Abstract
Objective
Previously we demonstrated anthrax toxin receptor 2 knockout (Antxr2−/−) mice are fertile but fail to deliver their pups at term. This parturition defect is associated with over-accumulation of extracellular matrix (ECM) proteins and decreased myometrial cell content in the uterus. Myometrial cell loss in Antxr2−/− uterine tissue prompted us to evaluate if ANTXR2 is essential for human uterine smooth muscle cell (HUSMC) viability and function.
Study Design
We subjected HUSMC to lentiviral-mediated knock down (R2KD) or retroviral-mediated over-expression (R2OE) of ANTXR2. Flow cytometry confirmed R2KD or R2OE in cell lines vs control (CTL). Cell behavior and function in CTL, R2KD and R2OE cells were evaluated for apoptosis via TUNEL assay, migration via Boyden chamber assay and with oxytocin-mediated collagen contraction assays. Matrix metalloproteinase (MMP) activity was evaluated using gelatin zymography. Cell lines and samples were run in duplicate. Student t-test was used for statistical analysis.
Results
ANTXR2 is expressed by HUSMC. HUMSC-R2KD cells exhibited increased apoptosis (p<0.05) and decreased migration (p<0.05) while HUSMC-R2OE cells exhibited no change in apoptosis (p=0.91) and increased migration (p=0.05) vs CTL. HUMSC-R2KD cells contracted significantly less than CTL while HUSMC-R2OE cells showed no difference in contractility vs CTL. MMP2 activity appeared slightly decreased in HUMSC-R2KD cells and increased in HUSMC-R2OE cells vs CTL.
Conclusion
ANTXR2 is expressed by HUSMC and appears important for normal HUSMC viability, migration and contractility. Further studies are needed to delineate if ANTXR2 is important for normal and abnormal labor patterns.
Keywords: anthrax toxin receptor 2, extracellular matrix, uterine smooth muscle cells, migration, apoptosis, contractility
INTRODUCTION
The Anthrax Toxin Receptor (ANTXR) proteins, ANTXR1 and ANTXR2 (also known as Tumor Endothelial Marker 8 (TEM8) and Capillary Morphogenesis Gene 2 (CMG2), respectively), are most commonly recognized for their ability to bind anthrax toxin. These transmembrane receptors are also known to be critical for angiogenic processes such as endothelial cell proliferation, migration and network formation in part by binding to and interacting with extracellular matrix (ECM) proteins.1–6
Recently, we demonstrated that Antxr2 is essential for parturition in mice. Antxr2 knockout (Antxr2−/−) mice are fertile and carry pregnancies to term, but then fail to deliver their fetuses which results in maternal death or fetal reabsorption.2,3 Gross analysis of the reproductive tracts revealed enlarged and fibrotic uterine and cervical tissue. Histological analysis of uterine and cervical tissue from the Antxr2−/− mice demonstrated excessive accumulation of ECM proteins, particularly, collagen. It also showed striking disruption of smooth muscle cell layers in the myometrium. It was unclear if the disruption of the smooth muscle cell layer was due to the direct effect of absent Antxr2 expression on the myometrial cells or whether it was secondary to the over-accumulation of collagen/ECM proteins. The loss of smooth muscle cells was so severe in aged mice that the myometrial layers could not be delineated. We hypothesized that the parturition defect in Antxr2−/− mice was due in part to decreased smooth muscle cell content and therefore possible dystocia due to inadequate contractile force.
To investigate the mechanism responsible for the aberrant ECM remodeling in Antxr2−/− mouse uterine tissue, we evaluated the activity of matrix metalloproteinases (MMPs), enzymes involved in ECM remodeling. MMP2 activity in uterine lysates and conditioned medium from Antxr2−/− mouse embryonic fibroblasts was reduced in the Antxr2 samples. ANTXR2 was found to form a complex with and enhance the activity of MT1-MMP (a membrane bound MMP responsible for MMP2 activation).3 These findings suggested that disruption of the Antxr2 gene in mice resulted in decreased MT1-MMP activity and thus decreased MMP2 activation. The lack of MMP2 activity in Antxr2−/− mouse likely led to decreased ECM remodeling, eventually caused fibrosis in the reproductive tracts of Antxr2−/− mice. Despite these findings, the role of Antxr2 in normal murine uterine smooth muscle cell function and viability remained unclear. In particular, it was unclear if the uterine smooth muscle cells in the myometrium of the Antxr2−/− mouse were being lost due to increased rates of apoptosis and if loss of Antxr2 disrupted the intrinsic function of smooth muscle cells which includes migration, contraction and ECM remodeling.
To date, no studies have evaluated ANTXR2 expression on human uterine smooth muscle cells (HUMSC) nor have studies addressed the role of ANTXR2 during normal HUSMC function. Studies have documented that individuals harboring a mutation in the ANTXR2 gene have a rare, autosomal recessive disease called Systemic Hyalinosis, which is further characterized as two syndromes, infantile systemic hyalinosis (ISH) and juvenile hyaline fibromatosis (JHF).7–9 Patients with ISH and JHF have abnormal collagen and glycosaminoglycan deposition in various tissues resulting in gingival hypertrophy, progressive joint contractures, osteolysis, osteoporosis, recurrent subcutaneous fibromas and hyaline depositions.10 However, studies are lacking on the role of ANTXR2 in human reproductive tissues and during parturition. We sought to evaluate ANTXR2 expression in HUSMC and delineate the role of ANTXR2 in HUSMC function and ECM homeostasis.
METHODS
DNA constructs
For ANTXR2 knockdown, lentiviral pLKO.1 vectors encoding scrambled control shRNA and shRNA targeting ANTXR2 transcripts were purchased from Sigma Aldrich. The pLKO.1 vector carries puromycin resistance allowing for selection of shRNA expressing cells. The ANTXR2 siRNA target sense sequence was 5′ – CCTGCACCTATCCTGAATAAA – 3′. For ANTXR2 overexpression, we used a retroviral vector engineered to express recombinant receptor-EGFP fusion protein as previously described.2,11 Human uterine smooth muscle cells (Promocell, Heidelberg, Germany) were cultured in smooth muscle cell growth medium 2 (Promocell, Heidelberg, Germany) according to manufacturer’s instructions. Cells were cultured under standard conditions in a humidified incubator at 37°C, 5% CO2.
ANTXR2/CMG2 Gene Silencing and Overexpression
Lentivirus was produced in transfected 293T cells and retrovirus was produced in GP2-293 cells and used to transduce HUSMCs as previously described.2,12 Briefly, lentiviral-containing supernatants and retroviral-containing supernatants were collected 24 hours after transfection, filtered, diluted 1:1 with HUSMC growth medium 2 and added to low passage HUSMC. This process was repeated at 24 h, 36 h and 48 h. At 48 h after the initial infection, lentiviral cell lines were selected for 5 days with puromycin (Invitrogen, Carlsbad, CA) at 1μg/ml and then maintained at 0.5μg/ml. Retroviral cell lines were selected for 5 days with 300μg/ml hygromycinB (Invitrogen, Carlsbad, CA). ANTXR2 gene silencing and overexpression were confirmed via flow cytometry as described previously described.2
Evaluation of Cell Viability – Apoptosis Assay
HUSMC cell lines were seeded at equivalent numbers on type I collagen-coated coverslips. Samples were run in duplicate. After allowing the cells to adhere to the plates, the medium was changed to basal medium. After 48 h, TUNEL (TdT mediated dUTP Nick End Labeling) staining of cells was carried out using the ApopTag® Red In Situ Apoptosis Detection Kit (Millipore) according to the manufacturer’s instructions. DAPI (Vector) was used to counterstain nuclei. Cells were visualized on a Nikon ECLIPSE E 800 microscope. Five pictures were taken at 20X magnification (top, middle, bottom, left and right) for each coverslip and the number of TUNEL-positive cells was counted per high power field. An average of the number of TUNEL positive cells in each of the five pictures per coverslip was obtained for each sample and used for analysis. Apoptotic cells were also detected using the Annexin V-FITC Apoptosis Detection Kit (BioVision, Mountain View, CA) according to the manufacturer’s instructions. Quantification of apoptotic cells was performed using flow cytometry. Samples were run in duplicate.
Evaluation of HUSMC Migration – Boyden Chamber Assay
We performed cell migration assays using a specialized Boyden migration chamber that included a 24-well tissue culture plate with cell culture inserts (BD Falcon, San Jose, CA). The inserts contained an 8-μm pore size polycarbonate membrane that was precoated with matrigel. Briefly, we seeded equal numbers of the HUSMC cell lines on the membrane of the culture inserts. Basal HUSMC medium supplemented with 10ng/mL of epidermal growth factor (EGF) was added on top of the cells in each insert. As a control in this migration test, the same medium (basal HUSMC medium supplemented with 10 ng/mL of EGF) was also added to the lower compartment of each of the cell lines. In order to test the migration of HUMSC-R2KD and HUSMC-R2OE cell lines, basal HUSMC medium supplemented with 10 ng/mL of EGF and 20 ng/mL of the chemoattractant, platelet-derived growth factor subunit B (PDGF-B), was added to the lower compartments in another set of each of the cell lines. After 48 h of incubation at 37 °C, the migrated cells were fixed in 4% PFA. The HUSMC from the upper surface of the membrane were removed using a moist cotton-tipped swab. We stained the migrating cells on the lower surface of the membrane, which had migrated through the polycarbonate membrane, with crystal violet staining solution for 20 min and rinsed them three times with distilled water. Three pictures were obtained at 10X (top, middle, bottom) of each membrane and the number of migrating cells per image was obtained. The average of the number of migrated cells was then calculated from each of the three images. This was repeated for each membrane in all cell lines. Samples were run in duplicate and the experiment was repeated to confirm results.
Evaluation of HUSMC Contractility – Collagen Contraction Assay
To measure myometrial cell contraction in response to oxytocin (OT) we adapted an assay based upon the technique described by Dallot et al.13 A collagen gel was made using 1 part RPMI (Sigma), 1 part H20, 7 parts type 1A collagen (Cellmatrix), 1 part neutralization buffer (Cellmatrix). HUSMC cell lines were mixed into the collagen gel at equal numbers and 300μL of HUSMC/collagen gel mixture was added to individual wells of a 24-well plate and left at 37°C for 30 minutes to allow gelling of the collagen mixture. 500μL of HUSMC growth medium 2 was then added to each well. At 48h, each collagen lattice was detached from the bottom of the well with a sterile pipette tip. 100 nM of oxytocin (Sigma) diluted in HUSCM growth medium 2 or HUSMC without oxytocin was added to each well and left overnight at 37°C. Cell contraction was stopped by fixing the lattices in PBS-4% paraformaldehyde. The lattices were photographed with a digital camera and the surface area of each lattice was calculated. For each lattice, collagen contraction was determined in triplicate and expressed as percentage contraction. Percentage contraction was taken as the percentage of lattice size diminution relative to the size area of the well. The experiment was repeated to confirm results.
Evaluation of MMP Activity - Gelatin Zymography
Gelatin Zymography analysis was performed as previously described.14,15 After 16–24 hours, the conditioned medium was harvested from CTL, R2KD and R2OE cells which were seeded at equal densities. The conditioned medium was cleared by centrifugation at 12,000 rpm for 10 minutes and subjected to analysis by SDS-substrate gel electrophoresis (zymography) under non-denaturing conditions in 8.0% SDS-polyacrylamide gels impregnated with 1 mg/ml gelatin as previously described.14,15 The gels were incubated at 37°C overnight in 50 mM Tris (pH 7.5), 5 mM CaCl2, 1 mM ZnCl2 and stained with Coomassie Brilliant Blue R25. Destained gel images were captured by Kodak EL Logic 100 Imaging System. Samples were tested in duplicate. All of the experiments were repeated twice. ImageJ 1.45 s (NIH) was used to quantify zymography band intensities.
Statistical Analysis
Statistical significance was evaluated using the unpaired Student’s t test with a p value less than 0.05 considered statistically significant.
RESULTS
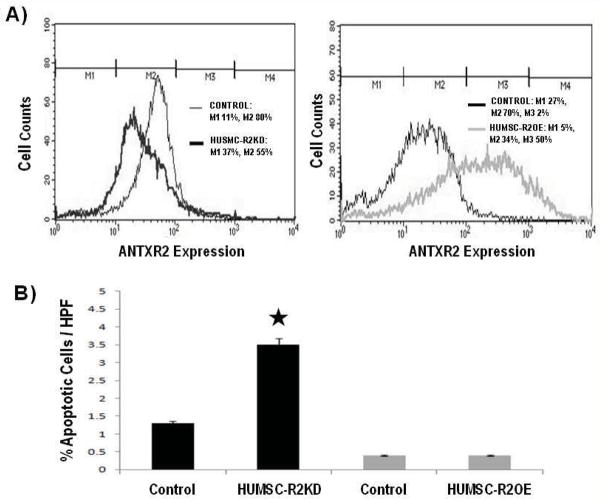
To delineate the function of ANTXR2 in HUSMC, we evaluated cell viability, migration and contractility upon ANTXR2 knockdown or over-expression. Flow cytometry confirmed the presence of ANTXR2 protein on the surface of HUSMC and we were able to achieve approximately 40% lentiviral-mediated knock down (KD) and 30% retroviral-mediated over-expression (OE) relative to control (CTL). Figure 1A represents results from one of two flow cytometry assessments that were performed.
Figure 1.
ANTXR2 is expressed on human uterine smooth muscle cells (HUSMC) and knockdown of ANTXR2 increased rates of HUSMC apoptosis. A) These graphs represent results from one of two flow cytometry assessments that were performed. Flow cytometry confirmed the presence of ANTXR2 protein on the surface of HUSMC. Marker 1 (M1) represents the ANTXR2 negative cells and Marker 2 and 3 (M2, M3) represents the CMG2 positive cells. On average, we were able to achieve approximately 40% lentiviral-mediated knock down (KD) and 30% retroviral-mediated over-expression (OE) relative to control (CTL). B) TUNEL (TdT mediated dUTP Nick End Labeling) staining results showed lentiviral-mediated KD of ANTXR2 in HUSMC increased apoptosis whereas retroviral-mediated OE of ANTXR2 did not affect the number of apoptotic cells.
We first asked whether HUSMC viability was affected when ANTXR2 expression was reduced or increased. HUSMC-R2KD cells exhibited increased apoptosis via TUNEL assay (p<0.05) as compared to CTL cells (Figure 1B). The HUSMC-R2OE cells exhibited no significant change in apoptosis (Figure 1B, p=0.9), relative to CTL. These findings were replicated using the ANNEXIN-V assay (data not shown), where R2KD displayed increased apoptosis. Higher rates of R2KD resulted in cell death and inability to sustain HUSMC-R2KD cell lines (data not shown).
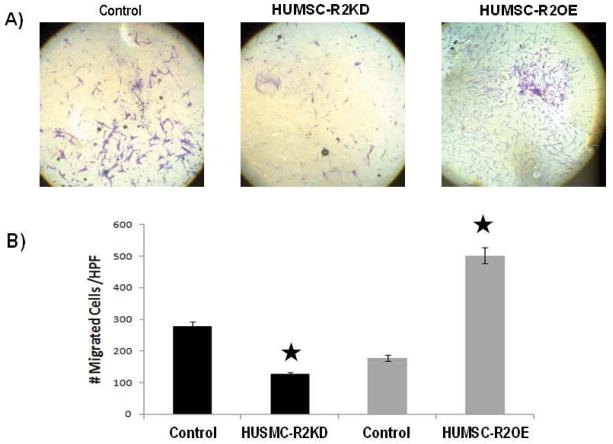
In pregnancy, it is well established that the uterus undergoes myometrial hyperplasia.16 Cell migration is required during this process for proper formation and function of the myometrial layers. As such, we assessed HUSMC migration towards a migratory signal using a Boyden chamber assay. HUSMC cell lines were allowed to migrate across a filter toward media supplemented with PDGF-B, a factor known to promote migration of smooth muscle cells.17 We found that HUSMC-R2KD cells migrated to PDGF-B to a lesser extent than CTL cells (p<0.05) indicating that ANTXR2 expression is necessary for chemokine-directed migration. (Figure 2). HUSMC-R2OE cells exhibited increased migration relative to CTL (p=0.05). (Figure 2)
Figure 2.
Knockdown of ANTXR2 in HUSMC (HUSMC-R2KD) decreased directed cell migration whereas over-expression of ANTXR2 in HUSMC (HUSMC-R2OE) increased directed cell migration. A) Images of HUSMC-R2KD, control (HUSMC-CTL) and HUSMC-OE migrating cells on the lower surface of the Boyden chamber migration assay membrane. Cells are stained with crystal violet and pictures were obtained at 10X. B) HUSMC-R2KD cells migrated to PDGF-B to a lesser extent than HUSMC-CTL cells (p<0.05) indicating that ANTXR2 expression is necessary for chemokine-directed migration. HUSMC-R2OE cells exhibited increased migration relative to CTL (p=0.05).
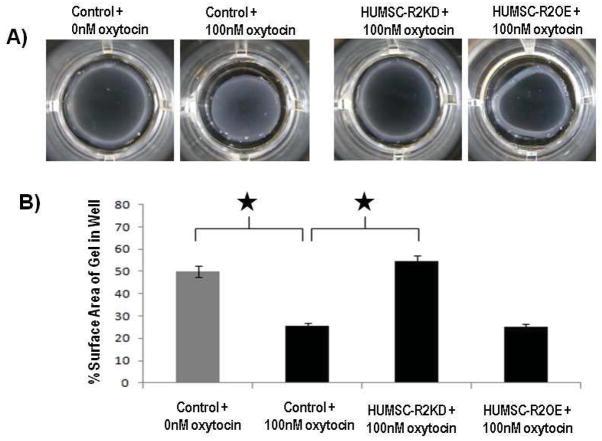
We reported that Antxr2−/− mice are fertile and carry pregnancies to term, but fail to deliver their fetuses resulting in maternal death or fetal reabsorption.3 As the predominant function of HUSMC is to produce uterine contractions, we used an oxytocin-mediated collagen contraction assay using cultured HUSMC. We sought to evaluate if the parturition defect seen in Antxr2−/− mice was in part due to a defect in myometrial contractility. HUSMC-CTL cells contracted upon oxytocin treatment and the surface area of the collagen gel was significantly reduced compared to HUSMC-CTL cells that were not treated with oxytocin (HUSMC-CTL without oxytocin: % gel surface area = 49%, HUSMC-CTL with oxytocin: % gel surface area = 26%, p<0.05). HUSMC-R2KD cells treated with oxytocin did not contract the collagen gel well, resulting in collagen gel surface areas similar to HUSMC-CTL cells that were not treated with oxytocin (HUSMC-R2KD with oxytocin: % gel surface area = 54% vs HUSMC-CTL with oxytocin: % gel surface area = 49%, p=0.24) HUSMC-R2OE cells did not contract any better than and HUSMC-CTL cells treated with oxytocin (HUSMC-R2OE with oxytocin: % gels surface area = 25% vs HUSMC-CTL with oxytocin = 26%, p=0.9). (Figure 3) This findings suggests that sufficient R2 levels are required to elicit a normal contraction response to oxytocin. (Figure 3)
Figure 3.
Decreasing ANTXR2 expression on human uterine smooth muscle cells (HUSMC) resulted in decreased oxytocin-induced HUSMC contractility. A) Images of detached collagen lattices 48h after addition of 0nM or 100nM oxytocin. B) HUSMC with decreased ANTXR2 expression (HUSMC-R2KD) did not contract the collagen gel well after oxytocin administration, resulting in collagen gel surface areas similar to control (HUSMC-CTL) cells that were not treated with oxytocin. HUSMC with increased ANTXR2 expression (HUSMC-R2OE) did not contract any better than and HUSMC-CTL cells treated with oxytocin.
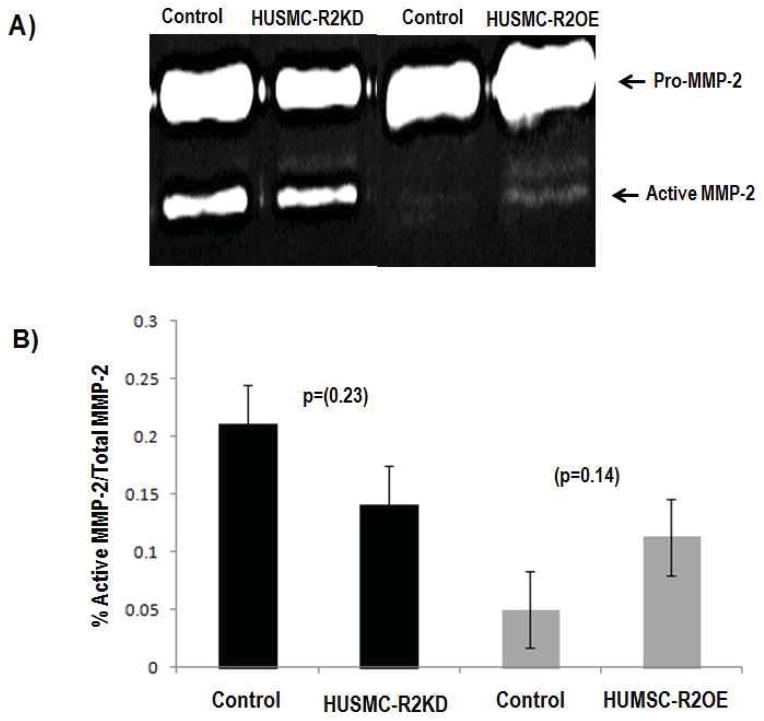
Smooth muscle cells regulate extracellular matrix formation and remodeling, partly through the production of matrix metalloproteinases (MMPs).18 In Antxr2−/− mice, the marked disruption of the myometrial layer coincided with the progressive appearance of a thickened, collagen dense, acellular stroma layer. One aspect of extracellular matrix formation and remodeling involves the production and degradation of collagen. As matrix metalloproteinases (MMPs) are known degrade collagen and are produced by uterine smooth muscle cells, particularly from leiomyomata cells,19 MMP2 and MMP9 activity was assessed by gelatin zymography in HUMSC-R2KD and HUSMC-R2OE cells. Although not statistically significant, we consistently observed a decrease in the ratio of active MMP2 to total MMP2 in the conditioned medium from HUMSC-R2KD cells compared to that of HUSMC-CTL cells. Conversely, we consistently observed an increase in the ratio of active MMP2 to total MMP2 in the conditioned medium from HUMSC-R2OE cells compared to that of HUSMC-CTL cells.
DISCUSSION
Antxr2−/− female mice exhibit a parturition defect that is in part due to loss of myometrial cells. Uteri of nonpregnant Antxr2−/− mice also lose myometrial layers over time and this loss is coincident with ECM accumulation.3 These discoveries led to the hypothesis that ANTXR2 is essential for normal uterine smooth muscle cell viability and function in the pregnant and nonpregnant uterus. The series of in vitro experiments presented in this study demonstrate that knockdown of ANTXR2 expression in human uterine smooth muscle cells (HUSMC) leads to decreased cell viability, directed migration and contractility. Conversely, overexpression of ANTXR2 in HUSMC leads to increased cell migration, but has no effect on HUSMC viability or contractility. We conclude that ANTXR2 is necessary for proper HUSMC viability and function.
The mechanism by which ANTXR2 promotes HUSMC viability, migration and contractility remains to be determined, as studies investigating these mechanisms specifically in uterine smooth muscle cells are lacking. However, literature evaluating airway smooth muscle cells shows that the attachment of smooth muscle cells to ECM components, particularly type IV collagen and fibronectin, is vital to maintaining normal cell growth patterns, cytoskeleton formation and cell spreading.20 Utilizing function blocking antibodies and peptides, Freyer et al demonstrated that the α5β1 integrin is an important transducer of the ECM-derived survival signal in smooth muscle cells.20 ANTXRs are known to share structural similarities with α-integrin subunits,11 and have been demonstrated to bind type IV collagen and laminin.6 Thus, HUMSCs lacking ANTXR2 may not attach to the ECM properly. This could explain the decrease in cell viability and migration we detected in HUSMC-R2KD cells. Like integrins, ANTXR2 may also have a role in outside-in signaling mechanisms that promote cell viability. Therefore, ANTXR2 deficient HUSMC may be more prone to undergoing apoptosis due to defective intracellular survival signals.
A recent study by Yang et al suggests that ANTXR interaction with the actin cytoskeleton may also be an important factor for normal cell function.21 This study demonstrated that ANTXR1, the closest homolog to ANTXR2, is highly expressed in HUSMC. A genetic screen utilizing cDNA from uterine tissue identified transgelin and alpha smooth muscle actin (α-SMA) as ANTXR1 binding proteins. Subsequent analysis demonstrated that ANTXR1 and α-SMA physically interact. As α-SMA is an integral component to the contractile apparatus of smooth muscle cells, this study demonstrated that proteins integral to regulating smooth muscle cell migration and contractility are linked to ANTXR1. The same may hold true for ANTXR2. As such, it is possible that loss of ANTXR2 in HUSMC could lead to disruption of the actin cytoskeleton. This would in turn affect cell migration as well as the contractile properties of HUSMC. If HUSMC are not properly situated within the extracellular matrix and have disrupted contractile properties, this may affect the contractile force or contraction pattern that is produced by the human myometrium. Further studies are needed to evaluate if ANTXR2 is involved in abnormal myometrial contractile states such as dysfunctional labor or preterm labor.
Normal cell migration requires remodeling of the ECM around the cell. Previously, we reported ANTXR2 forms a complex with a membrane-bound MMP, MT-1 MMP. We hypothesized that this complex then activates MMP2, an enzyme known to degrade and remodel the ECM.3 We also demonstrated that MMP2 activity was reduced in Antxr2−/− uterine lysates and conditioned medium from Antxr2−/− mouse embryonic fibroblasts. These findings suggest that disruption of the Antxr2 gene in mice results in decreased MT1-MMP activity and thus decreased MMP2 activation. Decreased MMP2 activity may result in defective ECM remodeling and therefore aberrant cell migration. Our current study demonstrates a trend toward decreased MMP2 activity in HUMSC-R2KD cells. One hypothesis as to why this trend did not reach statistical significance is that MMP2 activation is dependent on the amount of ANTXR2 present. In our study, we were able to achieve 30% knockdown of ANTXR2 in HUMSC. We believe that we generated higher levels of ANTXR2 knockdown, but these cells died before we could work with them further. Additional studies utilizing alternative approaches are needed to delineate the role of ANXTR2 during MMP activation in HUMSC.
In summary, we have documented that ANTXR2 is expressed on HUSMC and is intrinsic to normal HUSMC function. This suggests that the disappearance of myometrial layers in the uteri of pregnant and nonpregnant Antxr2−/− mice is in part due to loss of Antxr2 expression in the smooth muscle cells. In turn, loss of the muscle cell layers that are involved in ECM remodeling during the estrus cycle and pregnancy would account for the aberrant accumulation of multiple ECM proteins in the Antxr2−/− reproductive tracts. These studies highlight the importance of normal ANTXR2 function in HUSMCs and indicate that further studies are needed to delineate the role of ANTXR2 in obstetrics, namely preterm and dysfunctional labor. Results from these studies may expand options for tocolytics and labor induction or augmentation agents.
Figure 4.
MMP2 activity levels in human uterine smooth muscle cells with altered ANTXR2 expression. A) Gelatin zymography results showing HUSMC with decreased ANTXR2 expression (HUSMC-R2KD) exhibited slightly less MMP2 activity vs control (HUSMC-CTL), while HUSMC with increased ANTXR2 expression (HUSMC-R2OE) exhibited slightly more MMP2 activity vs HUMSMC-CTL. B) ImageJ 1.45 s (NIH) quantification of zymography band intensities of MMP2 activity in HUSMC-CTL, HUSMC-R2KD and HUSMC-R2OE cells.
Acknowledgments
Financial support: None
Footnotes
Disclosure statement: The authors report no conflict of interest.
Paper presentation: The abstract was presented at the 32nd Annual SMFM meeting in Dallas, TX (2/6/12–2/12/12).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hotchkiss KA, Basile CM, Spring SC, et al. TEM8 expression stimulates endothelial cell adhesion and migration by regulating cell-matrix interactions on collagen. Exp Cell Res. 2005;305:133–144. doi: 10.1016/j.yexcr.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 2.Reeves CV, Dufraine J, Young JA, Kitajewski J. Anthrax toxin receptor 2 is expressed in murine and tumor vasculature and functions in endothelial proliferation and morphogenesis. Oncogene. 2009;6:789–801. doi: 10.1038/onc.2009.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reeves CV, Wang X, Charles-Horvath PC, et al. Anthrax toxin receptor 2 functions in ECM homeostasis of the murine reproductive tract and promotes MMP activity. PLoS One. 2012;7(4):e34862. doi: 10.1371/journal.pone.0034862. Epub 2012 Apr 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rmali KA, Puntis MC, Jiang WG. TEM-8 and tubule formation in endothelial cells, its potential role of its vW/TM domains. Biochem Biophys Res Commun. 2005;334:231–238. doi: 10.1016/j.bbrc.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 5.Nanda A, Carson-Walter EB, Seaman S, et al. TEM8 interacts with the cleaved C5 domain of collagen alpha 3(VI) Cancer Res. 2004;64:817–820. doi: 10.1158/0008-5472.can-03-2408. [DOI] [PubMed] [Google Scholar]
- 6.Bell SE, Mavila A, Salazar R, et al. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J Cell Sci. 2001;114:2755–2773. doi: 10.1242/jcs.114.15.2755. [DOI] [PubMed] [Google Scholar]
- 7.Dowling O, Difeo A, Ramirez MC, et al. Mutations in capillary morphogenesis gene-2 result in the allelic disorders juvenile hyaline fibromatosis and infantile systemic hyalinosis. Am J Hum Genet. 2003;73:957–966. doi: 10.1086/378781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanks S, Adams S, Douglas J, et al. Mutations in the gene encoding capillary morphogenesis protein 2 cause juvenile hyaline fibromatosis and infantile systemic hyalinosis. Am J Hum Genet. 2003;73:791–800. doi: 10.1086/378418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stucki U, Spycher MA, Eich G, et al. Infantile systemic hyalinosis in siblings: clinical report, biochemical and ultrastructural findings, and review of the literature. Am J Med Genet. 2001;100:122–129. doi: 10.1002/1096-8628(20010422)100:2<122::aid-ajmg1236>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka K, Ebihara T, Kusubata M, et al. Abnormal collagen deposition in fibromas from patient with juvenile hyaline fibromatosis. J Dermatol Sci. 2009;55:197–200. doi: 10.1016/j.jdermsci.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Scobie HM, Rainey GJ, Bradley KA, Young JA. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci U S A. 2003;100:5170–5174. doi: 10.1073/pnas.0431098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tung JJ, Hobert O, Berryman M, Kitajewski J. Chloride intracellular channel 4 is involved in endothelial proliferation and morphogenesis in vitro. Angiogenesis. 2009;12:209–220. doi: 10.1007/s10456-009-9139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dallot E, Pouchelet M, Gouhier N, Cabrol D, Ferre F, Breuiller-Fouche M. Contraction of cultured human uterine smooth muscle cells after stimulation with endothelin-1. Biol Reprod. 2003;68:937–942. doi: 10.1095/biolreprod.102.008367. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Wilson MJ, Slaton JW, et al. Increased aggressiveness of human prostate PC-3 tumor cells expressing cell surface localized membrane type-1 matrix metalloproteinase (MT1-MMP) J Androl. 2009;30:259–274. doi: 10.2164/jandrol.108.006494. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Yi J, Lei J, Pei D. Expression, purification and characterization of recombinant mouse MT5-MMP protein products. FEBS Lett. 1999;462:261–266. doi: 10.1016/s0014-5793(99)01534-3. [DOI] [PubMed] [Google Scholar]
- 16.Katzenellenbogen BS, Bhakoo HS, Ferguson ER, et al. Estrogen and anti-estrogen action in reproductive tissues and tumors. Rec Prog Horm Res. 1979;35:259–300. doi: 10.1016/b978-0-12-571135-7.50010-2. [DOI] [PubMed] [Google Scholar]
- 17.Rossi MJ, Chegini N, Masterson BJ. Presence of epidermal growth factor, platelet-derived growth factor, and their receptors in human myometrial tissue and smooth muscle cells: their action in smooth muscle cells in vitro. Endocrinology. 1992;130:1716–1727. doi: 10.1210/endo.130.3.1311246. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Zhang J, Fu W, Guo D, Jiang J, Wang Y. Association of smooth muscle cell phenotypes with extracellular matrix disorders in thoracic aortic dissection. J Vasc Surg. 2012 Dec;56(6):1698–709. doi: 10.1016/j.jvs.2012.05.084. [DOI] [PubMed] [Google Scholar]
- 19.Dou Q, Tarnuzzer RW, Williams RS, Schultz GS, Chegini N. Differential expression of matrix metalloproteinases and their tissue inhibitors in leiomyomata: a mechanism for gonadotrophin releasing hormone agonist-induced tumour regression. Mol Hum Reprod. 1997 Nov;3(11):1005–14. doi: 10.1093/molehr/3.11.1005. [DOI] [PubMed] [Google Scholar]
- 20.Freyer AM, Johnson SR, Hall IP. Effects of growth factors and extracellular matrix on survival of human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2001 Nov;25(5):569–76. doi: 10.1165/ajrcmb.25.5.4605. [DOI] [PubMed] [Google Scholar]
- 21.Yang M, Chaudhary A, Seaman S, et al. The Cell Surface Structure of Tumor Endothelial Marker 8 (TEM8) is Regulated by the Actin Cytoskeleton. Biochim Biophys Acta. 2011;1813(1):39–49. doi: 10.1016/j.bbamcr.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]