Abstract
Scientific evidence based on neuroimaging approaches over the last decade has demonstrated the efficacy of physical activity improving cognitive health across the human lifespan. Aerobic fitness spares age-related loss of brain tissue during aging, and enhances functional aspects of higher order regions involved in the control of cognition. More active or higher fit individuals are capable of allocating greater attentional resources toward the environment and are able to process information more quickly. These data are suggestive that aerobic fitness enhances cognitive strategies enabling to respond effectively to an imposed challenge with a better yield in task performance. In turn, animal studies have shown that exercise has a benevolent action on health and plasticity of the nervous system. New evidence indicates that exercise exerts its effects on cognition by affecting molecular events related to the management of energy metabolism and synaptic plasticity. An important instigator in the molecular machinery stimulated by exercise is brain-derived neurotrophic factor, which acts at the interface of metabolism and plasticity. Recent studies show that exercise collaborates with other aspects of lifestyle to influence the molecular substrates of cognition. In particular, select dietary factors share similar mechanisms with exercise, and in some cases they can complement the action of exercise. Therefore, exercise and dietary management appear as a noninvasive and effective strategy to counteract neurological and cognitive disorders.
Introduction
A growing body of evidence supports the influence of exercise in vitality and function of the central nervous system (CNS) and promoting resistance against neurological disorders. According to these studies, exercise has the extraordinary capacity to enhance mental health, and current efforts are being devoted to use this capacity to reduce cognitive decay in aging and psychiatric disorders. In this article, we discuss recent studies in humans and animals substantiating the ability of exercise to promote cognitive health across the lifespan. It is our intention to have a comprehensive account about the effects of exercise in the brain through bridging the results of human studies with their underlying molecular mechanisms as studied in animals. We expect that this review can serve as basic logic for future studies in this important area of research. Innovations in neuroimaging have been critical to document the relationship between the cognitive gain and the activity of specific neural networks in the cerebral cortex and hippocampus in individuals who practice exercise. Recent advances in imaging of the human hippocampus stand to more directly bridge the gap between human and animal models (e.g., Pereira et al., 2007) (137). In turn, advances in the field of molecular biology have been important to elucidate some of the molecular mechanisms involved with the effects of exercise in animals. Even further, as discussed later in this article, exciting new evidence indicates that some of the influences of exercise in the brain can reach the genome with the potential to promote epigenetic modifications.
The understanding of the mechanisms by which exercise affects cognitive abilities has been nourished from several fronts. In particular, exercise has demonstrated an extraordinary aptitude to influence molecular pathways involved with synaptic function underlying learning and memory. Given the intrinsic relationship between exercise and energy metabolism, it is not that surprising that modulation of energy-related molecular systems seems a pivotal mechanism by which exercise affects synaptic plasticity and cognition. These findings harmonize with an emerging line of studies showing that the metabolism of energy at the cellular level is closely associated with regulation of synaptic plasticity and neuronal excitability. The story that is being unfolded is that proper energy metabolism involving the mitochondria is crucial for supporting neuronal signaling events through the plasma membrane. Within this territory, molecular systems such as those involving brain-derived neurotrophic factor (BDNF), which are at the interface of metabolism and synaptic plasticity, can play a crucial role on exercise-induced cognitive enhancement. Accordingly, we discuss the aptitude of exercise to impact molecular systems involved with metabolism and synaptic plasticity that can sustain mental function. These mechanisms play a crucial role in mediating exercise effects on aging and in neurodegenerative diseases.
Biological rationale for the effects of exercise on cognition
The brain has a remarkable capacity for modifying its structure and function according to the influences of the environment and experience. Physical activity has played one of the most vital roles during biological adaptation and survival of the species through thousands of years, in a process in which the modern brain was developed. During these events, exploration, defense, foraging as well as cognitive skills were tightly integrated to motor operations for survival. Thus, the hippocampus, a structure that has a fundamental role in memory processing is one of the main brain regions influenced by physical activity. In addition, development of brain regions that warrant energy efficiency such as the hypothalamus likely evolved with centers that control cognitive abilities, and this has introduced the concept of the “metabolic brain.” These adaptations enabled enough energy saving to support the development of a larger and more complex brain having the capacity to walk upright; all of these features obviously require coordination with cognitive strategies centered in successful survival. In this article, we first discuss evidence demonstrating the profound influence of exercise on cognitive function as assessed in human studies. Discussion will follow on the molecular mechanisms by which exercise affects cognitive function as gathered from animal studies.
Physical Activity, Brain, and Cognition in Humans
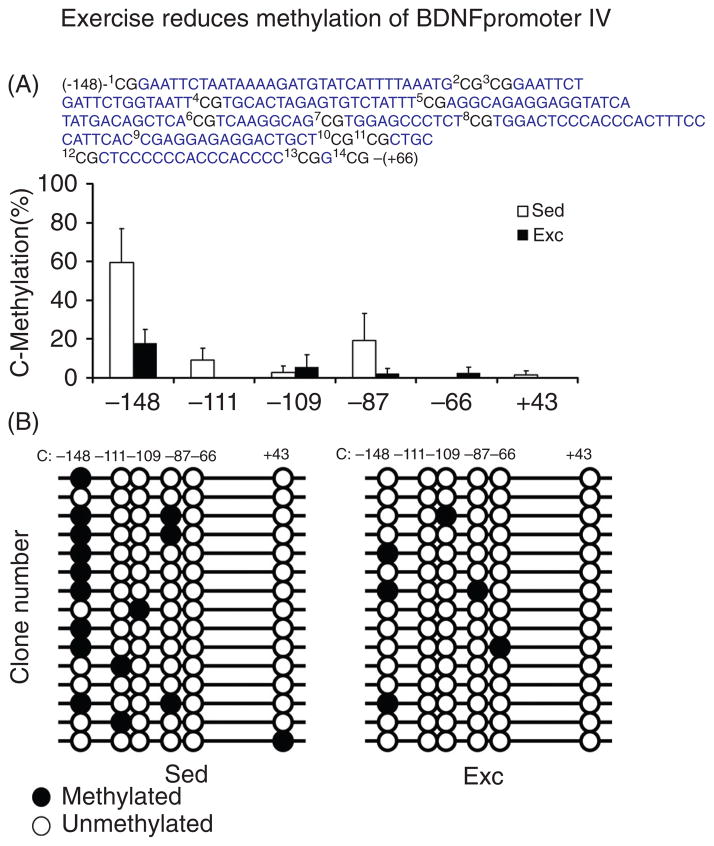
With recent innovations in neuroimaging, the physical activity-human cognition literature has pursued exercise-induced changes in networks involving the prefrontal cortex and associated cognitive processes. Studies in the hippocampus stand to more directly bridge the gap between the human and animal models. Recent studies have bridged this gap by imaging the hippocampus in older humans and examined differences in the volume of this structure and related cognitive performance as a function of aerobic fitness (16, 59, 60). Specifically, Erickson et al. (60) investigated 165 cognitively healthy older adults between 59 to 81 years had their cardiorespiratory fitness assessed via a maximal graded exercise test, and their hippocampal volume measured using functional magnetic resonance imaging (fMRI) during performance on a spatial memory task. Findings indicated that higher fitness was associated with larger bilateral hippocampal volume, and greater fitness and hippocampal volume were associated with better spatial memory performance (59). In addition, hippocampal volume partially mediated the association between fitness and spatial memory performance. Given that the hippocampus demonstrates disproportionately lager degradation during aging, these findings suggest that aerobic fitness may be an effective means for preventing age-related cortical decay and cognitive impairment (59). A second study (61), utilized a randomized control design with 120 older adults and found that the exercise treatment group increased their hippocampal volume by 2%. In addition, the exercise group demonstrated higher levels of serum BDNF and improvements in spatial memory. As expected, the control group did not demonstrate such changes in brain structure or cognitive performance; thus, providing definitive evidence to suggest that aerobic exercise training increases hippocampal volume during late life, and has positive implications for memory performance (61).
Another particularly compelling study (137) investigated the role of exercise on neurogenesis in the hippocampus using both human and mouse models. Specifically, two magnetic resonance imaging (MRI) studies were conducted, with the first study imaging cerebral blood volume in the hippocampal formation of exercising mice. The findings revealed that exercise selectively upregulated cerebral blood volume in the dentate gyrus, which is the only region of the hippocampus that has been observed to support adult neurogenesis; and further, these increases were found to correlate with measures of neurogenesis collected post mortem (137). In their second study, cerebral blood volume was measured in adult humans (mean age = 33 years) following 12 weeks of aerobic exercise training. Similar to the mouse model, exercise selectively influenced the dentate gyrus, with cerebral blood volume changes correlating with cardiorespiratory fitness changes (137). These findings were further supported by improved cognitive performance on the Rey Auditory Verbal Learning Test following the exercise intervention, which also correlated with changes in cardiorespiratory fitness and cerebral blood volume (137). Across species, these data suggest that within the hippocampus, the dentate gyrus is uniquely susceptible to exercise intervention, with an increase in exercise-relate behavior relating to neurogenesis. As described in more detail in the mechanisms section, BDNF has been related to hippocampal volume, which as noted, has implications for learning and memory. Specific to humans, circulating BDNF has been related to hippocampal volume, with aerobic fitness related to the upregulation of BDNF serum levels and greater hippocampal volume among older adults (3, 60, 61, 111). Lastly, recent work has begun to extend these intriguing findings to younger populations, with cross-sectional evidence suggesting that aerobic fitness relates to larger hippocampal volume (23) and better relational memory performance (24), during preadolescent childhood.
Historical prospective
Despite these initial advances in the understanding of how physical activity affects hippocampal structure and function, and memory, the human literature has mostly pursued exercise as a means to ameliorate or protect against the mal-adaptive effects of cognitive aging related to other aspects of cognition. Though sporadic publications have appeared on the topic of exercise and cognition since at least the 1930s (6, 18, 112, 138), Spirduso was the first to programmatically study the relation of physical activity to cognitive aging. Spirduso investigated the association of exercise to cognition in older adults, and suggested that aerobic exercise was beneficial to the initiation and execution of action [i.e., reaction time (RT); (5, 164, 165)]. However, no such relationship was observed in younger adults. Later, Etnier and her colleagues (62) conducted a systematic review of the exercise-cognition literature using meta-analytic technique, and suggested that physical activity had a small, but positive effect on perceptual, cognitive, and motor skills. As discussed below, advances in the understanding of how physical activity translates to cognitive processing in animals have been crucial for the acceptance of the concept that exercise can improve brain function.
Exercise during aging
More recently, Kramer and colleagues (109) extended these earlier findings through the examination of the effects of aerobic fitness training on older adults using a randomized control design. That is, 124 older adults between the ages of 60 and 75 years were randomly assigned to either a 6-month intervention of walking (i.e., aerobic training) or flexibility (i.e., nonaerobic) training. Results indicated that the walking group, but not the flexibility group, improved their performance (i.e., shorter RT) across a series of tasks that tapped different aspects of cognitive control, indicating that physical activity is beneficial to cognitive performance during aging (109).
Cognitive control refers to a subset of goal-directed, self-regulatory operations involved in the selection, scheduling, and coordination of computational processes underlying perception, memory, and action. Core cognitive processes collectively termed “cognitive control” or “executive control” include; inhibition, working memory, and cognitive flexibility (45). Inhibition (or inhibitory control) refers to the ability to override a strong internal or external pull to appropriately act within the demands imposed by the environment (42). Working memory refers to the ability to mentally represent information, manipulate stored information, and act upon it (42). Finally, cognitive flexibility refers to the ability to quickly and flexibly switch perspectives, focus attention, and to adapt behavior for the purposes of goal directed action (11, 42, 45).
Based on their earlier findings of aerobic training-induced changes in cognitive control, Colcombe and Kramer (30) performed a meta-analysis to examine the relationship between aerobic training and cognition in older adults between 55 and 80 years of age using data from 18 randomized exercise interventions that included control groups. Results showed that exercise training increased cognitive performance by half a standard deviation (which is considered to be a moderate effect) when compared to the pretest and the control group (30). Further, the most robust behavioral change following the intervention was observed for tasks requiring greater amounts of cognitive control compared to other cognitive tasks requiring smaller amounts of cognitive control. Their findings suggested that aerobic training is associated with general benefits in cognition that are selectively and disproportionately larger for tasks or task components requiring greater amounts of cognitive control. A second recent meta-analysis (162) corroborated Colcombe and Kramer’s (30) findings, in that aerobic exercise was related to attention, processing speed, memory, and cognitive control. It should be noted, however, that smaller effects were observed in some studies included in the meta-analyses. Accordingly, Hillman et al. (90) examined the relationship between self-reported physical activity and inhibition (one aspect of cognitive control) using a modified flanker task in 241 individuals between 15 and 71 years of age. Results indicated that increases in the amount of physical activity was related to decreases in response speed across conditions of the flanker task requiring variable amounts of inhibition, suggesting a generalized relationship between physical activity and response speed. In addition, physical activity was related to better accuracy for both congruent and incongruent trials in older adults, while no such relationship was observed for younger adults. Interestingly, this relationship was disproportionately larger for trials requiring greater amounts of inhibition in the older adults, suggesting that physical activity has both a general and selective association with task performance (90).
Exercise during childhood
Other data with school age children has corroborated the findings in adults and extended them to developing populations. Specifically, Sibley and Etnier (160) conducted a meta-analysis and found a positive relationship between physical activity and cognitive function in school age children (age 4–18 years), suggesting that physical activity may be related to cognition during development. Examination of the findings revealed that physical activity participation was related to cognitive performance along eight measurement categories (i.e., perceptual skills, intelligent quotient, achievement, verbal tests, mathematics tests, memory, developmental level/academic readiness, and other), with results indicating a beneficial relationship of physical activity on all cognitive categories, with the exception of memory (160). Although this effect was found for all age groups, it was stronger for children in the 4 to 7 and 11 to 13 year groupings, compared to the 8 to 10 and 14 to 18 year groupings.
Cross-sectional research has also supported the association between fitness and cognition during preadolescent development. An initial study examined 7 to 12-year-old children who completed a paper and pencil version of the Stroop Color-Word task, which requires cognitive control functioning among other cognitive processes, and the Fitnessgram; a field test of physical fitness (14). Results indicated that better performance on each of the three Stroop conditions (i.e., congruent, neutral, and incongruent) was associated with better performance on the aerobic capacity test (i.e., the number of laps run on the PACER test) of the Fitnessgram. That is, higher levels of aerobic fitness were associated with correctly naming more colors during each Stroop condition, independently of other factors such as age, gender, or intelligence. These initial findings suggested that increased levels of aerobic fitness may benefit cognitive processes underlying cognitive control during preadolescent development. Taken together, the data suggest a beneficial relationship between physical activity and behavioral indices of cognitive performance across the human lifespan.
One additionally interesting aspect of research is the translation of these physical activity and fitness effects on cognition to ecologically valid settings. School-age children provide an excellent means by which to examine this relationship in the real world, as performance on fitness tests and academic achievement tests are routinely assessed as part of school curriculum. A growing literature base is beginning to develop on this topic, with the available data indicating that fitness has either a positive relationship or no relationship to scholastic measures of cognition (22, 88, 176, 179). Regardless, the data suggest that time spent engaged in physical activities is beneficial as it does not detract from scholastic performance, and can in fact improve overall health and function.
Imaging the relation of fitness to brain and cognition
With advancements in neuroimaging techniques, the understanding of the effects of aerobic fitness on brain structure and function has rapidly advanced over the last decade. In particular, a series of studies (16,31–33,60,67,88,108,147,152) have been conducted on older humans to better understand the relation of aerobic fitness to brain and cognition. Normal aging results in the loss of brain tissue (31), with markedly larger tissue loss evidenced in the frontal, temporal, and parietal cortices (16, 58, 149). As such, cognitive functions subserved by these brain regions (such as those involved in cognitive control and memory) are expected to decay more dramatically than other aspects of cognition. Specifically, age-related decreases in gray matter volume have been associated with decrements in a variety of cognitive control processes. For instance, age-related differences in gray matter volume (brain tissue composed of neuronal cell bodies and supporting structures) of the frontal cortex were related to a greater number of preservative errors on the Wisconsin Card Sorting Test (150) and better memory encoding on the Auditory Verbal Learning Test (67). Decreases in gray matter volume may result from several factors including loss in the number of neurons, neuronal shrinkage, reduction in dendritic arborization, and alterations in glia (158). Further, decreases in white matter (brain tissue composed primarily of myelinated nerve fibers) volume, which represent changes in connectivity between neurons, also occur as a result of aging. Loss of white matter volume further relates to performance decrements on a host of cognitive tasks (41, 131, 167) and may result from the demyelination of axons, reducing the rapid and effective conduction of electrical signals through the nervous system. However, based on studies of non-human animals outlined within this paper, and the aerobic fitness-induced benefits to cognitive control observed in behavioral studies in humans, scientists have speculated that an active lifestyle may serve to spare age-related loss in regions of the brain that support top-down cognitive control. Interestingly, related research has posited similar benefits of exercise to cognition and brain health in a variety of special populations afflicted with various diseases including dementia (153), Alzheimer’s disease (94, 161), and schizophrenia (132).
Indeed, Colcombe and his colleagues (31) examined the relation of aerobic fitness to gray and white matter tissue loss using high resolution MRI in 55 healthy older adults between 55 to 79 years of age. They observed robust age-related decreases in tissue density in frontal, temporal, and parietal structures using voxel-based morphometry, a technique used to assess brain volume. Interestingly, substantial reductions in the amount of tissue loss in these structures were observed as a function of aerobic fitness (as measured via a cardiorespiratory stress test to assess maximal oxygen consumption) (31). Given that the brain structures most affected by aging also demonstrated the greatest fitness-related sparing, these initial findings provide a biological basis for aerobic fitness-related benefits to brain health during aging.
Additionally, Colcombe et al. (32) conducted a second study to examine the effect of aerobic fitness training on brain structure using a randomized control design with 59 sedentary healthy adults between 60 to 79 years of age. Approximately half the participants received a 6-month aerobic exercise (i.e., walking) intervention and the other half received a stretching and toning intervention. Twenty younger adults were also included to assess changes in brain structure over the course of a 6-month period, but did not participate in an exercise intervention. Results indicated that brain volume increased for both gray and white matter in adults who participated in the aerobic fitness training. However, no such findings were observed for those older adults in the nonaerobic training group or for the younger adult comprising the control group (32). Specifically, those assigned to the aerobic training group demonstrated increases in gray matter in the frontal lobes, including the dorsal anterior cingulate cortex (ACC), supplementary motor area, middle frontal gyrus, dorsolateral region of the right inferior frontal gyrus, and the left superior temporal lobe (32). White matter volume changes were also evidenced for the aerobic fitness group with increases in white matter tracts within the anterior third of the corpus callosum (32). These areas are important for cognition, as they have been implicated in top-down control of attention and memory processes. Importantly, these findings suggest that aerobic training not only spares age-related loss of brain structures but also may in fact enhance the structural health of specific brain areas.
Complementing the structural changes noted above, corroborating research has examined the relationship between aerobic fitness and functional changes in brain. That is, aerobic fitness training has also been observed to induce changes in patterns of functional activation using fMRI, such that it is possible to image activity in the brain while an individual is performing a task. This approach involves inferring changes in neuronal activity from alteration in blood flow or metabolic activity in the brain. Colcombe and his colleagues (33) examined the relation of aerobic fitness to brain and cognition across two studies with older adults. In the first study, 41 participants (mean age ~66 years) were divided into higher and lower fitness groups based on their performance on a car-diorespiratory stress test. In the second study, 29 participants (age range = 58–77 years) were recruited and randomly assigned to either a fitness-training (i.e., walking) or a control (i.e., stretching and toning) group. In both studies, participants were given a modified flanker task requiring variable amounts of inhibitory control. Results indicated that fitness (study 1) and fitness-training (study 2) was related to greater activation in the middle frontal gyrus and superior parietal cortex, regions involved in attentional control and inhibitory functioning (33). The authors concluded that increased recruitment of relevant brain regions for higher fit individuals may reflect an increase in the ability of the frontal attentional networks to bias task-related activation in the parietal cortex (33). In addition, they observed reduced activation in the rostral ACC in higher fit and aerobically trained older adults compared to their sedentary and untrained counterparts, respectively, indicating decreased behavioral conflict is related to increases in aerobic fitness (33). Importantly, these changes in patterns of activation were related to significant and substantial improvements in performance on the flanker task.
More recently, neuroimaging studies have employed a number of different approaches to understand functional connectivity among various brain regions and networks (17, 191, 192). Such approaches have been aimed at determining how the entire brain works during cognitive challenge and while deactivated (i.e., at rest or independent of specific cognitive demands). Specifically, functional connectivity using fMRI affords researchers a measure of temporal coherence between spatially remote regions of the brain. Although the default mode network (i.e., a large network including the posterior cingulate cortex, frontal medial cortex, and bilateral hippocampal and parahippocampal cortices) has been most widely studied relative to aerobic fitness, other large-scale networks including the frontal executive and fronto-parietal networks have also received attention (192). Such studies have demonstrated interesting effects of aerobic fitness on frontal, temporal, and parietal brain regions (191, 192), as well as a network involving the hippocampus and ACC (17), during rest. These results suggest that increased functional connectivity is related to better cognitive control and memory in older adults. Such an approach is promising, as it provides new insight into the role of aerobic fitness on brain function during aging.
Taken together, the findings across the human neuroimaging studies indicate that increases in aerobic fitness, derived from physical activity participation, is related to improvements in the integrity of brain structure and function, and may underlie improvements in cognition across tasks requiring top-down cognitive control. It should be noted that, to date, all neuroimaging research examining the role of fitness on brain and cognition has employed older adults. Future research endeavors need to consider earlier periods of the human lifespan to better determine the underlying mechanisms for the beneficial relation of fitness to behavioral performance in children and young adults. Regardless, these findings utilize some of the most sophisticated techniques available to image the human brain and have advance the knowledge base on the relation of physical activity to brain and cognition.
Fitness and brain electrophysiological activity
Although neuroimaging (i.e., MRI and fMRI) has been valuable in understanding aspects of the physical activity-cognition relationship due to the relatively high spatial resolution provided by these measures, information about the impact of fitness on neuronal activity has provided additional insight into the temporal magnitude for the effects of physical activity on cognition. First reported by Hans Berger in 1929, neural activity in the cerebral cortex and subcortical regions produces electrical potentials at the level of the scalp, and the electroencephalogram (EEG) can be recorded as a time series of the fluctuating voltages. That is, EEG activity is a recording of the difference in electrical potentials between various locations on the scalp. When electrodes are placed on the scalp, the EEG reflects activity of large populations of neurons firing in synchrony (96). Further, the dipoles from the individual neurons must be spatially aligned perpendicular to the scalp to be detected (114). Thus, EEG measurement is most likely to result from cortical pyramidal cells, which are aligned perpendicular to the cortex surface (114).
The recorded neuroelectric activity can be decomposed along the basic dimensions of frequency and amplitude. The amplitude of EEG, which is the topic of the current section, is measured in microvolts (μV) and is indicative of the relative size of the neuroelectric signal. Research on the temporal dynamics of the neuroelectric system has further focused on a class of EEG activity, known as event-related brain potentials (ERPs), which have been found to be particularly susceptible to physical activity and cardiorespiratory fitness. ERPs refer to a class of EEG activity that occurs in response to, or in preparation for, a stimulus or response (34). This neuroelectric activity is indicative of the synchronous activity of large populations of neurons (96), and may be classified as either exogenous (i.e., obligatory responses dependent upon the physical properties of the eliciting stimulus) or endogenous (i.e., higher order cognitive processes that often require active participation from the subject, but are independent of the physical properties of the stimulus environment) (96).
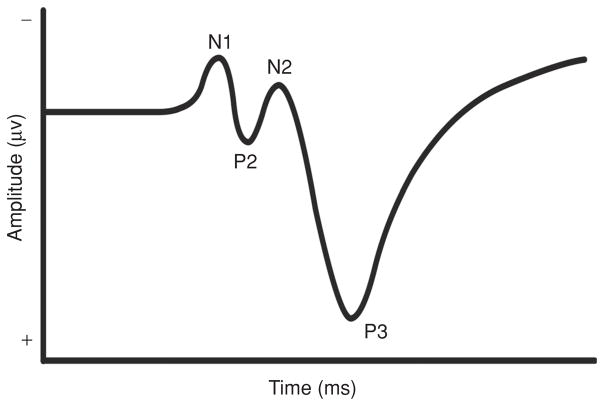
As indicated above, ERPs may be collected to either a stimulus or response. Stimulus-locked ERP components are described based on their polarity (i.e., positive or negative deflections in the EEG waveform) and ordinal position along the waveform. A standard ERP to a visual stimulus comprises a series of several components, with earlier components (P1, N1, and P2) relating to aspects of spatial attention and later components (N2 and P3) relating to aspects of cognitive function (35, 114). Response-locked ERP components, including the error-related negativity (ERN) and the error positivity (Pe) are thought to index neural correlates of action monitoring and error awareness, respectively.
Electrophysiological indices of action monitoring
Recent ERP investigations have complemented the neuroimaging studies described above. Specifically, one ERP component following error commission is the ERN (70) or Ne (64), which is a negative-going component observed in a response-locked averages of incorrect responses. It is maximal over fronto-central recording sites (i.e., FCz) (64, 70) and has been localized to the dorsal ACC using dipole localization techniques (44, 186), fMRI (21), and magneto-encephalography (120). Though the ERN is generally believed to reflect a cognitive learning mechanism used to correct an individual’s responses during subsequent environmental interaction, the specific functional significance of the ERN remains unresolved. Two distinct theories have suggested differential processes that relate to ERN activation. One theory, the reinforcement learning model (93), holds that the ERN measures error detection and monitoring (9,64,157). The other theory posits that the ERN reflects a conflict monitoring process (13) that is part of a system involving the ACC, with the purpose of detecting (or monitoring) levels of response conflict. Although there is debate about the nature of the ERN, there is consensus that the ACC is the primary structure driving the ERN signal (21, 120).
With regard to the relation of physical activity and fitness to the ERN component, several studies have been conducted in child and adult populations. Specifically, Themanson, Hillman, and Curtin (173) assessed the relationship between self-reported physical activity, ERN amplitude, and posterror task performance in older (60–71 years) and younger (18–21 years) adults during a task requiring variable amounts of cognitive control (i.e., task switching). Physical activity was assessed using the Yale Physical Activity Survey (50), and participants were instructed to respond as quickly as possible on each trial. The results indicated that older adults exhibited greater slowing of RT during task conditions requiring more extensive amounts of cognitive control and smaller ERN amplitude compared to younger adults. When physical activity was considered, decreases in ERN amplitude and greater response slowing following errors was found for older and younger physically active participants, relative to their sedentary counterparts (173). Given that posterror response slowing is a behavioral indicator of increased recruitment and implementation of additional top-down control to improve behavioral performance on subsequent environmental interactions (70, 103), the findings suggest increased top-down control among physically active individuals during tasks requiring action monitoring (173). This increase in control not only improves behavioral performance, but also decreases the conflict-related activation of action-monitoring processes, resulting in a more efficient neuroelectric profile, and increases behavioral adjustments used to correct behavior after error commission.
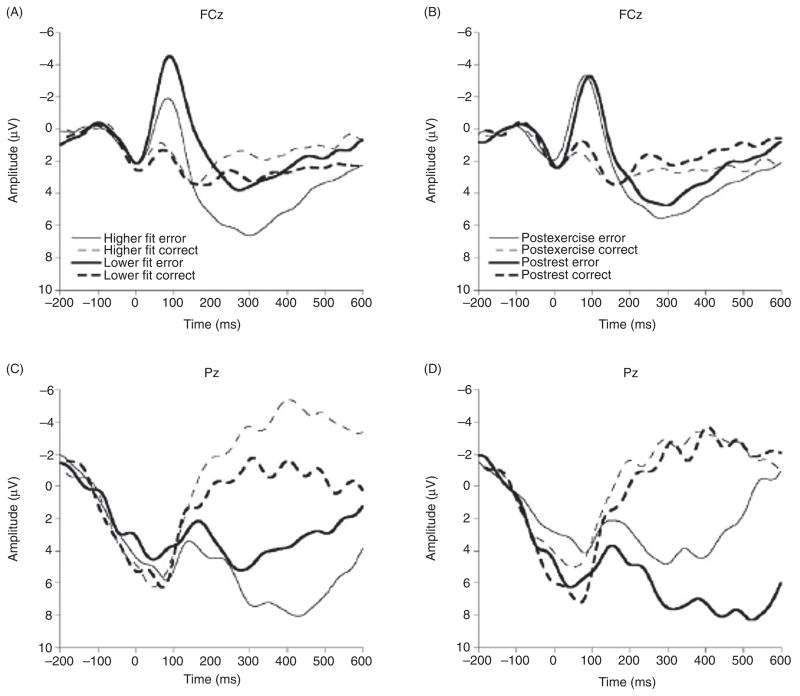
A second study examining the influence of cardiorespiratory fitness on action monitoring was conducted using two groups of young adults (mean age = 20.4 years) that exhibited large differences in their respective fitness levels (172). Neuroelectric (i.e., ERN) and behavioral (i.e., posterror slowing) indices of action monitoring were acquired during the completion of a flanker task requiring variable amounts of inhibitory control. Similar to the initial study (173), participants were instructed to respond as quickly as possible. Findings demonstrated a relationship between fitness level and indices of action monitoring, with the higher fit group exhibiting smaller ERN amplitude than the lower fit group (see Fig. 1). These data corroborate the initial study (173) and suggest that increases in aerobic fitness are associated with reduced activation of the neuroelectric index associated with action monitoring during error responses. Further, greater posterror response slowing was found for higher fit, relative to lower fit, individuals, suggesting an increase in both neural and behavioral posterror adjustments in top-down control (172). Recently, these findings have been extended to preadolescent children, who also demonstrated reductions in ERN amplitude and better task performance on speeded tasks with greater amounts of fitness (91).
Figure 1.
Grand averaged response-locked waveforms for subjects exposed to cardiorespiratory fitness (left side) and acute exercise (right side) showing error and correct trials at the FCz and Pz electrode sites. Reprinted, with permission, from reference (172), p. 763.
Collectively, findings from these studies are consonant with the concept that individuals with increased fitness and/or physical activity participation may exert greater levels of top-down control during task instructions requiring a rapid response. Such a response strategy appears to be associated with greater efficiency of the neuroelectric system (i.e., reduced ERN amplitude) designed to respond to indicants of task performance problems (172,173). These findings also are consonant with those of Colcombe and his colleagues (2004) who observed fitness-related reductions in ACC activation using fMRI, and suggest that fitness may enhance brain structure and function, resulting in benefits in cognitive function.
Recent evidence derived from young adults (18–25 years) has indicated that increased fitness may not only relate to more efficiency in action monitoring processes during rapid responding but also greater flexibility in the allocation of these processes during tasks requiring variable response strategies (174). Specifically, participants were instructed to perform a flanker task under conditions stressing either response speed or response accuracy. Prior research had indicated that ERN amplitude increases during accuracy, compared to speed, instructions due to motivational factors associated with enhanced importance or salience of errors (70, 79) or due to an increase in attentional focus (197). When cardiorespiratory fitness was considered, those individuals exhibiting higher levels of fitness demonstrated increased ERN amplitude and greater posterror accuracy during accuracy instructions relative to those exhibiting lower levels of fitness. Further, when the difference in ERN amplitude between the accuracy and speed conditions was assessed, higher fit participants indicated larger differences in both ERN amplitude and posterror accuracy, suggesting greater flexibility in the allocation of action monitoring resources as a function of task demands. This flexibility resulted in superior task performance as a function of fitness as well (174). Similar ERN and task performance findings were observed between higher and lower fit preadolescent children (9–10 years) using a task that manipulated the amount of cognitive control required, suggesting that fitness may benefit action monitoring processes across the lifespan (144).
Taken together, the findings of these investigations indicate that higher cardiorespiratory fitness is associated with greater flexibility in the allocation of action monitoring resources to meet desired outcomes. This titration of neuroelectric activation to meet task demands appears functional to the successful evaluation of action monitoring processes following error commission. Given that both superior task performance and alterations in task performance as a function of task demands were associated with greater levels of fitness, the data suggest that fitness may serve to enhance top-down control of processes to support the regulation of behavior. Accordingly, convergent evidence has emerged from multiple investigations (33,91,144,172–174) to suggest that fitness is beneficial to the ACC and the neural network subserving top-down control of action monitoring processes.
Electrophysiological indices of stimulus evaluation
Embedded within the stimulus-locked ERP potential (see Fig. 2), the P3 component has been among the most widely studied since it was first discovered by Sutton and his colleagues in the mid-1960s (168). The P3 (also referred to as the “P300” or “P3b”) is a positive deflection in an ERP that occurs approximately 300 to 800 ms after stimulus onset, depending on task complexity and individual characteristics of the participant. Because of its relation to aspects of information processing as described below, this endogenous component has captured considerable attention in the literature. Although the P3 may be recorded using a variety of paradigms, it has most commonly been observed during simple discrimination tasks (e.g., oddball task) in which individuals must discern between two stimuli that are randomly presented in a series. The participants’ task is to respond to one or both stimuli, which occur with differing probabilities (139).
Figure 2.
Characterization of a stimulus-locked event-related potential denoting the N1, P2, N2, and P3 components.
The precise neural structures giving rise to the P3 are unknown, because this component results from a network of neural circuitry and generators. However, discrimination tasks requiring attentional focus are thought to elicit frontal lobe activation (145, 146), with ERP (i.e., the P3a subcomponent) and fMRI studies demonstrating greater frontal lobe activity in response to infrequent or alerting stimuli, which are oftentimes uninstructed (139). The ACC is activated when working memory representations of the stimulus environment change, which in turn signals the inferior-temporal lobe for stimulus maintenance (i.e., memory storage). The P3 (i.e., P3b) component is elicited when attentional resources are allocated for subsequent memory updating after stimulus evaluation. This is thought to occur when memory storage operations that are initiated in the hippocampal formation are transmitted to the parietal cortex, which is typically where the P3 exhibits maximal amplitude in the scalp distribution (107, 166). Thus, the neuroelectric events that underlie the generation of the P3 occur from the interaction between frontal lobe and hippocampal/temporal-parietal function (107, 139) to modulate attentional control over the contents of working memory (139).
The P3 has been extensively examined to investigate aspects of information processing related to stimulus engagement. Specifically, the P3 is believed to reflect the allocation of attentional resources involved in working memory operations during context updating of the stimulus environment once sensory information has been analyzed (51, 52), the amplitude of the P3 component is, therefore, thought to reflect changes in the neural representation of the stimulus environment and is proportional to the amount of attentional resources needed to engage a given stimulus or task, with greater attentional allocation increasing P3 amplitude (141). The latency of the P3 component is a measure of stimulus evaluation or cognitive processing speed (53), with longer latencies reflecting increased processing time.
Physical activity and the P3 potential
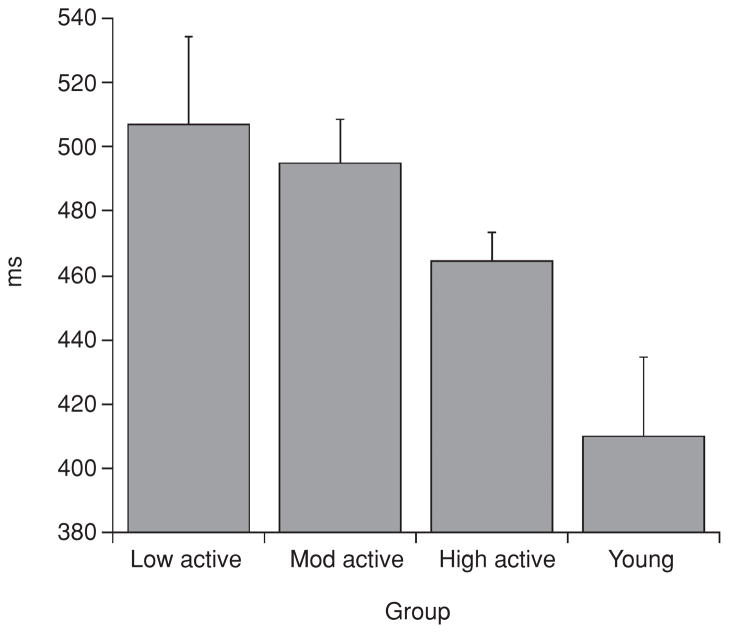
The P3 component was first observed to modulate as a function of aerobic fitness by Dustman and his colleagues (55), who compared older (50–62 years) and younger (20–31 years), high and low fit individuals using a Latin square design. Participants performed a graded exercise test (VO2max) to assess cardiorespiratory fitness and a visual oddball task to measure the P3 potential. Findings revealed that older high fit individuals had faster P3 latencies than their sedentary counterparts, and that their latencies did not differ from either young adults group (i.e., both high and low fit). No differences were observed between fitness groups for young adults (see Fig. 3). P3 amplitude was not reported. Given that P3 latency is believed to index cognitive processing or stimulus evaluation speed (Duncan-Johnson, 1981), the findings suggest that lower levels of fitness are detrimental to cognitive functioning during middle-to-older adulthood, due to delays in the speed of cognitive operations. These findings were the first to demonstrate that fitness had a relationship with the neural underpinnings involved in higher order cognitive function, and suggested that fitness may, in part, lead to sparing of cognitive aging. Since Dustman’s (55) seminal work, a number of findings have appeared in the literature that have better characterized this relationship and extended the knowledge base in several directions.
Figure 3.
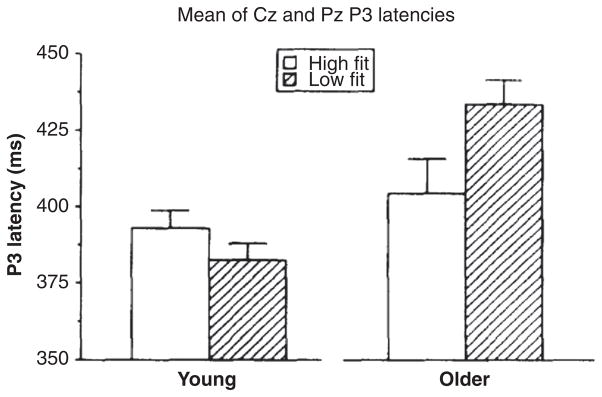
P3 latencies for young and older men undergoing low and high aerobic fitness. While P3 latency occurred significantly later for the older as compared to young men, the age effect was mostly due to very long P3 latencies of the older low fit men. Each mean was based on data for 15 subjects. The error bars are standard errors of the mean. Reprinted, with permission, from reference (55), p. 198.
P3 latency
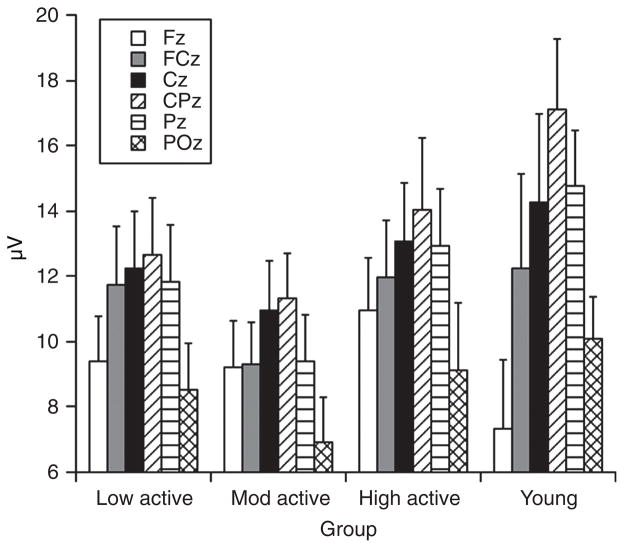
As a function of Dustman’s research (55, 56), matched with those of Spirduso and her colleagues’ (5, 164, 165) on task performance, the study of physical activity and P3 focused on aging populations to determine whether physical activity and/or fitness was a useful tool in minimizing the impact of aging on cognition. For example, Hillman and his colleagues (92) replicated the findings of Dustman et al. (55) by demonstrating selectively faster P3 latency for older higher fit compared to older lower fit individuals (60–70 years), with no such findings observed for lower fit groups (18–28 years). However, follow up work by Hillman and his colleagues (86) extended these findings in two meaningful ways. First, the relationship between physical activity and P3 latency were extended to the realm of cognitive control, as prior research employed stimulus discrimination tasks with relatively small cognitive control requirements. Accordingly, Hillman et al. (86) used a modified flanker task to determine the influence of physical activity on inhibition and observed that physical activity was related to faster cognitive processing speed, as indexed by shorter P3 latencies, across conditions requiring variable amounts of inhibition. Secondly, Hillman et al. (86) recruited low, moderate, and high active older adults (60–70 years) along with a control group consisting of younger adults (18–21 years) to investigate whether the relationship between physical activity and P3 latency was linear, such that greater amounts of physical activity were related to increases in processing speed. The findings suggested that this was indeed the case, as lesser amounts of physical activity were related to longer P3 latencies across the older groups. In addition, the most active of the older adult groups did not differ statistically from the younger adult control group, but they also did not differ from the moderately active group (see Fig. 4). As such, these preliminary data indicate that the relationship between physical activity and P3 latency may be linear in older adults, but additional research is necessary to determine the extent to which the relationship may be described by such a pattern.
Figure 4.
P3 latency by group across both conditions of the Eriksen flankers task. Reprinted, with permission, from reference (86), p. 180.
A second study from Hillman’s laboratory (89) corroborated the findings of physical activity influences on P3 latency using a different cognitive control task that taps inhibition, working memory, and cognitive flexibility. Specifically, older (60–70 years) and younger (18–21 years) physically active and sedentary adults performed a task-switching paradigm requiring them to perform a single task repeatedly or switch back and forth randomly between two tasks. The latter condition requires greater amounts of cognitive control as individuals must hold two rule sets in working memory, inhibit one rule set while performing the other, and flexibly switch between rule sets based on a cue. Results of the study indicated that, regardless of age, physical activity was related to faster P3 latency during the switch task, but not during the single item task. That is, physical activity related to faster cognitive processing speed only during condition requiring greater amounts of cognitive control (89). This pattern of results was the first to suggest that physical activity may influence cognitive processing speed during earlier periods of the human lifespan; however, it should be noted that not all researchers have observed this finding (159), though methodological differences between the paradigms may account for the disparate results.
Accordingly, studies of P3 latency have begun to investigate whether differences as a function of fitness may be evidenced during earlier periods in the human lifespan. Specifically, Kamijo and Takeda (100) investigated 40 young adults (mean age 21.1 years) to determine the role of physical activity on cognitive control using a spatial priming task. The findings indicated a larger negative priming effect on P3 and RT latency in the active, relative to the sedentary, group (100). Given that negative priming reflects inhibitory function to prevent interference from distracters on working memory, this pattern of results suggests greater inhibitory control was associated with physical activity.
Other work has investigated the role of fitness during even earlier periods of the lifespan, with several recent cross-sectional studies examining the potentially beneficial influence of fitness during preadolescent development (87, 88, 91, 144). Hillman and colleagues (87) used a visual oddball paradigm to investigate the role of fitness on P3 latency in young adults (18–21 years) and children (7–12 years). Regardless of age, more fit individuals exhibited faster P3 latency and shorter RT relative to their sedentary peers. Importantly, across age groups, the relationship of fitness to cognition was observed after controlling for age, intelligence, socioeconomic status, and body composition, factors known to have independent relationships with either fitness or cognitive function (87).
Taken together, with few exceptions (159), the available literature suggests a beneficial relation of physical activity and cardiorespiratory fitness to neuroelectric indices of cognitive processing speed (i.e., P3 latency), suggesting that this characteristic is related to better cognitive health and effective functioning. Although only a modest literature base is present at this time, there appears to be considerable consistency, with more activity and/or fitness relating to better cognitive performance. The P3 latency data suggest that one benefit stemming from participation in physical activity leading to higher fitness is a decrease in the time needed to capture information in the stimulus environment. Given this decreased time for stimulus engagement, it is likely that additional time is available for other cognitive operations occurring downstream from stimulus evaluation. As such, physical activity and fitness appear beneficial to stimulus capture, affording additional time for processes involved in decision making and response selection.
P3 amplitude
In addition to the beneficial relationship of physical activity to cognitive processing speed (i.e., P3 latency) described above, evidence also exists to suggest that the amount of attentional resources available for allocation toward the stimulus environment (i.e., P3 amplitude) are also benefited by physical activity. Polich, who has extensively investigated the P3, as well as factors influencing the modulation of the P3, was the first to indicate that physical activity participation was related to the size of the component amplitude. Specifically, Polich and Lardon (142) compared high (mean age = 30 years) and low (mean age = 34.7 years) physically active individuals using visual and auditory oddball paradigms to determine whether physical activity was related to the allocation of attentional resources during stimulus engagement. They observed larger P3 amplitude for high active relative to low active adults, indicating that physical activity indeed was related to greater attentional resources allocation during stimulus capture. Such a finding, though cross-sectional, spurred considerable interest in determining the relationship of physical activity on the neural underpinnings of cognitive functioning.
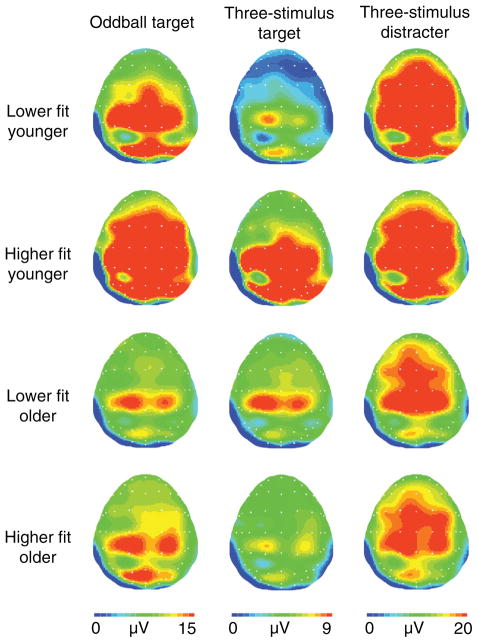
Following Polich’s (142) initial findings, other researchers have replicated the physical activity-P3 amplitude relationship, and extended it to include older adult populations using visual (118) and somatosensory (84) oddball tasks. More recently, Pontifex, Hillman, and Polich (143) assess the relationship of age and fitness to P3 amplitude during a three stimuli oddball task. This task provides the requisite discrimination between stimuli to elicit a P3 (i.e., P3b) component with a parietal scalp topography, but also includes rare, alerting stimuli (i.e., distracter stimuli) that are uninstructed. Such distracter stimuli elicit a frontal-central topographic P3a component due to the need to engage greater focal attention to orient within the stimulus environment (140). Accordingly, the three stimuli oddball task allows for a greater understanding of which aspects of attention may be influenced by cardiorespiratory fitness through the examination of the P3a and P3b components. Participants included older (60–70 years) and younger (18–22 years) adults bifurcated based on VO2max values, and two oddball tasks were provided to manipulate discrimination difficulty. Results indicated that under easy discrimination conditions, both older and younger high fit individuals exhibited larger P3b amplitude and shorter RT latency. However, under difficult discrimination conditions, only younger high fit participants displayed elevated P3b amplitude. Further, fitness was not found to modulate P3a amplitude (see Fig. 5) (143). Thus, the findings suggest selective influences of fitness on the attentional system such that it may be beneficial to the allocation of attentional resources, but not attentional orienting. Further, the findings also suggest that fitness may not protect against cognitive-aging during tasks requiring difficult visual discrimination, as only high fit young adults showed larger P3 amplitude during this condition (143). However, it is unknown at this time whether this null finding resulted from deficits in the visual system or an inability to allocate attentional resources in demanding visual environments.
Figure 5.
Topographical amplitude maps for the P3a and P3b components for each age and fitness from each stimulus. Note different voltage scales for each task. Reprinted, with permission, from reference (143), p. 384.
Beyond stimulus discrimination tasks, Hillman and colleagues have investigated the P3 potential in a series of studies involving individuals across the human lifespan. That is, two studies focusing on physical activity influences on older adults’ P3 amplitude during tasks requiring variable amounts of cognitive control have been pursued (86, 89). As described in the P3 latency section, two cross-sectional studies examining variable amounts of physical activity participation in older adults, with younger adult comparison groups, were undertaken to better understand the role of physical activity on neuroelectric indices of attention. In both studies, results indicated that greater amounts of physical activity were related to larger P3 amplitude, suggesting increased attentional resource allocation during stimulus engagement. In the Hillman et al. (86) study, increased P3 amplitude was observed only over frontal scalp sites in the moderate and high active older adults relative to the younger adult group (see Fig. 6). In the Hillman et al. (89) study, both older and younger high active adults demonstrated larger P3 amplitude relative to their sedentary counterparts. Further, the pattern of results suggested that high active older adults displayed larger P3 amplitude over frontal scalp sites and high active younger adults displayed larger amplitude over parietal scalp sites, with both groups exhibiting equivocally larger P3 amplitude over central sites, indicating that although physical activity was related to greater P3 amplitude across groups, the scalp topography differed as a function of age (89).
Figure 6.
Distribution of P3 amplitude across both conditions of the Eriksen flankers at midline sites by group. Reprinted, with permission, from reference (86), p. 181.
This frontal shift in scalp topography with aging is not surprising as multiple previous reports have demonstrated such a pattern of results for the P3 component. Researchers have used dipole-modeling techniques to demonstrate increased frontal lobe involvement during advanced aging (68). As such, age-related decline in processes reflected by P3 scalp distribution are suggestive of changes in cortical integrity. That is, the observed similarity across electrodes sites on the scalp (i.e., frontal shift) that accompany older adulthood may relate to the decreased efficiency of cognitive processes giving rise to the P3. Thus, it has been speculated that the frontal shift in the topographic distribution is indicative of some compensatory mechanism to aid older adults in meeting the demands of the imposed challenge. These aging differences are expected to emerge more frequently when greater amounts of cognitive control are necessary, due to the involvement of the frontal lobes in this aspect of cognition. However, when physical activity is considered, these age-related topographic shifts may not only reflect compensation, but rather more effective cognitive functioning, as older active adults demonstrated larger P3 amplitude over frontal sites (86,89) and better task performance (89) relative to their less active counterparts.
Interestingly, fitness also appears to benefit neuroelectric indices of cognitive function in preadolescent children. Several recent cross-sectional reports have demonstrated that P3 amplitude is modulated by cardiorespiratory fitness across tasks requiring both stimulus discrimination (87) and cognitive control (91, 144) such that higher fit preadolescent children exhibit larger P3 amplitude and better task performance than their lower fit peers. These findings are especially encouraging given that fitness has also been positively linked with academic performance, as outlined above. As such, future research might endeavor to examine potential links between fitness, basic neuroelectric measures of cognition, and school-based academic achievement.
Collectively, the literature-base examining the influence of physical activity and fitness on P3 amplitude is encouraging, with the vast majority of reports indicating a positive relation of these factors on neuroelectric indices of attentional resource allocation. Interestingly, the relation of physical activity/fitness to P3 amplitude has been demonstrated across the human lifespan with preadolescent children, young and older adults all indicating better cognitive function with greater amounts of activity or fitness. However, it remains an open question whether these relationships are governed by similar neural mechanisms across the lifespan, or whether differential effects of physical activity on brain may provide similar benefits in attention during different periods of the lifespan. In other words, the mechanisms that underlie the physical activity-cognition relationship are, at present, unclear and might differ as a function of ongoing developmental organization and age-related cortical decay. Future research should address whether the mechanisms that support the physical activity-cognition relationship differ across the lifespan.
Summary of physical activity and human cognition
The available data suggest that physical activity effectively improves cognitive health across the human lifespan. According to neuroimaging data, cardiorespiratory fitness not only spares age-related loss of brain tissue (i.e., gray and white matter) during aging but also appears to enhance the structural health of specific brain areas (31, 32). Importantly, the areas of brain most influenced by aerobic fitness are higher order regions involved in the control of cognition and memory. In addition, aerobic fitness has been shown to promote better functioning of brain, especially in neural networks involved in cognitive control of inhibition and attention (33). Related electrophysiological studies have provided convergent evidence to indicate functional improvements in cognition as a result of cardiorespiratory fitness or the adoption of a physically active lifestyle. From these data, a picture has emerged suggesting that more active or higher fit individuals are capable of allocating greater attentional resources toward the environment and process perceived information more quickly. Accordingly, fewer resources are required to monitor their actions for these individuals. On the other hand, less active and lower fit individual appear unable to allocate attentional resources during stimulus engagement, and thus require more resources to monitor their actions. These data are suggestive of differential cognitive strategies to respond effectively to an imposed challenge. Lastly, activity- and fitness-related differences in the neural underpinnings of cognition and action are meaningful because higher levels of activity and fitness promote more effective task performance.
Mechanisms Involved in the Effects of Exercise on Cognitive Function: Animal Studies
Overview of molecular mechanisms: Synaptic plasticity
Abundant progress has been achieved in the last decade unraveling the cellular and molecular mechanisms responsible for the influence of exercise enhancing cognition. It has become known that exercise activates the neural circuitry important for learning and memory using molecular systems associated with synaptic plasticity and energy metabolism. Exercise appears to enhance the process by which information is transmitted across cells at the synapse, in which select neurotrophic factors such as BDNF play a major role. For example, exercise influences the production of BDNF in the area vital for learning and memory, the hippocampus (78, 128, 189). As discussed below, BDNF possesses the extraordinary capacity to enhance neuronal excitability and synaptic plasticity by interacting with energy metabolism, thereby supporting cognitive abilities. BDNF seems to orchestrate the action of other neurotrophic factors, neurotransmitter systems, and hormones. As discussed below, the interactions between BDNF and insulin-like growth factor 1 (IGF-1), and subsequent effects on downstream effectors of synaptic plasticity, neurogenesis, and metabolism are crucial for the action of exercise on learning and memory (190). Even more recent information indicates that exercise may affect neuronal signaling across the synapse by influencing homeostasis of the plasma membrane.
Neurotrophin downstream pathways, synaptic plasticity, and learning and memory
Multiple gene analyses using microarray technology have been instrumental to delineate the impact of exercise on a large variety of molecular systems in the brain. These studies have shown that voluntary exercise elevated the expression of genes that belong to the categories of synaptic modulation and signal transduction (125). As discussed later in the text, multiple protein analyses have extended these results at the gene expression level to the protein level (48). The majority of the upregulated genes are members of synaptic trafficking machinery (synapsin I and II, synaptotagmin, and syntaxin); part of signaling transduction pathways (CaM-KII, MAP-K/ERK, I and II; protein kinase C, PKC-δ); or transcription factor cAMP response element binding protein (CREB). Microarray studies have shown that exercise also affected genes related to neurotransmitter systems, that is, exercise elevated the expression of genes related to the N-methyl-D-aspartate (NMDA) glutamatergic receptor system while downregulated genes related to the GABAergic system. It is notable that the action of the GABAergic system generally opposes the action of glutamate such that exercise seems to function at modulating the equilibrium between glutamate and GABA. Most of the genes upregulated after exercise are associated with the actions of the BDNF and IGF systems in the brain (47). Given the strong involvement of BDNF on neuronal excitability and synaptic function, the results seem to indicate a predominant action of BDNF for the influence of exercise on the brain. The results of these gene and protein expression studies have been confirmed and complemented by studies (see below) showing the functional interaction of these intracellular signaling pathways under the action of exercise.
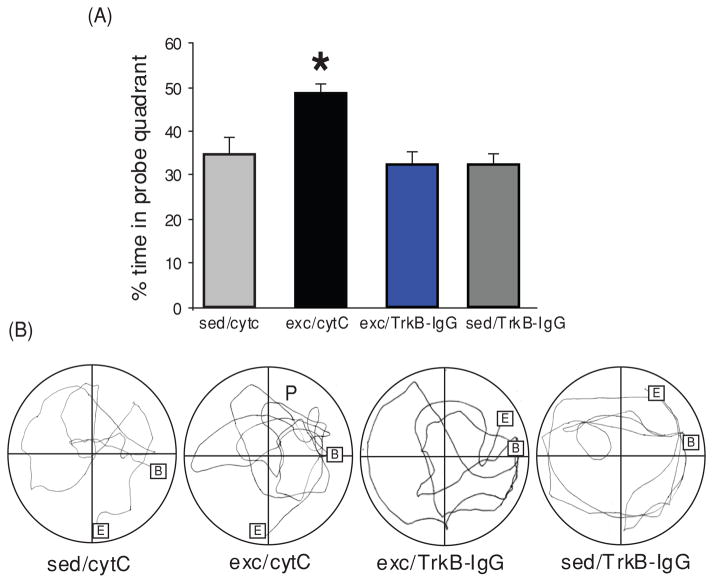
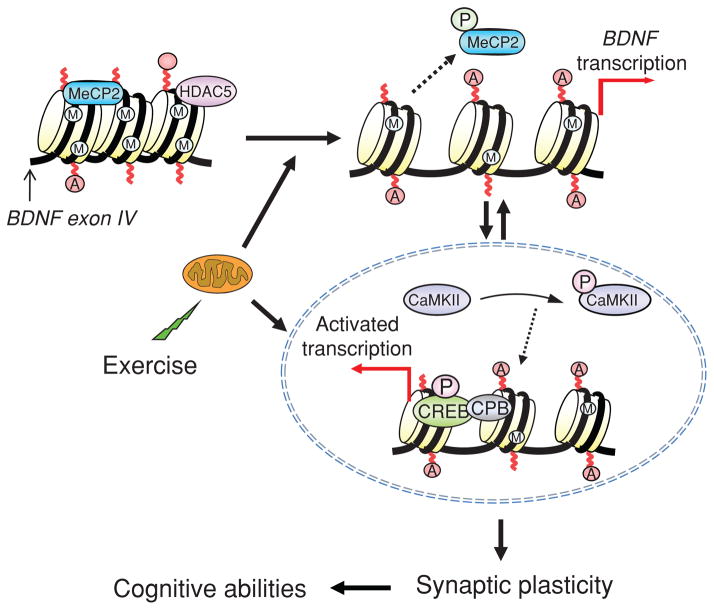
BDNF has emerged as a crucial mediator for the ability of exercise to enhance learning and memory, such that blocking BDNF signaling has been shown to abolish the effects of exercise on learning and memory (see Fig. 7) (190). The action of exercise on learning performance in the Morris water maze is coordinated with hippocampal synaptic proteins such as synaptophysin and synapsin (76, 130). Synapsin I is a phosphoprotein localized to the presynaptic membrane, and synaptophysin is a major integral protein on synaptic vesicles (175). The action of BDNF seems also coordinated with the function of IGF-1, such that blocking IGF-1 signaling receptors has been described to attenuate the effects of exercise on cognition and on BDNF and synaptic proteins (e.g., synapsin I). As discussed below, the involvements of IGF-1 in energy metabolism and synaptic plasticity make IGF-1 a suitable partner for BDNF to mediate the influence of exercise on cognition (see Fig. 8). Numerous studies support the function of BDNF in learning and memory and they range from demonstrations that hippocampal BDNF is increased during learning tasks (80,104) to demonstrations that genetic deletion of the BDNF gene impairs memory formation [Mizuno (116,121) and LTP (126,136)]. It is significant that an association between BDNF and learning and memory was found to exist when measuring the performance of rats to learn a spatial learning and memory task (123). These studies showed that hippocampal levels of BDNF were associated with the ability to learn and retain information.
Figure 7.
Role of brain-derived neurotrophic factor (BDNF) on the action of exercise on learning and memory assessed in the Morris Water Maze task. (A) Exercise improved learning ability as depicted by the enhanced aptitude of exercised animals to locate the platform in a significantly shorter time (shorter escape latencies in the exc/cytC group). Blocking BDNF action during exercise resulted in escape latency comparable to sedentary control animal (exc/TrkB-IgG vs. sed/cytC). The BDNF receptor blocker TrkB-IgG was injected into the hippocampus and cytochrome C (cytC) was used as a vehicle control. Data are expressed as mean ± SEM (ANOVA; Fischer test; Scheffe Fischer test; *, P < 0.05; **,‡‡, P < 0.01; * represents comparison between groups, ‡‡ represents comparison within groups). (B) Exercise increased the memory retention as indicated by significantly more time in quadrant P than sedentary controls (exc/cytC vs. sed/cytC). Blocking BDNF action during exercise abolished this exercise-induced preference for the P quadrant (exc/TrkB-IgG vs. exc/cytC), to sedentary control levels (exc/TrkB-IgG vs. sed/cytC). Representative samples of trials traveled during the probe test (B, begin, E, end, P, quadrant which previously housed the platform). Each value represents the mean ± SEM (ANOVA; Fischer test; *, P < 0.05). Reprinted, with permission, from reference (190), pp. 2582, 2584.
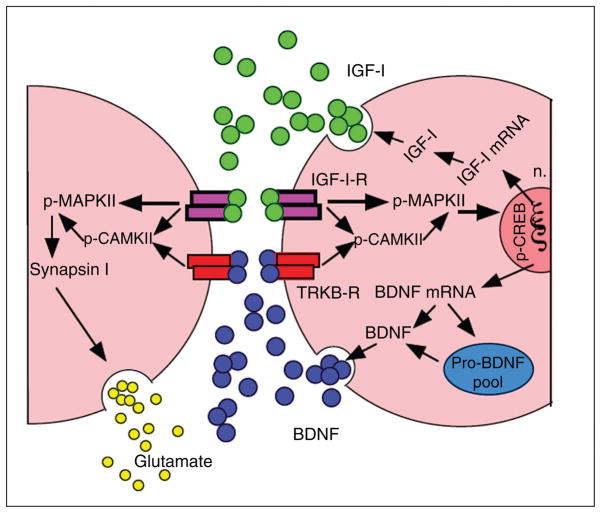
Figure 8.
Potential mechanism through which insulin-like growth factor 1 (IGF-1) may interface with brain-derived neurotrophic factor (BDNF)-mediated synaptic plasticity in the hippocampus during exercise. Exercise can induce IGF-1 production in the hippocampus. IGF-1 and BDNF are shown to have similar downstream signaling mechanisms, incorporating both p-CAMKII and p-MAPKII signaling cascades. In turn, these affect the state of vesicular release and gene expression by modulating synapsin I and CREB, respectively. IGF-1 may modulate BDNF possibly at the pro-BDNF level. The regulation of IGF-1 and BDNF mRNA expression, BDNF, and pro-BDNF protein is illustrated on the postsynaptic membrane for concise purposes, although this type of regulation likely occurs on the presynaptic neuron as well. Reprinted, with permission, from reference (47), p. 831.
Although 1 week of daily running in a wheel has been shown to promote detectable elevations in BDNF in conjunction with improvements in spatial learning performance (190), longer periods of exercise progressively increase levels of BDNF and make the BDNF response steadier overtime. To evaluate the effects of exercise on neurological applications, it is important to understand the relationship between the length of the exercise regimen and the stability of the response after exercise is stopped. Assessment of the time course of hippocampal BDNF availability following 3 weeks of exercise revealed the highest elevations of BDNF immediately after the exercise period, moderate 2 weeks after exercise ended, with levels returning to baseline by 3 to 4 weeks (7). In addition, prior exposure to exercise seems to prime the system to respond to exercise incurred in a later occasion. For example, previous exercise facilitates BDNF increases (8) such that reducing the frequency of exercise (alternating days) has been shown to be as effective as daily exercise. The results of these studies showing that the effects of exercise are somehow saved in the brain circuits for some time after its completion are significant for human applications of exercise-based therapies.
BDNF function blocking experiments have been useful to elucidate the contribution of different pathways and mechanisms responsible for mediating the effects of exercise in hippocampal synaptic plasticity (74, 77). Accordingly, it was determined that exercise employs several different conduits of signal transduction, such as mitogen-activated protein kinase (MAPK), calcium/calmodulin protein kinase II (CAMKII), and the NMDA receptor (NMDA-R), to mediate its effects on hippocampal synaptic plasticity. MAPK, CAMKII, and the NMDA-R have been found to interact with downstream effectors of BDNF action on synaptic transmission, that is, CREB and synapsin I, respectively (77). CREB is critical for long-term neuronal plasticity requisite for the formation of long-term memory (98, 155). BDNF can potentiate synaptic transmission through the NMDA-R (117), such that NMDA-R activation can also influence CAMKII to converge on the MAPK cascade (69). A large body of evidence showing that NMDA-R is critical for long-term potentiation (LTP) and learning and memory (122) supports the influence of exercise on cognition.
Genetic studies in humans reveal that variations in the BDNF genotype can have profound effects on cognitive function. The Val66Met BDNF polymorphism is a common single nucleotide polymorphism, consisting of a nonconservative amino acid substitution of valine to methionine at codon 66 in the human BDNF gene (Val66Met). This BDNF polymorphism has been implicated in abnormal hippocampal function and memory processing (57, 82), as well as with abnormal cerebral cortical morphology (106) and function. In particular, the polymorphism in the cerebral cortex has been associated with reduced activity-dependent release of BDNF and abnormalities of the cortex to respond to short-term motor stimulation (106). Evidence thus far indicates that the Val66Met polymorphism in BDNF play significant roles in structural and functional plasticity in mood disorders such as schizophrenia (15), elevated risk of depression (180), and even on the capacity of the brain for cognitive recovery after TBI (110). The overall evidence with regards to the Val66Met BDNF polymorphism in humans emphasizes the crucial role that BDNF plays on maintaining brain structure and function, as variations in the BDNF genotype appears to increase risk for various cognitive and mood disorders.
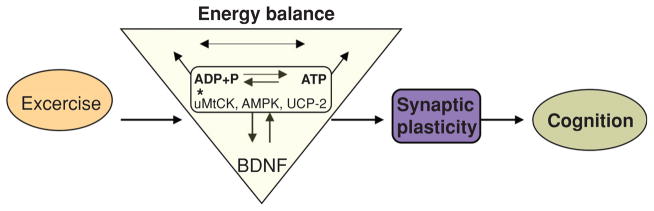
BDNF is at the interface of cellular energy metabolism and synaptic plasticity
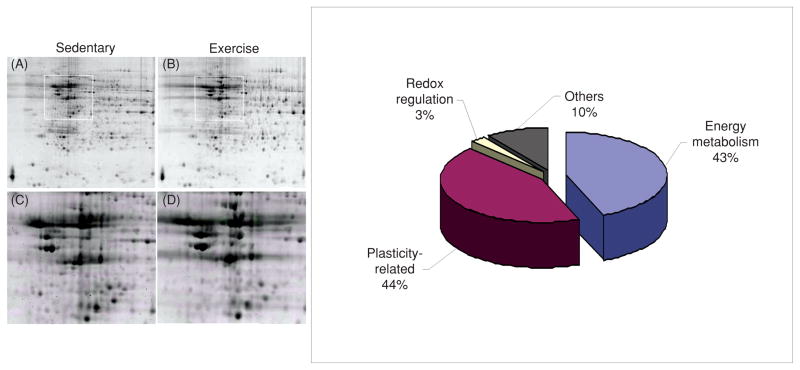
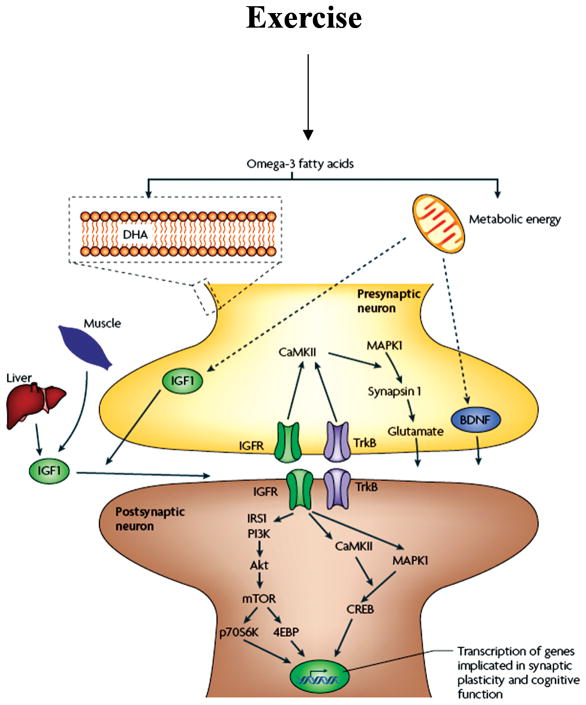
One of the most fundamental biological necessities is to conserve energy, which appears to contrast with the soaring energy demands of the brain. Although the brain mass is only 2% of that of the body, the metabolic demands of the brain accounts for 20% of the total energy consumed (65). The nervous system seems to have developed the ability to efficiently use energy by keeping tight control of body resources. In particular, exercise appears to play an action on the brain by coordinating peripheral events with higher order function using metabolic signals. These ideas have received experimental evidence by the use of multiple protein analysis, which has shown at the molecular level the association between metabolic processes and synaptic plasticity during exercise. These proteomic studies have been important to define the effect of voluntary exercise on the expression pattern and posttranslational modification of multiple protein classes in the rat hippocampus (48). For example, a mass spectrometry analysis on two-dimensional gels of multiple protein spots of relatively high abundance revealed that the majority of the proteins identified were associated with energy metabolism and synaptic plasticity (see Fig. 9). Given that most of the proteins found elevated are implicated in cognitive function, these findings seem to support the concept that exercise uses processes of energy metabolism and synaptic plasticity to benefit the brain. Recent findings show that in conjunction with BDNF, IGF-1 plays a role in synaptic plasticity (148), neurotransmitter synthesis, and release (2), and can support cognitive function (20, 154). IGF-1 can also be produced in peripheral tissue such as skeletal muscle and liver in response to meals and exercise. Accordingly, IGF-1 plays a major role in regulating the different aspects of general body metabolism such as plasma lipid concentration (198) and insulin action (39), in conjunction with its roles on synaptic plasticity and learning and memory.
Figure 9.
Proteomic analysis showing preponderant action of exercise on proteins associated with energy metabolism and synaptic plasticity. Representative two-dimensional gels of the hippocampus from sedentary (panel A) and exercise (panel B) rats. The boxes in A and B represent the areas enlarged in C and D showing the position of protein spots. The diagram on the right illustrates the relative proportion of protein types stimulated by voluntary exercise. Modified, with permission, from reference (48), p. 1270
Early studies reporting that transgenic mice heterozygous for BDNF suffer from hyperphagia, obesity, and hyperinsulinemia provided original support to the notion that BDNF is involved in energy metabolism (101, 115). The peripheral or central administration of BDNF has been found to reduce body weight and to improve blood glucose control in obese diabetic rodents (177). As a complement to these earlier observations, new studies indicate that control of energy metabolism is an integral aspect for the actions of BDNF on synaptic plasticity and cognitive function. For example, blocking the action of BDNF during the exercise period has been shown to counteract the enhancing effects of exercise on levels of energy proteins in the hippocampus and on the performance of the animal to learn a spatial learning task (see Fig. 10) (74). This research suggests that the ability of exercise to enhance cognitive function involves the action of BDNF on metabolic processes, such that BDNF may function as a metabotrophin in the hippocampus. Exercise modulates molecular systems in the brain associated with the balance and transduction of energy, which also influence learning and memory, that is, AMPK, ghrelin, ubiquitous mitochondrial creatine kinase, uncoupling protein 2 (UCP2), and IGF-1 (see Fig. 10). It is significant that exercise regulates elements by which the body signals the brain about aspects of energy homeostasis that are crucial for regulation of cognitive function. In addition, disruption of energy homeostasis using a high dose of vitamin D3 injected into the hippocampus during voluntary wheel running has been shown to decrease exercise-induced BDNF and to abolish the effects of exercise on end products of BDNF action that are important for learning and memory (188). These findings support a mechanism by which exercise uses processes of energy metabolism and synaptic plasticity to support cognitive processes.
Figure 10.
Proposed mechanism by which exercise enhances cognitive function by engaging aspects of cellular energy metabolism. There is a crucial association between metabolic energy and synaptic plasticity, in which brain-derived neurotrophic factor (BDNF) plays a crucial role. The effects of exercise on hippocampal BDNF would activate several molecular systems involved in the metabolism of energy, thereby modulating the capacity of the synapse to process information relevant to cognitive function. In particular, molecular systems such as uMtCK, AMPK, and UCP-2 may work at the interface between energy and synaptic plasticity. Energy related-molecules can interact with BDNF to modulate synaptic plasticity and cognitive function. Therefore, BDNF appears to be a central integrator for the effects of exercise on synaptic markers and energy metabolic processes to affect cognitive function. Reprinted, with permission, from reference (74), p. 2284.
Energy homeostasis mediators
AMPK is a serine-threonine kinase, which is described as a “fuel gauge” for cellular metabolism (81) due its ability to sense low energy levels and to reestablish the proper energy balance of the cell. Ghrelin is secreted from an empty stomach and can bind hippocampal receptors with profound effects on hippocampal synaptic plasticity, altering LTP, and hippocampal-dependent learning and memory (46,119). Central and peripheral ghrelin production contributes to modulating energy balance (38, 184), and intravenous injections of ghrelin given to human subjects increase appetite and energy intake (195). UmtCK is involved in energy maintenance and transduction, and may function to modulate aspects of cognitive function possibly by interacting with the BDNF system. UCP2 is a mitochondrial uncoupling protein that is suggested to play an important role in the regulation of energy metabolism via its ability to uncouple mitochondrial electron transport from ATP synthesis by permitting a proton leak across the mitochondrial membrane.
Exercise during brain development
Exposure to exercise during development has been shown to benefit the brain during the adult life such that exercise during pregnancy can enhance the ability of the offspring to learn a spatial learning task. Specifically, the pups whose mothers run on a treadmill during pregnancy (20 m/min for 30 min/d for 5 consecutive days a week) had increased hippocampal BDNF mRNA and performed better than sedentary counterparts on a spatial learning task (135). Studies in humans have shown that exercise during pregnancy maintains the aerobic fitness of the mother, reduces pregnancy-associated discomforts (85, 183), and can improve placental and fetal growth (28). Based on studies that the placenta may be a source of neurotrophic factors for the developing fetus (71, 182), it is possible that neurotrophic factors produced by the mother may permeate the placenta to influence the fetus (71, 182). In turn, rat pups exposed to exercise immediately after weaning have shown to be beneficial for the animals when they grow older, in terms of enhanced ability for learning (72).
Exercise, neurogenesis, and angiogenesis—BDNF, IGF-1, and VGF
Specific regions in the adult mammalian brain such as the olfactory bulb and hippocampal formation have the extraordinary capacity to produce new neurons in a process also known as neurogenesis. Abundant research in the last decade has shown that exercise is one of the strongest promoters of neurogenesis in the brain of adult rodents (97, 102) and humans (1,61), and this has introduced the possibility that proliferating neurons could contribute to the cognitive enhancement observed with exercise. In addition to BDNF, the actions of IGF-1 and vascular endothelial growth factor (VEGF) (54) are considered essential for the angiogenic and neurogenic effects of exercise in the brain. Although the action of exercise on brain angiogenesis has been known for many years (10), it is not until recently that neurovascular adaptations in the hippocampus have been associated with cognitive function (29). Exercise enhances the proliferation of brain endothelial cells throughout the brain (113), hippocampal IGF gene expression (47), and serum levels of both IGF (178) and VEGF (63). IGF-1 and VEGF, apparently produced in the periphery, support exercise induced neurogenesis and angiogenesis, as corroborated by blocking the effects of exercise using antibodies against IGF-1 (47) or VEGF (63). Therefore, the interactive effects of IGF-1 with VEGF, and BDNF, involving peripheral and central sources are important to orchestrate the action of exercise on neurogenesis and angiogenesis. The fact that factors induced by exercise such as BDNF, can also facilitate synaptic function and other aspects of neuronal plasticity, makes it difficult to isolate neurogenesis as a single variable for the effects of exercise on cognition.
Regulation of BDNF in the brain
A newly discovered aspect on the regulation of BDNF by exercise is that exercise can modulate BDNF at the transcriptional level by using mechanisms of epigenetic regulation (see below), and at the translational level by using the tissue-type plasminogen activator (tPA). There are at least two types of BDNF in the brain, the precursor (proBDNF) and its mature product (mBDNF)—these forms preferentially bind specific receptors and exert distinct functions. It has been found that 1 week of voluntary exercise stimulates both pro and mature BDNF in the rat hippocampus (49). An important aspect of the BDNF regulation is the action of tPA, a serine proteinase shown to facilitate proBDNF cleavage into mBDNF. The blockade of tPA activity reduced the exercise effects on proBDNF and mBDNF, and inhibited the TrkB signaling downstream effectors phospho-ERK, phospho-Akt, and phospo-CaMKII, and plasticity markers phospho-synapsin I and GAP-43. These results indicate that the effects of exercise on hippocampal plasticity are dependent on BDNF processing and BDNF receptor signaling, with implications for neuronal function. Based on the described effects of tPA for regulation of LTP and memory formation (133, 134), it is likely that the regulation of tPA by exercise may be part of the loop by which exercise influences synaptic plasticity and cognition.
Exercise and epigenetics
Epigenetic mechanisms allow for lasting modifications in the genome with important functional consequences, and exciting new evidence indicates that they may be involved in control of cognitive function and emotions. Chromatin refers to the complex of histones (proteins) and DNA that is tightly wound up in the nucleus and this conformation can regulate the expression of genes. When chromatin is tightly wound up, it is expressionally silent, while open chromatin tends to be more functional, that is, conducive for gene expression. Epigenetic mechanisms play an important role in the silencing of genes, primarily through DNA methylation and deacetylation of histones (19). Epigenetic mechanisms involving postreplication modifications of DNA and nuclear proteins have been shown to modulate BDNF gene. An exercise regimen known for its capacity to elevate hippocampal levels of BDNF mRNA and protein, and enhance learning and memory, has recently been shown to promote remodeling of chromatin containing the BDNF gene. Exercise affects histone acetylation (see Fig. 11) and DNA methylation (see Fig. 12) localized to the promoter IV region of the BDNF gene. Transcription involving promoter IV (formerly promoter III) can mediate synapse plasticity and learning and memory, and is subjected to epigenetic regulation (66). Promoter IV transcription is suppressed by methyl-CpG-binding protein (MeCP2), which belongs to a family of methylcytosine-binding proteins that contribute to the gene silencing effect of DNA methylation (25). Exercise also affected levels of p-MeCP2. It is known that neuronal depolarization dissociates MeCP2 from the BDNF promoter resulting in demethylation within the promoter and BDNF transcription (26). The effects of exercise were also sufficient to elevate the levels of p-CaMKII and p-CREB—molecules intimately involved in the pathways by which neural activity engage mechanisms of epigenetic regulation to stimulate BDNF transcription. Results are consistent with the notion that exercise influences epigenetic mechanisms to promote stable elevations in BDNF gene expression, which may have important implications for regulation of synaptic plasticity and behavior (see Fig. 13). Furthermore, a new line of studies indicates that the pathobiology of several brain disorders may reside in epigenetic modifications in the genome (129,170). For example, depression-like behavior in mice results in methylation of histone H3 and long-lasting suppression of BDNF transcription through promoters IV and VI (181). In turn, exercise and BDNF have been associated with reducing depression and promoting cognitive enhancement. This implies the fascinating possibility that epigenetic regulation of the BDNF gene can be a biological mechanism by which exercise can promote mental health (i.e., reduce depression) and resistance to neurological disorders. These studies showing the influence of exercise on the epigenome open new avenues and therapeutic prospects in the wage against neurological and psychiatric disorders. The original concept of epigenetics implies the idea that modifications in DNA expression and function can contribute to inheritance of information (193). Although this principle has not been fully demonstrated in mammals, exercise remains as a crucial candidate for promoting stable heritable biological adaptations.
Figure 11.
Exercise regulates brain-derived neurotrophic factor (BDNF) using epigenetic mechanisms. (A) Voluntary exercise increased histone H3 acetylation in hippocampi of rats assessed using chromatin immunoprecipitation (ChIP) assay. Primers specific to BDNF promoter IV were used to amplify the DNA from the AceH3 immunoprecipitates, and the relative enrichments of the BDNF promoter IV in the AceH3 immunoprecipitates were measured using real-time PCR. Equal amounts of DNA from sedentary (Sed) or exercised (Exc) rat hippocampi were used for immunoprecipitation. Data are presented as means ± SEM; *, P < 0.05. (B) Levels of AceH3 and H3 were assessed by Western blot analysis in the same hippocampal tissue used for the ChIP assay, and found a significant (**, P < 0.01) increase in the ratio AceH3/H3 in the exercise group compared to sedentary rats. Reprinted, with permission, from reference (75), p. 387.
Figure 12.
Exercise regulates brain-derived neurotrophic factor (BDNF) using epigenetic mechanisms. Exercise reduced DNA methylation of BDNF exon IV promoter in rat hippocampus. (A) The bar graph shows the DNA methylation levels of exercise and sedentary control animals on six CpG sites. Bisulfite sequencing analysis showed that the DNA methylation level was less in animals exposed to exercise, −148 CpG site showing the most dramatic DNA demethylation. (B) The number on top of the diagram labels the position of CpG sites relative to the transcription starting site (+1), and each horizontal line represents result for one clone (opened circles: unmethylated CpGs, filled circles: methylated CpGs). The DNA methylation level was calculated by the number of methylated CpG divided by the total number of CpGs analyzed, values represent the mean ± SEM; *, P < 0.05. (Sed: Sedentary; Exc: exercise). Reprinted, with permission, from reference (75), p. 385.
Figure 13.
Proposed mechanism by which exercise impacts synaptic plasticity and cognitive abilities by engaging aspects of epigenetic regulation. As discussed in the text, changes in energy metabolism may be an important mediator for the effects of exercise on synaptic plasticity, in a process engaging mechanisms of epigenetic regulation. Exercise promotes DNA demethylation in BDNF promoter IV, involving phosphorylation of methyl CpG binding protein 2 (MeCP2), and acetylation of histone H3. These events may result in dissociation of MeCP2 and chromatin remodeling events leading to BDNF gene transcription. The effects of exercise on brain-derived neurotrophic factor (BDNF) regulation may also involve the action of histone deacetylases (HDACs) such as HDAC5 implicated in the regulation of BDNF gene (Tsankova et al., 2006). Exercise elevates the activated stages of calcium/calmodulin-dependent protein kinase II (p-CaMKII) and cAMP response element binding protein (p-CREB), which in turn can contribute to regulate BDNF transcription, as well as participate in the signaling events by which BDNF influences synaptic plasticity and cognitive abilities. The impact of exercise on the remodeling of chromatin containing the BDNF gene emphasizes the importance of exercise on the control of gene transcription in the context of brain function and plasticity. Reprinted, with permission, from reference (75), p. 388.
Diet and exercise as indissoluble collaborators
Exercise likely orchestrates its function with other components of the daily living. In particular, locomotion is closely integrated to feeding as a survival strategy in most animal species, such that it is important to understand how feeding and exercise interact on the brain. New evidence indicates that exercise and dietary factors play complementary actions during the control of energy homeostasis and synaptic plasticity with important implications for the modulation of cognitive abilities (see Fig. 14) (74). Probably the best demonstrated interaction between diet and exercise is observed during the consumption of the omega-3 polyunsaturated fatty acid, docosahexaenoic (22:6n-3, DHA), in which DHA dietary supplementation can affect hippocampal plasticity and cognitive function in similar ways to exercise (27,196). DHA is abundant in fish, particularly salmon. The DHA diet and exercise enhanced cognitive function, and these effects were paralleled by elevations in BDNF-related synaptic proteins. Levels of the Akt signaling system were also elevated in proportion to BDNF levels. Interestingly, the effects of DHA diet and exercise were additive (see Fig. 15). More in-depth studies have shown that the DHA diet and exercise can complement their actions by exerting differential effects on molecular systems controlling important aspects of brain homeostasis and cognition in the hippocampus and hypothalamus (196). For example, exercise elevated levels of glucocorticoids receptors in the hypothalamus but the DHA diet had opposite effects, while the concurrent application of diet and exercise suppressed the sole effects of diet or exercise. These studies were important to define the roles of the hypothalamus and hippocampus to integrate the effects of diet and exercise for the regulations of brain plasticity and cognitive function. Flavonoids generally abundant in vegetables and fruits have also been shown to interact with exercise. The combination of a flavonoid enriched diet and exercise increased the expression of genes that have a positive effect on neuronal plasticity while decreased the expression of genes involved with deleterious functions such as inflammation and cell death (185). Exercise has also proven to be effective in reducing the effects of unhealthy diets (see Fig. 16). For example, studies have shown that a diet high in saturated fat and sucrose reduces the levels of BDNF-related synaptic plasticity and cognitive function, while the concurrent exposure to exercise compensated for the effects of the diet (124). The results of these studies are significant to define the potential of healthy diets and exercise to be used to overcome neurological disorders affecting cognitive abilities.
Figure 14.
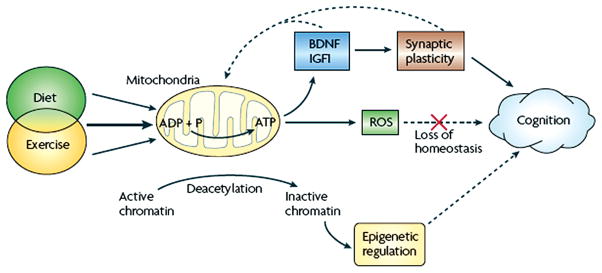
Hypothetical mechanism by which the interaction of exercise with other aspects of lifestyle such as feeding would affect cognitive abilities. Exercise activates molecular systems involved in energy metabolism and synaptic plasticity, and the interaction between these systems influences cognitive function. The same type of interaction may involve epigenetic mechanisms with long-lasting effects on cognition. Diet and exercise can affect mitochondrial energy production, which is important for maintaining neuronal excitability and synaptic function. The combined applications of select diets and exercise can have synergistic effects on synaptic plasticity and cognitive function. Specific energy events may regulate the activation of molecules such as BDNF and IGF-1 that support synaptic plasticity and cognitive function. The mitochondrion manages the balance of energy so that excess energy production caused by high caloric intake or strenuous exercise results in formation of reactive oxygen species (ROS). When ROS levels exceed the buffering capacity of the cell, synaptic plasticity and cognitive function are compromised. Failure to maintain energy homeostasis can gradually affect the cellular machinery associated with cognitive function, and increase the risk for mental disorders. Healthy diets and physiological levels of exercise, which have the capacity to reestablish cellular homeostasis, that is, energy metabolism and buffer ROS, can help to maintain cognitive function under challenging situations. Reprinted, with permission, from reference (73), p. 571.
Figure 15.
Brain-derived neurotrophic factor (BDNF) works at the interface of energy and cognition. Dietary omega-3 fatty acids can affect synaptic plasticity and cognition. The omega-3 fatty acid DHA that is mainly found in fish, can affect synaptic function and cognitive abilities by providing plasma membrane fluidity at synaptic regions. The fact that docosahexaenoic (DHA) constitutes more than the 30% of the total phospholipids composition in brain plasma membranes, makes DHA crucial for maintaining neuronal excitability and synaptic function that rely on membrane integrity. Dietary DHA is indispensable for maintaining membrane ionic permeability and function of transmembrane receptors that support synaptic transmission and cognitive abilities. Omega-3 fatty acids also activate energy-generating metabolic pathways that subsequently affect molecules such as BDNF and insulin-like growth factor 1 (IGF-1). IGF-1 can also be produced in the gastrointestinal system (liver) and skeletal muscle such that IGF-1 can convey peripheral messages to the brain in the context of diet and exercise. BDNF and IGF-1 signaling can activate pathways associated with learning and memory such as the mitogen-activated protein (MAP) kinase, and CaMKII signaling systems and modulations of synapsin I and cAMP response element binding protein (CREB). BDNF has also been involved in modulating synaptic plasticity and neuronal function through the PI3K/Akt and the mTOR-PI3K signaling systems. The activity of the mTOR and Akt signaling pathways are also modulated by metabolic signals such as insulin and leptin. Reprinted, with permission, from reference (73), p. 572.
Figure 16.
Cartoonish representation that illustrates the interaction between exercise and diet on the regulation of brain plasticity and cognitive function. (A) Based on experimental evidence (73), exercise or a diet rich in the omega-3 fatty acid docosahexaenoic can increase the expression of genes involved in synaptic plasticity and function while a high-saturated fat and sucrose (HF) diet has the opposite effects. Dietary supplementation with omega-3 fatty acids elevates levels of brain-derived neurotrophic factor (BDNF)-mediated synaptic plasticity in the hippocampus, a brain region important for learning and memory. Molecular changes are associated with an enhancement in hippocampal-dependent spatial learning performance in the Morris water maze (MWM). (B) In turn, animals exposed for three weeks to a HF diet showed opposite effects to the omega-3 fatty acid diet on BDNF levels and cognitive capacity. Concomitant exposure of the animals to voluntary running wheel exercise enhanced the effects of the omega-3 fatty acid diet, while counteracted the effects of the HF diet on synaptic markers and cognitive ability. Values are expressed as a percentage of control (regular diet, no exercise). Modified, with permission, from reference (73), p. 575
Housekeeping action of exercise on neurons supports cognitive function: effects on plasma membrane
The broad action of exercise is significant to support fundamental aspects of the maintenance and function of neurons. The integrity of the plasma membrane is critical for neuronal signaling, and the lack of continuous maintenance may exacerbate the effects of various neurological disorders (73). DHA is an important constituent of neuronal plasma membrane phospholipids, reaching up to a 17% concentration of the total fatty acids (95, 156). DHA provides cell membranes with the fluidity (83,169) required for proper neuronal signaling (171) and processing of cognitive information. Although DHA is critical for brain function, most mammals are inefficient at synthesizing DHA (105) forcing the brain to depend on dietary consumption of DHA. Emerging research indicates that exercise is a viable strategy to preserve membrane DHA by acting on molecular systems important for the metabolism and function of DHA in the hippocampus. It has been shown that exercise modulates syntaxin 3, a plasma membrane-bound protein associated with the action of DHA on cell membrane expansion (see Fig. 17) (40). Additionally, exercise can affect the NR2B subunit of the NMDA receptor, which provides support to synaptic growth and plasticity and learning. Illustrating the effects of exercise on NMDA function, it has been shown that the application of NR2B subunit antagonists abolishes the effects of exercise on receptor-dependent LTP in the mouse dentate gyrus (187). Exercise may help to maintain synaptic and cognitive function by supporting membrane stability, which is required for synaptic function and processing of higher order information. The results of these studies are important to illustrate how exercise can support important aspects of neuronal maintenance, which are fundamental for neuronal function.
Figure 17.
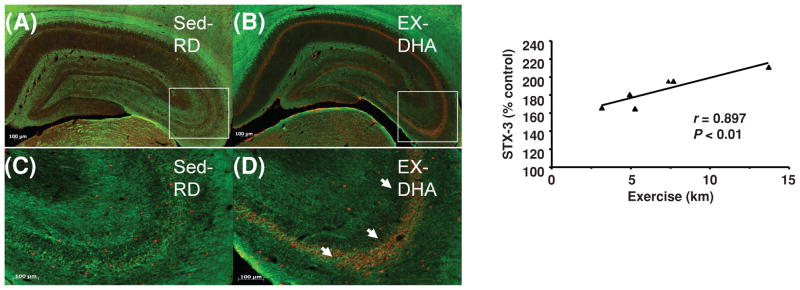
Exercise contributes to the action of an omega-3 diet by supporting plasma membrane homeostasis. Exercise enhanced the effects of docosahexaenoic (DHA) dietary supplementation on syntaxin 3 (STX-3), a protein associated with synaptic membrane (A). The values were converted to percent of RD-Sed controls (mean ± SEM; ANOVA; **, P < 0.01). (B) The levels of STX-3 changed proportionally to the amount of exercise in animals fed the DHA diet (DHA-Exc). (C–F) Immunofluorescence for STX-3 in coronal sections of the hippocampus after DHA diet combined with exercise. Representative sections show STX-3 red fluorescence label (Cy3 secondary antibody) in RD-Sed (C and E) controls and DHA-Exc (D and F) rats. High magnification photomicrographs of CA3 hippocampal areas highlighted in E and F show a marked increase in STX-3 immunofluorescence (white arrows) in a (F) DHA-Exc rat compared to a (E) RD-Sed control. Immunofluorescence for myelin-associated glycoprotein was performed in the same brain sections to label myelinated axons in green (FITC secondary antibody). Reprinted, with permission, from reference (27), p. 34.
Future directions: Exercise and disease prevention
As discussed above, abundant evidence supports the role of exercise enhancing cognitive function in young subjects and reducing cognitive decay in aging. Studies performed in humans indicate that exercise has the potential to reduce the risk for various neurological diseases including Alzheimer, Huntington’s and Parkinson’s, up to the point to attenuate functional decline after the onset of neurodegeneration. Exercise has also been shown to improve quality of life in individuals with Alzheimer’s disease, in terms of improving cognitive, mood, and physical functions on the daily living. Clinical intervention studies in individuals with Parkinson’s disease demonstrate that aerobic training improves movement initiation and aerobic capacity, and improves activities of daily living. Exercise is therapeutic and protective in depression, and its effects are proportional to the amount of exercise. Randomized and crossover clinical trials demonstrate the efficacy of aerobic or resistance training exercise (2–4 months) as a treatment for depression in both young and older individuals. Although exercise seems to have both preventative and therapeutic effects on the course of depression, the underlying mechanisms are poorly understood. Other proposed mechanisms include exercise-driven changes in the hypothalamic-pituitary-adrenal axis that regulates the stress response. In addition to this, the immune system seems to play a critical role in influence of exercise on the body and brain. A recent study (4) shows that exercise appears to enhance protective mechanisms in the aging brain by promoting homeostasis following an induced immune challenge. It appears that aging produces cognitive vulnerability to peripheral immune challenge by displaying an exaggerated brain proinflammatory response, which may interfere with BDNF function and learning. These studies suggest an important role of the immune system in mediating exercise induced brain plasticity. The interaction of exercise and the immune system may be an important factor in the control of brain plasticity and disease. In addition, based on the encouraging results of epidemiological studies indicating that exercise may reduce the risk for genetic predisposition for dementia (43), interventional studies are necessary in this fertile area of research. A positive outcome in this prospect is supported by the results of new animal studies indicating that exercise has the capacity to influence epigenetic mechanisms that modulate cognitive abilities at the molecular level.
Conclusions and research demands
The evidence accumulated so far indicates that exercise is a strong promoter of cognitive health in humans. Several animal studies support the view that exercise can influence molecular events via epigenetic mechanisms that can modulate cognitive abilities (36,75,151). The active lifestyle of our early ancestors using locomotion and foraging may have demanded development of cognitive abilities for survival. The intrinsic ability of locomotion to engage energy transactions at the cellular level seems to have evolved simultaneously with molecular adaptations serving cognitive function. In the modern age where industrialization has dramatically transformed lifestyle, it is ever more so important to realize the dependency that the brain holds on physical activity and healthy dietary choices. The amount of physical activity practiced in modern age has been largely reduced below the level of genetic predisposition (37). It has been estimated that at least 70% of the US population gets less than 30 min of moderate-intensity physical activity a day (US department of Health and Human Services, 1996). The mismatch between levels of physical activity and our genetics may, therefore, contribute to the prevalence of several metabolic diseases such as obesity (12, 194) and derived metabolic dysfunctions such as type II diabetes, hypertension, and cardiovascular disease (12, 99, 127). Even further, as discussed in this article, metabolic dysfunction can be the starting point for several neurological disorders resulting in protracted cognitive function. Recent findings illustrate the interdependency of energy metabolic processes and synaptic plasticity, and this may provide a mechanism to explain how exercise can affect mental health. The fact that exercise contributes to reduce the risk of various psychiatric disorders associated with abnormal metabolism appears to support the latter possibility.
Acknowledgments
This work was supported by National Institutes of Health Awards NS50465-06, NS068473, and NS56413. The authors thank Cameron Feng, B.S. for helpful editorial assistance.
References
- 1.Ang ET, Tai YK, Lo SQ, Seet R, Soong TW. Neurodegenerative diseases: Exercising toward neurogenesis and neuroregeneration. Front Aging Neurosci. 2010;2 doi: 10.3389/fnagi.2010.00025. pii. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anlar B, Sullivan KA, Feldman EL. Insulin-like growth factor-I and central nervous system development. Horm Metab Res. 1999;31:120–125. doi: 10.1055/s-2007-978708. [DOI] [PubMed] [Google Scholar]
- 3.Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Fishel MA, Watson GS, Cholerton BA, Duncan GE, Mehta PD, Craft S. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch Neurol. 2010;67:71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HE, Campeau S, Watkins LR, Patterson SL, Maier SF. Little exercise, big effects: Reversing aging and infection-induced memory deficits, and underlying processes. J Neurosci. 2011;31:11578–11586. doi: 10.1523/JNEUROSCI.2266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baylor AM, Spirduso WW. Systematic aerobic exercise and components of reaction time in older women. J Gerontol. 1988;43:121–126. doi: 10.1093/geronj/43.5.p121. [DOI] [PubMed] [Google Scholar]
- 6.Beise D, Peaseley V. The relationship of reaction time, speed, and agility of big muscle groups and certain sport skills. Research Quarterly. 1937;8:133–142. [Google Scholar]
- 7.Berchtold NC, Castello N, Cotman CW. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167:588–597. doi: 10.1016/j.neuroscience.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein PS, Scheffers MK, Coles MG. “Where did I go wrong?” A psychophysiological analysis of error detection. J Exp Psychol Hum Percept Perform. 1995;21:1312–1322. doi: 10.1037//0096-1523.21.6.1312. [DOI] [PubMed] [Google Scholar]
- 10.Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blair C, Zelazo PD, Greenberg MT. The measurement of executive function in early childhood. Dev Neuropsychol. 2005;28:561–571. doi: 10.1207/s15326942dn2802_1. [DOI] [PubMed] [Google Scholar]
- 12.Booth FW, Chakravarthy MV, Gordon SE, Spangenburg EE. Waging war on physical inactivity: Using modern molecular ammunition against an ancient enemy. J Appl Physiol. 2002;93:3–30. doi: 10.1152/japplphysiol.00073.2002. [DOI] [PubMed] [Google Scholar]
- 13.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 14.Buck SM, Hillman CH, Castelli DM. The relation of aerobic fitness to stroop task performance in preadolescent children. Med Sci Sports Exerc. 2008;40:166–172. doi: 10.1249/mss.0b013e318159b035. [DOI] [PubMed] [Google Scholar]
- 15.Buckley PF, Pillai A, Howell KR. Brain-derived neurotrophic factor: Findings in schizophrenia. Curr Opin Psychiatry. 2011;24:122–127. doi: 10.1097/YCO.0b013e3283436eb7. [DOI] [PubMed] [Google Scholar]
- 16.Bugg JM, Head D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol Aging. 2011;32:506–514. doi: 10.1016/j.neurobiolaging.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burdette JH, Laurienti PJ, Espeland MA, Morgan A, Telesford Q, Vechlekar CD, Hayasaka S, Jennings JM, Katula JA, Kraft RA, Rejeski WJ. Using network science to evaluate exercise-associated brain changes in older adults. Front Aging Neurosci. 2010;2:23. doi: 10.3389/fnagi.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burpee RH, Stroll W. Measuring reaction time of athletes. Research Quarterly. 1936;7:110–118. [Google Scholar]
- 19.Burzynski SR. Gene silencing–a new theory of aging. Med Hypotheses. 2003;60:578–583. doi: 10.1016/s0306-9877(03)00050-1. [DOI] [PubMed] [Google Scholar]
- 20.Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. 2001;21:5678–5684. doi: 10.1523/JNEUROSCI.21-15-05678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 22.Castelli DM, Hillman CH. Physical activity, cognition, and school performance: From neurons to neighborhoods. In: Meyer A, Gulotta T, editors. Physical Activity as Interventions: Application to Depression, Obesity, Drug Use, and Beyond. (in press) [Google Scholar]
- 23.Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, Vanpatter M, Pontifex MB, Raine LB, Konkel A, Hillman CH, Cohen NJ, Kramer AF. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010;1358:172–183. doi: 10.1016/j.brainres.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaddock L, Hillman CH, Buck SM, Cohen NJ. Aerobic fitness and executive control of relational memory in preadolescent children. Med Sci Sports Exerc. 2011;43:344–349. doi: 10.1249/MSS.0b013e3181e9af48. [DOI] [PubMed] [Google Scholar]
- 25.Chao HT, Zoghbi HY. The yin and yang of MeCP2 phosphorylation. Proc Natl Acad Sci U S A. 2009;106:4577–4578. doi: 10.1073/pnas.0901518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- 27.Chytrova G, Ying Z, Gomez-Pinilla F. Exercise contributes to the effects of DHA dietary supplementation by acting on membrane-related synaptic systems. Brain Res. 2009;1341:32–40. doi: 10.1016/j.brainres.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clapp JF, Kim H, Burciu B, Schmidt S, Petry K, Lopez B. Continuing regular exercise during pregnancy: Effect of exercise volume on fetoplacental growth. Am J Obstet Gynecol. 2002;186:142–147. doi: 10.1067/mob.2002.119109. [DOI] [PubMed] [Google Scholar]
- 29.Clark PJ, Brzezinska WJ, Puchalski EK, Krone DA, Rhodes JS. Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus. 2009;19:937–950. doi: 10.1002/hipo.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 31.Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 32.Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 33.Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coles MGH, Gratton G, Fabiani M. Event-related potentials. In: Cacioppo JT, Tassinary LG, editors. Principles of Psychophysiology: Physical, Social, and Inferential Elements. New York, NY: Cambridge University Press; 1990. pp. 413–455. [Google Scholar]
- 35.Coles MGH, Rugg MD. Event-related brain potentials: An introduction. In: Rugg MD, Coles MGH, editors. Electrophysiology of Mind: Event-Related Brain Potentials and Cognition. New York, NY: Oxford University Press; 1995. pp. 1–26. [Google Scholar]
- 36.Collins A, Hill LE, Chandramohan Y, Whitcomb D, Droste SK, Reul JM. Exercise improves cognitive responses to psychological stress through enhancement of epigenetic mechanisms and gene expression in the dentate gyrus. PLoS One. 2009;4:e4330. doi: 10.1371/journal.pone.0004330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cordain L, Gotshall RW, Eaton SB. Physical activity, energy expenditure and fitness: An evolutionary perspective. Int J Sports Med. 1998;19:328–335. doi: 10.1055/s-2007-971926. [DOI] [PubMed] [Google Scholar]
- 38.Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 39.Cusi K, DeFronzo R. Recombinant human insulin-like growth factor I treatment for 1 week improves metabolic control in type 2 diabetes by ameliorating hepatic and muscle insulin resistance. J Clin Endocrinol Metab. 2000;85:3077–3084. doi: 10.1210/jcem.85.9.6827. [DOI] [PubMed] [Google Scholar]
- 40.Darios F, Davletov B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature. 2006;440:813–817. doi: 10.1038/nature04598. [DOI] [PubMed] [Google Scholar]
- 41.Davatzikos C, Resnick SM. Degenerative age changes in white matter connectivity visualized in vivo using magnetic resonance imaging. Cereb Cortex. 2002;12:767–771. doi: 10.1093/cercor/12.7.767. [DOI] [PubMed] [Google Scholar]
- 42.Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deeny SP, Poeppel D, Zimmerman JB, Roth SM, Brandauer J, Witkowski S, Hearn JW, Ludlow AT, Contreras-Vidal JL, Brandt J, Hatfield BD. Exercise, APOE, and working memory: MEG and behavioral evidence for benefit of exercise in epsilon4 carriers. Biol Psychol. 2008;78:179–187. doi: 10.1016/j.biopsycho.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychol Sci. 1994;5:303–305. [Google Scholar]
- 45.Diamond A. The early development of executive functions. In: Bialystok EF, editor. Lifespan Cognition: Mechanisms of Change. New York: Oxford University Press; 2006. pp. 70–95. [Google Scholar]
- 46.Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschöp MH, Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 47.Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140:823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- 48.Ding Q, Vaynman S, Souda P, Whitelegge JP, Gomez-Pinilla F. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. Eur J Neurosci. 2006;24:1265–1276. doi: 10.1111/j.1460-9568.2006.05026.x. [DOI] [PubMed] [Google Scholar]
- 49.Ding Q, Ying Z, Gómez-Pinilla F. Exercise influences hippocampal plasticity by modulating brain-derived neurotrophic factor processing. Neuroscience. 2011;192:773–780. doi: 10.1016/j.neuroscience.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER. A survey for assessing physical activity among older adults. Med Sci Sports Exerc. 1993;25:628–642. [PubMed] [Google Scholar]
- 51.Donchin E. Presidential address, 1980. Surprise!…Surprise? Psychophysiology. 1981;18:493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- 52.Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behav Brain Sci. 1988;11:355–372. [Google Scholar]
- 53.Duncan-Johnson CC. Young Psychophysiologist Award address, 1980. P300 latency: A new metric of information processing. Psychophysiology. 1981;18:207–215. doi: 10.1111/j.1469-8986.1981.tb03020.x. [DOI] [PubMed] [Google Scholar]
- 54.During MJ, Cao L. VEGF, a mediator of the effect of experience on hippocampal neurogenesis. Curr Alzheimer Res. 2006;3:29–33. doi: 10.2174/156720506775697133. [DOI] [PubMed] [Google Scholar]
- 55.Dustman RE, Emmerson RY, Ruhling RO, Shearer DE, Steinhaus LA, Johnson SC, Bonekat HW, Shigeoka JW. Age and fitness effects on EEG, ERPs, visual sensitivity, and cognition. Neurobiol Aging. 1990;11:193–200. doi: 10.1016/0197-4580(90)90545-b. [DOI] [PubMed] [Google Scholar]
- 56.Dustman RE, Emmerson RY, Shearer DE. Physical activity, age, and cognitive-neurophysiological function. J Aging Phys Activ. 1994;2:143–181. [Google Scholar]
- 57.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 58.Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, Pence BD, Martin SA, Vieira VJ, Woods JA, McAuley E, Kramer AF. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2010;30:5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wójcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, Gach HM, Thompson PM, Ho AJ, Kuller LH. Physical activity predicts gray matter volume in late adulthood: The Cardiovascular Health Study. Neurology. 2010;75:1415–1422. doi: 10.1212/WNL.0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Etnier JL, Salazar W, Landers DM, Petruzello SJ, Han M, Nowell P. The influence of physical fitness and exercise upon cognitive functioning: A meta-analysis. J Sports Exerc Psychol. 1997;19:249–277. [Google Scholar]
- 63.Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 64.Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of cross-modal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- 65.Fehm HL, Kern W, Peters A. The selfish brain: Competition for energy resources. Prog Brain Res. 2006;153:129–140. doi: 10.1016/S0079-6123(06)53007-9. [DOI] [PubMed] [Google Scholar]
- 66.Feng J, Fouse S, Fan G. Epigenetic regulation of neural gene expression and neuronal function. Pediatr Res. 2007;61:58R–63R. doi: 10.1203/pdr.0b013e3180457635. [DOI] [PubMed] [Google Scholar]
- 67.Flöel A, Ruscheweyh R, Krüger K, Willemer C, Winter B, Völker K, Lohmann H, Zitzmann M, Mooren F, Breitenstein C, Knecht S. Physical activity and memory functions: Are neurotrophins and cerebral gray matter volume the missing link? Neuroimage. 2010;49:2756–2763. doi: 10.1016/j.neuroimage.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 68.Friedman D, Simpson G, Hamberger M. Age-related changes in scalp topography to novel and target stimuli. Psychophysiology. 1993;30:383–396. doi: 10.1111/j.1469-8986.1993.tb02060.x. [DOI] [PubMed] [Google Scholar]
- 69.Fukunaga K, Muller D, Miyamoto E. CaM kinase II in long-term potentiation. Neurochem Int. 1996;28:343–358. doi: 10.1016/0197-0186(95)00097-6. [DOI] [PubMed] [Google Scholar]
- 70.Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- 71.Gilmore JH, Jarskog LF, Vadlamudi S. Maternal infection regulates BDNF and NGF expression in fetal and neonatal brain and maternal-fetal unit of the rat. J Neuroimmunol. 2003;138:49–55. doi: 10.1016/s0165-5728(03)00095-x. [DOI] [PubMed] [Google Scholar]
- 72.Gomes da Silva S, Unsain N, Mascó DH, Toscano-Silva M, de Amorim HA, Silva Araújo BH, Simões PS, da Graça Naffah-Mazzacoratti M, Mortara RA, Scorza FA, Cavalheiro EA, Arida RM. Early exercise promotes positive hippocampal plasticity and improves spatial memory in the adult life of rats. Hippocampus. 2010;22:347–358. doi: 10.1002/hipo.20903. [DOI] [PubMed] [Google Scholar]
- 73.Gomez-Pinilla F. Brain foods: The effects of nutrients on brain function. Nat Rev Neurosci. 2008;9:568–578. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008;28:2278–2287. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gomez-Pinilla F, Zhuang Y, Feng J, Ying Z, Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur J Neurosci. 2011;33:383–390. doi: 10.1111/j.1460-9568.2010.07508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Griesbach GS, Hovda DA, Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–115. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gómez-Pinilla F, Huie JR, Ying Z, Ferguson AR, Crown ED, Baumbauer KM, Edgerton VR, Grau JW. BDNF and learning: Evidence that instrumental training promotes learning within the spinal cord by up-regulating BDNF expression. Neuroscience. 2007;148:893–906. doi: 10.1016/j.neuroscience.2007.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gómez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- 79.Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42:151–160. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- 80.Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- 81.Hardie DG. AMP-activated protein kinase: A key system mediating metabolic responses to exercise. Med Sci Sports Exerc. 2004;36:28–34. doi: 10.1249/01.MSS.0000106171.38299.64. [DOI] [PubMed] [Google Scholar]
- 82.Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hashimoto M, Hossain S, Shimada T, Shido O. Docosahexaenoic acid-induced protective effect against impaired learning in amyloid beta-infused rats is associated with increased synaptosomal membrane fluidity. Clin Exp Pharmacol Physiol. 2006;33:934–939. doi: 10.1111/j.1440-1681.2006.04467.x. [DOI] [PubMed] [Google Scholar]
- 84.Hatta A, Nishihira Y, Kim SR, Kaneda T, Kida T, Kamijo K, Sasahara M, Haga S. Effects of habitual moderate exercise on response processing and cognitive processing in older adults. Jpn J Physiol. 2005;55:29–36. doi: 10.2170/jjphysiol.R2068. [DOI] [PubMed] [Google Scholar]
- 85.Heffernan AE. Exercise and pregnancy in primary care. Nurse Pract. 2000;25:42, 49, 53–46. passim. [PubMed] [Google Scholar]
- 86.Hillman CH, Belopolsky AV, Snook EM, Kramer AF, McAuley E. Physical activity and executive control: Implications for increased cognitive health during older adulthood. Res Q Exerc Sport. 2004;75:176–185. doi: 10.1080/02701367.2004.10609149. [DOI] [PubMed] [Google Scholar]
- 87.Hillman CH, Castelli DM, Buck SM. Aerobic fitness and neurocognitive function in healthy preadolescent children. Med Sci Sports Exerc. 2005;37:1967–1974. doi: 10.1249/01.mss.0000176680.79702.ce. [DOI] [PubMed] [Google Scholar]
- 88.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 89.Hillman CH, Kramer AF, Belopolsky AV, Smith DP. A cross-sectional examination of age and physical activity on performance and event-related brain potentials in a task switching paradigm. Int J Psychophysiol. 2006;59:30–39. doi: 10.1016/j.ijpsycho.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 90.Hillman CH, Motl RW, Pontifex MB, Posthuma D, Stubbe JH, Boomsma DI, de Geus EJ. Physical activity and cognitive function in a cross-section of younger and older community-dwelling individuals. Health Psychol. 2006;25:678–687. doi: 10.1037/0278-6133.25.6.678. [DOI] [PubMed] [Google Scholar]
- 91.Hillman CH, Pontifex MB, Raine LB, Castelli DM, Hall EE, Kramer AF. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience. 2009;159:1044–1054. doi: 10.1016/j.neuroscience.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hillman CH, Weiss EP, Hagberg JM, Hatfield BD. The relationship of age and cardiovascular fitness to cognitive and motor processes. Psychophysiology. 2002;39:303–312. doi: 10.1017/s0048577201393058. [DOI] [PubMed] [Google Scholar]
- 93.Holroyd CB, Coles MG. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- 94.Honea RA, Thomas GP, Harsha A, Anderson HS, Donnelly JE, Brooks WM, Burns JM. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:188–197. doi: 10.1097/WAD.0b013e31819cb8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Horrocks LA, Farooqui AA. Docosahexaenoic acid in the diet: Its importance in maintenance and restoration of neural membrane function. Prostaglandins Leukot Essent Fatty Acids. 2004;70:361–372. doi: 10.1016/j.plefa.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 96.Hugdahl K. Psychophysiology: The Mind-Body Perspective. Cambridge, MA: Harvard University Press; 1995. p. 429. [Google Scholar]
- 97.Itoh T, Imano M, Nishida S, Tsubaki M, Hashimoto S, Ito A, Satou T. Exercise increases neural stem cell proliferation surrounding the area of damage following rat traumatic brain injury. J Neural Transm. 2011;118:193–202. doi: 10.1007/s00702-010-0495-3. [DOI] [PubMed] [Google Scholar]
- 98.Josselyn SA, Nguyen PV. CREB, synapses and memory disorders: Past progress and future challenges. Curr Drug Targets CNS Neurol Disord. 2005;4:481–497. doi: 10.2174/156800705774322058. [DOI] [PubMed] [Google Scholar]
- 99.Jung RT. Obesity as a disease. Br Med Bull. 1997;53:307–321. doi: 10.1093/oxfordjournals.bmb.a011615. [DOI] [PubMed] [Google Scholar]
- 100.Kamijo K, Takeda Y. General physical activity levels influence positive and negative priming effects in young adults. Clin Neurophysiol. 2009;120:511–519. doi: 10.1016/j.clinph.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 101.Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kernie SG, Parent JM. Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol Dis. 2010;37:267–274. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 104.Kesslak JP, So V, Choi J, Cotman CW, Gomez-Pinilla F. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: A mechanism to facilitate encoding and circuit maintenance? Behav Neurosci. 1998;112:1012–1019. doi: 10.1037//0735-7044.112.4.1012. [DOI] [PubMed] [Google Scholar]
- 105.Kim HY. Novel metabolism of docosahexaenoic acid in neural cells. J Biol Chem. 2007;282:18661–18665. doi: 10.1074/jbc.R700015200. [DOI] [PubMed] [Google Scholar]
- 106.Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, Cramer SC. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9:735–737. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- 107.Knight RT. Distributed cortical networks for visual attention. J Cogn Neurosci. 1997;9:75–91. doi: 10.1162/jocn.1997.9.1.75. [DOI] [PubMed] [Google Scholar]
- 108.Kramer AF, Erickson KI. Capitalizing on cortical plasticity: Influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11:342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 109.Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 110.Krueger F, Pardini M, Huey ED, Raymont V, Solomon J, Lipsky RH, Hodgkinson CA, Goldman D, Grafman J. The role of the Met66 brain-derived neurotrophic factor allele in the recovery of executive functioning after combat-related traumatic brain injury. J Neurosci. 2011;31:598–606. doi: 10.1523/JNEUROSCI.1399-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Laske C, Banschbach S, Stransky E, Bosch S, Straten G, Machann J, Fritsche A, Hipp A, Niess A, Eschweiler GW. Exercise-induced normalization of decreased BDNF serum concentration in elderly women with remitted major depression. Int J Neuropsychopharmacol. 2010;13:595–602. doi: 10.1017/S1461145709991234. [DOI] [PubMed] [Google Scholar]
- 112.Lawther JD. Psychology of Coaching. Englewood Cliffs, NJ: Prentice Hall, Inc; 1951. p. 333. [Google Scholar]
- 113.Lopez-Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci U S A. 2004;101:9833–9838. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Luck SJ. An Introduction to the Event-Related Potential Technique. Cambridge, MA: The MIT Press; 2005. p. 388. [Google Scholar]
- 115.Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ma YL, Wang HL, Wu HC, Wei CL, Lee EH. Brain-derived neurotrophic factor antisense oligonucleotide impairs memory retention and inhibits long-term potentiation in rats. Neuroscience. 1998;82:957–967. doi: 10.1016/s0306-4522(97)00325-4. [DOI] [PubMed] [Google Scholar]
- 117.Martin JL, Finsterwald C. Cooperation between BDNF and glutamate in the regulation of synaptic transmission and neuronal development. Commun Integr Biol. 2011;4:14–16. doi: 10.4161/cib.4.1.13761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McDowell K, Kerick SE, Santa Maria DL, Hatfield BD. Aging, physical activity, and cognitive processing: An examination of P300. Neurobiol Aging. 2003;24:597–606. doi: 10.1016/s0197-4580(02)00131-8. [DOI] [PubMed] [Google Scholar]
- 119.McNay EC. Insulin and ghrelin: Peripheral hormones modulating memory and hippocampal function. Curr Opin Pharmacol. 2007;7:628–632. doi: 10.1016/j.coph.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 120.Miltner WH, Lemke U, Weiss T, Holroyd C, Scheffers MK, Coles MG. Implementation of error-processing in the human anterior cingulate cortex: A source analysis of the magnetic equivalent of the error-related negativity. Biol Psychol. 2003;64:157–166. doi: 10.1016/s0301-0511(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 121.Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Molnár E. Long-term potentiation in cultured hippocampal neurons. Semin Cell Dev Biol. 2011;22:506–513. doi: 10.1016/j.semcdb.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 123.Molteni R, Barnard RJ, Ying Z, Roberts CK, Gómez-Pinilla F. A high- fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–814. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 124.Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gómez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–440. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 125.Molteni R, Zheng JQ, Ying Z, Gómez-Pinilla F, Twiss JL. Voluntary exercise increases axonal regeneration from sensory neurons. Proc Natl Acad Sci U S A. 2004;101:8473–8478. doi: 10.1073/pnas.0401443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mu JS, Li WP, Yao ZB, Zhou XF. Deprivation of endogenous brain-derived neurotrophic factor results in impairment of spatial learning and memory in adult rats. Brain Res. 1999;835:259–265. doi: 10.1016/s0006-8993(99)01592-9. [DOI] [PubMed] [Google Scholar]
- 127.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 128.Neeper SA, Gómez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- 129.Nestler EJ. Epigenetic mechanisms in psychiatry. Biol Psychiatry. 2009;65:189–190. doi: 10.1016/j.biopsych.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 130.Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5:287–294. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- 132.Pajonk FG, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, Kierer A, Müller S, Oest M, Meyer T, Backens M, Schneider-Axmann T, Thornton AE, Honer WG, Falkai P. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010;67:133–143. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- 133.Pang PT, Lu B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: Role of secreted proteins tPA and BDNF. Ageing Res Rev. 2004;3:407–430. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 134.Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- 135.Parnpiansil P, Jutapakdeegul N, Chentanez T, Kotchabhakdi N. Exercise during pregnancy increases hippocampal brain-derived neurotrophic factor mRNA expression and spatial learning in neonatal rat pup. Neurosci Lett. 2003;352:45–48. doi: 10.1016/j.neulet.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 136.Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 137.Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pierson WR, Montoye HJ. Movement time, reaction time, and age. J Gerontol. 1958;13:418–421. doi: 10.1093/geronj/13.4.418. [DOI] [PubMed] [Google Scholar]
- 139.Polich J. Clinical application of the P300 event-related brain potential. Phys Med Rehabil Clin N Am. 2004;15:133–161. doi: 10.1016/s1047-9651(03)00109-8. [DOI] [PubMed] [Google Scholar]
- 140.Polich J. Updating P300: An integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Polich J, Heine MR. P300 topography and modality effects from a single-stimulus paradigm. Psychophysiology. 1996;33:747–752. doi: 10.1111/j.1469-8986.1996.tb02371.x. [DOI] [PubMed] [Google Scholar]
- 142.Polich J, Lardon MT. P300 and long-term physical exercise. Electroencephalogr Clin Neurophysiol. 1997;103:493–498. doi: 10.1016/s0013-4694(97)96033-8. [DOI] [PubMed] [Google Scholar]
- 143.Pontifex MB, Hillman CH, Polich J. Age, physical fitness, and attention: P3a and P3b. Psychophysiology. 2009;46:379–387. doi: 10.1111/j.1469-8986.2008.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pontifex MB, Raine LB, Johnson CR, Chaddock L, Voss MW, Cohen NJ, Kramer AF, Hillman CH. Cardiorespiratory fitness and the flexible modulation of cognitive control in preadolescent children. J Cogn Neurosci. 2011;23:1332–1345. doi: 10.1162/jocn.2010.21528. [DOI] [PubMed] [Google Scholar]
- 145.Posner MI. Attention as a cognitive neural system. Curr Dir Psychol Sci. 1992;1:11–14. [Google Scholar]
- 146.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 147.Prakash RS, Voss MW, Erickson KI, Lewis JM, Chaddock L, Malkowski E, Alves H, Kim J, Szabo A, White SM, Wójcicki TR, Klamm EL, McAuley E, Kramer AF. Cardiorespiratory fitness and attentional control in the aging brain. Front Hum Neurosci. 2011;4:229. doi: 10.3389/fnhum.2010.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ramsey MM, Adams MM, Ariwodola OJ, Sonntag WE, Weiner JL. Functional characterization of des-IGF-1 action at excitatory synapses in the CA1 region of rat hippocampus. J Neurophysiol. 2005;94:247–254. doi: 10.1152/jn.00768.2004. [DOI] [PubMed] [Google Scholar]
- 149.Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik F, Salthouse T, editors. Handbook of Aging and Cognition, II. Mahwah, NJ: Erlbaum; 2000. pp. 1–90. [Google Scholar]
- 150.Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: Evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- 151.Reul JM, Hesketh SA, Collins A, Mecinas MG. Epigenetic mechanisms in the dentate gyrus act as a molecular switch in hippocampus-associated memory formation. Epigenetics. 2009;4:434–439. doi: 10.4161/epi.4.7.9806. [DOI] [PubMed] [Google Scholar]
- 152.Rosano C, Venkatraman VK, Guralnik J, Newman AB, Glynn NW, Launer L, Taylor CA, Williamson J, Studenski S, Pahor M, Aizenstein H. Psychomotor speed and functional brain MRI 2 years after completing a physical activity treatment. J Gerontol A Biol Sci Med Sci. 2010;65:639–647. doi: 10.1093/gerona/glq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rovio S, Spulber G, Nieminen LJ, Niskanen E, Winblad B, Tuomilehto J, Nissinen A, Soininen H, Kivipelto M. The effect of midlife physical activity on structural brain changes in the elderly. Neurobiol Aging. 2010;31:1927–1936. doi: 10.1016/j.neurobiolaging.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 154.Saatman KE, Contreras PC, Smith DH, Raghupathi R, McDermott KL, Fernandez SC, Sanderson KL, Voddi M, McIntosh TK. Insulin-like growth factor-1 (IGF-1) improves both neurological motor and cognitive outcome following experimental brain injury. Exp Neurol. 1997;147:418–427. doi: 10.1006/exnr.1997.6629. [DOI] [PubMed] [Google Scholar]
- 155.Sakamoto K, Karelina K, Obrietan K. CREB: A multifaceted regulator of neuronal plasticity and protection. J Neurochem. 2011;116:1–9. doi: 10.1111/j.1471-4159.2010.07080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 157.Scheffers MK, Coles MG, Bernstein P, Gehring WJ, Donchin E. Event-related brain potentials and error-related processing: An analysis of incorrect responses to go and no-go stimuli. Psychophysiology. 1996;33:42–53. doi: 10.1111/j.1469-8986.1996.tb02107.x. [DOI] [PubMed] [Google Scholar]
- 158.Scheibel AB. Structural and functional changes in the aging brain. In: Birren JE, Schaie DW, editors. Handbook of the Psychology of Aging. San Diego: Academic Press; 1996. pp. 105–128. [Google Scholar]
- 159.Scisco JL, Leynes PA, Kang J. Cardiovascular fitness and executive control during task-switching: An ERP study. Int J Psychophysiol. 2008;69:52–60. doi: 10.1016/j.ijpsycho.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 160.Sibley BA, Etnier JL. The relationship between physical activity and cognition in children: A meta-analysis. Pediatr Exerc Sci. 2003;15:243–256. [Google Scholar]
- 161.Smith JC, Nielson KA, Woodard JL, Seidenberg M, Durgerian S, Antuono P, Butts AM, Hantke NC, Lancaster MA, Rao SM. Interactive effects of physical activity and APOE-ε4 on BOLD semantic memory activation in healthy elders. Neuroimage. 2011;54:635–644. doi: 10.1016/j.neuroimage.2010.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Spirduso WW. Reaction and movement time as a function of age and physical activity level. J Gerontol. 1975;30:435–440. doi: 10.1093/geronj/30.4.435. [DOI] [PubMed] [Google Scholar]
- 164.Spirduso WW. Physical fitness, aging, and psychomotor speed: A review. J Gerontol. 1980;35:850–865. doi: 10.1093/geronj/35.6.850. [DOI] [PubMed] [Google Scholar]
- 165.Spirduso WW, Clifford P. Replication of age and physical activity effects on reaction and movement time. J Gerontol. 1978;33:26–30. doi: 10.1093/geronj/33.1.26. [DOI] [PubMed] [Google Scholar]
- 166.Squire LR, Kandel ER. Memory from Mind to Molecules. New York: W.H. Freeman and Co; 1999. p. 235. [Google Scholar]
- 167.Sullivan EV, Pfefferbaum A, Adalsteinsson E, Swan GE, Carmelli D. Differential rates of regional brain change in callosal and ventricular size: A 4-year longitudinal MRI study of elderly men. Cereb Cortex. 2002;12:438–445. doi: 10.1093/cercor/12.4.438. [DOI] [PubMed] [Google Scholar]
- 168.Sutton S, Braren M, Zubin J, John ER. Evoked-potential correlates of stimulus uncertainty. Science. 1965;150:1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- 169.Suzuki H, Park SJ, Tamura M, Ando S. Effect of the long-term feeding of dietary lipids on the learning ability, fatty acid composition of brain stem phospholipids and synaptic membrane fluidity in adult mice: A comparison of sardine oil diet with palm oil diet. Mech Ageing Dev. 1998;101:119–128. doi: 10.1016/s0047-6374(97)00169-3. [DOI] [PubMed] [Google Scholar]
- 170.Sweatt JD. Experience-dependent epigenetic modifications in the central nervous system. Biol Psychiatry. 2009;65:191–197. doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Teague WE, Fuller NL, Rand RP, Gawrisch K. Polyunsaturated lipids in membrane fusion events. Cell Mol Biol Lett. 2002;7:262–264. [PubMed] [Google Scholar]
- 172.Themanson JR, Hillman CH. Cardiorespiratory fitness and acute aerobic exercise effects on neuroelectric and behavioral measures of action monitoring. Neuroscience. 2006;141:757–767. doi: 10.1016/j.neuroscience.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 173.Themanson JR, Hillman CH, Curtin JJ. Age and physical activity influences on action monitoring during task switching. Neurobiol Aging. 2006;27:1335–1345. doi: 10.1016/j.neurobiolaging.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 174.Themanson JR, Pontifex MB, Hillman CH. Fitness and action monitoring: Evidence for improved cognitive flexibility in young adults. Neuroscience. 2008;157:319–328. doi: 10.1016/j.neuroscience.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Thiel G. Synapsin I, synapsin II, and synaptophysin: Marker proteins of synaptic vesicles. Brain Pathol. 1993;3:87–95. doi: 10.1111/j.1750-3639.1993.tb00729.x. [DOI] [PubMed] [Google Scholar]
- 176.Tomporowski PD, Davis CL, Miller PH, Naglieri JA. Exercise and children’s intelligence, cognition, and academic achievement. Educ Psychol Rev. 2008;20:111–131. doi: 10.1007/s10648-007-9057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tonra JR, Ono M, Liu X, Garcia K, Jackson C, Yancopoulos GD, Wiegand SJ, Wong V. Brain-derived neurotrophic factor improves blood glucose control and alleviates fasting hyperglycemia in C57BLKS-Lepr(db)/lepr(db) mice. Diabetes. 1999;48:588–594. doi: 10.2337/diabetes.48.3.588. [DOI] [PubMed] [Google Scholar]
- 178.Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Trudeau F, Shephard RJ. Physical education, school physical activity, school sports and academic performance. Int J Behav Nutr Phys Act. 2008;5:10. doi: 10.1186/1479-5868-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tsai SJ, Hong CJ, Liou YJ. Effects of BDNF polymorphisms on antidepressant action. Psychiatry Investig. 2010;7:236–242. doi: 10.4306/pi.2010.7.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 182.Uchida S, Inanaga Y, Kobayashi M, Hurukawa S, Araie M, Sakuragawa N. Neurotrophic function of conditioned medium from human amniotic epithelial cells. J Neurosci Res. 2000;62:585–590. doi: 10.1002/1097-4547(20001115)62:4<585::AID-JNR13>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 183.Uzendoski AM, Latin RW, Berg KE, Moshier S. Physiological responses to aerobic exercise during pregnancy and post-partum. J Sports Med Phys Fitness. 1990;30:77–82. [PubMed] [Google Scholar]
- 184.van der Lely AJ, Tschöp M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004;25:426–457. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- 185.van Praag H. Exercise and the brain: Something to chew on. Trends Neurosci. 2009;32:283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. J Cogn Neurosci. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- 187.Vasuta C, Caunt C, James R, Samadi S, Schibuk E, Kannangara T, Titterness AK, Christie BR. Effects of exercise on NMDA receptor subunit contributions to bidirectional synaptic plasticity in the mouse dentate gyrus. Hippocampus. 2007;17:1201–1208. doi: 10.1002/hipo.20349. [DOI] [PubMed] [Google Scholar]
- 188.Vaynman S, Gomez-Pinilla F. Revenge of the “sit”: How lifestyle impacts neuronal and cognitive health through molecular systems that interface energy metabolism with neuronal plasticity. J Neurosci Res. 2006;84:699–715. doi: 10.1002/jnr.20979. [DOI] [PubMed] [Google Scholar]
- 189.Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122:647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 190.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 191.Voss MW, Erickson KI, Prakash RS, Chaddock L, Malkowski E, Alves H, Kim JS, Morris KS, White SM, Wójcicki TR, Hu L, Szabo A, Klamm E, McAuley E, Kramer AF. Functional connectivity: A source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia. 2010;48:1394–1406. doi: 10.1016/j.neuropsychologia.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, Alves H, Heo S, Szabo AN, White SM, Wójcicki TR, Mailey EL, Gothe N, Olson EA, McAuley E, Kramer AF. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2:1–17. doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Waddington CH. Canalization of development and the inheritance of acquired characters. Nature. 1942;150:563–565. doi: 10.1038/1831654a0. [DOI] [PubMed] [Google Scholar]
- 194.Wendorf M, Goldfine ID. Archaeology of NIDDM. Excavation of the “thrifty” genotype. Diabetes. 1991;40:161–165. doi: 10.2337/diab.40.2.161. [DOI] [PubMed] [Google Scholar]
- 195.Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 196.Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. 2008;155:751–759. doi: 10.1016/j.neuroscience.2008.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychol Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- 198.Zenobi PD, Holzmann P, Glatz Y, Riesen WF, Froesch ER. Improvement of lipid profile in type 2 (non-insulin-dependent) diabetes mellitus by insulin-like growth factor I. Diabetologia. 1993;36:465–469. doi: 10.1007/BF00402285. [DOI] [PubMed] [Google Scholar]