Abstract
Background
Patient websites with secure access to shared electronic medical records (SMR) may support care of patients with HIV, particularly during heightened need. However, groups disproportionately affected by HIV may be less likely to use them.
Objective & Design
We performed an observational cohort study to compare use of seven SMR features by adult patients with HIV. Automated data from the 36 months following SMR implementation were assessed in two integrated delivery systems.
Participants, Main Measures, Key Results
Most (3888/7398) patients used the SMR at least once. Users were most likely to view medical test results (49%), use secure messaging (43%), or request appointments (31%) or medication refills (30%). Initial use was associated with a new prescription for antiretroviral therapy [rate ratio (RR) 1.65, p <0.001], a recent change to CD4+ count <200 cells/μL (RR 1.34, p <0.02), a new HIV RNA ≥75 copies/mL (RR 1.63, p <0.001), or a recent increase in non-HIV comorbidity score (RR 1.49, p = 0.0001). In age-, sex-, and comorbidity-adjusted analyses, users were less likely to be women (RR 0.49, p=0.0001), injection drug users (RR 0.59, p = 0.0001), or from lower-socioeconomic neighborhoods (RR 0.68, p = 0.0001). Compared with nonusers, users were less likely to be Black (RR 0.38, p = 0.0001), Hispanic (RR 0.52, p = 0.0001) or Asian/Pacific Islander (RR 0.59, p = 0.001).
Conclusions
SMR use was higher among those with HIV who had indicators of recent increases in health care need and lower among several vulnerable populations. Health care providers and systems should support SMR use among patients with HIV as part of broader efforts to improve overall access to care.
Keywords: HIV, personal health record, health care disparities
BACKGROUND
Ongoing collaboration with providers is essential for care of patients with HIV. To reduce HIV-related morbidity and mortality, patients and providers monitor CD4+ T-cell counts and HIV viral loads and initiate and adjust antiretroviral therapy. Patients on antiretroviral therapy must adhere tightly to the regimen for benefits, while managing the medications’ frequent side effects.1 Health care systems that enable good communication with providers and access to services such as laboratory monitoring and medication refills are critical for patients to manage HIV infection successfully.
Patient websites providing secure access to electronic medical records that are shared between patients and health care providers may help meet the ongoing care needs of many patients with HIV. Also known as integrated personal health records, these web-based shared electronic medical records (SMR) can provide a constellation of services for patients, typically including exchanging secure electronic messages and scheduling appointments with health care providers, ordering medication refills, and viewing care plans, medical test results, and other portions of the electronic medical record.2–4 Proposed federal “meaningful use” criteria for electronic health records support patients’ use of the SMR including secure messaging with providers.5 Many of these services may help patients with HIV, particularly during heightened need, such as starting new antiretroviral medications or having a significant CD4+ count decrease.
Despite the SMR’s potential, some groups of patients disproportionately affected by HIV may be less likely to use it. HIV is estimated to be nine times more common in blacks and nearly three times more common in Hispanics6 than in whites; black and Hispanic populations are also less likely to receive highly active antiretroviral therapy(HAART)7 and experimental treatments. Individuals with low socioeconomic status (SES) are twice as likely to have HIV8 and are more likely to die of HIV9 and not receive HAART. Older patients with HIV have faster progression of disease, with treatment often complicated by coexisting chronic health conditions.10 All these sociodemographic groups are also less likely to use the Internet11 and patient websites.12–15 An initial study of personal health record use among patients with HIV receiving care at San Francisco General Hospital also found that users were more likely be Caucasian and non-Hispanic.16 Further understanding potential differences in SMR use by patients with HIV who belong to vulnerable populations is essential to ensure health care is designed to meet the needs of all patients with HIV. These potential differences are particularly important if the SMR is being used to support care at critical times, such as initiation of ART or a drop in CD4+ cell count.
METHODS
We performed a cohort study of adult HIV+ patients in the first 36 months following implementation of the SMR at Group Health (GH) (8/1/03–7/31/06) and Kaiser Permanente Northern California (KPNC) (11/1/05–10/31/08). GH and KPNC are large, integrated, health care delivery organizations providing multidisciplinary care, including HIV specialty care. The study population included enrollees aged 18 years or older in either institution’s HIV registry. Patients were followed from the date they met eligibility criteria (≥18, HIV+, enrolled in health plan) until the earliest of disenrollment, death, or the end of the study period.
Beginning in 2003 at GH and 2005 at KPNC, all patients could access an SMR (www.ghc.org or www.kp.org) with seven features common to both sites: secure messaging with health care providers; requesting medication refills; requesting in-person appointments; and viewing after-visit summaries, allergies, immunizations, and test results (excluding CD4+ and HIV RNA results at KPNC). Detailed descriptions of the patient websites at GH3, 17 and KPNC4 were previously reported. Patients verified their identity to GH or KPNC before using these features.17 We hypothesized that SMR use would be higher among those with a recent heightened need for care but lower among racial and ethnic minorities, older patients, and those from lower socioeconomic groups.
DATA SOURCES
The KPNC HIV registry18, 19 includes all known cases since the early 1980s and the GH registry since 1997. Registry data include sex; birth date; race/ethnicity; dates of known HIV infection and AIDS diagnoses; and, at the KPNC only, HIV transmission risk factors. KPNC and GH also have historical databases on member demographics, prescriptions, hospitalizations, outpatient visits, and laboratory tests, including CD4+ T-cell count and HIV RNA test results, health insurance status, and Zip code. Date of deaths was identified from hospitalizations, membership files, California and Washington state death certificates, and Social Security Administration data sets.
MEASURES
Use of the shared medical record
Primary outcome of interest was any SMR use defined as using at least one of the seven SMR features during the study period. Secondary outcome was continued SMR use (mean days of SMR use/month). Rates of use were measured as the number of days per month in which patients used any of the SMR features.
Variables potentially associated with shared medical record use
Primary predictors were recent increase in health care need, race/ethnicity, neighborhood SES, and age. We defined recent increase in health care need as one of the following clinical events occurring within the prior three months: start or restart of antiretroviral therapy (ART); new CD4+ <200 cells/μL; newly quantifiable HIV RNA ≥75 copies/mL; or worsening comorbidity unrelated to HIV. Non-HIV morbidity was measured using a modified Charlson index, excluding HIV/AIDS diagnoses.20 Neighborhood SES was categorized as “low” for a patient if at least 20% of 2000 Census block residents had an income below $20,000 or at least 25% of residents over age 25 had not completed high school.14, 21, 22 Secondary predictors included sex, HIV risk factors, insurance status, time with health plan, and specific comorbid conditions (depression and hepatitis B and C). Predictor selection was based on prior studies of SMR use in other populations13, 23, 24 and prior studies of access to care in people with HIV.7 Depression was defined by outpatient diagnosis in prior 12 months. HIV transmission risk factors and history of hepatitis B and C were from the HIV and the HCV/HBV registries at each site.
STATISTICAL METHODS
We used Cox proportional hazards analysis to identify the factors associated with any use of the SMR. Outcome was time to first use; rate ratios (RRs; hazard ratios) compared the rate of initial use (percentage of patients per month who first used SMR) with that of a reference group. Separate Cox models were fit to each variable in Table 3, first adjusting for site only, and then for site, sex, age, and non-HIV related morbidity.
Table 3.
Predictors of Any Use of Shared Electronic Medical Record During the 36 Months After Initial Shared Medical Record Availability among Patients with HIVa
| Predictors | Unadjusted HR of shared medical record Useb | P | Adjusted HR of shared medical record Usec | P |
|---|---|---|---|---|
| Site: GH vs. KPNC | 1.06 | 0.37 | 1.13 | 0.15 |
| Age, y | ||||
| 18–29 | Reference | |||
| 30–39 | 1.34 | 0.001 | 1.31 | 0.01 |
| 40–49 | 1.18 | 0.05 | 1.17 | 0.13 |
| 50+ | 1.18 | 0.06 | 1.15 | 0.18 |
| Male sex | 2.05 | 0.0001 | 2.06 | 0.0001 |
| Race/ethnicityd | ||||
| Caucasian | Reference | |||
| Black | 0.35 | 0.0001 | 0.38 | 0.0001 |
| Hispanic | 0.54 | 0.0001 | 0.52 | 0.0001 |
| Asian/Pacific Islander | 0.62 | 0.0001 | 0.59 | 0.0001 |
| Native American | 0.30 | 0.04 | 0.30 | 0.04 |
| Other | 0.85 | 0.37 | 0.86 | 0.45 |
| Low neighborhood SES | 0.66 | 0.0001 | 0.68 | 0.0001 |
| Charlson Comorbidity Score (excluding HIV) | ||||
| 0: Low | Reference | |||
| 1: Medium | 0.98 | 0.68 | 1.01 | 0.83 |
| 2+: High | 0.77 | 0.0001 | 0.82 | 0.004 |
| ART | 1.30 | 0.0001 | 1.16 | 0.0001 |
| CD4+ count <200 cells per microliter | 0.83 | 0.002 | 0.90 | 0.11 |
| HIV RNA <75 copies per milliliter | 0.99 | 0.84 | 0.97 | 0.45 |
| Risk Factor for HIV | ||||
| Men sex with men | Reference | Reference | ||
| Intravenous drug user | 0.60 | 0.0001 | 0.59 | 0.0001 |
| Heterosexual | 0.43 | 0.0001 | 0.46 | 0.0001 |
| Hepatitis B Infection: monthly | 1.16 | 0.10 | 1.11 | 0.28 |
| Hepatitis C Infection: monthly | 0.76 | 0.0002 | 0.77 | 0.001 |
| History of depression: monthly | 1.24 | 0.0001 | 1.22 | 0.0001 |
| Tenure with health plan <1 year | 1.53 | 0.0001 | 1.36 | 0.0001 |
| Insurance | ||||
| Commercial | Reference | Reference | ||
| Medicare | 0.83 | 0.001 | 0.94 | 0.28 |
| Medicaid | 0.35 | 0.0001 | 0.45 | 0.0001 |
ART indicates receipt of 1 or more antiretroviral medications; GH, Group Health; HR, hazard ratio; KPNC, Kaiser Permanente Northern California; SES, socioeconomic status.
Pairwise P value versus reference group for variables with multiple categories. All analyses control for site by stratifying on site in Cox proportional hazards regression model.
Rate ratio/HR compares rates of initial use (number of initial users per day) relative to reference group (from time-varying Cox proportional hazards analysis). P tests if rate were same as those of reference group (rate ratio = 1). The following variables were updated monthly: non-HIV-related morbidity, antiretroviral use, CD4 count, viral load, hepatitis B infection, hepatitis C infection, continuity of care, depression diagnosis. Stratified on site (KPNC vs. GH).
Stratified on site and adjusted for age, sex and non-HIV Charlson Comorbidity Index Score.
Race/ethnicity was missing for 67 at GH, 455 at KPNC.
Race/ethnicity analyses adjusted for age, sex, and a modified Charlson index (without HIV/AIDS);25 this allowed potential mediation of SMR use by racial/ethnic group through SES factors26 and is consistent with the Institute of Medicine recommendation for handling potential health care disparities (defined as any difference not due to clinical need or preferences) when comparing groups defined by race/ethnicity.25 Fixed and time-varying characteristics potentially associated with SMR use were identified before the analyses. We looked at baseline factors that did not change during follow-up and examined how the following time-varying factors (updated monthly) were related to use: non-HIV-related morbidity, antiretroviral use, CD4+ count, viral load, hepatitis B infection, hepatitis C infection, and depression diagnosis.
We tested for interactions between sites and each potential predictor of SMR use. To minimize the risk of false-positive interactions, interaction tests used a 0.01 significance level. We also tested for interactions between racial groups and HIV risk factor, sex, and overall HIV healthcare need in past 3 months (any start of antiretroviral therapy, new CD4+ count fewer than 200 cells per microliter, new HIV RNA of 75 or more copies per milliliter, or increase in Charlson Comorbidity Index score).
Cox models assessed the short-term effect of markers of increased health care need on the likelihood of initial SMR use. We defined short-term as three months and assessed whether individuals were more likely to start using the SMR in the three months following one of these events: worsening non-HIV-related morbidity, start of antiretroviral therapy, CD4+ count below 200 cells/μL, viral load exceeding 75 copies/mL.
We also examined which of these factors were correlated with more frequent use of the SMR among those who used the SMR at least once. We used negative binomial regression to compare rates of SMR use following initial use. To account for over-dispersion typical of count data, we used robust standard errors. We fit two negative binomial regression models for each variable, first adjusting only for site and then for site, age, sex, and the non-HIV Charlson index.
RESULTS
The study population consisted of 6644 KPNC and 754 GH patients (Table 1). Compared with GH patients, KPNC patients were more likely to be black (KPNC 17.7% vs. 10.9% GH), Hispanic (KPNC 14.7% vs. GH 5.4%), older (mean age KPNC 45.9 vs. GH 42.8 years), living in a low-SES neighborhood (KPNC 26.6% vs. GH 18.1%), and HIV+ for longer (KPNC 9.3 vs. GH 3.5 years). Race/ethnicity was missing for 455 (6.6%) at KPNC and 67 (8.9%) patients at GH.
Table 1.
Baseline Characteristics of Kaiser Permanente Northern California and Group Health Populations with HIV.
| KPNC | GH | Total | ||||
|---|---|---|---|---|---|---|
| N | 6644 | 754 | 7398 | |||
| Age, mean (SD), y | 45.9 | (10.1) | 42.8 | (10.0) | 45.6 | (10.1) |
| 18–29 | 5.6% | 8.6% | 5.9% | |||
| 30–39 | 21.2% | 30.4% | 22.1% | |||
| 40–49 | 40.3% | 35.1% | 39.8% | |||
| 50+ | 32.9% | 25.9% | 32.2% | |||
| Male | 89.5% | 91.1% | 89.7% | |||
| Race/Ethnicitya | ||||||
| Caucasian | 61.5% | 80.1% | 63.3% | |||
| Black | 17.7% | 10.9% | 17.0% | |||
| Hispanic/Latino | 14.7% | 5.4% | 13.8% | |||
| Asian/Pacific Islander | 5.0% | 2.9% | 4.8% | |||
| Native American | 0.2% | 0.4% | 0.2% | |||
| Other | 0.9% | 0.3% | 0.8% | |||
| Low neighborhood SES2 | 26.6% | 18.1% | 25.7% | |||
| Charlson Comorbidity Score (excluding HIV)b | ||||||
| 0 to <1 | 77.6% | 82.2% | 78.0% | |||
| 1 to <2 | 12.6% | 10.4% | 12.4% | |||
| ≥2 | 9.7% | 7.5% | 9.5% | |||
| ARTb | 57.0% | 52.8% | 56.6% | |||
| AIDS diagnosisb | 53.9% | 31.3% | 51.6% | |||
| CD4+ count <200 cells per microliterb | 19.0% | 16.9% | 18.8% | |||
| HIV RNA <75 copies per milliliter | 53.6% | 42.6% | 52.5% | |||
| HIV Transmission Risk Behavior | ||||||
| Men sex with men | 73.4% | NA | 73.4% | |||
| Injection drug use | 7.8% | NA | 7.8% | |||
| Heterosexual | 17.4% | NA | 17.4% | |||
| Other | 1.4% | NA | 1.4% | |||
| Known Hepatitis B Infectionb | 3.3% | 0.4% | 3.0% | |||
| Known Hepatitis C Infection | 6.7% | 2.1% | 6.2% | |||
| Documented History of Depressionb | 31.0% | 38.7% | 31.8% | |||
| Health Plan Tenure, y, mean (SD) | 8.3 | (7.9) | 10.0 | (10.3) | 8.5 | (8.2) |
| Insurance | ||||||
| Commercial | 86.7% | 85.9% | 86.7% | |||
| Medicare | 10.6% | 11.0% | 10.6% | |||
| Medicaid | 2.7% | 3.2% | 2.7% | |||
| Ambulatory encounters, annual, mean (SD)b | 5.4 | (5.5) | 3.2 | (4.1) | 5.2 | (5.4) |
| Phone Encounters, annual, mean (SD)b | 0.1 | (0.5) | 0.8 | (2.2) | 0.2 | (0.9) |
ART indicates receipt of 1 or more antiretroviral medications; GH, Group Health; KPNC, Kaiser Permanante Northern California; NA, not available; SD standard deviation; SES, socioeconomic status.
Race/ethnicity missing for 67 at GH, 455 at KPNC.
Based on 24 months of data before baseline.
Overall use of the shared medical record by health plan
Over the 36-month study period, 3,411 (51.3%) KPNC participants and 477 (63.6%) at GH (p = 0.01 between sites) used the SMR. Among the SMR’s seven available functions, the highest proportion of enrollees requested medication refills (27.9% KPNC, 58.9% GH), used secure messaging (41.1% KPNC, 57.6% GH), viewed medical test results (47.4% KPNC, 62.1% GH), and requested appointments (30.3% KPNC, 39.6% GH) (Table 2).
Table 2.
Proportion of HIV-Positive Enrollees Using Functions of the Online Shared Electronic Medical Record, First 36 Months of Service at Group Health and Kaiser Permanentea
| Shared Electronic Record Function | KPNC, % | GH, % |
|---|---|---|
| Any Use | 51.3% | 63.6% |
| Pharmacy refills and list of medications | 27.9% | 58.9% |
| Secure messaging to and from healthcare team | 41.1% | 57.6% |
| Medical test results | 47.4% | 62.1% |
| After-visit summaries | 10.2% | 52.2% |
| List of allergies | 5.1% | 30.6% |
| Immunization history | 20.0% | 39.3% |
| Appointment requests | 30.3% | 39.6% |
GH indicates Group Health; KPNC, Kaiser Permanente Northern California.
Patient websites for Kaiser Permanente Northern California and Group Health are www.kp.org and www.ghc.org, respectively.
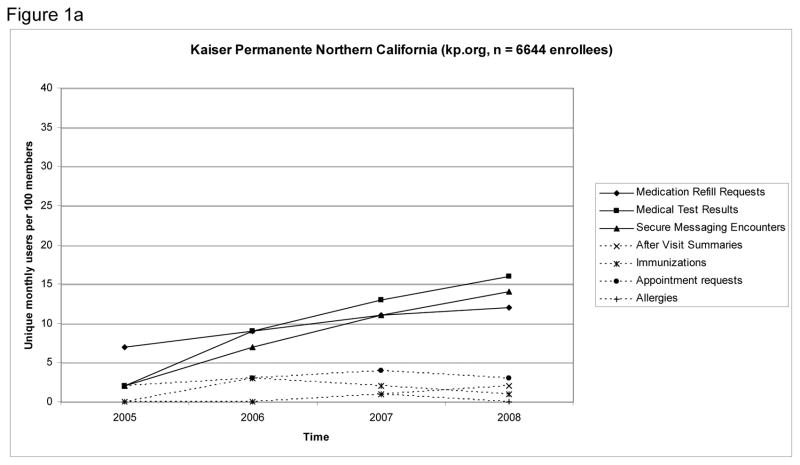
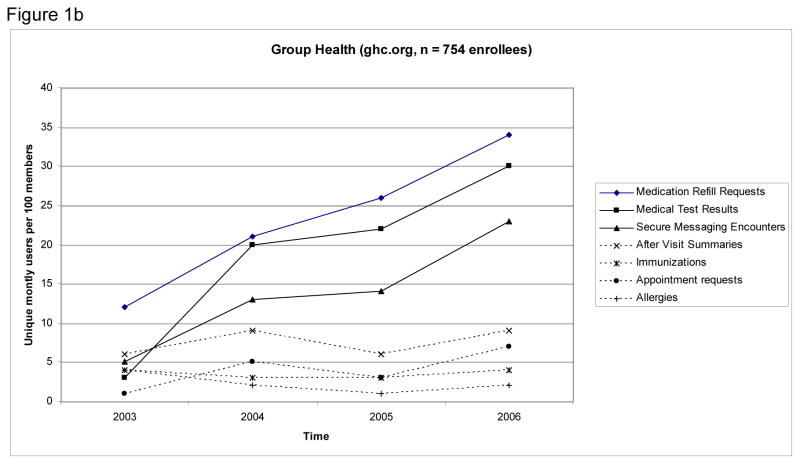
Figure 1 shows rates of use for SMR functions over the study period. In the final month of the study, the most frequently used features were viewing medical test results (16 unique users per 100 enrollees KPNC; 28 unique users per 100 enrollees GH), requesting medication refills (12 unique users per 100 enrollees KPNC; 34 unique users per 100 enrollees GH), and using secure messaging (14 unique users per 100 enrollees KPNC; 23 unique users per 100 enrollees GH).
Figure 1.
Figures 1 a & b: Monthly Use Rate of Functions of Shared Electronic Medical Records Among Patients with HIV, First 36 Months of Service at Kaiser Permanente Northern California (Panel A) and Group Health (Panel B)a
Initial shared medical record use
We show results of analyses combining participants at KPNC and GH (Table 3). To see if there was evidence that the factors associated with SMR use varied by site, we tested for interactions between each factor and site with SMR use as the outcome. None of the interactions were significant so we report only the overall hazard ratio for each factor. Concordant with hypotheses, initial users were less likely to be black [ratio of signup rates (RR) 0.35, p = 0.0001], Hispanic (RR 0.54, p = 0.0001), Asian/Pacific Islander (RR 0.62, p = 0.0001), or Native American (RR 0.30, p = 0.04) than to be of white, non-Hispanic origin. These differences persisted in analyses adjusting for age, sex, and non-HIV morbidity. The relationship between initial SMR use and race/ethnicity was similar across risk factors for HIV (p = 0.10 for interaction test) and across sex (p = 0.76). These racial differences were present whether or not someone had recently experienced one or more of the HIV-related clinical events listed in table 4, such as starting on ART or having their viral load rise above the detectable limit (p=.50 to test for race by event interaction). As shown in Table 3, SMR users were also less likely to live in lower-SES neighborhoods (RR 0.66, p = 0.0001). Contrary to our hypothesis, SMR users were not more likely to be younger (RR 1.18, p = 0.06 comparing those 50 years and older with those 18–29 years).
Table 4.
Relationship Between Change in Clinical Status and Initial Use of Shared Electonic Medical Record in the Subsequent 3 Months.
| Changes | Unadjusted HR of shared medical record Usea | P | Adjusted HR of shared medical record Usea | P |
|---|---|---|---|---|
| Change to higher Charlson Comorbidity Score (excluding HIV)b | 1.29 | 0.01 | 1.49 | 0.0001 |
| New to ART (1st time) | 1.69 | 0.0001 | 1.65 | 0.0001 |
| CD4+ count <200 cells per microliter | 1.33 | 0.02 | 1.34 | 0.02 |
| HIV RNA ≥75 copies per milliliter | 1.69 | 0.0001 | 1.63 | 0.0001 |
ART indicates receipt of 1 or more antiretroviral medications; HR, hazard ratio.
Rate ratio/HR compares rates of initial use (number of initial users per day) relative to reference group (from Cox proportional hazards analysis stratified on site). Adjusted for age, sex, and non-HIV-related morbidity and, stratified on site. All variables in table were time-varying and updated monthly.
Change to higher level of monthly non-AIDS Charlson Comorbidity Index score.
SMR users were more likely to be men (RR 2.05, p = 0.0001) (Table 3). Compared to men with a history of sex with men, SMR use was lower among those with a history of injection drug use (RR 0.60, p = 0.0001) and heterosexual activity (RR 0.43, p = 0.0001). SMR users were more likely to be older than 18 to 29 years (RR 1.34, p = 0.001 for 30 to 39 years; RR 1.18, p = 0.05 for 40 to 49 years), have a lower non-HIV morbidity score (RR 0.77, p = 0.0001 comparing high to low Charlson Score), be taking antiretroviral therapy (RR 1.30, p = 0.0001), have CD4+ count <200 cells/μL (RR 0.83, p = 0.002), have hepatitis C (RR 0.76, p = 0.0002), have depression (RR 1.24, p = 0.0001), have been with a health plan less than a year (RR 1.53, p = 0.0001), and have Medicare (RR 0.83, p = 0.001) or Medicaid (RR 0.35, p = 0.0001) compared to commercial insurance. Those 50 years of age and older had a trend toward higher use compared with the 18 to 29 year old population (RR 1.18, p = 0.06). All tests of the proportional hazards assumption were non-significant, indicating the association of each variable with initial SMR use was similar in all three years.
Recent health care need and initial shared medical record use
Concordant with our hypotheses, higher initial use of SMR was associated with a new prescription for antiretroviral therapy (RR 1.69, p <0.0001), a recent change to HIV RNA ≥75 copies/mL (RR 1.69, p <0.0001), a recent change to CD4+ count <200 cells/μL, and a recent change to a higher non-HIV morbidity score (Table 4).
Amount of shared medical record use
Among those who used the SMR at least once, black SMR users on average used the SMR 20% less frequently than did white SMR-users (RR 0.80, p = 0.0001) (Table 5). Hispanic and Asian/Pacific Islander SMR users used it about 15% less (p = 0.02, p = 0.04, respectively). Adjustment for age, sex, site, and health care need minimally changed results. Using the SMR more often was associated with a recent change to detectable HIV RNA ≥75 copies/mL (RR 1.10, p <0.03) (Table 6).
Table 5.
Comparison of Frequency of Use of the Shared Electronic Medical Record (Mean Times per Month) Among Those Who Used the Shared Electronic Medical Record at Least Oncea
| Characteristic | Unadjusted Ratio of Mean Use Ratesb | P | Adjusted Ratio of Mean Use Ratesc | P |
|---|---|---|---|---|
| Site | ||||
| KPNC | Reference | Reference | ||
| GH | 1.74 | 0.0001 | 1.93 | 0.0001 |
| Age, y | ||||
| 18–29 | Reference | Reference | ||
| 30–39 | 1.05 | 0.45 | 1.06 | 0.51 |
| 40–49 | 1.14 | 0.06 | 1.11 | 0.22 |
| 50+ | 1.30 | 0.0001 | 1.19 | 0.04 |
| Male sex | 1.08 | 0.18 | 1.02 | 0.77 |
| Race/ethnicityd | ||||
| Caucasian | Reference | Reference | ||
| African American | 0.80 | 0.0001 | 0.81 | 0.0002 |
| Hispanic | 0.86 | 0.02 | 0.89 | 0.04 |
| Asian/Pacific Islander | 0.82 | 0.04 | 0.92 | 0.32 |
| Native American | 0.58 | 0.38 | 0.90 | 0.86 |
| Other | 0.90 | 0.67 | 1.07 | 0.69 |
| Low neighborhood SES | 0.92 | 0.03 | 0.94 | 0.13 |
| Charlson Comorbidity Score (excluding HIV) | ||||
| 0: Low | Reference | Reference | ||
| 1: Medium | 1.14 | 0.01 | 1.12 | 0.02 |
| 2+: High | 1.33 | 0.0001 | 1.30 | 0.0001 |
| ART | 0.98 | 0.58 | 0.95 | 0.15 |
| CD4+ count <200 cells per microliter | 1.17 | 0.001 | 1.20 | 0.002 |
| HIV RNA <75 copies per microliter | 0.97 | 0.32 | 0.91 | 0.02 |
| Risk factor for HIV | ||||
| Men sex with men | Reference | Reference | ||
| Intravenous Drug User | 1.16 | 0.03 | 1.25 | 0.006 |
| Heterosexual | 0.87 | 0.01 | 0.88 | 0.13 |
| Other | 1.18 | 0.27 | 1.22 | 0.24 |
| Hepatitis B Infection | 1.14 | 0.09 | 1.12 | 0.22 |
| Hepatitis C Infection | 1.28 | 0.0001 | 1.30 | 0.0002 |
| History of Depression | 1.10 | 0.90 | 1.11 | 0.02 |
| Tenure with Health plan <1 year | 1.03 | 0.55 | 1.05 | 0.25 |
| Insurance | ||||
| Commercial | Reference | Reference | ||
| Medicare | 1.06 | 0.23 | 0.94 | 0.25 |
| Medicaid | 0.98 | 0.91 | 1.00 | 0.99 |
ART indicates receipt of one or more antiretroviral medications; GH, Group Health; HR, hazard ratio; KPNC, Kaiser Permanente Northern California; SES, socioeconomic status.
P tests if rates were same as those of reference group (ie, if ratio = 1). Time-varying covariates were assessed as of the month a person first used the shared electronic medical record and included non-HIV-related morbidity, antiretroviral use, CD4+ count, viral load, hepatitis B infection, hepatitis C infection, continuity of care, depression diagnosis, and Charlson Comorbidity Index score(excluding HIV).
Ratio of mean use rates (mean number of times shared electronic medical record was used per month) relative to reference group (from negative binomial regression that adjusted for site only).
Adjusted for site, age, sex and Charlson non-HIV morbidity index.
Race/ethnicity was missing for 67 at GH, 455 at KPNC.
Table 6.
Relationship between Change in Clinical Status and Frequency of Use of Shared Electronic Medical Record (Mean Times per Month) in the Subsequent 3 Months Among Those Who Used the Shared Electronic Medical Record at Least Once.
| Clinical Status | Unadjusted Ratio of Mean Use Ratesa | P | Adjusted Ratio of Mean Use Ratesb | P |
|---|---|---|---|---|
| Change to a higher Charlson Comorbidity Score (excluding HIV)c | 1.15 | 0.09 | 1.12 | 0.15 |
| New to ART | 1.01 | 0.91 | 1.15 | 0.06 |
| CD4+ count <200 cells per microliter | 1.13 | 0.27 | 1.22 | 0.10 |
| HIV RNA ≥75 copies per milliliter | 1.10 | 0.03 | 1.17 | 0.007 |
ART indicates receipt of one or more antiretroviral medications
Rate of use rates (mean number of times shared electronic medical record is used per month) relative to reference group (from negative binomial regression that adjusted for site only). P tests if rates were the same as those of reference group (ie, if ratio = 1).
Ratio of use rates adjusted for site, age, sex, non-HIV Charlson Comorbidity index (analysis of the comorbidity index adjusted for only 1st 3 variables). All variables in the table were time-varying and were assessed as of the month a person first used the SMR (non-HIV Charlson Comorbidity index also assessed as of this date).
Change to higher level of monthly non-AIDS Charlson Comorbidity index.
DISCUSSION
In the first three years after implementation, a little more than half of all patients with HIV used at least one SMR feature. Patients were most likely to use the SMR to communicate electronically with providers, obtain medication refills, schedule appointments, and view medical test results. Compared with other SMR features, these four services support more frequent patient activities in managing HIV care. Patients with HIV used these four online features two to five times more often each month than the general enrollee population.3, 4 In 2006, for example, the general population at Group Health had 4 unique users of secure messaging each month for every 100 enrollees3 compared with 23 unique users for every 100 patients with HIV. Use of an SMR was also more likely in patients with a recent decline in CD4+ count, a newly elevated HIV RNA level, and recent initiation of antiretroviral therapy. These events often mark a heightened need for care that may include communication with health care providers between office visits and medication adjustment or monitoring. To help meet these needs, providers have traditionally used phone calls between office visits; but for many patients, the SMR may now be more efficient and effective. Although no trials have reported using the SMR to improve care of individuals with HIV, trials using the SMR as part of planned care interventions in other chronic conditions have found improved clinical outcomes, including glycemic control in patients with type 2 diabetes,27 blood pressure control in patients with hypertension,28 and easing of depression in patients recently started on antidepressant medications.29 Our results suggest the online features of the SMR may help health care providers and systems better support many patients with HIV at key times of need.
SMR adoption, however, was not uniform. Major groups of patients disproportionately affected by HIV were less likely to use the SMR. Compared with the white population, blacks were two-thirds less likely, and Hispanics about half as likely, to use the SMR at all. Among those who had used the SMR at least once, blacks used the SMR 19% less often and Hispanics 11% less often than whites. SMR users were also less likely to be from low-SES neighborhoods or have Medicaid insurance. Our results extend findings of other studies among patients with HIV, in people with diabetes and in the general population, showing less use of patient websites among minority racial and ethnic groups12–16 and among people with lower socioeconomic status.12, 15, 23 For race and ethnicity, two of these studies found lower use among blacks regardless of education level,13, 15 suggesting the reasons for the differences transcend socioeconomic clustering;13–15, 30 for some patients who have experienced discrimination or have lower trust in health care providers, the additional social distance associated with the SMR may be a barrier to use. These minority groups have a higher prevalence of HIV and are less likely to receive combination ART,6–9 raising concerns about the possibility of widening disparities in access associated with SMR implementations.
Use of the SMR differed by a few other important patient factors. In contrast to studies in the general population24, 31 and those with diabetes15, 23 showing lower use in the older population, older age was associated with a trend toward higher adoption of the SMR. This may be because there are relatively few individuals over the age of 65 years in the HIV populations studied or to unmeasured patient characteristics attenuating the effect of age in this population. Among SMR users, those 50 years or older also used the SMR more compared with those 18 to 29 years, suggesting that older individuals may be using the SMR to support their overall higher need for care. Patients with depression were more likely to use the SMR, which is in agreement with a study showing mental health and substance abuse conditions were not barriers to engagement in personal health record use.32 Compared with men, women were about half as likely to use the SMR which is also consistent with a prior study of personal health record use among patients with HIV.16 Women have worse HIV-related health outcomes,33 lower use of combination ART,7, 34 and greater likelihood of discontinuing ART.33–35 Use of SMR was also less likely among those with a history of intravenous drug use or heterosexual exposure; both of these populations are also less likely to receive antiretroviral therapy compared with the population of men who have sex with men.7 Further research is needed to identify the causes behind these differences in SMR use and to alleviate potential disparities in access to care. For those individuals from lower income populations, including many intravenous drug users,36 text messaging or mobile health applications may enable further reach of some SMR features. We did not find differences in patient demographic characteristics to account for these site differences in overall rate of initial use.
The study has several limitations. We evaluated use of the SMR among patients with HIV receiving care in two integrated health care delivery systems using advanced electronic medical record systems. Although use of SMRs and advanced electronic medical records is increasing, the generalizability of these results is currently limited. We could not measure participants’ individual-level education or income, both of which are associated with the digital divide.37 KPNC patients were not able to view CD4 or HIV RNA results in the SMR. We also could not measure patient preferences or abilities for communicating with health care providers. We did not account for potential provider factors38 and did not have complete information on different marketing efforts by the healthcare organizations that may have played a role in patients’ SMR use. Examining the relationship between SMR use and other health care utilization, including phone and in person visits, was also beyond the scope of this analysis. Finally, we examined the first three years of use of the SMR. Although the pattern of adoption by racial and ethnic minorities did not change over this time period, longer studies may show different patterns of adoption over time, especially as both organizations cronduct specific outreach to minority groups.
We found higher use of the SMR among patients with a recent increase in need for healthcare and lower use across 4 racial and ethnic minority groups, among women, and patients with a history of injection drug use and lower SES. Healthcare systems should continue efforts to enable SMR use by all patients, while integrating SMR access into broader efforts to better meet patients needs and preferences for access to care, whether in person, online, or by phone. Patients and physicians must be able to communicate freely through the best means possible for each patient and healthcare need. Future studies should seek to better understand and address the needs and care preferences of vulnerable HIV populations with respect to the SMR.
Take-away Points.
Observational cohort study comparing use of seven features of an online shared medical record by adult patients with HIV.
Use was higher among those with HIV who had indicators of recent increases in health care need and lower among several vulnerable populations.
Health care providers and systems should support use of online shared medical records among patients with HIV as part of broader efforts to improve overall access to care.
Acknowledgments
The authors thank Gwen Schweitzer for her help in manuscript preparation. The authors of this study have no disclosures related to this study. This research was supported by National Institutes of Health grant: 5R01MH081750-03 (principal investigator, Sheryl L. Catz) and grant K01AI071725 (principal investigator, Michael Silverberg).
Funding: This study was funded by National Institute of Mental Health grant # R01-MH081750 (PI, Sheryl L. Catz)
Footnotes
Financial Disclosure: None. The authors have no financial disclosures related to the study.
CONFLICTS OF INTEREST
The authors have no conflict of interest with the study.
Contributor Information
Michael J. Silverberg, Email: Michael.J.Silverberg@kp.org.
Wendy A. Leyden, Email: Wendy.Leyden@nsmtp.kp.org.
Michael Horberg, Email: Michael.Horberg@nsmtp.kp.org.
Sheryl L. Catz, Email: catz.s@ghc.org.
References
- 1.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008 Aug 6;300(5):555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 2.Tang PC, Lansky D. The missing link: bridging the patient-provider health information gap. Electronic personal health records could transform the patient-provider relationship in the twenty-first century. Health Aff (Millwood) 2005 Sep-Oct;24(5):1290–1295. doi: 10.1377/hlthaff.24.5.1290. [DOI] [PubMed] [Google Scholar]
- 3.Ralston JD, Coleman K, Reid RJ, Handley MR, Larson EB. Patient experience should be part of meaningful-use criteria. Health Aff (Millwood) 2010 Apr;29(4):607–613. doi: 10.1377/hlthaff.2010.0113. [DOI] [PubMed] [Google Scholar]
- 4.Silvestre AL, Sue VM, Allen JY. If you build it, will they come? The Kaiser Permanente model of online health care. Health Aff (Millwood) 2009 Mar-Apr;28(2):334–344. doi: 10.1377/hlthaff.28.2.334. [DOI] [PubMed] [Google Scholar]
- 5.HIT Policy Committee. Meaningful Use Workgroup Request for Comments Regarding Meaningful Use Stage 22011. [Google Scholar]
- 6.CDC. HIV/AIDS Surveillance Report. 2009. [Google Scholar]
- 7.Andersen R, Bozzette S, Shapiro M, et al. Access of vulnerable groups to antiretroviral therapy among persons in care for HIV disease in the United States. HCSUS Consortium. HIV Cost and Services Utilization Study. Health Serv Res. 2000 Jun;35(2):389–416. [PMC free article] [PubMed] [Google Scholar]
- 8.Denning PDE. Communities in Crisis: is there a generalized HIV epidemic in impoverished urban areas of the United States. Paper presented at: XVIII International AIDS Conference; July 18–23, 2010; Vienna. 2010. [Google Scholar]
- 9.Cunningham WE, Hays RD, Duan N, et al. The effect of socioeconomic status on the survival of people receiving care for HIV infection in the United States. J Health Care Poor Underserved. 2005 Nov;16(4):655–676. doi: 10.1353/hpu.2005.0093. [DOI] [PubMed] [Google Scholar]
- 10.Kirk JB, Goetz MB. Human immunodeficiency virus in an aging population, a complication of success. J Am Geriatr Soc. 2009 Nov;57(11):2129–2138. doi: 10.1111/j.1532-5415.2009.02494.x. [DOI] [PubMed] [Google Scholar]
- 11.PEW Internet and American Life Project. [Accessed May 17, 2011];Demographics of internet users. 2010 http://www.pewinternet.org/Static-Pages/Trend-Data/Whos-Online.aspx.
- 12.Yamin CK, Emani S, Williams DH, et al. The digital divide in adoption and use of a personal health record. Arch Intern Med. 2011 Mar 28;171(6):568–574. doi: 10.1001/archinternmed.2011.34. [DOI] [PubMed] [Google Scholar]
- 13.Roblin DW, Houston TK, 2nd, Allison JJ, Joski PJ, Becker ER. Disparities in use of a personal health record in a managed care organization. J Am Med Inform Assoc. 2009 Sep-Oct;16(5):683–689. doi: 10.1197/jamia.M3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu J, Huang J, Kinsman J, et al. Use of e-Health services between 1999 and 2002: a growing digital divide. J Am Med Inform Assoc. 2005 Mar-Apr;12(2):164–171. doi: 10.1197/jamia.M1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarkar U, Karter AJ, Liu JY, et al. Social disparities in internet patient portal use in diabetes: evidence that the digital divide extends beyond access. J Am Med Inform Assoc. 2011 Jan 24; doi: 10.1136/jamia.2010.006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahn JS, Hilton JF, Van Nunnery T, et al. Personal health records in a public hospital: experience at the HIV/AIDS clinic at San Francisco General Hospital. J Am Med Inform Assoc. 2010 Mar-Apr;17(2):224–228. doi: 10.1136/jamia.2009.000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ralston JD, Carrell D, Reid R, et al. Patient web services integrated with a shared medical record: patient use and satisfaction. J Am Med Inform Assoc. 2007 Nov-Dec;14(6):798–806. doi: 10.1197/jamia.M2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horberg MA, Hurley LB, Silverberg MJ, Kinsman CJ, Quesenberry CP. Effect of clinical pharmacists on utilization of and clinical response to antiretroviral therapy. J Acquir Immune Defic Syndr. 2007 Apr 15;44(5):531–539. doi: 10.1097/QAI.0b013e318031d7cd. [DOI] [PubMed] [Google Scholar]
- 19.Silverberg MJ, Leyden W, Horberg MA, et al. Older age and the response to and tolerability of antiretroviral therapy. Arch Intern Med. 2007 Apr 9;167(7):684–691. doi: 10.1001/archinte.167.7.684. [DOI] [PubMed] [Google Scholar]
- 20.Hartzell JD, Spooner K, Howard R, Wegner S, Wortmann G. Race and mental health diagnosis are risk factors for highly active antiretroviral therapy failure in a military cohort despite equal access to care. J Acquir Immune Defic Syndr. 2007 Apr 1;44(4):411–416. doi: 10.1097/QAI.0b013e31802f83a6. [DOI] [PubMed] [Google Scholar]
- 21.Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research: one size does not fit all. JAMA. 2005 Dec 14;294(22):2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 22.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992 May;82(5):703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weppner WG, Ralston JD, Koepsell TD, et al. Use of a shared medical record with secure messaging by older patients with diabetes. Diabetes Care. 2010 Nov;33(11):2314–2319. doi: 10.2337/dc10-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ralston JD, Rutter CM, Carrell D, et al. Patient use of secure electronic messaging within a shared medical record: a cross-sectional study. J Gen Intern Med. 2009 Mar;24(3):349–355. doi: 10.1007/s11606-008-0899-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smedley BD, Stith AY, Nelson AR Institute of Medicine (U.S.) Unequal treatment : confronting racial and ethnic disparities in health care. Washington, D.C: National Academy Press; 2003. Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. [PubMed] [Google Scholar]
- 26.Le Cook B, McGuire TG, Zuvekas SH. Measuring trends in racial/ethnic health care disparities. Med Care Res Rev. 2009 Feb;66(1):23–48. doi: 10.1177/1077558708323607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ralston JD, Hirsch IB, Hoath J, et al. Web-based collaborative care for type 2 diabetes: a pilot randomized trial. Diabetes Care. 2009 Feb;32(2):234–239. doi: 10.2337/dc08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green BB, Cook AJ, Ralston JD, et al. Effectiveness of home blood pressure monitoring, Web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA. 2008 Jun 25;299(24):2857–2867. doi: 10.1001/jama.299.24.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon GE, Ralston JD, Savarino J, et al. Randomized trial of depression follow-up care by online messaging. J Gen Intern Med. 2011 Jul;26(7):698–704. doi: 10.1007/s11606-011-1679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalichman SC, Weinhardt L, Benotsch E, et al. Internet access and Internet use for health information among people living with HIV-AIDS. Patient Educ Couns. 2002 Feb;46(2):109–116. doi: 10.1016/s0738-3991(01)00134-3. [DOI] [PubMed] [Google Scholar]
- 31.Weingart SN, Rind D, Tofias Z, Sands DZ. Who uses the patient internet portal? The PatientSite experience. J Am Med Inform Assoc. 2006 Jan-Feb;13(1):91–95. doi: 10.1197/jamia.M1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hilton JF, Barkoff L, Chang O, et al. A cross-sectional study of barriers to personal health record use among patients attending a safety-net clinic. PLoS One. 2012;7(2):e31888. doi: 10.1371/journal.pone.0031888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Losina E, Schackman BR, Sadownik SN, et al. Racial and sex disparities in life expectancy losses among HIV-infected persons in the united states: impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. Clin Infect Dis. 2009 Nov 15;49(10):1570–1578. doi: 10.1086/644772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood E, Montaner JS, Tyndall MW, et al. Prevalence and correlates of untreated human immunodeficiency virus type 1 infection among persons who have died in the era of modern antiretroviral therapy. J Infect Dis. 2003 Oct 15;188(8):1164–1170. doi: 10.1086/378703. [DOI] [PubMed] [Google Scholar]
- 35.Gebo KA, Fleishman JA, Conviser R, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005 Jan 1;38(1):96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- 36.Schrager L, Friedland G, Feiner C, Kahl P. Demographic characteristics, drug use, and sexual behavior of i.v. drug user with AIDS in Bronx, New York. Public Health Rep. 1991 Jan-Feb;106(1):78–84. [PMC free article] [PubMed] [Google Scholar]
- 37.Mehra B, Merkel C, Bishop AP. The internet for empowerment of minority and marginalized users. New Media & Society. 2004;6(6):781–802. [Google Scholar]
- 38.Wald JS. Variations in patient portal adoption in four primary care practices. AMIA Annu Symp Proc. 2010;2010:837–841. [PMC free article] [PubMed] [Google Scholar]