Abstract
Intranasal exposure to cadmium has been related to olfactory dysfunction in humans and to nasal epithelial damage and altered odorant-guided behavior in rodent models. The pathophysiology underlying these deficits has not been fully elucidated. Here we use optical imaging techniques to visualize odorant-evoked neurotransmitter release from the olfactory nerve into the brain’s olfactory bulbs in vivo in mice. Intranasal cadmium chloride instillations reduced this sensory activity by up to 91% in a dose-dependent manner. In the olfactory bulbs, afferents from the olfactory epithelium could be quantified by their expression of a genetically-encoded fluorescent marker for olfactory marker protein. At the highest dose tested, cadmium exposure reduced the density of these projections by 20%. In a behavioral psychophysical task, mice were trained to sample from an odor port and make a response when they detected an odorant against a background of room air. After intranasal cadmium exposure, mice were unable to detect the target odor. These experiments serve as proof of concept for a new approach to the study of the neural effects of inhaled toxicants. The use of in vivo functional imaging of the neuronal populations exposed to the toxicant permits the direct observation of primary pathophysiology. In this study optical imaging revealed significant reductions in odorant-evoked release from the olfactory nerve at a cadmium chloride dose two orders of magnitude less than that required to induce morphological changes in the nerve in the same animals, demonstrating that it is a more sensitive technique for assessing the consequences of intranasal neurotoxicant exposure. This approach is potentially useful in exploring the effects of any putative neurotoxicant that can be delivered intranasally.
Keywords: Cadmium, Olfaction, Imaging, Psychophysics, Pathophysiology, SynaptopHluorin
1. Introduction
The olfactory system is the only point in the mammalian nervous system in which neurons are physically exposed to the organism’s external environment. This position makes the olfactory epithelium uniquely vulnerable to environmental neurotoxicants (Genter et al., 2009; Hastings and Miller, 2003). The olfactory nerve can also serve as a vector for neurotoxicants to be transported into the central nervous system, bypassing the blood-brain barrier (Baker and Genter, 2003; Bondier et al., 2008; Dorman et al., 2002; Genter et al., 2009; Shipley, 1985; Sunderman, 2001; Tjalve et al., 1996; Tjalve and Henriksson, 1999). The olfactory system is thus of direct importance in evaluating the consequences of exposure to aerosolized neurotoxicants, but may also be valuable as a model in which to evaluate the physiological effects of such exposures on neural tissue in vivo (Hastings, 1990). Here we use a combination of optical imaging, genetically-identified tract tracing, and behavioral psychophysical methods to investigate the pathophysiological effects of acute exposure to cadmium in this system.
The mouse olfactory system consists of a sheet of nasal epithelium containing approximately 20 million olfactory receptor neurons (ORNs), each of which expresses just one out of a repertoire of about one thousand olfactory receptors (Buck and Axel, 1991). The ORN axons project via the olfactory nerve to the olfactory bulb, where they segregate such that all of the axons from ORNs expressing each type of receptor project to one or two specific glomeruli on the surface of the bulb (Mombaerts et al., 1996). Odorants reaching the olfactory epithelium in the nasal cavity stimulate an odorant-specific subset of olfactory receptors, thus driving synaptic input from an odorant-specific subset of ORN populations into a corresponding subset of olfactory bulb glomeruli. In a strain of transgenic mice expressing the genetically encoded, fluorescent exocytosis indicator synaptopHluorin (spH) in ORN axon terminals, we have previously demonstrated that this odorant-evoked neurotransmitter release from the olfactory nerve can be visualized in vivo through a cranial window (Bozza et al., 2004; McGann et al., 2005). This technique can provide an extremely sensitive metric of ORN function.
Cadmium is a heavy metal frequently found in aerosolized form in cigarette smoke and in some industrial workplaces, most notably smelting and battery manufacture (ATSDR 2008). Cadmium is known to transport to the central nervous system after intranasal exposure in rodent models (Bondier et al., 2008; Evans and Hastings, 1992; Hastings and Evans, 1991; Sun et al., 1996; Tjalve et al., 1996), where it can have a range of harmful effects on neurons, including the blockade of calcium channels (Chow, 1991; Wang, S.H. et al., 2008) and the induction of apoptosis (Wang, S. et al., 2008). Exposure to aerosolized cadmium compounds has been associated with olfactory dysfunction in humans (Adams and Crabtree, 1961; Mascagni et al., 2003; Rose et al., 1992; Sułkowski et al., 2000).Rose et al. (1992) found that workers in a brazing facility chronically exposed to aerosolized cadmium were more likely to exhibit dysosmias than a reference group, and that workers with the highest levels of urinary cadmium and tubular proteinuria (evidence of kidney damage associated with cadmium exposure) had the most elevated olfactory thresholds.Rydzewski et al. (1998) and Sułkowski et al. (2000) studied olfactory function in workers in nickel-cadmium battery plants in Poland and reported significant correlations between olfactory impairment and blood and urine cadmium concentrations. Mascagni et al. (2003) demonstrated that workers exposed to more modest concentrations of cadmium fumes in an Italian foundry also exhibited elevated olfactory thresholds compared to controls. While strongly suggestive, these data must be viewed with caution because occupational exposures typically include confounding exposures (such as nickel dust) and such studies cannot rigorously control for exposure concentration and duration.
In rodent models, intranasal instillation of 400 µg cadmium chloride has been reported to cause nasal epithelial damage and altered odorant-guided passive avoidance behavior in the mouse (Bondier et al., 2008), but in another study chronic exposure to aerosolized cadmium oxide at up to 500 µg/m3 did not measurably impair odorant-cued conditioned suppression in the rat (Sun et al., 1996). Each of these studies had significant limitations. They both used gross histological measures (such as epithelial thickness) to assess the impact of cadmium exposure on the olfactory epithelium, finding differing results. However, a morphologically-intact epithelium may not respond normally to odorants, and it may not sustain normal projections to the olfactory bulbs. In each study the only metric of altered neurophysiology was a behavioral change (or lack thereof).Bondier et al. (2008) showed that cadmium-exposed mice did not spontaneously avoid the butanol-scented arm of a Y-maze, but this approach does not unambiguously support their conclusion that these mice were hyposmic – cadmium exposure might alternatively have changed the perceptual quality of butanol such that it was no longer aversive (i.e. parosmia; see Doty, 2009). A behavioral signal detection task could provide a more sensitive metric for assessing sensory function (Bushnell et al., 2007).Sun et al. (1996) found no evidence of a cadmium-induced change in absolute olfactory threshold using a task in which odorant presentation signals an impending footshock, thus spontaneously suppressing conditioned licking for a water reward. However, rodents can sometimes perform simple olfactory tasks even after large lesions to the olfactory epithelium and bulb (Slotnick and Bodyak, 2002), so the absence of a behavioral deficit in these highly-trained animals does not by itself demonstrate normal function. Physiological recording of odorant-evoked neural responses in vivo could provide a more direct measure of cadmium-induced pathophysiology.
2. Material and methods
2.1 Subjects
Subjects in the imaging experiments were male and female OMP-spH mice, which express the spH construct (Miesenböck et al., 1998) from the locus for OMP (Bozza et al., 2004). These mice were derived from our previously reported strain (Jackson Labs stock #004946), which were on a mixed C57Bl/6 and 129 background (Bozza et al., 2004). Mice were backcrossed for seven generations to a strain of albino C57Bl/6J-Tyrc-2J/J mice (Jackson Labs stock # 000058) to generate a line of homozygous OMP-spH mice on a pure albino C57Bl/6 background. Imaging experiments were conducted on both homozygous and heterozygous OMP-spH mice (from an F1 cross to 129) aged 6 to 10 weeks. As previously reported (Bozza et al., 2004; McGann et al., 2005), we observed no differences between mice that were homozygous and heterozygous for spH and pooled data across both genotypes in presenting our results. Subjects in behavioral experiments were female C57Bl/6 mice initially aged 6 weeks, purchased from Charles River Laboratories. All animals were group housed with a 12:12 h light:dark cycle. Mice used for imaging procedures had food and water available ad libitum. Animals used in behavioral testing were water restricted and maintained at 90% of original body weight. All procedures were performed in accordance with protocols approved by the Rutgers University Animal Care and Use Committee.
2.2 Intranasal infusions
Mice in cadmium-exposed groups received intranasal instillations of 6 µL of pH 7.4 buffer solution containing 200 mM HEPES, 0.9% NaCl, and either 18.18 mM, 1.818 mM, or 0.182 mM CdCl2 as noted. These concentrations yield individual infusions of 20µg, 2 µg, and 0.2 µg of cadmium chloride, respectively. Mice used in imaging experiments received an infusion of cadmium solution into one external naris and vehicle solution (without CdCl2) into the contralateral naris, with side randomly counterbalanced across subjects. Because the nasal passages on the left and right sides are separated by a nasal septum and the ORN projections are strictly ipsilateral, this design permitted within-subjects, left vs. right comparisons. Mice used in behavioral experiments received bilateral infusions of either cadmium or vehicle solution. Reflux of the instillate was not observed.
For the intranasal instillation procedure, animals were lightly anesthetized with 1000µg/kg metatomadine and 70mg/kg ketamine administered intraperitoneally (i.p). An Eppendorf microloader attached to a 10µL Hamilton syringe containing the infusate was inserted 7mm into one naris, the mouse was placed on its back, and the infusion was delivered. The animal remained on its back for five min and was then rotated to its side for another 20 min to ensure coverage across the epithelium. The procedure was repeated for the other naris. Anesthesia was then reversed by subcutaneous (s.c.) injection of 1mg/kg atipamezole hydrochloride. The mouse was maintained on a heating pad and given food and water ad libitum during overnight recovery from anesthesia. The experimenter was blind to the contents of all infusates.
2.3 In vivo imaging of neurotransmitter release
2.3.1 Surgical implantation of cranial window
Two days after intranasal cadmium exposure, mice were anesthetized with 0.01 mL/g of 10 mg/mL pentobarbital (i.p.), with 0.05 mL boosters as needed to maintain anesthetic plane. Body temperature was maintained using a rectal temperature probe and feedback-regulated heating pad. Mice were administered 0.005mL/g 0.1% atropine (s.c.) to reduce nasal secretions and ~0.25mL of 0.25% bupivacaine (s.c.) as a local anesthetic along the scalp. The scalp was shaved, then surgically opened with a midline incision. The periosteal membrane was removed and the skull dried with a 70% ethanol solution. A headbar was fixed to the skull using dental acrylic to rigidly mount the mouse’s skull to a custom headholder. Using a micromotor dental handpiece, the skull overlying both olfactory bulbs was thinned until transparent when wet. Ringer’s solution containing 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM HEPES and 10 mM dextrose was applied over this cranial window, then topped with a glass coverslip.
2.3.2 Imaging apparatus
Optical imaging was performed using a custom apparatus composed of an Olympus epifluorescence illuminator coupled to an Olympus XLFluor4X macro objective (0.28 NA). Illumination was provided by an Opti-Quip 150W Xenon arc lamp with a 25% neutral density filter and controlled by a Uniblitz shutter. Macro-format fluorescence filter cubes included an HQ480/40 excitation filter, Q505LP dichroic mirror, and HQ535/50 emission filter (Chroma Technology). Images were acquired using a RedShirtImaging monochrome, back-illuminated CCD camera (NeuroCCD SM256) at 256 × 256 pixel resolution and frame acquisition rate of 7 Hz. The mouse was positioned under the microscope using a custom 3-axis optomechanical stage. The entire apparatus floated on a TMC vibration isolation table.
Odorants were presented by a custom built eight-channel, air dilution olfactometer controlled by a computer running software written for MatLab (Mathworks). Nitrogen was passed through vials of pure odorant to produce a saturated carrier vapor that was then diluted into ~500 mL/minute ultrazero-humidity compressed air by computer-controlled mass flow controllers at a user-specified ratio. Wetted parts downstream of the odorants were made of PTFE or PEEK, and source gases were filtered by a hydrocarbon/moisture gas purification system (Chromatography Research Services). Stimulus onset and offset were controlled by a computer controlled valve that shunted a vacuum from and to an odorant-removal tube concentric with the odorant delivery tube after the manner of Kauer and Moulton (1974). The odorant delivery tube was placed within 2cm of the mouse’s nose. Odorants included methyl valerate, 2-methyl 2-butanal, hexanone and butyl acetate, which are known to evoke transmitter release on the dorsal aspect of the olfactory bulb (Bozza et al., 2004; Wachowiak and Cohen, 2001). They were presented in 6 sec trials at a 2–6% dilution of saturated vapor. The minimum intertrial interval was 60 sec. The experimenter was blind to the experimental condition of the mouse.
2.3.3 Imaging data analysis
Imaging data were analyzed as described previously (McGann et al., 2005). Briefly, blank trials on which no odorants were presented were subtracted from each odorant trial to correct for bleaching. Odorant-evoked glomerular responses were measured as the average of 15 frames centered on the peak of the fluorescence increase minus the average of 15 baseline frames immediately prior to odorant onset. Trials were treated individually for amplitude measurements and averaged within odorants to create spatial maps of odorant-evoked responses. Candidate regions of interest corresponding to olfactory glomeruli were initially selected by hand and then confirmed statistically. A glomerulus was operationally defined as responding to an odorant if its average response across trials of that odorant was greater than zero by three standard errors or more. Analysis was performed using custom software written in MatLab and exported to Excel, SPSS, and SigmaPlot for statistical analysis.
2.4 Olfactory bulb histology
2.4.1 Histological procedures
Immediately after imaging, animals were intracardially perfused with 0.1M phosphate-buffered solution (PBS) followed by 4% paraformaldehyde. Brains were removed and post-fixed in 4% paraformaldehyde. Tissue was transferred to PBS at least 24 h before sectioning. Brains were blocked to include both olfactory bulbs and the frontal cortex and sectioned horizontally at 50 µm on a vibratome. Slices were mounted in ProLong Gold antifade agent (Invitrogen) containing DAPI on glass slides and sealed under a glass coverslip.
2.4.2 Quantification of glomerular afferents
Photos of olfactory bulb sections were taken at a resolution of 1360 × 1024 pixels and 14 bit analog-to-digital conversion with a Jenoptik MFcool Peltier-cooled CCD camera mounted on an Olympus BX41 microscope at 4X (0.16 NA). Images were collected with both DAPI (D350/50x excitation filter, T400LP dichroic mirror, and ET460/50m emission) and GFP (ET470/40x excitation filter, T495LP dichroic mirror, and ET525/50m emission) filter sets. Images were opened in ImageJ (NIH) and the glomerular layer of each olfactory bulb was selected as a region of interest based on the rings of periglomerular interneurons visualized by the DAPI stain. The optical density of these regions of interest was then measured in the corresponding image taken using the GFP-appropriate optical filter. Optical densities were recorded in Excel and exported to SPSS for statistical analysis. Experimenters were blind to the experimental condition of the animal until after the quantification was completed.
2.5 Behavioral experimentation
2.5.1 Apparatus
Mice were trained in operant conditioning chambers (Coulbourn Apparatus Habitest system) enclosed within sound attenuating cubicles (Med Associates or Coulbourn Apparatus). Reinforcements were delivered through a reward port, where 0.01 mL of 2% sucrose solution was delivered by a liquid dipper when the mouse broke a nosepoke photobeam on rewarded trials. Olfactory stimuli were presented through a custom controlled-access odor port consisting of a nose poke operandum with odorant and vacuum ports and a guillotine door on the front to prevent odorant access during the intertrial interval. A house light and ventilation fan were also included. Odorants were presented using custom computer controlled liquid dilution olfactometers, which passed room air through odorant vials containing a 1:100 dilution of the odorant in mineral oil and then on to the odor port. The rewarded olfactory stimulus was butyl acetate. Actual concentrations in the odor ports were measured and standardized across chambers and days of training using an UltraRae photoionization detector (Rae Systems). Each chamber and floor was washed with 70% ethanol after every session.
2.5.2 Training and testing protocols
Mice underwent two to three days of water restriction prior to initial training to achieve 90% of initial weight. Training began with conventional magazine training, in which mice received a liquid reward upon poking into the dipper port, as cued by the magazine light. The odor port remained closed throughout magazine training. Mice completed magazine training after 60 successful trials. In the second phase, mice were trained to nose poke into the odor port (in the absence of odorant) when the door opened, and then move to the reward port for reinforcement. Over at least 4 training sessions, mice were shaped to hold the initial nose poke for at least 1 sec (based on the break of a photobeam across the odor port) in order to receive a reinforcement. Each session lasted 60 successful trials or 60 min, whichever came first. After the mice achieved 60 successful trials in a single session, they were advanced to the odorant detection training. In this final phase of the training, mice were trained to nose poke in the odor port when the door opened and then to poke for reward if and only if they received the odorant butyl acetate. The intertrial interval after correct responses was 5 sec, and incorrect responses triggered a “time-out” punishment of 25 sec added to the intertrial interval. All training was performed daily. Mice received their daily ration of water at the conclusion of training to maintain body weight.
2.5.3 Testing and data analysis
Detection performance was measured by the d’ discrimination metric, defined as the proportion of trials in which the mouse poked at the reward port when the odor was presented (hits) minus the proportion of trials in which the mouse poked at the reward port when the odor was absent (false alarms). Mice were considered to have reached criterion performance when they exhibited a d’ of 0.5 or greater (equivalent to 75% correct performance) on three consecutive days. The average d’ of these three days to criterion served as the baseline measurement. Immediately after reaching criterion, mice received bilateral intranasal instillations of either cadmium or vehicle solution. Mice received ad libitum water access overnight following intranasal infusion to support their recovery from anesthesia, and were given a single recovery day on water restriction after the infusion. They were then tested for performance on the second day post-infusion. A mixed model ANOVA was run with discrimination index as the dependent variable using time point (baseline measurement, test measurement) as a within-subjects independent variable and instillation group (cadmium, vehicle) as a between-subjects independent variable.
3. Results
3.1 Cadmium exposure disrupts odorant-evoked neurotransmitter release from the olfactory nerve
To assess the effects of intranasal cadmium exposure on olfactory physiology, we used optical imaging techniques to visualize odorant-evoked neurotransmitter release from the olfactory nerve in vivo in a line of transgenic mice (Bozza et al., 2004). These mice express the spH construct (Miesenböck et al., 1998), which sequesters pH-sensitive Green Fluorescent Protein (GFP) inside the lumen of synaptic vesicles. The rapid increase in intravesicular pH during exocytosis causes a rapid and proportional increase in GFP fluorescence. In this line of mice, spH is expressed under the control of the promoter for olfactory marker protein (OMP). OMP is normally expressed exclusively and at high levels in all ORNs (Keller and Margolis, 1975; Monti-Graziadei et al., 1977; Slotnick et al., 2001). Consequently, spH signals in the olfactory bulbs of these mice linearly indicate transmitter release from the olfactory nerve into olfactory bulb glomeruli (Wachowiak et al., 2005). This odorant-evoked transmitter release can be visualized in vivo through a cranial window or in brain slices (Bozza et al., 2004; McGann et al., 2005).
As previously reported (Bozza et al., 2004), odorant presentation in control mice evoked large, odorant-specific increases in the fluorescence of individual olfactory bulb glomeruli. Consistent with previous results (Bozza et al., 2004; Soucy et al., 2009), the patterns of odorant-evoked activity were typically bilateral and approximately symmetric. In five control mice, averaging across a panel of four odorants, the number of glomeruli receiving synaptic input was not different between the left and right olfactory bulbs (average ratio 0.95 ± 0.10, one-sample t-test, p = 0.66) nor was the distribution of glomerular response amplitudes (two-sample Kolmogorov-Smirnov test, p = 0.07).
To assess the effects of intranasal cadmium exposure, we measured odorant-evoked neurotransmitter release from the olfactory nerve in twelve mice that received intranasal instillations of cadmium chloride in one naris and vehicle in the other, thus allowing for a within-subjects left-right comparison. We varied the dose of cadmium chloride across mice to include 20, 2, or 0.2 µg. For each odorant tested in each mouse, we measured the number of olfactory bulb glomeruli receiving synaptic input from ORNs and computed the ratio of glomeruli receiving input on the cadmium-exposed side to that on the vehicle-exposed side. We then averaged these ratios across odorants to generate an overall metric of asymmetry for each mouse. We also compared the amplitude distributions of the observed glomerular inputs between cadmium-exposed and control-bulbs bulbs across odorants.
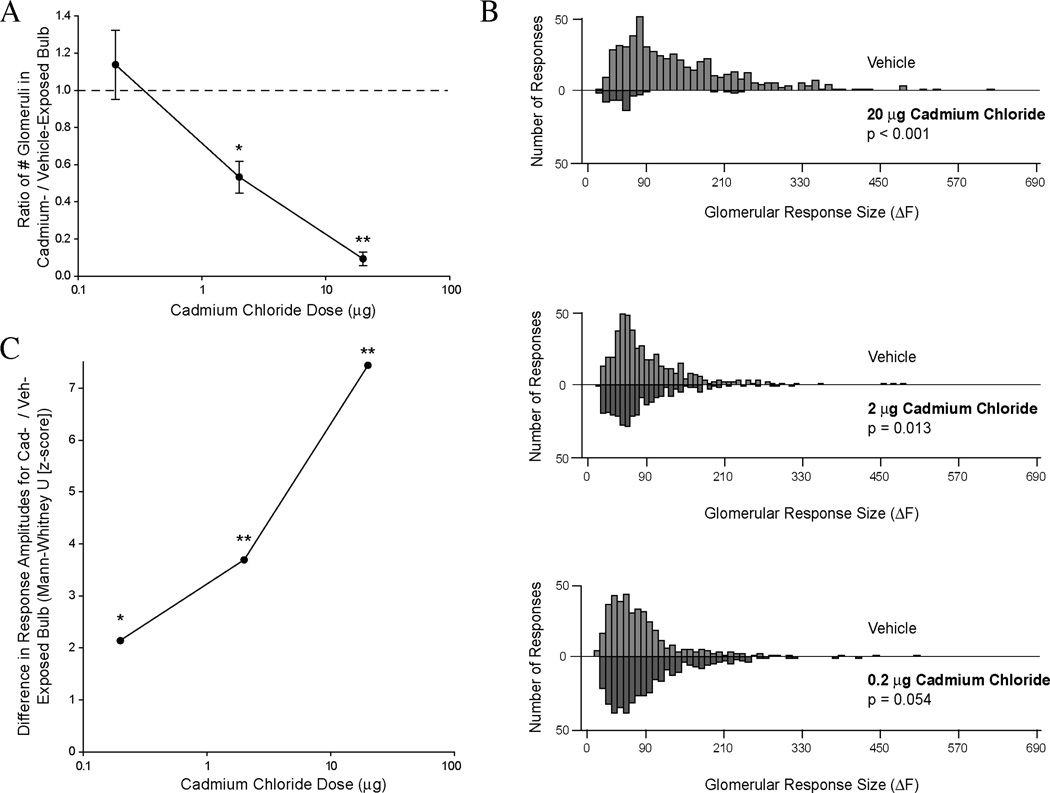
As illustrated in Figures 1 and 2, intranasal exposure to 20 µg cadmium chloride greatly reduced both the number of odorant-responsive glomeruli and the amplitude of the observed responses. As shown in Fig. 2B (upper), the overall distribution of glomerular response amplitudes was significantly different between the olfactory bulbs on the cadmium-exposed and vehicle-exposed sides (two-sample Kolmogorov-Smirnov test, p < 0.001). On average, the olfactory bulb on the cadmium-exposed side included only 9.3 ± 3.8% as many glomeruli receiving measurable input from ORNs as the contralateral, vehicle-exposed bulb (Fig. 1 left, and Fig. 2A), a significant decrease (one-sample t-test, p < 0.001). Among glomeruli that did receive measurable ORN input, the response amplitudes were significantly smaller on the cadmium-exposed side (Mann-Whitney U-test, p < 0.001).
Figure 1. Intranasal cadmium instillation reduces neurotransmitter release from the olfactory nerve into olfactory bulb glomeruli in a dose dependent manner.
(Upper) Baseline fluorescence images of the dorsal olfactory bulbs, viewed through a cranial window in vivo. (Lower) Pseudocolored response maps showing the increase in fluorescence during odorant presentation relative to the pre-odorant baseline. Each spot corresponds to a single olfactory bulb glomerulus. Callouts indicate individual traces showing the change in fluorescence in the corresponding glomerulus during the baseline and odorant presentation part of each trial.
Figure 2. Intranasal cadmium instillation reduces the magnitude of odorant-evoked synaptic input to the olfactory bulb glomeruli and the number of glomeruli receiving sensory input.
(A) Ratio of the number of glomeruli receiving synaptic input from the olfactory nerve in the olfactory bulb on the cadmium-exposed side to the corresponding number on the vehicle-exposed side, shown as a function of cadmium chloride dose. The dashed line at 1 denotes no difference between the olfactory bulbs. (B) Frequency-distribution of glomerular response amplitudes in vehicle-exposed (above the x-axis) and cadmium-exposed (below the x-axis) olfactory bulbs. Data are shown for the 20µg (top), 2 µg (middle), and 0.2 µg (bottom) cadmium chloride doses. (C) Plot of the Kolmogorov-Smirnov z-scores for the paired distributions shown in part B for each cadmium dose. * denotes p < 0.05, ** denotes p < 0.001.
The pathophysiological effects of cadmium exposure were strongly dose-dependent (one-way ANOVA, p = 0.001). As shown in Figures 1 and 2A, intranasal exposure to 2 µg cadmium chloride significantly changed the distribution of glomerular responses between cadmiumexposed and vehicle-exposed bulbs (Fig. 2B, middle; K-S test, p = 0.013). The number of responsive glomeruli in the cadmium-exposed bulb was significantly reduced to 53% ± 9% of the number on the vehicle-exposed side (one-sample t-test, p = 0.012). The amplitude of the observed responses was also significantly reduced (Mann-Whitney U-test, p < 0.001). Exposure to 0.2 µg cadmium chloride did not significantly change the overall distribution of responses, which takes into account both the number and size of the observed responses (Fig. 2B, lower; KS test, p = 0.054). The number of glomeruli receiving ORN input was not reduced (one-sample t-test, p = 0.51). However, the amplitudes of these responses were slightly but significantly reduced (Mann-Whitney U-test, p = 0.032).
3.2 Cadmium exposure damages the connectivity between the olfactory epithelium and olfactory bulb
In histological sections the vesicular pH gradient is neutralized, permitting the use of spH fluorescence as a simple indicator of OMP expression and thus a useful marker for ORN axonal projections (Slotnick et al., 2001). After each imaging session, the mice from experiment 3.1 were perfused and fixed. Their olfactory bulbs were sectioned at 50 µm on a vibratome and mounted in an antifade agent containing the nuclear stain DAPI. Approximately 20 sections from each olfactory bulb were photographed under fluorescence microscopy with both DAPI and GFP fluorescence in registration for quantitative analysis of GFP fluorescence. As shown in Fig. 3, in mice exposed to 20 µg cadmium chloride, the optical density of GFP fluorescence in the glomeruli of cadmium-exposed olfactory bulbs was significantly reduced to 80 ± 4% of that in the contralateral, vehicle-exposed bulbs (one-sample t-test, p = 0.003). No significant reductions were observed in mice exposed to the 2.0µg (p = 0.185) or 0.2µg (p = 0.253) doses (Fig. 3B). These results suggest that at our highest concentration, intranasal cadmium exposure can induce a reduction in axonal projections from the olfactory epithelium to the olfactory bulb.
Figure 3. Axonal projection density from the olfactory epithelium to the olfactory bulb is moderately reduced at the highest cadmium dose.

(A) Representative sections of cadmium- and vehicle-exposed olfactory bulbs across decreasing cadmium chloride doses. The DAPI nuclear stain marks rings of periglomerular interneurons, while the GFP is selectively expressed in the afferents from the olfactory epithelium into olfactory bulb glomeruli. (B) The ratio of GFP optical density in glomeruli from cadmium-exposed olfactory bulbs to that from vehicle-exposed olfactory bulbs, plotted relative to cadmium chloride dose. Dashed line at 1 represents no difference in GFP expression between bulbs. ** denotes p < 0.01.
3.3 Intranasal cadmium exposure profoundly impairs odor detection
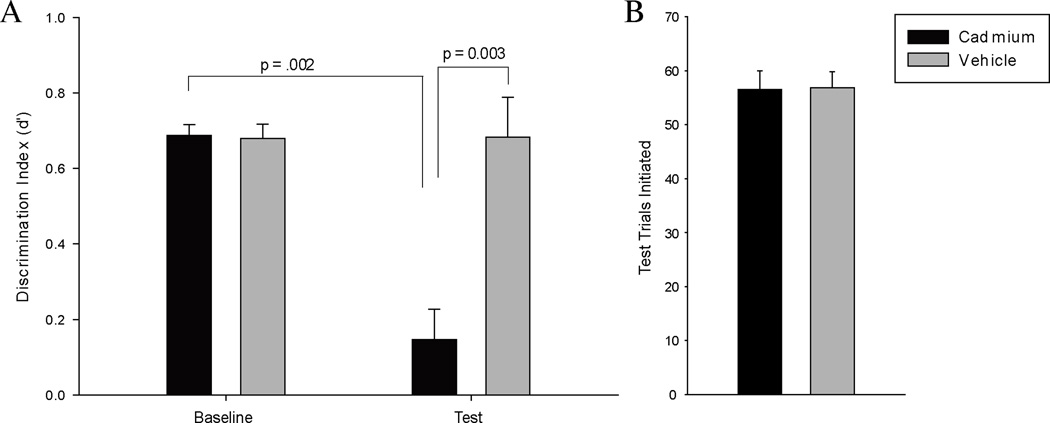
To investigate the functional and perceptual significance of the cadmium-induced pathophysiology, we evaluated the effects of intranasal cadmium exposure on mice trained to perform an odor detection task. Eleven wild type C57Bl/6 mice were successfully trained to perform a go-no go operant conditioning task in which the presentation of an odorant cued the availability of a liquid reward in a nearby reward port, while the absence of an odorant signaled that entry into the reward port would trigger a time-out punishment that delayed the next potential reward. Performance on the task was scored using the d’ metric, calculated as the fraction of odor trials in which the mouse correctly entered the reward port (“hits”) minus the fraction of no-odor trials in which the mouse incorrectly entered the reward port (“false alarms”). After reaching a criterion d’ of 0.5 (equivalent to 75% correct) for three consecutive days, each mouse was randomly assigned to receive a bilateral intranasal infusion of either 20 µg CdCl2 or vehicle. Mice were returned to the operant task on the second day after the infusion, and their detection performance was recorded.
As shown in Fig. 4A, intranasal exposure to cadmium chloride reduced performance on the detection task from an average pre-instillation d’ of 0.69 ± 0.03 to an average of 0.15 ± 0.08, while vehicle infusion had no effect. A mixed model ANOVA on the results (test day within subjects, instillation group between subjects) revealed the hypothesized test day by instillation group interaction (F = 10.43, p = 0.01, ηp2= 0.54) and simple main effects of instillation group (F = 25.38, p = 0.001, ηp2 = 0.74) and test day (F = 10.24, p = 0.011, ηp2 = .53). To follow up on the interaction, an independent samples t-test revealed that the average baseline discrimination index of the cadmium animals was not significantly different (p = 0.87) from the baseline average performance of control animals. On test day after instillation, the average d’ of the cadmium-treated animals was significantly lower (p = 0.003) than the average performance of control animals indicating impaired odor detection. This represents a significant reduction in cadmium-treated animal’s odor detection performance on test day compared to their own average baseline performance (paired t-test, p = 0.002). Vehicle instilled animals did not show this reduction on test day compared to baseline (paired t-test, p = 0.985). The performance of the cadmium-infused animals was not significantly different from zero (one-sample t-test, p = 0.11), demonstrating a complete lack of discrimination between odorant-present and odorant-absent cues.
Figure 4. Performance on an olfactory detection task approaches zero in mice receiving intranasal cadmium instillations.
(A) Performance cadmium- and vehicle-exposed mice on an odorant detection task before and after cadmium chloride instillations. (B) Average number of odorant detection trials initiated during the test session two days after instillations.
In principle, our experimental design controls for global changes in motivation because each odor discrimination trial only occurs when the mouse initiates it by nose-poking into the odorant-delivery port. We also observed no significant differences in the number of trials initiated by cadmium-infused mice compared to vehicle controls (independent samples t-test, p = 0.96), see Fig. 4B. Because the cadmium-infused mice were just as engaged in the discrimination task but entirely failed to discriminate despite being punished for wrong answers, we conclude that they were no longer able to detect the odorant.
4. Discussion
In these experiments, we demonstrated that intranasal instillations of cadmium chloride induced significant reductions in odorant-evoked neurotransmitter release from the olfactory nerve in vivo at doses of 0.2, 2 and 20 µg. At the highest dose, this reduction was accompanied by a reduction in axonal projections from the olfactory epithelium to the olfactory bulb and by the complete abolition of odorant discrimination in a psychophysical detection task. These results demonstrate the utility of optical imaging in the olfactory system to assess the pathophysiological effects of neurotoxicant exposure in the intact animal. They also have implications for estimates of the neurotoxic effects of cadmium and maximum safe occupational exposures.
To our knowledge, this is the first use of optical imaging to assess the pathophysiological effects of an environmental toxicant on neural function in vivo. This method is sensitive, revealing robust effects with a cadmium chloride dose 1/2000th of that previously reported to be effective using purely histological and behavioral techniques (Bondier et al., 2008). Even within our own study, the imaging experiments revealed a significant effect of cadmium at a hundredfold lower dose than the histological examination of ORN projections patterns within the same animals. The left-right symmetry of the olfactory system permits powerful within-subjects designs that can potentially demonstrate toxicant effects using smaller numbers of subjects than conventional between-subjects assays. Transport via the olfactory nerve is a candidate vector for neurotoxicants in a range of clinical disorders, including Parkinson’s disease (Costello et al., 2009), chronic solvent-induced neuropathy (Visser et al., 2008), and Alzheimer’s disease (Calderón-Garcidueñas et al., 2008), so exposure-induced changes in rodent olfactory physiology is a clinically-relevant model. Imaging from the mouse olfactory system may also be convenient for exploring the mechanisms of action of neurotoxicants in vivo, even those that are not typically exposed via aerosols. In this study we confined ourselves to visualizing transmitter release from the olfactory nerve (Bozza et al., 2004), but tools also exist for visualizing calcium dynamics in ORNs (Wachowiak and Cohen, 2001), glutamate reuptake in the olfactory glomerulus (Gurden et al., 2006), oxygen metabolism in the olfactory glomerulus (Lecoq et al., 2009), olfactory bulb hemodynamics (Tiret et al., 2009), and interneuron activity (Homma et al., 2009; Garaschuk and Konnerth, 2010) in vivo in this system, as appropriate to the toxicant and hypothesis. Using a genetically-encoded indicator, we have previously demonstrated repeated imaging from the same mouse over a period of a week or more (Bozza et al., 2004), which may permit even more powerful within-subjects designs for studying chronic toxicant exposures. Overall, these methods may have broad applicability within neurotoxicology.
Intranasal instillation of cadmium significantly reduced the amplitude of odorant evoked neurotransmitter release from the olfactory nerve (Fig. 2B and C), but the mechanisms underlying this effect remain to be elucidated. Cadmium has been shown to reduce action-potential evoked calcium influx in presynaptic terminals, consequently reducing glutamate release in hippocampal synapses (Wang et al., 2008a). We have previously characterized the strongly non-linear relationship between presynaptic calcium influx and glutamate exocytosis at ORN synapses (Wachowiak et al., 2005), suggesting that even modest reductions in calcium conductance could produce disproportionately large decreases in transmitter release. However, mesencephalic trigeminal neurons exposed to the same concentration of cadmium exhibited the opposite response, such that cadmium caused an increase in intracellular calcium concentrations that led in turn to necrosis (Yoshida, 2001). These conflicting results suggest that the effects of cadmium on intracellular calcium dynamics may depend not only on concentration but also on the particular complement of calcium conductances in the class of neurons under study. Further work is needed to explore whether the cadmium-induced pathophysiology and sensory impairments derive from a peripheral action (such as the disruption of olfactory transduction), a disruption of presynaptic function, or some other mechanism.
Our results showed not only a broad decrease in the amplitude of olfactory nerve input to the olfactory bulb, but also dose-dependent reductions in the number of glomeruli receiving that input (see Fig. 2A). Because each glomerulus receives projections from a molecularly-defined subset of ORNs, this suggests that ORNs expressing different olfactory receptors may be differentially sensitive to cadmium’s effects. We are unaware of any evidence linking cadmium susceptibility to G-protein coupled receptors. However, with a larger panel of odorants to identify the odorant-preference “fingerprint” of each glomerulus (Soucy et al., 2009; Wachowiak and Cohen, 2001) it may be possible to determine whether cadmium is preferentially disrupting the same set of glomeruli in each mouse. If so, then reverse-transcriptase polymerase chain reaction of gene products from individual glomeruli (Oda et al., 2006) should permit the identification of the specific olfactory receptors that are expressed in the most vulnerable ORNs, potentially yielding valuable insight into cadmium’s mechanism of action. Moreover, because the chemical identity of the odorant is represented by the differential pattern of odorant responses across ORNs expressing different odorant receptors (Malnic et al., 1999), it is even imaginable that selective destruction of a subset of ORN types could underlie the surprising increase in parosmia (a pathological change in the perceptual quality of odorants) reported after workplace exposure to aerosolized cadmium (Rydzewski et al., 1998).
The current study used acute intranasal instillations of cadmium chloride and found significant pathophysiological effects with doses as small as 0.2 µg. However, the most clinically relevant exposures typically occur via chronic inhalation of cadmium aerosols from industrial fumes, with an OSHA eight-hour Permissible Exposure Limit of 5 µg/m3, and via cigarette smoking, which exposes the smoker to about 1.7 µg of cadmium per cigarette of which about 10% is inhaled (ATSDR, 2008). We did not initially undertake an aerosolized exposure in part because the left-right within-subjects design is not possible with an aerosol, but further work in that vein could better test the consequences of such exposure for olfactory neurophysiology. We also note that the impairment in olfactory neurophysiology reported here with modest single doses of cadmium is potentially consistent with the impaired olfactory function observed in human smokers (Frye et al., 1990).
Acknowledgements
This work was supported by grant R00 DC009442 from the National Institute on Deafness and Other Communication Disorders to JPM, grants P30 ES005022 and T-32 ES07148 from the National Institute of Environmental Health Sciences, and a grant from the Busch Biomedical Research fund to JPM. We thank Matt Wachowiak, in whose laboratory the C57Bl/6 background OMP-spH mice were developed, and Jason Richardson and Ken Reuhl for their advice throughout this project.
References
- Adams RG, Crabtree N. Anosmia in alkaline battery workers. Br J Ind Med. 1961;18:216–221. doi: 10.1136/oem.18.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agency for Toxic Substances & Disease Registry. Toxicological profile for cadmium.. Chemical Abstract Service Registry No.7440-43-49. Atlanta, GA: Division of Toxicology and Environmental Medicine; 2008. [Google Scholar]
- Baker H, Genter MB. The Olfactory System and the Nasal Mucosa as Portals of Entry of Viruses, Drugs, and Other Exogenous Agents into the Brain. In: Doty RL, editor. Handbook of 13 Olfaction and Gustation. 2nd edition. New York: Marcel Dekker; 2003. pp. 909–950. [Google Scholar]
- Bondier JR, Michel G, Propper A. Harmful effects of cadmium on olfactory system in mice. Inhalation Toxicology. 2008;20(13):1169–1177. doi: 10.1080/08958370802207292. [DOI] [PubMed] [Google Scholar]
- Bozza T, McGann JP, Mombaerts P, Wachowiak M. In vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron. 2004;42:9–21. doi: 10.1016/s0896-6273(04)00144-8. [DOI] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode for odor receptors: A molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Oshiro WM, Samsam TE, Benignus VA, Krantz QT, Kenyon EM. A Dosimetric Analysis of the Acute Behavioral Effects of Inhaled Toluene in Rats. Toxico. Sci. 2007;99:181–189. doi: 10.1093/toxsci/kfm146. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Solt AC, Henríquez-Roldán C, Torres-Jardón R, Nuse B, Herritt L, Villarreal-Calderón R, Osnaya N, Stone I, García R, Brooks DM, González-Maciel A, Reynoso-Robles R, Delgado-Chávez R, Reed W. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain-barrier, ultrafine particulate deposition, and accumulation of amyloid {beta}-42 and {alpha}-synuclein in children and young adults. Toxicol Patho. 2008;l36:289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- Chow RH. Cadmium block of squid calcium currents. J of Gen Physiol. 1991;98:751–770. doi: 10.1085/jgp.98.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson’s disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol. 2009;169:919–926. doi: 10.1093/aje/kwp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman DC, Brenneman KA, McElveen AM, Lynch SE, Robers KC, Wong BA. Olfactory transport: A direct route of delivery of inhaled manganese phosphate to the rat brain. J Toxicol Environ Health. 2002;A65:1493–511. doi: 10.1080/00984100290071630. [DOI] [PubMed] [Google Scholar]
- Doty RL. The olfactory system and its disorders. Seminars in Neurology. 2009;29:74–81. doi: 10.1055/s-0028-1124025. [DOI] [PubMed] [Google Scholar]
- Evans J, Hastings L. Accumulation of Cd(II) in the CNS depending on the route of administration: Intraperitoneal, intratracheal, or intranasal. Fundam of Appl Toxicol. 1992;19:275–278. doi: 10.1016/0272-0590(92)90161-a. [DOI] [PubMed] [Google Scholar]
- Frye RE, Schwartz BS, Doty RL. Dose-related effects of cigarette smoking on olfactory function. JAMA. 1990;263:1233–1236. [PubMed] [Google Scholar]
- Garaschuk O, Konnerth A. In vivo two-photon calcium imaging using multicell bolus loading. Cold Spring Harbor Protocols. 2010 doi: 10.1101/pdb.prot5482. [DOI] [PubMed] [Google Scholar]
- Genter MB, Kendig EL, Knutson MD. Uptake of materials from the nasal cavity into the blood and brain: Are we finally beginning to understand these processes at the molecular level? Ann NY Acad Sci. 2009;1170:623–628. doi: 10.1111/j.1749-6632.2009.03877.x. [DOI] [PubMed] [Google Scholar]
- Gurden H, Uchida N, Mainen ZF. Sensory-evoked intrinsic optical signals in the olfactory bulb are coupled to glutamate release and uptake. Neuron. 2006;52:335–345. doi: 10.1016/j.neuron.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Hastings L. Sensory neurotoxicology: use of the olfactory system in the assessment of toxicity. Neurotoxicol and Teratol. 1990;12:455–459. doi: 10.1016/0892-0362(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Hastings L, Evans JE. Olfactory primary neurons as a route of entry for toxic agents into the CNS. Neurotoxicology. 1991;12:707–714. [PubMed] [Google Scholar]
- Hastings L, Miller ML. Influence of Environmental Toxicants on Olfactory Function. In: Doty RL, editor. Handbook of Olfaction and Gustation. 2nd edition. New York: Marcel Dekker; 2003. pp. 951–980. [Google Scholar]
- Homma R, Baker BJ, Jin L, Garaschuk O, Konnerth O, Cohen LB, Bleau CX, Canepari M, Djurisic M, Zecevic D. Wide-field and two-photon imaging of brain activity with voltage- and calcium-sensitive dyes. Methods Mol Biol. 2009;489:43–79. doi: 10.1007/978-1-59745-543-5_3. [DOI] [PubMed] [Google Scholar]
- Kauer JS, Moulton DG. Responses of olfactory bulb neurones to odour stimulation of small nasal areas in the salamander. J Physiol (Lond) 1974;243:717–737. doi: 10.1113/jphysiol.1974.sp010773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Margolis FL. Immunological studies of the rat olfactory marker protein. J Neurochem. 1975;24:110–116. doi: 10.1111/j.1471-4159.1975.tb03883.x. [DOI] [PubMed] [Google Scholar]
- Lecoq J, Tiret P, Najac M, Shepherd GM, Greer CA, Charpak S. Odor-evoked oxygen consumption by action potential and synaptic transmission in the olfactory bulb. J Neurosci. 2009;29:1424–1433. doi: 10.1523/JNEUROSCI.4817-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- Mascagni P, Consonni D, Bregante G, Chiappino G, Toffoletto F. Olfactory function in workers exposed to moderate airborne cadmium levels. Neurotoxicology. 2003;24:717–724. doi: 10.1016/S0161-813X(03)00024-X. [DOI] [PubMed] [Google Scholar]
- McGann JP, Pirez N, Gainey MA, Muratore C, Elias AS, Wachowiak M. Odorant representations are modulated by feedback but not lateral presynaptic inhibition of olfactory sensory neurons. Neuron. 2005;48:1039–1053. doi: 10.1016/j.neuron.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Monti-Graziadei GA, Margolis FL, Harding JW, Graziadei PP. Immunocytochemistry of the olfactory marker protein. J Histochem Cytochem. 1977;25(12):131–136. doi: 10.1177/25.12.336785. [DOI] [PubMed] [Google Scholar]
- Oda Y, Katada S, Omura M, Suwa M, Yoshihara Y, Touhara K. Odorant receptor map in the mouse olfactory bulb: in vivo sensitivity and specificity of receptordefined olfactory glomeruli. Neuron. 2006;52:857–869. doi: 10.1016/j.neuron.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Rose CS, Heywood PG, Costanzo RM. Olfactory impairment after chronic occupational cadmium exposure. J Occup Med. 1992;34:600–605. [PubMed] [Google Scholar]
- Rydzewski B, Sułkowski W, Miarzyñska M. Olfactory disorders induced by cadmium exposure: a clinical study. Int J of Occup Med and Environ Health. 1998;11:235–245. [PubMed] [Google Scholar]
- Shipley MT. Transport of molecules from nose to brain: Transneuronal anterograde and retrograde labeling in the rat olfactory system by wheat agglutinin-horseradish peroxidase applied to the nasal epithelium. Brain Res Bull. 1985;15:129–142. doi: 10.1016/0361-9230(85)90129-7. [DOI] [PubMed] [Google Scholar]
- Slotnick B, Bodyak N. Odor discrimination and odor quality perception in rats with disruption of connections between the olfactory epithelium and olfactory bulbs. J Neurosci. 2002;22:4205–4216. doi: 10.1523/JNEUROSCI.22-10-04205.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick B, Bodyak N, Davis BJ. Olfactory marker protein immunohistochemistry and the anterograde transport of horseradish peroxidase as indicies of damage to the olfactory epithelium. Chem Senses. 2001;26:605–610. doi: 10.1093/chemse/26.6.605. [DOI] [PubMed] [Google Scholar]
- Soucy ER, Albeanu DF, Fantana AL, Murthy VN, Meister M. Precision and diversity in an odor map on the olfactory bulb. Nat Neurosci. 2009;12:210–220. doi: 10.1038/nn.2262. [DOI] [PubMed] [Google Scholar]
- Sułkowski W, Rydzewski B, Miarzyñska M. Smell impairment in workers occupationally exposed to cadmium. Acta Oto-Laryngol. 2000;120:316–318. doi: 10.1080/000164800750001161. [DOI] [PubMed] [Google Scholar]
- Sun TJ, Miller ML, Hastings L. Effects of Inhalation of cadmium on the rat olfactory system: Behavior and morphology. Neurotoxicol Teratol. 1996;18:89–98. doi: 10.1016/0892-0362(95)02013-6. [DOI] [PubMed] [Google Scholar]
- Sunderman FW., Jr Nasal toxicity, carcinogenicity, and olfactory uptake of metals. Ann Clin Lab Sci. 2001;31:3–24. [PubMed] [Google Scholar]
- Tiret P, Chaigneau E, Lecoq J, Charpak S. Two-photon imaging of capillary blood flow in olfactory bulb glomeruli. Methods in Mol Biol. 2009;489:81–91. doi: 10.1007/978-1-59745-543-5_4. [DOI] [PubMed] [Google Scholar]
- Tjälve H, Henriksson J. Uptake of metals in the brain via olfactory pathways. Neurotoxicology. 1999;20:181–195. [PubMed] [Google Scholar]
- Tjälve H, Henriksson J, Tallkvist J, Larsson BS, Lindquist NG. Uptake of manganese and cadmium from the nasal mucosa into the central nervous system via olfactory pathways in rats. Pharmacol Toxicol. 1996;79:347–356. doi: 10.1111/j.1600-0773.1996.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Visser I, Lavini C, Booij J, Reneman L, de Boer AGEM, Wekking EM, de Joode EA, van der Laan G, van Dijk FJH, Schene AH, Den Heeten GJ. Cerebral impairment in chronic solvent-induced encephalopathy. Ann Neurol. 2008;63:572–580. doi: 10.1002/ana.21364. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron. 2001;32:723–735. doi: 10.1016/s0896-6273(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, McGann JP, Heyward P, Shao Z, Puche AC, Shipley M. Inhibition of olfactory receptor neuron input to olfactory bulb glomeruli mediated by suppression of presynaptic calcium influx. J Neurophysiol. 2005;94:2700–2712. doi: 10.1152/jn.00286.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Shih YL, Ko WC, Wei YH, Shih CM. Cadmium-induced autophagy and apoptosis are mediated by a calcium signaling pathway. Cell Mol Life Sc. 2008a;65:3640–3652. doi: 10.1007/s00018-008-8383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Hu P, Wang HL, Wang M, Chen JT, Tang JL, Ruan DY. Effects of Cd(2+) on AMPA receptor-mediated synaptic transmission in rat hippocampal CA1 area. Toxicol Lett. 2008b;176:215–222. doi: 10.1016/j.toxlet.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Yoshida S. Re-evaluation of acute neurotoxic effects of Cd2+ on mesencephalic trigeminal neurons of the adult rat. Brain Res. 2001;892:102–110. doi: 10.1016/s0006-8993(00)03240-6. [DOI] [PubMed] [Google Scholar]