Abstract
Object
The pathogenesis of cervical spondylotic myelopathy (CSM) is related to both primary mechanical and secondary biological injury. The authors of this study explored a novel, noninvasive method of promoting neuroprotection in myelopathy by using curcumin to minimize oxidative cellular injury and the capacity of omega-3 fatty acids to support membrane structure and improve neurotransmission.
Methods
An animal model of CSM was created using a nonresorbable expandable polymer placed in the thoracic epidural space, which induced delayed myelopathy. Animals that underwent placement of the expandable polymer were exposed to either a diet rich in docosahexaenoic acid and curcumin (DHA-Cur) or a standard Western diet (WD). Twenty-seven animals underwent serial gait testing, and spinal cord molecular assessments were performed after the 6-week study period.
Results
At the conclusion of the study period, gait analysis revealed significantly worse function in the WD group than in the DHA-Cur group. Levels of brain-derived neurotrophic factor (BDNF), syntaxin-3, and 4-hydroxynonenal (4-HNE) were measured in the thoracic region affected by compression and lumbar enlargement. Results showed that BDNF levels in the DHA-Cur group were not significantly different from those in the intact animals but were significantly greater than in the WD group. Significantly higher lumbar enlargement syntaxin-3 in the DHA-Cur animals combined with a reduction in lipid peroxidation (4-HNE) indicated a possible healing effect on the plasma membrane.
Conclusions
Data in this study demonstrated that DHA-Cur can promote spinal cord neuroprotection and neutralize the clinical and biochemical effects of myelopathy.
Keywords: myelopathy, docosahexaenoic acid, curcumin, membrane, spinal cord injury, rat
Cervical spondylotic myelopathy is a potentially debilitating disorder caused by both primary mechanical and secondary biological SCI. Its surgical management has undergone significant evolution in the past few decades, improving our ability to address the primary mechanical injury. Despite advancements in spinal instrumentation and operative technique, however, neurological recovery can still be relatively limited in many patients following surgery.
One of our greatest current challenges in treating patients with CSM is an inability to directly treat the insidious secondary biological injury that ubiquitously occurs with this disorder. It is likely that this barrier is primarily responsible for the lack of meaningful neurological recovery in some patients after surgery as well as the propagation of SCI and progression of disease in those treated nonoperatively Thus, there is a distinct need to develop therapies and treatment algorithms that directly address the secondary biological injury associated with chronic SCI and that can enhance repair processes following nonoperative treatment or surgical decompression.
In recent years there has been increasing interest in the influence of dietary factors on specific molecular systems and mechanisms within the CNS. In particular, the omega-3 fatty acid DHA has shown therapeutic potential based on its effects in reducing inflammation and providing structural material to plasma membranes and on its effects on overall neuronal function.3 In turn, curcumin possesses strong antiinflammatory and antioxidant capacity20, 23 such that the combined actions of DHA and curcumin could be a potential strategy to counteract the biological injury encountered in CSM.
Most attention has been directed toward the brain, and a number of studies have shown the benefit of dietary supplementation on cerebral function, such as memory and learning, as well as in enhancing the neural repair of damage caused by pathological processes, including Alzheimer disease,4 epilepsy,8 and traumatic brain injury.20, 23 In contrast, comparatively little information has been published regarding the use of dietary supplementation to enhance neural repair in disorders affecting the spine, and this represents a fertile area for study. The goal of the present study was to investigate a novel, noninvasive method of promoting neuroprotection in an animal model of myelopathy by using the combined application of DHA and curcumin.
Methods
Experimental Design
Twenty-seven male Sprague-Dawley rats (Charles River) weighing between 200 and 250 g were housed 2–3 per cage and maintained in environmentally controlled rooms (22°–24°C) with a 12-hour light/dark cycle. After 1 week of acclimation, the rats were randomly assigned to 3 groups consisting of 9 animals each: spinal compression and a diet supplemented with saturated fats designed to replicate a Western society diet9 (62% saturated fats, 34% sucrose; D12079B, Research Diets, Inc.; WD group), spinal compression and a diet (102566, Dyets, Inc.) supplemented with DHA (1.2%, Nordic Naturals, Inc.) and curcumin (500 ppm, Sigma Corp.; DHA-Cur group), and intact with a standard diet (control group). Spinal compression animals underwent placement of a thin epidural nonresorbable polymer in the midthoracic spine, designed to induce a delayed neurological deficit. The assigned diets were administered to the rats after the surgery, and the animals were allowed to feed ad libitum for 6 weeks. Examiners of the molecular and functional testing were blinded to the treatment arms.
Experiments were performed in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. The UCLA Chancellor’s Animal Research Committee approved all procedures.
Surgical Procedures
The surgical procedures were performed under sterile conditions. Animals were given an analgesic (Buprenex) subcutaneously to alleviate pain associated with the procedure. Rats were maintained in a deep anesthetic state with isoflurane gas throughout the surgery. The caudal-most ribs were palpated, and the midthoracic region was localized. Under a magnification loupe (× 3.5), a 1.5-cm midline incision was created and a self-retaining retractor was placed. The spinous processes were identified, and 2 adjacent vertebral levels were further skeletonized. The spinous process of the superjacent level was grasped with a clamp and gently elevated to open the intralaminar space. At this point, a needle-nose rongeur was used to create a bilateral partial laminectomy. Hemostasis was established, and the spinal canal and dura mater were identified. At this point a precut 2 × 4 × 1–mm piece of dry expandable polyvinyl alcohol sponge (Merocel, Medtronic, Inc.) was gently inserted through the intralaminar defect and seated beneath the cephalad lamina. Care was taken to insert the sponge in an atraumatic fashion without causing a neurological deficit. The polyvinyl alcohol sponge expands to approximately 3 times its size after absorbing liquid. The sponge was kept dry until seated in the epidural space. The self-retaining retractors were then removed, and the skin was closed with 4–0 nylon sutures. The rat was injected with analgesics and saline and then recovered on a warming pad.
Magnetic Resonance Imaging Procedure
Three animals underwent thoracic spine MRI 2 weeks postoperatively to obtain radiographic confirmation that spinal canal compression was induced by the model (Fig. 1). A high-field, small-bore Bruker MRI Bio-Spec 70/30 (Bruker BioSpin) 7-T magnet was used. It has a 30-cm bore with Avance electronics and is controlled by ParaVision 5.0 operating software. Images were obtained using a 166-mm BGA gradient system and a 60-cm-volume transceiver.
Fig. 1.
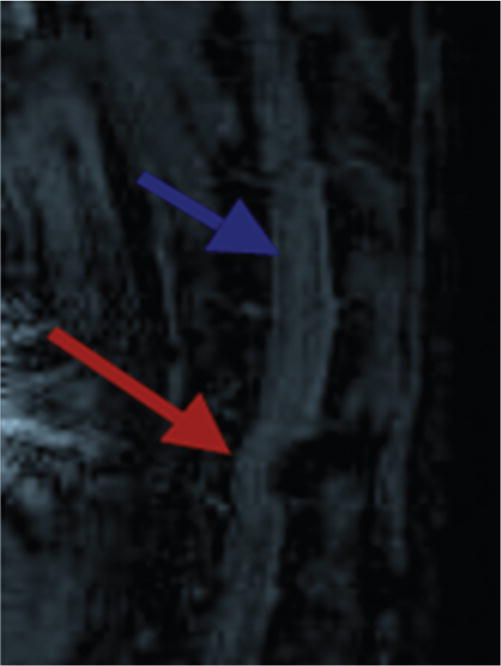
Sagittal thoracic MR image showing spinal cord compression caused by the posteriorly placed sponge (red arrow). The blue arrow indicates an area of normal spinal cord caliber rostral to the area of compression.
Behavioral Testing
CatWalk Test
Prior to surgery the animals underwent baseline gait testing utilizing the CatWalk automated gait analysis system (Noldus Information Technology). The apparatus is made of a 1.3-m-long glass plate with dim fuorescent light beamed into the glass from the side. In a darkened environment (below 1 lux of illumination), the light is reflected downward, and when the animal’s paws come in contact with the glass surface, images of the footprints are recorded by the camera under the walkway (Fig. 2). The images from each trial were converted into digital signals and processed with a threshold set at 30 au (range 0–225 au), meaning that all pixels brighter than 30 au were used. The imaging setting used in this study defined 1 pixel as 0.085 mm. Rats were subjected to 3 consecutive runs in each test. Data were collected in the computer and analyzed using CatWalk XT software.
Fig. 2.
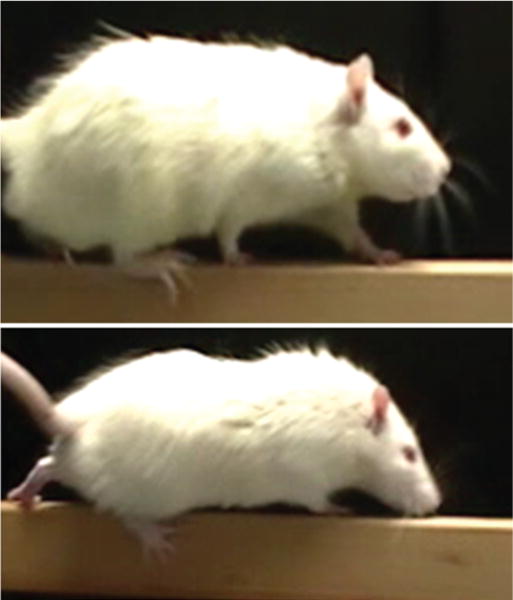
Upper: Photograph of an animal from the DHA-Cur group showing feet appropriately placed on the balance beam while walking during behavioral testing. Lower: Photograph of an animal from the WD group demonstrating a foot fault with the right hind leg slipping over the side of the beam.
Beam-Walk Test
The beam-walk test was performed as previously described.25 This test was used to evaluate the fine motor coordination of all animals. The device consists of a narrow wooden beam 10 mm wide and 180 mm in length and is suspended 400 mm from the table-top. Rats were videotaped as they walked both directions of the beam. The foot faults (missing or grasping the sides of the beam) over 50 steps were counted in either direction on the beam.
Molecular Assessment
All rats were euthanized 42 days after the surgical procedure, even the intact ones. The midthoracic region (approximately 5 mm cephalad and caudal to the indented region of spinal cord where the sponge was placed) and the lumbar enlargement region were dissected and saved at −70 C° for protein analysis. Protein extracts were prepared in lysis buffer (137 mM NaCl; 20 mM Tris-HCl, pH 8.0; 1% NP-40; 10% glycerol; 1 mM phenylmethylsulfonyl fluoride; 10 mg/ml aprotinin; 1 mg/ml leupeptin; and 0.5 mM sodium vanadate). Homogenates were processed in a centrifuge, and supernatants were collected. Total proteins were quantified according to the MicroBCA procedure (Pierce).
Twenty-five micrograms of protein from each sample was used for Western blot analysis. The membranes were incubated overnight with the following primary antibodies: anti-BDNF (1:1000, Santa Cruz Biotechnology), anti–4-HNE (1:1000), antisyntaxin-3 (1:1000), and antiactin (1:2000) used as an internal standard. Secondary antibodies were anti–goat or anti–rabbit immunoglobulin G–horseradish peroxidase (Santa Cruz Biotechnology). After rinsing with buffer (0.1% Tween-20 in TBS), immunocomplexes were visualized by chemiluminescence using the Amersham ECL Plus Western blotting detection kit (GE Healthcare Life Sciences) according to the manufacturer’s instructions. Film signals were digitally scanned using a Hewlett-Packard scanner (HP Scanjet 3970) and quantified with NIH Image software, normalized for actin levels.
Statistical Analysis
All statistical analyses were performed using SPSS 16.0 software (SPSS, Inc.). Group differences were considered significant at p < 0.05. Results were expressed as the mean percent of intact control values and represent the mean ± standard error of the mean for protein measurements. All data were analyzed with 1-way ANOVA. Post hoc tests were conducted using Bonferroni comparisons.
Results
Behavioral Assessment
The CSM model was successful in creating a delayed neurological deficit. In the WD group, worsening gait function was initially observed at the postoperative Day 21 time point. This worsening was manifested by an increase in foot faults while attempting to ambulate on the beam walk, which were not observed immediately postoperatively. Animals fed the diet supplemented with DHA and Cur had fewer foot faults than those fed the WD (Fig. 2 and Videos 1 and 2).
Video 1. Clip of an animal from the WD group demonstrating significant gait difficulty while walking across the beam at the postoperative Day 42 time point. Click here to view with Media Player. Click here to view with Quicktime.
Video 2. Clip of an animal from the DHA-Cur group am bulating across the beam without any foot faults at the postoperative Day 42 time point. Click here to view with Media Player. Click here to view with Quicktime.
The gait decline was confirmed further by using CatWalk XT software data analysis. The WD group demonstrated significant worsening in specific ambulation parameters, such as cadence (p < 0.02), mean stepping intensity (p < 0.03; Fig. 3), and print width (p < 0.04; Fig. 4), at Day 42 as compared with baseline (Table 1). The gait decline in this group became significantly worse than baseline at the postoperative Day 21 time point and continued to be significantly worse throughout the remainder of the study. In contrast, there was no significant difference in the baseline and postoperative Day 42 gait parameters for the DHA-Cur animals. Additionally, although the 2 groups started with statistically equivalent performance in these parameters, the DHA-Cur group had significantly better gait function than the WD group at the final postoperative Day 42 time point (p < 0.05 ).
Fig. 3.
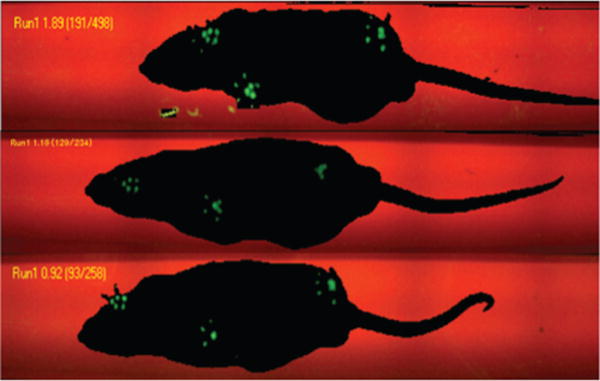
Noldus CatWalk image showing gait patterns of representative animals from each of the 3 groups. The print area size and stepping intensity fluorescence of the right hind leg are similar in the control (upper) and DHA-Cur groups (lower) but significantly less in the WD group (center).
Fig. 4.
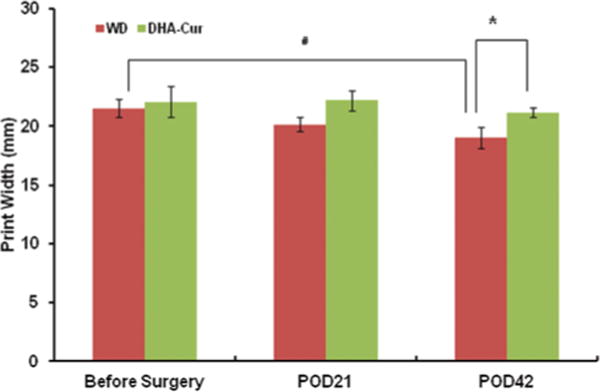
Bar graph showing print width data obtained using the Noldus CatWalk software. There is statistically significant worsening between the print width baseline and postoperative Day 42 (POD42) in the WD group (p < 0.04) but not in the DHA-Cur group. There is a significant difference between the WD and DHA-Cur groups on POD42 (p < 0.04).
TABLE 1.
Effect of diet on gait spatial parameters
| Cadence (step/second)
|
Mean Intensity (au)
|
Print Width (mm)
|
||||
|---|---|---|---|---|---|---|
| Group | Before Surgery | Postop Day 42 | Before Surgery | Postop Day 42 | Before Surgery | Postop Day 42 |
| Western diet | 6.92 ± 1.04 | 4.08 ± 0.59* | 94.25 ± 6.05 | 80.33 ± 1.58* | 21.49 ± 0.76 | 19.05 ± 0.90* |
| diet along w/DHA + Cur | 6.89 ± 0.47 | 5.84 ± 0.79† | 96.96 ± 4.22 | 98.09 ± 2.41† | 22.06 ± 1.32 | 21.14 ± 0.40† |
Intragroup comparison for Western diet before surgery versus postoperative Day 42 shows significant difference in all 3 parameters.
Intergroup comparison for Western diet versus DHA-Cur diet at postsurgery Day 42 shows significant difference in all 3 parameters.
Molecular Assessment
Effects of Diet in the Thoracic Region
The 4-HNE levels in the thoracic region at the site of compression were significantly elevated in the WD group (180% of intact animals) as compared with those in both the intact animals (p < 0.01) and the rats that had received DHA-Cur (89% of intact animals, p < 0.01), indicating severe cellular membrane damage. In comparison, rats in the DHA-Cur group had thoracic spinal cord 4-HNE levels that were not significantly different from those in the intact control animals (p > 0.05; Fig. 5A). Syntaxin-3 levels in the thoracic region were significantly lower in the WD group (61% of intact) than in both the control group (p < 0.01) and the DHA-Cur group (91% of intact, p < 0. 05 ). There was no statistical difference in syntaxin-3 levels between the DHA-Cur and intact groups (Fig. 5B). Thoracic spinal cord BDNF levels were significantly lower in the WD group (79% of intact) than in both the intact group (p < 0.05) and the DHA-Cur group (106% of intact, p < 0.05). The thoracic spinal cord BDNF levels in the DHA-Cur group were not significantly different from those in the intact control animals (p > 0.05; Fig. 5C).
Fig. 5.
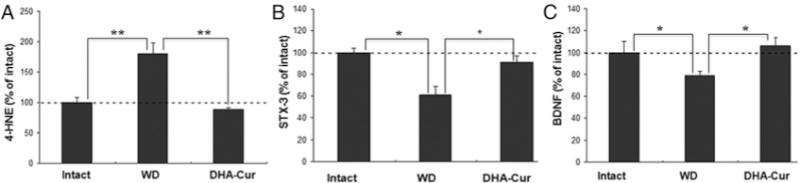
Bar graphs showing results of thoracic region molecular analysis. A: The 4-HNE levels in the thoracic region at the site of compression were significantly elevated in the WD group as compared with both the intact and the DHA-Cur groups (**p < 0.01). B: Syntaxin-3 (STX-3) levels were significantly lower in the WD group than in both the intact group (p < 0.01, larger asterisk at left) and the DHA-Cur group (p < 0.05, smaller asterisk at right). C: Spinal cord BDNF levels were significantly lower in the WD group than in both the intact group and the DHA-Cur group (*p < 0.05).
Effects of Diet in the Lumbar Enlargement Region
The 4-HNE levels in lumbar enlargement were also significantly higher in the WD group (154% of intact) than in both the intact control group (p < 0.05) and the DHA-Cur group (93% of intact, p < 0.05; Fig. 6A). There was no significant difference in lumbar 4-HNE levels between the DHA-Cur group and the intact group (p > 0.05). The lumbar syntaxin-3 levels were significantly lower in the WD group (67% of control) than in both the intact group (p < 0.05) and the DHA-Cur group (112% of control, p < 0.05). There was no significant difference in syntaxin-3 levels between the DHA-Cur group and the intact group (p > 0.05; Fig. 6B). The lumbar enlargement BDNF levels were significantly lower in the WD group (65% of control) than in both the intact group (p < 0.05) and the DHA-Cur group (108% of control, p < 0.05). There was no significant difference in BDNF levels between the DHA-Cur group and the intact group (p > 0.05; Fig. 6C).
Fig. 6.
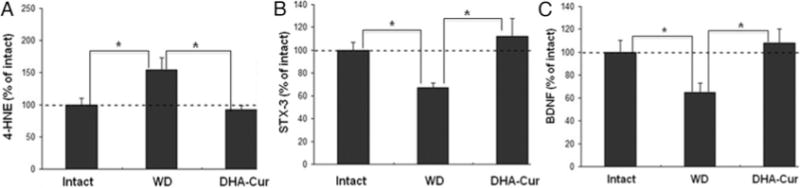
Bar graphs showing results of lumbar enlargement molecular analysis. A: The 4-HNE levels in the region of lumbar enlargement were significantly higher in the WD group than in both the intact and the DHA-Cur groups (*p < 0.05). B: Syn-taxin-3 levels were significantly lower in the WD group as compared with both the intact and the DHA-Cur group (*p < 0.05). C: Lumbar enlargement BDNF levels were significantly lower in the WD group than in both the intact group and the DHA-Cur group (*p < 0.05).
Discussion
Dietary Supplementation
In recent years, there has been rapidly increasing interest in the utility of dietary supplementation to augment CNS function. Most attention has been directed at the brain, and, in fact, many adults regularly ingest supplemental nutrients to enhance important brain functions such as memory and cognition. Some of the most commonly used nutritional supplements for this purpose are the omega-3 fatty acids, which have been demonstrated to upregulate genes critical for maintaining synaptic function and plasticity in rodents.24 Of the omega-3 fatty acids, DHA has been one of the most used clinically and has been extensively studied in a variety of animal models. Docosahexaenoic acid is essential for CNS structure and function, as it is an important constituent of neuronal membrane phospholipids.6, 18 In addition, DHA reduces inflammation and improves neurotransmission.1, 6, 21
Curcumin is one of the principle components of the Indian curry spice turmeric and has been used medicinally for its antiinflammatory and antibacterial properties for centuries. Recent studies have also demonstrated antioxidant properties, and therapeutic applications of curcumin within the CNS have been described in the treatment of Alzheimer disease, subarachnoid hemorrhage,22 and ischemic stroke.14 As with DHA, the neuroprotective effects of curcumin have been predominantly studied in the brain, where it has been shown to promote neural repair by restoring energy homeostasis that is critical for synaptic function.23
Cervical Spondylotic Myelopathy Secondary Biological Injury and the Role of BDNF
Our injury model reduced levels of BDNF protein while DHA-Cur dietary treatment counteracted these effects. The described actions of neurotrophins to limit neurological injury and enhance neural repair have a potentially strong application in the treatment of CSM. One of the main characteristics of the secondary neuronal injury in chronic SCI is the decreased concentration of critical axonal growth factors that could enhance functional spinal circuit repair. Of the key growth factors involved in neural repair, BDNF has demonstrated particular promise, as it has been shown to have important roles in axonal regeneration, promoting neuronal survival, regulating synapse formation and stabilization, increasing dendritic complexity, and enhancing synaptic efficacy.15 Brain-derived neurotrophic factor has also been shown to suppress the delayed apoptosis of oligodendrocytes after SCI in a rodent model.13 Apoptotic oligodendrocyte cell loss occurs in the evolution of CSM, and the extent of this loss correlates with the severity of neurological injury.11 Even more importantly, the powerful action of BDNF in promoting neuronal excitability is crucial to enhance neuronal function and the transmission of information across the synapse.
Kasahara et al.10 progressively compressed the spinal cords of rats by placing a thin expanding polymer sheet under the T-7 lamina. Parallel cohorts underwent laminectomy with removal of the polymer at 6, 9, and 12 weeks postcompression, were euthanized, and underwent histopathological examination. In the nonlaminectomy group, BDNF protein levels were elevated 6 weeks after compression but declined after 12 weeks. The decompressive procedure was effective in preventing neuronal loss in the 6-week group but was not effective in the 9- and 12-week groups. The authors suggested that the initial upregulation of BDNF expression probably served as a compensatory mechanism to protect against neural injury and that the decreasing level of BDNF may be one of the mechanisms of compression-induced spinal cord neuronal death.
These findings were supported in part by Xu et al.,26 who evaluated adenovirus-mediated BDNF (AdV-BDNF) gene transfer in and around the area of mechanical compression in the twy/twy hyperostotic mouse model. Retrograde spinal cord delivery of either AdV-LacZ or AdV-BDNF was performed via sternocleidomastoid injection, and 4 weeks later the upper cervical segments were harvested and subjected to pathohistological analysis. Immunoreactivity to BDNF and the number of surviving anterior horn neurons were much higher in the AdV-BDNF cohort. The overall results seem to suggest a protective action of BDNF that mitigates the effects of chronic SCI.
Dietary Supplementation in Myelopathy
In the present study we used a model similar to that used by Kasahara et al.10 and Kim et al.;12 we placed a thin expandable nonresorbable polymer, polyvinyl alcohol, underneath the thoracic lamina of laboratory animals to induce delayed myelopathy with resultant neurological symptomatology. The polymer was carefully placed in an atraumatic fashion to ensure that an acute SCI was not induced. Development of the neurological deficit was principally related to the stretch injury across the narrowed segment during animal motion over time, akin to the etiology of dynamic myelopathy encountered in human CSM.5
The rationale for using curcumin and DHA to combat the secondary biological injury in myelopathy is severalfold. We have previously shown that dietary treatment using a curcumin derivative resulted in the elevation of spinal cord BDNF levels as compared with levels in animals not receiving the supplementation.25 Moreover, studies in vitro or in vivo have shown that DHA promotes the growth of CNS neurons by involving the action of BDNF.24 Thus, one of the postulated mechanisms of neural repair in the present study was to use curcumin and DHA to maintain levels of BDNF within the damaged spinal cord and potentiate its effects.
Additionally, oxidative membrane damage is a major cause of neuronal death and a reduction in the viability and function of remaining neurons after CNS injury.17 Plasma membranes are particularly susceptible to oxidative damage, which can significantly impair neurotransmission and cellular function. Docosahexaenoic acid is a major component of the phospholipidic structure of plasma membranes and has been demonstrated to reduce oxidative cellular injury following acute SCI.7 As free radical–mediated cell damage is a leading mechanism of SCI in CSM,11, 27 we hypothesized that curcumin and DHA could have substantial beneficial effects in limiting oxidative membrane injury and promoting neural repair. Cellular membrane oxidative damage at the site of compression in this model was confirmed by the elevation of spinal cord 4-HNE levels in the animals that did not receive dietary supplementation. Neuronal injury has been associated with oxidative damage to membranes, which involves reactions of free radicals with double bonds of fatty acids that produce α,β-unsaturated aldehydes such as 4-HNE.19 Similarly, the 4-HNE levels were significantly higher within the lumbar enlargement in the animals that did not have dietary supplementation, indicating significant lipid peroxidation. The significance of the lumbar enlargement is that this is the location of the motor neurons and nerves fibers that innervate the hind limbs. Thus, injury to the primary corticospinal tract fibers also resulted in downstream cellular membrane damage within the lumbar enlargement.
We further analyzed the cellular membrane injury by measuring syntaxin-3 levels in the region of lumbar enlargement. This protein is localized to the presynaptic plasma membrane, which plays a crucial role in the docking and fusion of vesicles during neurotransmitter re-lease.16 Given that syntaxin-3 has been shown to be under the influence of DHA2 and is located in areas of synaptic contacts, it is a suitable marker to assess the effects of our dietary treatment on the plasma membrane. Accordingly, our data demonstrated significantly higher lumbar syntaxin-3 in the DHA-Cur group and, in conjunction with a reduction in lipid peroxidation (4-HNE), indicated a possible healing effect on the plasma membrane.
The animals that received DHA-Cur maintained significantly higher levels of spinal cord BDNF both at the level of compression and in the region of lumbar enlargement than those that did not receive dietary supplementation. Based on the aforementioned animal CSM studies and other laboratory investigations of BDNF, it is likely that the maintained levels of spinal cord BDNF, combined with decreased cellular membrane injury, were critical factors in the preserved neurological function observed in the DHA-Cur group.
Conclusions
There is a great need for the development of neural repair strategies to help neutralize the secondary biological injury encountered during both the nonoperative and operative treatment of patients with CSM. Dietary supplementation has proven to be successful in the treatment of a variety of pathological conditions throughout the CNS. In an animal model of myelopathy, we have demonstrated that DHA and curcumin can counteract the effects of chronic spinal cord compression through several molecular mechanisms, resulting in the preservation of neurological function.
Supplementary Material
http://mfile.akamai.com/21490/wmv/digitalwbc.download.akamai.com/21492/wm.digitalsource-na-regional/spine12-16_video_1.asx (Media Player).
http://mfile.akamai.com/21488/mov/digitalwbc.download.akamai.com/21492/qt.digitalsource-global/spine12-16_video_1.mov (Quicktime).
http://mfile.akamai.com/21490/wmv/digitalwbc.download.akamai.com/21492/wm.digitalsource-na-regional/spine12-16_video_2.asx (Media Player).
http://mfile.akamai.com/21488/mov/digitalwbc.download.akamai.com/21492/qt.digitalsource-global/spine12-16_video_2.mov (Quicktime).
Acknowledgments
The authors thank Mr. William Reeves and Mrs. Deborah Berger for their generous research support.
Disclosure
This work was supported by NIH Grant No. RO1 NS056413 and the Craig H. Neilsen Foundation.
Abbreviations used in this paper
- AdV-BDNF
adenovirus-mediated BDNF
- BDNF
brain-derived neurotrophic factor
- CSM
cervical spondylotic myelopathy
- Cur
curcumin
- DHA
docosahexaenoic acid
- SCI
spinal cord injury
- WD
Western diet
- 4-HNE
4-hydroxynonenal
Footnotes
Author contributions to the study and manuscript preparation include the following. Conception and design: Holly, Blaskiewicz, Ying, Gomez-Pinilla. Acquisition of data: Holly, Blaskiewicz, Wu, Feng, Ying. Analysis and interpretation of data: all authors. Drafting the article: all authors. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Holly. Statistical analysis: Wu, Feng, Ying.
References
- 1.Calderon F. Kim HY Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem. 2004;90:979–988. doi: 10.1111/j.1471-4159.2004.02520.x. [DOI] [PubMed] [Google Scholar]
- 2.Darios F, Davletov B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature. 2006;440:813–817. doi: 10.1038/nature04598. [DOI] [PubMed] [Google Scholar]
- 3.Gómez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008;9:568–578. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamaguchi T, Ono K, Yamada M. REVIEW: Curcumin and Alzheimer’s disease. CNS Neurosci Ther. 2010;16:285–297. doi: 10.1111/j.1755-5949.2010.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson FC, Geddes JF, Vaccaro AR, Woodard E, Berry KJ, Benzel EC. Stretch-associated injury in cervical spondylotic myelopathy: new concept and review. Neurosurgery. 2005;56:1101–1113. [PubMed] [Google Scholar]
- 6.Horrocks LA, Farooqui AA. Docosahexaenoic acid in the diet: its importance in maintenance and restoration of neural membrane function. Prostaglandins Leukot Essent Fatty Acids. 2004;70:361–372. doi: 10.1016/j.plefa.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Huang WL, King VR, Curran OE, Dyall SC, Ward RE, Lal N, et al. A combination of intravenous and dietary docosahexaenoic acid significantly improves outcome after spinal cord in jury. Brain. 2007;130:3004–3019. doi: 10.1093/brain/awm223. [DOI] [PubMed] [Google Scholar]
- 8.Jyoti A, Sethi P, Sharma D. Curcumin protects against electrobehavioral progression of seizures in the iron-induced experimental model of epileptogenesis. Epilepsy Behav. 2009;14:300–308. doi: 10.1016/j.yebeh.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103:59–68. doi: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasahara K, Nakagawa T, Kubota T. Neuronal loss and expression of neurotrophic factors in a model of rat chronic compressive spinal cord injury. Spine (Phila Pa 1976) 2006;31:2059–2066. doi: 10.1097/01.brs.0000231893.21964.f2. [DOI] [PubMed] [Google Scholar]
- 11.Kim DH, Vaccaro AR, Henderson FC, Benzel EC. Molecular biology of cervical myelopathy and spinal cord injury: role of oligodendrocyte apoptosis. Spine J. 2003;3:510–519. doi: 10.1016/s1529-9430(03)00117-7. [DOI] [PubMed] [Google Scholar]
- 12.Kim P, Haisa T, Kawamoto T, Kirino T, Wakai S. Delayed myelopathy induced by chronic compression in the rat spinal cord. Ann Neurol. 2004;55:503–511. doi: 10.1002/ana.20018. [DOI] [PubMed] [Google Scholar]
- 13.Koda M, Murakami M, Ino H, Yoshinaga K, Ikeda O, Hashimoto M, et al. Brain-derived neurotrophic factor suppresses delayed apoptosis of oligodendrocytes after spinal cord injury in rats. J Neurotrauma. 2002;19:777–785. doi: 10.1089/08977150260139147. [DOI] [PubMed] [Google Scholar]
- 14.Lapchak PA. Neuroprotective and neurotrophic curcuminoids to treat stroke: a translational perspective. Expert Opin Investig Drugs. 2011;20:13–22. doi: 10.1517/13543784.2011.542410. [DOI] [PubMed] [Google Scholar]
- 15.Maier IC, Schwab ME. Sprouting, regeneration and circuit formation in the injured spinal cord: factors and activity. Philos Trans R Soc Lond B Biol Sci. 2006;361:1611–1634. doi: 10.1098/rstb.2006.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMahon HT, Südhof TC. Synaptic core complex of synaptobrevin, syntaxin, and SNAP25 forms high affinity alpha-SNAP binding site. J Biol Chem. 1995;270:2213–2217. doi: 10.1074/jbc.270.5.2213. [DOI] [PubMed] [Google Scholar]
- 17.Merenda A, Gugliotta M, Holloway R, Levasseur JE, Alessandri B, Sun D, et al. Validation of brain extracellular glycerol as an indicator of cellular membrane damage due to free radical activity after traumatic brain injury. J Neurotrauma. 2008;25:527–537. doi: 10.1089/neu.2007.0359. [DOI] [PubMed] [Google Scholar]
- 18.Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 19.Sharma S, Ying Z, Gomez-Pinilla F. A pyrazole curcumin derivative restores membrane homeostasis disrupted after brain trauma. Exp Neurol. 2010;226:191–199. doi: 10.1016/j.expneurol.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma S, Zhuang Y, Ying Z, Wu A, Gomez-Pinilla F. Dietary curcumin supplementation counteracts reduction in lev els of molecules involved in energy homeostasis after brain trauma. Neuroscience. 2009;161:1037–1044. doi: 10.1016/j.neuroscience.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stillwell W, Shaikh SR, Zerouga M, Siddiqui R, Wassall SR. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod Nutr Dev. 2005;45:559–579. doi: 10.1051/rnd:2005046. [DOI] [PubMed] [Google Scholar]
- 22.Wakade C, King MD, Laird MD, Alleyne CH, Jr, Dhandapani KM. Curcumin attenuates vascular inflammation and cerebral vasospasm after subarachnoid hemorrhage in mice. Antioxid Redox Signal. 2009;11:35–45. doi: 10.1089/ars.2008.2056. [DOI] [PubMed] [Google Scholar]
- 23.Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp Neurol. 2006;197:309–317. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. 2008;155:751–759. doi: 10.1016/j.neuroscience.2008.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu A, Ying Z, Schubert D, Gomez-Pinilla F. Brain and spinal cord interaction: a dietary curcumin derivative counteracts locomotor and cognitive deficits after brain trauma. Neurorehabil Neural Repair. 2011;25:332–342. doi: 10.1177/1545968310397706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu K, Uchida K, Nakajima H, Kobayashi S, Baba H. Targeted retrograde transfection of adenovirus vector carrying brain-derived neurotrophic factor gene prevents loss of mouse (twy/twy) anterior horn neurons in vivo sustaining mechanical compression. Spine (Phila Pa 1976) 2006;31:1867–1874. doi: 10.1097/01.brs.0000228772.53598.cc. [DOI] [PubMed] [Google Scholar]
- 27.Yamaura I, Yone K, Nakahara S, Nagamine T, Baba H, Uchida K, et al. Mechanism of destructive pathologic changes in the spinal cord under chronic mechanical compression. Spine (Phila Pa 1976) 2002;27:21–26. doi: 10.1097/00007632-200201010-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
http://mfile.akamai.com/21490/wmv/digitalwbc.download.akamai.com/21492/wm.digitalsource-na-regional/spine12-16_video_1.asx (Media Player).
http://mfile.akamai.com/21488/mov/digitalwbc.download.akamai.com/21492/qt.digitalsource-global/spine12-16_video_1.mov (Quicktime).
http://mfile.akamai.com/21490/wmv/digitalwbc.download.akamai.com/21492/wm.digitalsource-na-regional/spine12-16_video_2.asx (Media Player).
http://mfile.akamai.com/21488/mov/digitalwbc.download.akamai.com/21492/qt.digitalsource-global/spine12-16_video_2.mov (Quicktime).


