Introduction
T lymphocytes are central to the induction and maintenance of the allergic inflammatory response with emphasis on the role of CD4+ T cells and their ability to produce Th2 cytokines such as IL-4, IL-5, and IL-13 (1). CD8+ T cells have also been demonstrated in the bronchoalveolar lavage (BAL) fluid and sputum of asthmatics (2,3) and in lung tissue of fatal asthma (4), but their specific role in asthma pathogenesis has been somewhat controversial. Both enhancing (5) and protective (6) roles for CD8+ T cells have been described
Among the many mediators released during an asthmatic response, leukotriene levels, including leukotriene B4 (LTB4) were increased in the BAL fluid and breath condensates of asthmatics (7) and are potent mediators of asthma (8,9). LTB4 is an important regulator of neutrophil chemotaxis to the lung (10). We and others showed the importance of LTB4 interacting with its high affinity receptor, BLT1, in the accumulation of T cells in the lung (11–14). We found in a mouse model of experimental asthma that BLT1-expressing effector memory CD8+ T cells were a potent source of IL-13 and required for the development of lung allergic responses (15). Moreover, these CD8+BLT1+ T cells were more resistant to corticosteroids than CD4+ T cells and corticosteroids enhanced their activation and effector function by upregulating BLT1 expression through increased IL-2 receptor expression (16). In asthmatics, the numbers of CD8+BLT1+ T cells were increased in the tissue and bronchoalveolar (BAL) fluid and numbers of CD8+BLT1+ T cells producing IL-13 correlated with diminished lung function (17,18). In severe asthmatics, comparison of transcriptome analyses showed large changes in circulating CD8+ but not CD4+ T cells compared to patients with non-severe asthma or controls (19).
To further define potential differences in peripheral blood CD4+ and CD8+ T cells that may contribute to asthma pathogenesis, we compared responses of these T cell subsets from steroid-sensitive (SS) and steroid-resistant (SR) asthmatics. The results identified important differences in the responses of CD4+ and CD8+ T cells to cell activation and between SS and SR asthmatics in the induction of BLT1 expression, steroid sensitivity and cytokine production in these subsets.
Methods
Subjects
Subjects with a diagnosis of asthma according to American Thoracic Society criteria were selected. To qualify for study, baseline FEV1 was less than or equal to 80% predicted. All subjects were non-smokers (no smoking for >1 year with a total of less than 10 pack/year) and had not experienced an upper respiratory tract infection for at least 6 weeks prior to enrollment. Asthmatic patients had a PC20 of 8 mg/ml or lower if steroid-naive or 16 mg/ml if on an inhaled corticosteroid (ICS); patients otherwise demonstrated a 12% or greater improvement in FEV1 after albuterol. After baseline characterization, all subjects received prednisolone (40 mg) orally for 7 days and were categorized as having SS asthma if the FEV1 value improved by 10% or greater or as having SR asthma if the FEV1 improved by less than 10%. For controls, healthy adults with no history of atopic or respiratory disease were enrolled. None of the subjects received systemic corticosteroid therapy for at least 6 weeks before study. This study was approved by the Institutional Review Board of National Jewish Health (Denver, CO).
Human peripheral blood mononuclear cell (PBMC) purification, isolation of CD4+ and CD8+ T cells, and culture procedures
Human PBMCs were isolated from heparinized venous blood by density gradient centrifugation and CD4+ and CD8+ T cells were isolated by negative selection using the magnetic bead human CD4+ T cell isolation kit II and human CD8+ T cell isolation kit II (Miltenyi Biotec, Auburn, CA), respectively. Isolated CD4+ and CD8+ T cells (>95% purity) were stimulated in culture with anti-human CD3 mAb (2 μg/mL, BD Pharmingen, San Jose, CA)/anti-human CD28 mAb (2 μg/mL, BD Pharmingen) and human recombinant IL-2 (100 U/mL, Peprotech, Rocky Hill, NJ) in the presence or absence of 100 nM dexamethasone (Dex, Sigma-Aldrich, St. Louis, MO). Cells were cultured for 8 days at 37°C and 5% CO2 in 12-well culture plates (Becton Dickinson, Franklin Lakes, NJ) at 1×106 cells/mL per well. Medium containing IL-2, with or without Dex, was changed every other day and analysis of BLT1 surface expression was carried out at day 0 and day 8 of culture, the day of peak BLT1 expression (16). At all time points, cell viability remained >90% as assessed by trypan blue dye exclusion.
Flow cytometry
For staining of BLT1, cells were blocked with 10% human IgG for 15 min at 4°C. FITC conjugated anti-hLTB4 receptor (BLT1) mAb (AbD Serotec, Raleigh, NC) or isotype control was added and incubated for 30 min at 4°C and washed three times. BLT1 surface receptor staining was analyzed by flow cytometry using FlowJo software (Tree Star, Inc., Ashland, OR). Allophycocyanin-conjugated anti-CD3, PerCP-conjugated CD4, PE-conjugated, and PerCP Cy5.5-conjugated CD8 were used for staining of CD4+ and CD8+ T cells.
Intracellular Ca2+ monitoring
Activation of CD8+ T cells following ligation of BLT1 by LTB4 was monitored by changes in intracellular Ca2+ concentrations. Isolated CD8+ T cells (5 ×106 cells/mL) were incubated for 45 min at 37°C with 5 μM indo-1 acetoxymethyl ester (Invitrogen, Carlsbad, CA). Cells were resuspended and subjected to analysis by flow cytometry (LSR II; Becton Dickinson, Franklin Lakes, NJ) with FlowJo software (16). After a baseline was established, cells were stimulated with LTB4 (100 nM, 1000 nM, Sigma-Aldrich) and intracellular Ca2+ concentrations were determined by measurement of the median fluorescence ratio of indo-1 acetoxymethyl ester at 390 nm/490 nm emission.
Cytokine levels in cell culture supernatant fluid
Equal numbers of isolated CD4+ and CD8+ T cells were stimulated in culture with anti-CD3/anti-CD28 in the presence of IL-2 for 48 hrs. Culture supernates were harvested for analysis of cytokine levels (IL-4, IL-5, IL-10, IL-13, and IFN-γ) by ELISA (R&D systems, Minneapolis, MN). The detection limits were 8.0 pg/mL for IFN-γ, 10 pg/mL for IL-4, 3.0 pg/mL for IL-5, 3.9 pg/mL for IL-10, and 32 pg/mL for IL-13.
Statistical analysis
Patient clinical variables were analyzed using descriptive statistics, including proportions or means where appropriate. Data were expressed as means (±SEM or SD). The paired t test was used to compare functional responses in the presence or absence of Dex from the same donors. Between group (control, SS, and SR asthma) comparisons were performed using one-way ANOVA. P values of 0.05 or less were considered significant. Analysis for statistically significant differences for ELISA data was performed using the unpaired 2-tailed t test and the nonparametric Mann-Whitney test, respectively. Graph Pad Prism, version 4.01 (San Diego, CA) was used for all statistical calculations.
Results
Demographics of enrolled subjects
A total of 19 subjects, including 9 asthmatics and 10 healthy controls, were enrolled (Table 1). Patients in the asthma group fulfilled the American Thoracic Society definition of asthma (>12% bronchodilator response) and were atopic as demonstrated by multiple positive immediate skin prick responses to allergens. Serum IgE levels were highest and lung function the lowest in SR asthmatics when compared to controls (P<0.05). The percentage of total lymphocytes in BAL fluid expressing both CD8 and BLT1 and those expressing CD8, BLT1, and IL-13 were higher in asthmatics than in controls with the highest percentages in SR asthmatics (Table 1).
Table 1.
Patient Demographics and Pulmonary Function
| Group Parameter |
Control (CTL) | Steroid-Sensitive (SS) | Steroid-Resistant (SR) | P value |
|---|---|---|---|---|
| No. of subjects | 10 | 5 | 4 | |
| Age (yrs) (mean±SD) | 32.7±11.0 | 30.6±6.5 | 35.5±9.9 | |
| Sex (M/F) | 5:5 | 3:2 | 2:2 | |
| Race | Cauc (8), Asian (2) |
Cauc (4), Hisp(1) |
Cauc (2), Hisp(1), AFAM(1) |
|
| Average positive skin prick tests (range) | 0 | 5.6+ (4+~9+) | 5.0+ (1+~10+) | |
| Serum total IgE (IU/mL) (range) | 76 (2~377) | 177 (86~409) | 333 (56~589)* | <0.05* |
| Prebronchodilator FEV1 % predicted (mean±SD) Postbronchodilator |
95.1±10.2 | 75.8±8.2* | 65.8±8.2* | <0.05* |
| 104.4±10.6 | 89.8±9.9 | 79.5±13.0 | <0.05* | |
| FVC % predicted (mean±SD) Prebronchodilator Postbronchodilator |
99.2±3.9 | 90.8±10.1 | 88.5±19.8 | NS |
| 99.2±5.9 | 97.4±12.5 | 97.8±26.1 | NS | |
|
FEV1/FVC
Prebronchodilator Postbronchodilator |
0.82±0.03 | 0.70±0.05 | 0.63±0.06 | <0.05* |
| 0.86±0.05 | 0.78±0.08 | 0.69±0.08 | <0.05* | |
|
% Lvmphocvtes in BAL expressing:
BLT1+CD8+ BLT1+CD8+IL-13+ |
13.97±1.6
4.78±1.2 |
25.32±3.4
13.4±3.7 |
34.2±5.1
16.5±2.3 |
<0.05*
<0.05* |
Compared to controls.
Cauc: Caucasian, Hisp: Hispanic, AFAM: African American.
BLT1 expression on isolated CD4+ and CD8+ T cells
BLT1 expression on isolated, live CD3+CD4+ T cells and CD3+CD8+ T cells was analyzed at baseline and in the presence or absence of Dex in controls, SS and SR asthmatics. When CD4+ T cells from each group were compared, the percentages of BLT1-positive cells at baseline was low and there was only a modest increase following activation of the CD4+ T cells with anti-CD3/anti-CD28 (Fig. 1). Addition of Dex to the cultures had little effect on the percentages of BLT1 in CD4+ T cells. (P>0.05).
Figure 1.
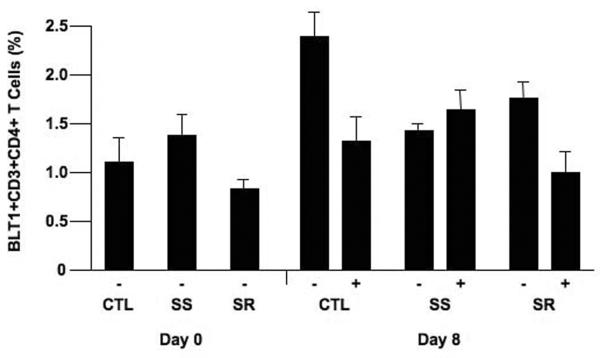
BLT1 expression on stimulated CD4+ T cells. Isolated CD4+ T cells were stimulated with anti-CD3/anti-CD28 for 8 days and BLT1 expression was assessed by flow cytometry. There were no significant differences between any of the groups.
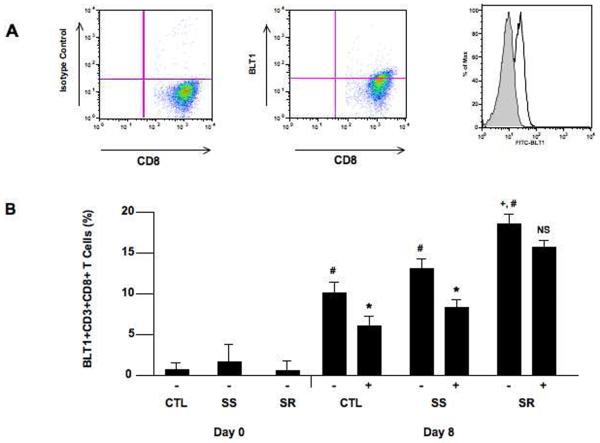
In contrast, when isolated CD8+ T cells were examined, the following was shown: in all groups, activation with anti-CD3/anti-CD28 resulted in an increase in the percent of CD8+ T cells expressing BLT1. The increases were higher in isolated CD8+ T cells than in isolated CD4+ T cells after stimulation (Fig. 2). The increases in BLT1-staining CD8+ T cells was higher in asthmatics than in controls (P<0.05) and these increases were higher in CD8+ T cells from SR than in CD8+ T cells from SS asthmatics (P<0.05). In SS asthmatics (and controls), the percentages of BLT1-expressing CD8+ T cells on day 8 was lower when cells were cultured in the presence of corticosteroid unlike SR CD8+ T cells, where numbers were maintained (P<0.05). Individual responses in CD4+ and CD8+ T cells from controls and SS and SR asthmatics are shown in Supplemental Figure 1.
Figure 2.
BLT1 expression on stimulated CD8+ T cells. Isolated CD8+ T cells were stimulated with anti-CD3/anti-CD28 for 8 days and BLT1 expression was assessed by flow cytometry. A) Representative plots are illustrated. Representative data showing isotype control staining (left panel) and BLT1 expression (center panel). The right panel shows the flow analysis of BLT1 staining and the discrimination between the FITC-isotype control and FITC-BLT1 monoclonal antibody staining. B) BLT1 expression on unstimulated and stimulated CD8+ T cells in the presence or absence of dexamethasone (Dex). #P<0.05 comparing unstimulated to stimulated group. *P<0.05 comparing steroid treated to non-treated group. +P<0.05 comparing SR to CTL and SS groups. −: no Dex, +: includes Dex.
Ca2+ influx in isolated CD8+ T cells
To determine the functional activity of the upregulated expression of BLT1 on the activated CD8+ T cells, we determined the effect of adding LTB4 to CD8+ T cells and monitored intracellular Ca2+ concentrations. When activated CD8+ T cells with upregulated BLT1 expression were exposed to two concentrations of LTB4, intracellular Ca2+ concentrations increased in a dose-dependent manner (Fig. 3). Activated CD8+ T cells expressing BLT1 from all groups showed similar increases in intracellular Ca2+ concentrations (data not shown).
Figure 3.
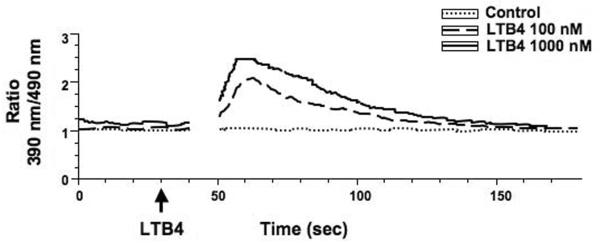
Addition of LTB4 to stimulated CD8+ T cells results in increased intracellular Ca2+ concentrations. Isolated CD8+ T cells stimulated with anti-CD3/anti-CD28 for 8 days were incubated with LTB4 (100 or 1,000 nM) and intracellular Ca2+ concentrations were monitored comparing the median fluoresense ratio of indo-1 to indo-2 as described in Methods.
Sensitivity to corticosteroids
To further delineate the differences in CD4+ and CD8+ T cells in SS and SR asthmatics, isolated subsets were stimulated and expanded with anti-CD3/anti-CD28 and IL-2 in the presence or absence of Dex. At the end of the culture period (day 8), cell counts were performed to determine the extent of expansion. When CD4+ T cells were stimulated in the presence of Dex, cell numbers on day 8 were significantly lower than in cultures not containing Dex. In cultures of CD8+ T cells, the attenuation of anti-CD3/anti-CD28 and IL-2-induced expansion was lowest (Table 2) and the expansion of CD8+ T cells in the SR group was reduced to the lowest extent.
Table 2.
Expansion of CD4+ and CD8+ T Cells and the Effect of DEX Treatment
| Group | CD4+ T cells | CD8+ T cells | ||
|---|---|---|---|---|
| Total cell count (x106) (mean±SD) | % Reduction of Cell Expansion | Total cell count (x106) (mean±SD) | % Reduction of Cell Expansion | |
| CTL Dex(−) | 24.4±11.7 | 31.7±14.8 | ||
| CTL Dex(+) | 8.5±8.6* | 65% | 16.0±8.2* | 50% |
| SS Dex(−) | 38.4±22.1 | 44.1±26.0 | ||
| SS Dex(+) | 7.8±4.4* | 80% | 25.5±15.5* | 60% |
| SR Dex(−) | 16.3±9.0 | 21.4±5.5 | ||
| SR Dex(+) | 3.3±1.9* | 80% | 17.6±5.3NS | 19%# |
P<0.05 compared to non-DEX-treated group.
P<0.05 compared to CTL and SS groups.
NS: Not significant.
Cytokine levels in cell culture supernatant fluid
We previously showed that in both mice and in asthmatics, CD8+ T cells were a source of the pro-allergic cytokine IL-13 (15,18). We compared cytokine production in CD4+ and CD8+ T cells after culture for 48 hrs in the presence of anti-CD3/anti-CD28 and IL-2. Levels of IL-13 were higher in supernates from cultured CD8+ T cells than from cultures of CD4+ T cells, whereas IL-10 levels were higher in CD4+ T cells from controls and SS asthmatics than from cultures of CD4+ T cells from SR asthmatics (P<0.05) (Fig. 4). In cultures of both CD4+ and CD8+ T cells, levels of IFN-γ were lowest from cells of SR asthmatics (P<0.05). IL-4 levels were at the level of detection and did not differ among the groups, nor did levels of IL-5 (data not shown).
Figure 4.
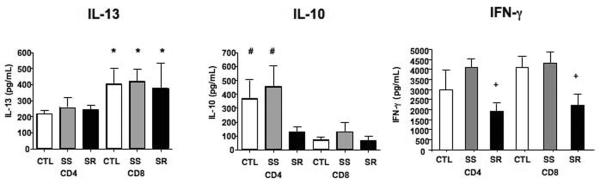
Cytokine production from cultures of CD4+ and CD8+ T cells. Isolated CD4+ and CD8+ T cells from CTL, SS, and SR patients were stimulated with anti-CD3/anti-CD28 for 48 hours and culture supernates were harvested and assayed by ELISA. *P<0.05 compared to CD4+ T cells. #P<0.05 compared to SR CD4+ T cells and CD8+ T cells. +P<0.05 comparing SR cells to CTL or SS cells.
Discussion
The contributions of CD4+ T helper cells in the development of allergic airway inflammation are well established (1). A growing body of evidence suggests that CD8+ T cells may also have the capacity to trigger allergic inflammatory processes in the lungs that contribute to clinically relevant immunopathological processes (1,5,18,20,21). A cross-sectional relationship between CD8+ T cells and asthma outcomes has been observed in fatal asthma (21). Studies examining a potential role for CD8+ T cells in asthma have noted a positive correlation between CD8+ T cell numbers, disease severity and the decline in lung function (5,18). We compared the phenotype and activation outcomes of CD4+ and CD8+ T cells in two subsets of asthmatics defined as SS or SR. The percentages of cells which expressed BLT1, the high affinity receptor for the lipid mediator LTB4, was higher in asthmatics than in non-asthmatics and in SR asthmatics, the highest percentages of BLT1-expressing cells were detected among activated CD8+ T cells. This increase in BLT1-expressing cells in SR asthmatic CD8+ T cells was maintained in the presence of corticosteroid and was functional as determined by the intracellular Ca2+ response to ligation of the receptor by LTB4. CD8+ T cells produced the highest levels of the cytokine IL-13, important in asthma and mucus production. SR asthmatic CD4+ T cells produced the lowest levels of the anti-inflammatory cytokine IL-10 and IFN-γ levels were the lowest in cultures of SR asthmatic CD4+ and CD8+ T cells. Importantly, CD8+ T cell expansion, especially in SR asthmatics, was relatively resistant to corticosteroid compared to CD4+ T cells.
Although LTB4 was initially described as a chemoattractant for myeloid leukocytes (23), it was shown that LTB4 could bind to a small proportion of human peripheral blood T cells (24). Consistent with these findings, LTB4 was shown to induce chemotaxis of human peripheral blood lymphocytes (25). Elevated levels of LTB4 have been reported in various allergic diseases and these levels have been related to disease activity and response to treatment. LTB4 levels were increased in the sputum, plasma, and bronchoalveolar lavage (BAL) fluid of asthmatic patients but not of healthy subjects (7,26). Many studies have shown that LTB4, although perhaps not a primary mediator of allergic disease, may be important in specific conditions such as severe persistent asthma (26). Both BAL LTB4 levels (8) and bronchial wall CD8+ T cell numbers have correlated with disease severity (5), supporting a potential link between these previously assumed independent findings.
Corticosteroids effectively suppress inflammatory responses through repression of many immune genes and are therefore widely used for the treatment of various allergic diseases. Th2 cytokine-producing CD4+ T cells and eosinophils play important roles in the pathogenesis of allergic diseases, and numbers of these cells, but not CD8+ T cells, were dramatically decreased in peripheral blood and inflamed bronchial tissues of patients with asthma after initiation of corticosteroid treatment (27). Although considered to be immunosuppressive and anti-inflammatory, several lines of investigation have now demonstrated that corticosteroids may increase immune/inflammatory responses and even enhance adaptive immune responses (28). As confirmed here, human (29) CD8+ T cells were shown to be relatively steroid-resistant compared to CD4+ T cells, similar to findings in mice (16). It has been well-established that a proportion of asthmatic patients suffer a decline in lung function despite high doses of inhaled or oral corticosteroid treatment and have been labeled as steroid-insensitive or steroid-refractory (30). Since the pathway leading to the generation of lipid mediators is activated in many inflammatory diseases, but is resistant to corticosteroid treatment (8), the LTB4-BLT1 axis in steroid-insensitive CD8+ T cells may be a major factor in steroid-refractory asthma.
In the present study, we confirmed this notion in a small group of SS and SR asthmatics and demonstrated, despite small numbers of patients, significant differences between CD4+ and CD8+ T cells. Activation with anti-CD3/anti-CD28 resulted in the expansion of BLT1-expressing CD8+ T cells in all groups, in contrast to CD4+ T cells. The numbers of BLT1+ CD8+ T cells were higher in asthmatics than in the controls and the increases were higher in CD8+ T cells from SR than from SS asthmatics. Expansion of activated CD4+ T cells with anti-CD3/anti-CD28 and IL-2 was significantly reduced on day 8 in the presence of Dex, whereas the effects of Dex on expansion of activated CD8+ T cells was lower and the smallest effects were seen in the CD8+ T cells from SR compared to SS asthmatics.
Although much of the previous work in asthma has focused on the role of CD4+ Th2 cells and their capacity for pro-allergic cytokine production, including IL-13, we previously showed that both mouse (15) and asthmatic (18) CD8+ T cells were a potent source of IL-13. When cytokine levels in cultures of CD4+ and CD8+ T cells activated with anti-CD3/anti-CD28 were assessed, levels of IL-13 were higher in CD8+ T cells than in CD4+ T cells. In contrast, levels of the anti-inflammatory cytokine, IL-10, were higher in CD4+ T cells from controls and SS asthmatics compared to CD4+ T cells from SR asthmatics. This inverse relationship between IL-13 and IL-10, increased IL-13 and decreased IL-10 levels in BAL fluid, has correlated with disease severity (31,32). Corticosteroids may exert some of their beneficial effects through the induction of IL-10 production (31,33). Levels of IFN-γ, which may downregulate Th2 cytokine production and asthmatic responses, were also shown to be lowest in both CD4+ and CD8+ T cells from SR asthmatics.
These studies extend previous findings implicating an important role for CD8+ T cells in a subset of asthmatics. The data presented identify impressive differences between CD4+ and CD8+ T cells and in SS and SR asthmatics. In asthmatics, numbers of BLT1+ CD8+ T cells were higher and demonstrated relative corticosteroid insensitivity and capacity for IL-13 production. These differences appeared enhanced in CD8+ T cells from SR asthmatics. Severe asthma has been defined as abnormal lung function despite treatment with high doses of corticosteroids (34). Because of differences in baseline lung function in the SR asthmatics in addition to differences in steroid sensitivity, it is difficult to distinguish at the present time if the differences in CD8+ T cell responses are related in part to disease severity as well as steroid responsiveness. Nonetheless, the findings identify the need for development of newer therapeutic approaches that target these pathogenic CD8+ T cells if we are to alter the course of severe asthma, especially when corticosteroids do not achieve asthma control.
Supplementary Material
Acknowledgments
Eun Hee Chung is currently affiliated with the Asthma Atopy Allergy Center, National Medical Center, Seoul, South Korea.
Grant Support: This work was supported by NIH grants HL-36577 and AI-77609. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: EHC carried out most of the studies, while YJ and KT helped erform some of the assays, DYML, ERS, AD, and RJM were involved in obtaining samples from patients and controls, EWG supervised the project, design and data analysis. All contributed to drafting of the manuscript.
References
- 1.Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003;111:450–463. doi: 10.1067/mai.2003.169. [DOI] [PubMed] [Google Scholar]
- 2.Walker C, Bauer W, Braun RK, et al. Activated T cells and cytokines in bronchoalveolar lavages from patients with various lung diseases associated with eosinophilia. Am J Respir Crit Care Med. 1994;150:1038–1048. doi: 10.1164/ajrccm.150.4.7921434. [DOI] [PubMed] [Google Scholar]
- 3.Lee SY, Kim SJ, Kwon SS, et al. Distribution and cytokine production of CD4 and CD8 T-lymphocyte subsets in patients with acute asthma attacks. Ann Allergy Asthma Immunol. 2001;86:659–664. doi: 10.1016/S1081-1206(10)62295-8. [DOI] [PubMed] [Google Scholar]
- 4.Faul JL, Tormey VJ, Leonard C, et al. Lung immunopathology in cases of sudden asthma death. Eur Respir J. 1997;10:301–307. doi: 10.1183/09031936.97.10020301. [DOI] [PubMed] [Google Scholar]
- 5.van Rensen EL, Sont JK, Evertse CE, et al. Bronchial CD8 cell infiltrate and lung function decline in asthma. Am J Respir Crit Care Med. 2005;172:837–841. doi: 10.1164/rccm.200504-619OC. [DOI] [PubMed] [Google Scholar]
- 6.Takeda K, Dow SW, Miyahara N, et al. Vaccine-induced CD8+ T cell-dependent suppression of airway hyperresponsiveness and inflammation. J Immunol. 2009;183:181–190. doi: 10.4049/jimmunol.0803967. [DOI] [PubMed] [Google Scholar]
- 7.Wardlaw AJ, Hay H, Cromwell O, Collins JV, Kay AB. Leukotrienes, LTC4 and LTB4, in bronchoalveolar lavage in bronchial asthma and other respiratory diseases. J Allergy Clin Immunol. 1989;84:19–26. doi: 10.1016/0091-6749(89)90173-5. [DOI] [PubMed] [Google Scholar]
- 8.Peters-Golden M, Henderson WR., Jr Leukotrienes. New Engl J Med. 2007;357:1841–54. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 9.Ohnishi H, Miyahara N, Gelfand EW. The role of leukotriene B(4) in allergic diseases. Allergol Int. 2008;57:291–298. doi: 10.2332/allergolint.08-RAI-0019. [DOI] [PubMed] [Google Scholar]
- 10.Martin TR, Pistorese BP, Chi EY, Goodman RB, Matthay MA. Effects of leukotriene B4 in the human lung. Recruitment of neutrophils into the alveolar spaces without a change in protein permeability. J Clin Invest. 1989;84:1609–1619. doi: 10.1172/JCI114338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyahara N, Takeda K, Miyahara S, et al. Leukotriene B4 (BLT1) is essential for allergen-mediated recruitment of CD8+ T cells and airway hyperresponsiveness. J Immunol. 2005;174:4979–4984. doi: 10.4049/jimmunol.174.8.4979. [DOI] [PubMed] [Google Scholar]
- 12.Miyahara N, Takeda K, Miyahara S, et al. Requirement for the leukotriene B4 receptor-1 in allergen-induced airway hyperresponsiveness. Amer J Resp Crit Care Med. 2005;172:161–167. doi: 10.1164/rccm.200502-205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taube C, Miyahara N, Ott V, et al. The leukotriene B4 receptor (BLT1) is required for effector CD8+ T-cell-mediated, mast cell-dependent airway hyperresponsiveness. J Immunol. 2006;176:3157–3164. doi: 10.4049/jimmunol.176.5.3157. [DOI] [PubMed] [Google Scholar]
- 14.Goodarzi K, Goodarzi M, Tager AM, Luster AD, von Andrian UH. Leukotriene B4 and BLT1 control cytotoxic effector T cell recruitment to inflamed tissues. Nature Immunol. 2003;4:965–973. doi: 10.1038/ni972. [DOI] [PubMed] [Google Scholar]
- 15.Miyahara N, Takeda K, Kodama T, et al. Contribution of antigen-primed CD8+ T cells to the development of airway hyperresponsiveness and inflammation is associated with IL-13. J Immunol. 2004;172:2549–2558. doi: 10.4049/jimmunol.172.4.2549. [DOI] [PubMed] [Google Scholar]
- 16.Ohnishi H, Miyahara N, Dakahama A, et al. Corticosteroids enhance CD8+ T cell-mediated airway hyperresponsiveness and allergic inflammation by upregulating leukotriene B4 receptor 1. J Allergy Clin Immunol. 2008;121:864–871. doi: 10.1016/j.jaci.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 17.Gelfand EW, Dakhama A. CD8+ T lymphocytes and leukotriene B4: novel interactions in the persistence and progression of asthma. J Allergy Clin Immunol. 2006;117:577–582. doi: 10.1016/j.jaci.2005.12.1340. [DOI] [PubMed] [Google Scholar]
- 18.Dakhama A, Collins ML, Ohnishi H, et al. IL-13-producing BLT1-positive CD8 cells are increased in asthma and are associated with airway obstruction. Allergy. 2013;68:666–673. doi: 10.1111/all.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsitsiou E, Williams AE, Moschos SA, et al. Transcriptome analysis shows activation of circulating CD8+ T cells in patients with severe asthma. J Allergy Clin Immunol. 2012;129:95–103. doi: 10.1016/j.jaci.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Cho SH, Stanciu LA, Holgate ST, Johnston SL. Increased interleukin-4, interleukin-5, and interferon-gamma in airway CD4+ and CD8+ T cells in atopic asthma. Am J Respir Crit Care Med. 2005;171:224–230. doi: 10.1164/rccm.200310-1416OC. [DOI] [PubMed] [Google Scholar]
- 21.O'Sullivan S, Cormican L, Faul JL, et al. Activated, cytotoxic CD8(+) T lymphocytes contribute to the pathology of asthma death. Am J Respir Crit Care Med. 2001;164:560–564. doi: 10.1164/ajrccm.164.4.2102018. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Z, Homer RJ, Wang Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subeptithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ford-Hutchinson AW, Bray MA, Doig MV, Shipley ME, Smith MJ, Leukotriene B. a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980;286:264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- 24.Payan DG, Missirian-Bastian A, Goetzl EJ. Human T-lymphocyte subset specificity of the regulatory effects of leukotriene B4. Proc Natl Acad Sci USA. 1984;81:3501–3505. doi: 10.1073/pnas.81.11.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bacon KB, Camp RD, Cunningham FM, Woollard PM. Contrasting in vitro lymphocyte chemotactic activity of the hydroxyl enantiomers of 12-hydroxy-5,8,10,14-eicosatetraenoic acid. Br J Pharmacol. 1988;95:966–974. doi: 10.1111/j.1476-5381.1988.tb11727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sampson AP, Castling DP, Green CP, Price JF. Persistent increase in plasma and urinary leukotrienes after acute asthma. Arch Dis Child. 1995;73:221–225. doi: 10.1136/adc.73.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoshino M, Nakamura Y, Sim JJ, Tomioka H. A comparative study of the effects of ketotifen, disodium cromoglycate, and beclomethasone dipropionate on bronchial mucosa and asthma symptoms in patients with atopic asthma. Respir Med. 1998;92:942–950. doi: 10.1016/s0954-6111(98)90194-9. [DOI] [PubMed] [Google Scholar]
- 28.Mittelstadt PR, Monteiro JP, Ashwell JD. Thymocyte responsiveness to endogenous glucocorticoids is required for immunological fitness. J Clin Invest. 2012;122:2384–2394. doi: 10.1172/JCI63067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L-B, Leung DYM, Strand MJ, Goleva E. ATF2 impairs glucocorticoid receptor-mediated transactivation in human CD8+ T cells. Blood. 2007;110:1570–1507. doi: 10.1182/blood-2007-01-070755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin RJ, Szefler SJ, King TS, et al. The Predicting Response to Inhaled Corticosteroid Efficacy (PRICE) trial. J Allergy Clin Immunol. 2007;119:73–80. doi: 10.1016/j.jaci.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borish L, Aarons A, Rumbyrt J, Cvietusa P, Negri J, Wenzel S. Interleukin-10 regulation in normal subjects and patients with asthma. J Allergy Clin Immunol. 1996;97:1288–1296. doi: 10.1016/s0091-6749(96)70197-5. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto K, Inoue H, Fukuyama S, et al. Decrease of interleukin-10-producing T cells in the peripheral blood of severe unstable atopic asthmatics. Intl Arch Allergy Immunol. 2004;134:295–302. doi: 10.1159/000079167. [DOI] [PubMed] [Google Scholar]
- 33.John M, Lim S, Seybold J, et al. Inhaled corticosteroids increase interleukin-10 but reduce macrophage inflammatory protein-1-alpha, granulocyte-macrophage colony-stimulating factor, and interferon-gamma release from alveolar macrophages in asthma. Amer J Resp Crit Care Med. 1998;157:256–262. doi: 10.1164/ajrccm.157.1.9703079. [DOI] [PubMed] [Google Scholar]
- 34.American Thoracic Society Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.