised, double-blind, placebo-controlled field trial. Lancet. 2008;371:2019–25. doi: 10.1016/S0140-6736(08)60839-9. [DOI] [PubMed] [Google Scholar]
- FULLER DH, CORB MM, BARNETT S, STEIMER K, HAYNES JR. Enhancement of immunodeficiency virus-specific immune responses in DNA-immunized rhesus macaques. Vaccine. 1997;15:924–6. doi: 10.1016/s0264-410x(96)00271-x. [DOI] [PubMed] [Google Scholar]
- GHOCHIKYAN A, DAVTYAN H, PETRUSHINA I, HOVAKIMYAN A, MOVSESYAN N, DAVTYAN A, KIYATKIN A, CRIBBS DH, AGADJANYAN MG. Refinement of a DNA based Alzheimer’s disease epitope vaccine in rabbits. Hum Vaccin Immunother. 2013;9:1002–1010. doi: 10.4161/hv.23875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHOCHIKYAN A, MKRTICHYAN M, PETRUSHINA I, MOVSESYAN N, KARAPETYAN A, CRIBBS DH, AGADJANYAN MG. Prototype Alzheimer’s disease epitope vaccine induced strong Th2-type anti-Abeta antibody response with Alum to Quil A adjuvant switch. Vaccine. 2006;24:2275–82. doi: 10.1016/j.vaccine.2005.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLENN GM, KENNEY RT, ELLINGSWORTH LR, FRECH SA, HAMMOND SA, ZOETEWEIJ JP. Transcutaneous immunization and immunostimulant strategies: capitalizing on the immunocompetence of the skin. Expert Rev Vaccines. 2003;2:253–67. doi: 10.1586/14760584.2.2.253. [DOI] [PubMed] [Google Scholar]
- GLENN GM, SCHARTON-KERSTEN T, VASSELL R, MATYAS GR, ALVING CR. Transcutaneous immunization with bacterial ADP-ribosylating exotoxins as antigens and adjuvants. Infect Immun. 1999;67:1100–6. doi: 10.1128/iai.67.3.1100-1106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLENN GM, THOMAS DN, POFFENBERGER KL, FLYER DC, ELLINGSWORTH LR, ANDERSEN BH, FRECH SA. Safety and immunogenicity of an influenza vaccine A/H5N1 (A/Vietnam/1194/2004) when coadministered with a heat-labile enterotoxin (LT) adjuvant patch. Vaccine. 2009;27(Suppl 6):G60–6. doi: 10.1016/j.vaccine.2009.10.031. [DOI] [PubMed] [Google Scholar]
- GREGORIADIS G, SAFFIE R, DE SOUZA JB. Liposome-mediated DNA vaccination. FEBS Lett. 1997;402:107–10. doi: 10.1016/s0014-5793(96)01507-4. [DOI] [PubMed] [Google Scholar]
- GUEBRE-XABIER M, HAMMOND SA, ELLINGSWORTH LR, GLENN GM. Immunostimulant patch enhances immune responses to influenza virus vaccine in aged mice. J Virol. 2004;78:7610–8. doi: 10.1128/JVI.78.14.7610-7618.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUERENA-BURGUENO F, HALL ER, TAYLOR DN, CASSELS FJ, SCOTT DA, WOLF MK, ROBERTS ZJ, NESTEROVA GV, ALVING CR, GLENN GM. Safety and immunogenicity of a prototype enterotoxigenic Escherichia coli vaccine administered transcutaneously. Infect Immun. 2002;70:1874–80. doi: 10.1128/IAI.70.4.1874-1880.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDY J, SELKOE DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- HARDY JA, HIGGINS GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- HEDLEY ML, CURLEY J, URBAN R. Microspheres containing plasmid-encoded antigens elicit cytotoxic T-cell responses. Nat Med. 1998;4:365–8. doi: 10.1038/nm0398-365. [DOI] [PubMed] [Google Scholar]
- HOLMES C, BOCHE D, WILKINSON D, YADEGARFAR G, HOPKINS V, BAYER A, JONES RW, BULLOCK R, LOVE S, NEAL JW, ZOTOVA E, NICOLL JA. Long-term effects of Abeta42 immunization in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–23. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- JONES DH, CORRIS S, MCDONALD S, CLEGG JC, FARRAR GH. Poly(DL-lactide-co-glycolide)-encapsulated plasmid DNA elicits systemic and mucosal antibody responses to encoded protein after oral administration. Vaccine. 1997;15:814–7. doi: 10.1016/s0264-410x(96)00266-6. [DOI] [PubMed] [Google Scholar]
- KIM JJ, NOTTINGHAM LK, SIN JI, TSAI A, MORRISON L, OH J, DANG K, HU Y, KAZAHAYA K, BENNETT M, DENTCHEV T, WILSON DM, CHALIAN AA, BOYER JD, AGADJANYAN MG, WEINER DB. CD8 positive T cells influence antigen-specific immune responses through the expression of chemokines. Journal of Clinical Investigation. 1998;102:1112–24. doi: 10.1172/JCI3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUTZLER MA, WEINER DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008;9:776–88. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGLEY JM, SALES V, MCGEER A, GUASPARINI R, PREDY G, MEEKISON W, LI M, CAPELLAN J, WANG E. A dose-ranging study of a subunit Respiratory Syncytial Virus subtype A vaccine with and without aluminum phosphate adjuvantation in adults > or =65 years of age. Vaccine. 2009;27:5913–9. doi: 10.1016/j.vaccine.2009.07.038. [DOI] [PubMed] [Google Scholar]
- LEMERE CA, MASLIAH E. Can Alzheimer disease be prevented by amyloid-beta immunotherapy? Nat Rev Neurol. 2010;6:108–19. doi: 10.1038/nrneurol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUXEMBOURG A, EVANS CF, HANNAMAN D. Electroporation-based DNA immunisation: translation to the clinic. Expert Opin Biol Ther. 2007;7:1647–64. doi: 10.1517/14712598.7.11.1647. [DOI] [PubMed] [Google Scholar]
- MASLIAH E, HANSEN L, ADAME A, CREWS L, BARD F, LEE C, SEUBERT P, GAMES D, KIRBY L, SCHENK D. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64:129–31. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- MCCRAY AN, UGEN KE, MUTHUMANI K, KIM JJ, WEINER DB, HELLER R. Complete regression of established subcutaneous B16 murine melanoma tumors after delivery of an HIV-1 Vpr-expressing plasmid by in vivo electroporation. Mol Ther. 2006;14:647–55. doi: 10.1016/j.ymthe.2006.06.010. [DOI] [PubMed] [Google Scholar]
- MKRTICHYAN M, GHOCHIKYAN A, MOVSESYAN N, KARAPETYAN A, BEGOYAN G, YU J, GLENN GM, ROSS TM, AGADJANYAN MG, CRIBBS DH. Immunostimulant adjuvant patch enhances humoral and cellular immune responses to DNA immunization. DNA Cell Biol. 2008;27:19–24. doi: 10.1089/dna.2007.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOVSESYAN N, DAVTYAN H, MKRTICHYAN M, PETRUSHINA I, TIRATURYAN T, ROSS TM, AGADJANYAN MG, GHOCHIKYAN A, CRIBBS DH. Low concentrations of anti-Abeta antibodies generated in Tg2576 mice by DNA epitope vaccine fused with 3C3d molecular adjuvant do not affect AD pathology. Hum Gene Ther. 2010;21:1569–1576. doi: 10.1089/hum.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOVSESYAN N, GHOCHIKYAN A, MKRTICHYAN M, PETRUSHINA I, DAVTYAN H, OLKHANUD PB, HEAD E, BIRAGYN A, CRIBBS DH, AGADJANYAN MG. Reducing AD-like pathology in 3xTg-AD mouse model by DNA epitope vaccine- a novel immunotherapeutic strategy. PLos ONE. 2008;3:e21–24. doi: 10.1371/journal.pone.0002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICOLL JA, WILKINSON D, HOLMES C, STEART P, MARKHAM H, WELLER RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9:448–52. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- PATTON RL, KALBACK WM, ESH CL, KOKJOHN TA, VAN VICKLE GD, LUEHRS DC, KUO YM, LOPEZ J, BRUNE D, FERRER I, MASLIAH E, NEWEL AJ, BEACH TG, CASTANO EM, ROHER AE. Amyloid-beta peptide remnants in AN-1792-immunized Alzheimer’s disease patients: a biochemical analysis. Am J Pathol. 2006;169:1048–63. doi: 10.2353/ajpath.2006.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETRUSHINA I, GHOCHIKYAN A, MKTRICHYAN M, MAMIKONYAN G, MOVSESYAN N, DAVTYAN H, PATEL A, HEAD E, CRIBBS DH, AGADJANYAN MG. Alzheimer’s Disease Peptide Epitope Vaccine Reduces Insoluble But Not Soluble/Oligomeric A{beta} Species in Amyloid Precursor Protein Transgenic Mice. J Neurosci. 2007;27:12721–12731. doi: 10.1523/JNEUROSCI.3201-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETRUSHINA I, TRAN M, SADZIKAVA N, GHOCHIKYAN A, VASILEVKO V, AGADJANYAN MG, CRIBBS DH. Importance of IgG2c isotype in the immune response to b-amyloid in APP/Tg mice. Neurosci Letters. 2003;338:5–8. doi: 10.1016/s0304-3940(02)01357-5. [DOI] [PubMed] [Google Scholar]
- PRICE DL, SISODIA SS. Cellular and molecular biology of Alzheimer’s disease and animal models. Annu Rev Med. 1994;45:435–446. doi: 10.1146/annurev.med.45.1.435. [DOI] [PubMed] [Google Scholar]
- SCHARTON-KERSTEN T, YU J, VASSELL R, O’HAGAN D, ALVING CR, GLENN GM. Transcutaneous immunization with bacterial ADP-ribosylating exotoxins, subunits, and unrelated adjuvants. Infect Immun. 2000;68:5306–13. doi: 10.1128/iai.68.9.5306-5313.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRES CA, IWASAKI A, BARBER BH, ROBINSON HL. Differential dependence on target site tissue for gene gun and intramuscular DNA immunizations. J Immunol. 1997;158:4529–432. [PubMed] [Google Scholar]
- WALTHER W, STEIN U, FICHTNER I, SCHLAG PM. Low-volume jet injection for efficient nonviral in vivo gene transfer. Mol Biotechnol. 2004;28:121–8. doi: 10.1385/MB:28:2:121. [DOI] [PubMed] [Google Scholar]