DISCUSSION OF PROBLEM/CLINICAL PRESENTATION
The prevalence of thyroid nodules is approximately 3% to 8% in the general population1–4 but increases to almost 50% after 65 years of age.5 With advances in technology, incidence of thyroid nodules on ultrasound (US) has increased to almost 60%; however, the incidence of malignancy in thyroid is low at 5% to 15%.6,7 In addition, B-mode and Doppler US have been found to have low accuracy. Fine needle aspiration (FNA) is the standard procedure to determine if a nodule is cancerous or not; however, FNA is an invasive procedure and would result in an inadequate sample in 10% to 20% of cases leading to rebiopsy.6 Palpation has been used in clinical examination to assess if a thyroid nodule is firm or palpable; however, palpation is subjective and will vary depending on the size and location of the nodule.8
IMAGING PROTOCOLS
Imaging Findings
US elastography is a promising new technique in the evaluation of the thyroid nodule. It allows for “virtual palpation” of the nodule, which may not be otherwise palpable. US elastography was developed to obtain information on tissue stiffness noninvasively.9–11 Due to the superficial location of the thyroid gland, it is feasible to obtain information regarding the stiffness in the organ or nodule.
Most malignant tumors are characterized by the presence of abnormally firm stroma due to the presence of collagen and myofibroblasts, which is the desmoplastic transformation. This tumor stroma promotes the proliferation of malignant cells (and could even initiate them).12 However, certain benign fibrous tumors can be very stiff as well (histiocytofibromas, for example). Previous ex vivo studies had suggested that there is considerable difference between the stiffness in normal thyroid tissue and thyroid tumors.13,14 Based on this observation, multiple in vivo studies were then performed to differentiate benign from malignant thyroid nodules. Various techniques exist for performing elastography as outlined in the other articles. A brief description of these techniques and their utility in thyroid elastography are mentioned here.
Quasi-static or strain elastography or sonoelastography
In quasi-static or strain elastography, the US probe is placed on the neck with gel interposition and compression is generated by pressure applied by the operator with the US probe on the skin. Improvement in the sensitivity of very small tissue movement detection has made it possible to use carotid pulsation to induce this tissue deformation.15 Classification using 4 or 5 visual categorical scores (Figs. 1 and 2), either color coded or in gray scale, has been proposed.16 Semi-quantitative analysis provides numerical values that correspond to the deformation ratios. The machine calculates a ratio between the zones of interest (regions of interest [ROI]) placed by the operator on the nodule and on the healthy tissue. The calculation can thus be made using the rates of deformation of the structure (“strain rate”) (Figs. 3 and 4).17
Fig. 1.
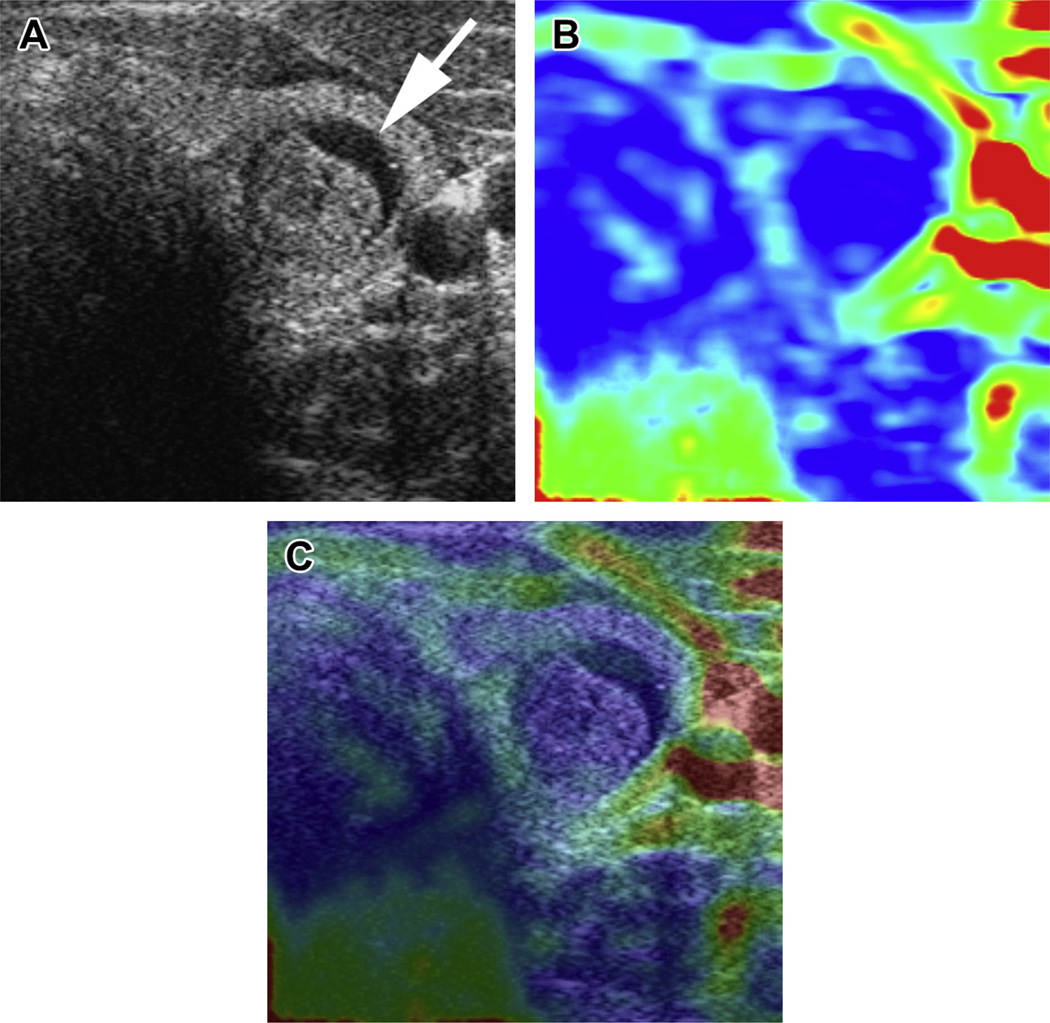
Strain elastography: B-mode US image (A) showing a nodule in the right thyroid gland (arrow). This lesion showed increased stiffness on elastography (B) and the overlay image (color overlayed on B-mode) (C) with predominantly blue color (high stiffness), which was shown to be a follicular adenoma on histopathology. C, carotid artery.
Fig. 2.
Strain elastography: B-mode US image (A) showing a nodule in the left thyroid gland with small areas of calcification in it (arrow). This lesion showed increased stiffness on elastography (B) and the overlay image (color overlayed on B-mode) (C) with a combination of blue and green color (moderate stiffness), which was shown to be a papillary carcinoma on histopathology.
Fig. 3.
Quantitative strain elastography: B-mode US image (A) showing a nodule in the left thyroid gland with cystic changes in it. ROIs were drawn on the lesion and adjacent to the carotid artery as shown in the elastography image (B). This lesion showed increased stiffness and had a thyroid stiffness index of 35, which was shown to be a papillary carcinoma on histopathology.
Fig. 4.
Quantitative strain elastography: B-mode US image (A) showing a solid nodule in the left thyroid gland. ROIs were drawn on the lesion and adjacent to the carotid artery as shown in the elastography image (B). This lesion showed intermediate stiffness and had a thyroid stiffness index of 13.5, which was shown to be a follicular lesion with low probability of malignancy on cytology.
A recent meta-analysis by Bojunga and colleagues,18 which included 639 nodules, reported a mean sensitivity of 92% and a specificity of 90% using strain elastography for the diagnosis of malignant thyroid nodules; however, the patient population was highly selected with a 24% prevalence of malignancy and many patients were sent to surgery.
Two recent studies19,20 with 309 and 97 patients, respectively, used strain values and ratios to determine thyroid nodule stiffness. All patients were referred to surgery. Vorländer and colleagues19 used a proprietary absolute measurement of strain value, which ranged from 1.0 (maximum soft) to 0.1 (maximum hard) and reported a negative predictive value (NPV) for malignancy of 100% using a strain ratio cutoff of greater than 0.31 and a positive predictive value (PPV) of 42% using a cutoff of less than 0.15. Cantisani and colleagues20 reported a sensitivity, specificity, PPV, and NPV of 97.3%, 91.7%, 87.8%, and 98.2%, respectively, for the prediction of malignancy using a strain ratio ≥2 (ratio of lesion strain to surrounding parenchyma). Elastography was more sensitive and specific than all conventional US features.
Another study compared strain elastography based on 4 point scores and the strain ratios between the nodule and the surrounding thyroid at the same depth.21 The diagnostic accuracy of the strain ratio evaluation was slightly higher (0.88 vs 0.79, P<.001) than that of the elastography score, with a higher specificity. Another prospective study22 evaluated strain elastography in 51 patients with small single solid nodules of 3 to 10 mm submitted to surgical resection. The 5-point scale developed by Itoh and colleagues16 for the breast elastography was used in this study; with a cutoff at 3/4, a sensitivity of 91%, specificity of 89%, PPV 94%, and NPV 85% for the diagnosis of malignant nodules was found. Thus, strain elastography seems to have a potential even in small nodules.
However, additional studies, including only a few follicular carcinomas, revealed inconclusive data on the value of elastography. Most malignant nodules missed by elastography were follicular carcinomas, which can be soft and difficult to differentiate from benign nodules.18 Strain elastography was evaluated in 102 patients with indeterminate cytology who went to surgery.23 Histology revealed 64 follicular adenomas, 32 follicular variants of papillary thyroid cancer, 4 follicular carcinomas, and 2 hyperplastic nodules. In this selected population, strain elastography (4-point scale) only reached a PPV of 34% and an NPV of 50%. Conversely, Cantisani and colleagues24 reported a study including 140 nodules with indeterminate cytology in which elastography with a strain ratio greater than 2.05 achieved sensitivity, specificity, NPV, PPV, and accuracy of 87.5%, 92%, 94.8%, 81.4%, 89.8%, respectively.
Acoustic radiation force imaging
In acoustic radiation force imaging (ARFI), an ROI is placed in the thyroid nodule while performing real-time B-mode imaging. The tissue at the ROI is mechanically excited using acoustic pulses to generate localized tissue displacements. These displacements result in shear-wave propagation away from the region of excitation and are tracked using ultrasonic, correlation-based methods.25 The maximum displacement is estimated for many US tracking beams and the shear wave speed of the tissue can be reconstructed26,27 along with the shear wave propagation velocity. Because there is no mechanical or external pressure used in ARFI and because shear wave propagation velocity is proportional to the square root of tissue elasticity, stiffness of the nodule can be calculated.28
A study by Bojunga and colleagues28 found that the median velocity of ARFI imaging in the healthy thyroid tissue, benign and malignant thyroid nodules was 1.76 m/s, 1.90 m/s, and 2.69 m/s, respectively. They did not find any significant difference in median velocity between healthy thyroid tissue and benign thyroid nodules; however, a significant difference was found between malignant thyroid nodules and healthy thyroid tissue and malignant thyroid nodules and benign thyroid nodules. A study by Azizi and colleagues29 found that the NPV of ARFI was better than the PPV; however, the PPV of ARFI was better than that of B-mode criteria of hypoechogenicity. Gu and colleagues30 in their study found that ARFI had a high sensitivity and specificity in evaluating benign and malignant nodules when they used a cutoff value of 2.555 m/s. Sporea and colleagues31 used ARFI in evaluating the utility of ARFI in diffuse thyroid disease. They found that the optimal cutoff value for the prediction of diffuse thyroid pathologic abnormality with ARFI was 2.36 m/s, which had a sensitivity of 62.5%, specificity of 79.5%, PPV of 87.6%, NPV of 55.5%, and accuracy of 72.7%.
Shear wave elastography
Shear wave elastography (SWE) is a user-independent method with no compressive maneuvers needed. This methodology captures the waves that propagate from the stimulated tissue in question with an ultrafast US tracking method, which displays real-time information in terms of velocity or estimated tissue stiffness expressed in kilopascals (Figs. 5 and 6). Sebag and colleagues32 reported that significantly higher elasticity index was noted in malignant thyroid nodules than benign nodules, and they reported the sensitivity and specificity of SWE were 85.2% and 93.9% using a cutoff level of 65 kPa. They reported higher diagnostic performance with a combined score (SWE+B-mode US) than that of B-mode US only. Other SWE studies33 showed variable cutoff values yielding a maximum sum of diagnostic performance to predict thyroid malignancy. Kim and colleagues34 in their study found similar results as the prior studies. Veyrieres and colleagues35 were able to confirm that the cutoff value of 66 kPa was the best US sign to rule out malignant thyroid nodules; however, they suggested that more work needed to be performed in calcified nodules and follicular tumors (Fig. 7).
Fig. 5.
SWE showing a nodule with an SWE value of 72 kPa on a transverse image (A) and an SWE ratio with the surrounding normal thyroid gland on the sagittal image (B) of 2.46, which was shown to be a papillary carcinoma in histopathology.
Fig. 6.
SWE showing a nodule with an SWE value of 7.2 kPa on a transverse image (A) and an SWE ratio with the surrounding normal thyroid gland on the sagittal image (B) of 0.92, which was shown to be a multinodular goiter on histopathology.
Fig. 7.
SWE showing a nodule with an SWE value of 13.8 kPa on a transverse image (A) and an SWE ratio with the surrounding normal thyroid gland on the sagittal image (B) of 0.42, which was shown to be a follicular adenoma on histopathology.
PEARLS, PITFALLS, AND VARIANTS
Elastography provides information about the stiffness in a particular tissue similar to what palpation of a lesion would do. Most malignant tumors have abnormally firm stroma because of the presence of collagen and myofibroblasts. This desmoplastic transformation promotes the proliferation of malignant cells (and could even initiate them).12 However, certain benign fibrous tumors can be very stiff as well (histiocytofibromas, for example). Thyroid US elastography could therefore make it possible to identify cancers with increased stiffness, such as papillary cancers; on the other hand, cancers with nonmodified elasticities will not be detected, as is the case in most follicular carcinomas (Fig. 8) and benign lesions with increased stiffness will fall into the false positive group with elastography (Fig. 9).
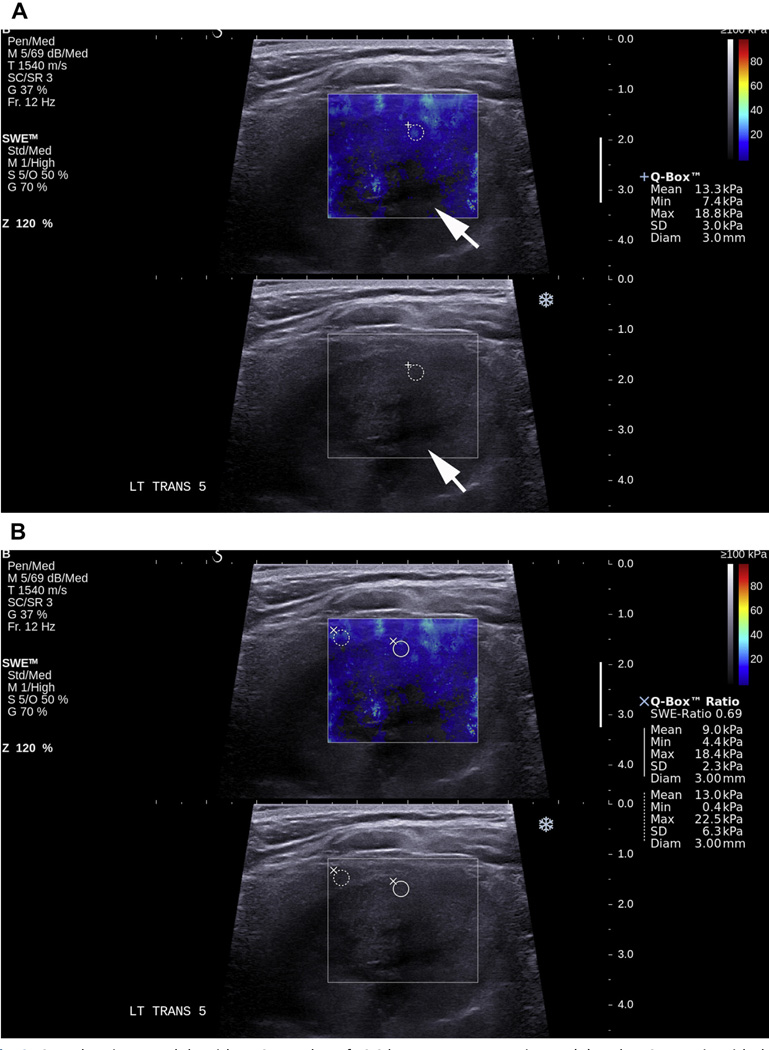
Fig. 8.
SWE showing a nodule with an SWE value of 13.3 kPa on a transverse image (A) and an SWE ratio with the surrounding normal thyroid gland on the transverse image (B) of 0.69, which was shown to be a follicular carcinoma on histopathology. Note the lack of shear wave information from the deeper part of the nodule (arrow) due to the presence of cystic areas.
Fig. 9.
Benign lesion with high stiffness on strain elastography: B-mode US image (A) showing a nodule in the left thyroid gland with cystic change in it (arrow). This lesion showed increased stiffness on elastography (B) and the overlay image (color overlayed on B-mode) (C) with predominantly blue color (high stiffness), which was shown to be a fibrotic nodule on histopathology.
Quasi-static elastography was first used and involved compression of tissue manually with the transducer. This compression would bring in variability in the amount of compression applied. To overcome this variability, machines then had a mechanism to show the amount of compression applied (mild, moderate, too much) on a scale displayed in real time on the image. Currently, the improvement in the sensitivity of very small tissue movement detection makes it possible to use the carotid pulsation to induce tissue deformation.36 Some of the limitations of quasi-static elastography include the need for an area of healthy tissue to be included in the elastography image, to be able to compare normal thyroid with the nodule. If all of the tissue in the image is abnormal, the nodule stiffness will vary with the obtained data, which could be a challenge especially in the case of a nodule in a background of an autoimmune thyroid disease.37 Similarly if the whole thyroid lobe is involved by a large nodule, there would not be any normal thyroid to compare. Tissue elasticity data obtained from ARFI are not translated in color-coded images as in other elastography methods and this method seems to be a transition technology from strain elastography to SWE.
Intralesional calcifications are common in thyroid nodules and may bias the stiffness of the lesion, as has been shown for nodules with peripheral calcifications.13,38,39 Elastography should also be interpreted with caution in extensive cystic areas because this may cause artifacts because of a loss of signal in the cystic region. Elastography shows high sensitivity, specificity, and NPV for the diagnosis of papillary carcinoma, which are known to be stiffer; however, the evaluations were performed in highly specialized centers with high incidences of carcinoma and data in follicular carcinomas, which are softer malignancies, have not been impressive.
A recent study using strain elastography, however, reported no additional value of elastography to experienced B-mode US.40
WHAT THE REFERRING PHYSICIAN NEEDS TO KNOW
A recent guideline published by the European Federation of Societies for Ultrasound in Medicine and Biology suggests that “elastography of thyroid lesions can be performed using either strain or shear wave elastography with many high-end systems using linear transducers. No patient preparation is required.” They recommend that “Elastography be used as an additional tool for thyroid lesion differentiation and based on expert opinion, elastography may be used to guide follow up of lesions negative for malignancy at FNA.”41 Elastography makes it possible to improve the PPV and the NPV of malignancy obtained from conventional US studies.20,42 The French Endocrinology Society recently specified in its “Consensus on the treatment of thyroid nodules” that elastography must therefore be integrated as a parameter of the US classification of the nodule; however, presently, it cannot replace it in any case.
SUMMARY
Elastography is undeniably a major technological advance in thyroid imaging in recent years. The anatomic characteristics of the thyroid (superficial organ) and the frequency of nodules in the thyroid make it an ideal organ for this technique. Static elastography is currently available in many machines. SWE is becoming the reference technique for thyroid; however, prospective studies (ongoing) will need to be performed before its routine use in the clinic. Elastography should not be considered as an alternative to conventional US, but like an additional parameter that optimizes the US imaging.43
KEY POINTS.
Techniques of elastography are available to evaluate the thyroid nodule.
Many reasons exist for false positive and false negative results in elastography.
Appropriate use of elastography is an adjunct to ultrasound and not a replacement.
Acknowledgments
Funding Sources: The author has received funding from NIH, R21 (Thyroid Elastography).
REFERENCES
- 1.Wiest PW, Hartshorne MF, Inskip PD, et al. Thyroid palpation versus high-resolution thyroid ultrasonography in the detection of nodules. J Ultrasound Med. 1998;17(8):487–496. doi: 10.7863/jum.1998.17.8.487. [DOI] [PubMed] [Google Scholar]
- 2.Tomimori E, Pedrinola F, Cavaliere H, et al. Prevalence of incidental thyroid disease in a relatively low iodine intake area. Thyroid. 1995;5(4):273–276. doi: 10.1089/thy.1995.5.273. [DOI] [PubMed] [Google Scholar]
- 3.Carroll BA. Asymptomatic thyroid nodules: incidental sonographic detection. AJR Am J Roentgenol. 1982;138(3):499–501. doi: 10.2214/ajr.138.3.499. [DOI] [PubMed] [Google Scholar]
- 4.Brander A, Viikinkoski P, Nickels J, et al. Thyroid gland: US screening in a random adult population. Radiology. 1991;181(3):683–687. doi: 10.1148/radiology.181.3.1947082. [DOI] [PubMed] [Google Scholar]
- 5.Mortensen JD, Woolner LB, Bennett WA. Gross and microscopic findings in clinically normal thyroid glands. J Clin Endocrinol Metab. 1955;15(10):1270–1280. doi: 10.1210/jcem-15-10-1270. [DOI] [PubMed] [Google Scholar]
- 6.Gharib H, Goellner JR. Fine-needle aspiration biopsy of the thyroid: an appraisal. Ann Intern Med. 1993;118(4):282–289. doi: 10.7326/0003-4819-118-4-199302150-00007. [DOI] [PubMed] [Google Scholar]
- 7.Hegedu¨s L. Clinical practice. The thyroid nodule. N Engl J Med. 2004;351(17):1764–1771. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- 8.Tan GH, Gharib H, Reading CC. Solitary thyroid nodule. Comparison between palpation and ultrasonography. Arch Intern Med. 1995;155(22):2418–2423. doi: 10.1001/archinte.155.22.2418. [DOI] [PubMed] [Google Scholar]
- 9.Ophir J, Alam SK, Garra B, et al. Elastography: ultrasonic estimation and imaging of the elastic properties of tissues. Proc Inst Mech Eng H. 1999;213(3):203–233. doi: 10.1243/0954411991534933. [DOI] [PubMed] [Google Scholar]
- 10.Gao L, Parker KJ, Lerner RM, et al. Imaging of the elastic properties of tissue-a review. Ultrasound Med Biol. 1996;22(8):959–977. doi: 10.1016/s0301-5629(96)00120-2. [DOI] [PubMed] [Google Scholar]
- 11.Greenleaf JF, Fatemi M, Insana M. Selected methods for imaging elastic properties of biological tissues. Annu Rev Biomed Eng. 2003;5:57–78. doi: 10.1146/annurev.bioeng.5.040202.121623. [DOI] [PubMed] [Google Scholar]
- 12.Mai KT, Perkins DG, Yazdi HM, et al. Infiltrating papillary thyroid carcinoma: review of 134 cases of papillary carcinoma. Arch Pathol Lab Med. 1998;122(2):166–171. [PubMed] [Google Scholar]
- 13.Rago T, Santini F, Scutari M, et al. Elastography: new developments in ultrasound for predicting malignancy in thyroid nodules. J Clin Endocrinol Metab. 2007;92(8):2917–2922. doi: 10.1210/jc.2007-0641. [DOI] [PubMed] [Google Scholar]
- 14.Lyshchik A, Higashi T, Asato R, et al. Elastic moduli of thyroid tissues under compression. Ultrason Imaging. 2005;27(2):101–110. doi: 10.1177/016173460502700204. [DOI] [PubMed] [Google Scholar]
- 15.Dighe M, Bae U, Richardson ML, et al. Differential diagnosis of thyroid nodules with US elastography using carotid artery pulsation. Radiology. 2008;248(2):662–669. doi: 10.1148/radiol.2482071758. [DOI] [PubMed] [Google Scholar]
- 16.Itoh A, Ueno E, Tohno E, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239(2):341–350. doi: 10.1148/radiol.2391041676. [DOI] [PubMed] [Google Scholar]
- 17.Luo S, Kim E, Dighe M, et al. Screening of thyroid nodules by ultrasound elastography using diastolic strain variation. Conf Proc IEEE Eng Med Biol Soc. 2009;1:4420–4423. doi: 10.1109/IEMBS.2009.5332744. [DOI] [PubMed] [Google Scholar]
- 18.Bojunga J, Herrmann E, Meyer G, et al. Real-time elastography for the differentiation of benign and malignant thyroid nodules: a meta-analysis. Thyroid. 2010;20(10):1145–1150. doi: 10.1089/thy.2010.0079. [DOI] [PubMed] [Google Scholar]
- 19.Vorla¨nder C, Wolff J, Saalabian S, et al. Real-time ultrasound elastography-a noninvasive diagnostic procedure for evaluating dominant thyroid nodules. Langenbecks Arch Surg. 2010;395(7):865–871. doi: 10.1007/s00423-010-0685-3. [DOI] [PubMed] [Google Scholar]
- 20.Cantisani V, D’Andrea V, Biancari F, et al. Prospective evaluation of multiparametric ultrasound and quantitative elastosonography in the differential diagnosis of benign and malignant thyroid nodules: preliminary experience. Eur J Radiol. 2012;81(10):2678–2683. doi: 10.1016/j.ejrad.2011.11.056. [DOI] [PubMed] [Google Scholar]
- 21.Ning CP, Jiang SQ, Zhang T, et al. The value of strain ratio in differential diagnosis of thyroid solid nodules. Eur J Radiol. 2012;81(2):286–291. doi: 10.1016/j.ejrad.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Dan HJ, Dan HY, et al. Differential diagnosis of small single solid thyroid nodules using real-time ultrasound elastography. J Int Med Res. 2010;38(2):466–472. doi: 10.1177/147323001003800210. [DOI] [PubMed] [Google Scholar]
- 23.Lippolis PV, Tognini S, Materazzi G, et al. Is elastography actually useful in the presurgical selection of thyroid nodules with indeterminate cytology? J Clin Endocrinol Metab. 2011;96(11):E1826–E1830. doi: 10.1210/jc.2011-1021. [DOI] [PubMed] [Google Scholar]
- 24.Cantisani V, Ulisse S, Guaitoli E, et al. Q-elastography in the presurgical diagnosis of thyroid nodules with indeterminate cytology. PLoS One. 2012;7(11):e50725. doi: 10.1371/journal.pone.0050725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nightingale K, Nightingale R, Stutz D, et al. Acoustic radiation force impulse imaging of in vivo vastus medialis muscle under varying isometric load. Ultrason Imaging. 2002;24(2):100–108. doi: 10.1177/016173460202400203. [DOI] [PubMed] [Google Scholar]
- 26.Nightingale K, Zhai L, Dahl J, et al. Proc 2006 IEEE Ultrasonics Symposium (IEEE) Piscataway, NJ: IEEE Operations center; 2006. Shear wave velocity estimation using acoustic radiation force impulsive excitation in liver in vivo; pp. 1156–1160. [Google Scholar]
- 27.Kasai C, Namekawa K, Koyano A, et al. Real-time two-dimensional blood flow imaging using an autocorrelation technique. IEEE Trans Son Ultrason. 1985;32:458–464. [Google Scholar]
- 28.Bojunga J, Dauth N, Berner C, et al. Acoustic radiation force impulse imaging for differentiation of thyroid nodules. PLoS One. 2012;7(8):e42735. doi: 10.1371/journal.pone.0042735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azizi G, Keller J, Lewis M, et al. Performance of elastography for the evaluation of thyroid nodules: a prospective study. Thyroid. 2013;23(6):734–740. doi: 10.1089/thy.2012.0227. [DOI] [PubMed] [Google Scholar]
- 30.Gu J, Du L, Bai M, et al. Preliminary study on the diagnostic value of acoustic radiation force impulse technology for differentiating between benign and malignant thyroid nodules. J Ultrasound Med. 2012;31(5):763–771. doi: 10.7863/jum.2012.31.5.763. [DOI] [PubMed] [Google Scholar]
- 31.Sporea I, Vlad M, Bota S, et al. Thyroid stiffness assessment by acoustic radiation force impulse elastography (ARFI) Ultraschall Med. 2011;32(3):281–285. doi: 10.1055/s-0029-1246048. [DOI] [PubMed] [Google Scholar]
- 32.Sebag F, Vaillant-Lombard J, Berbis J, et al. Shear wave elastography: a new ultrasound imaging mode for the differential diagnosis of benign and malignant thyroid nodules. J Clin Endocrinol Metab. 2010;95(12):5281–5288. doi: 10.1210/jc.2010-0766. [DOI] [PubMed] [Google Scholar]
- 33.Bhatia KS, Tong CS, Cho CC, et al. Shear wave elastography of thyroid nodules in routine clinical practice: preliminary observations and utility for detecting malignancy. Eur Radiol. 2012;22(11):2397–2406. doi: 10.1007/s00330-012-2495-1. [DOI] [PubMed] [Google Scholar]
- 34.Kim H, Kim JA, Son EJ, et al. Quantitative assessment of shear-wave ultrasound elastography in thyroid nodules: diagnostic performance for predicting malignancy. Eur Radiol. 2013;23(9):2532–2537. doi: 10.1007/s00330-013-2847-5. [DOI] [PubMed] [Google Scholar]
- 35.Veyrieres JB, Albarel F, Lombard JV, et al. A threshold value in shear wave elastography to rule out malignant thyroid nodules: a reality? Eur J Radiol. 2012;81(12):3965–3972. doi: 10.1016/j.ejrad.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Bae U, Dighe M, Dubinsky T, et al. Ultrasound thyroid elastography using carotid artery pulsation -preliminary study. J Ultrasound Med. 2007;26(6):797–805. doi: 10.7863/jum.2007.26.6.797. [DOI] [PubMed] [Google Scholar]
- 37.Di Pasquale M, Rothstein JL, Palazzo JP. Pathologic features of Hashimoto’s-associated papillary thyroid carcinomas. Hum Pathol. 2001;32(1):24–30. doi: 10.1053/hupa.2001.21138. [DOI] [PubMed] [Google Scholar]
- 38.Hong Y, Liu X, Li Z, et al. Real-time ultrasound elastography in the differential diagnosis of benign and malignant thyroid nodules. J Ultrasound Med. 2009;28(7):861–867. doi: 10.7863/jum.2009.28.7.861. [DOI] [PubMed] [Google Scholar]
- 39.Kim JK, Baek JH, Lee JH, et al. Ultrasound elastography for thyroid nodules: a reliable study? Ultrasound Med Biol. 2012;38(9):1508–1513. doi: 10.1016/j.ultrasmedbio.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 40.Moon HJ, Kim EK, Yoon JH, et al. Clinical implication of elastography as a prognostic factor of papillary thyroid microcarcinoma. Ann Surg Oncol. 2012;19(7):2279–2287. doi: 10.1245/s10434-011-2212-3. [DOI] [PubMed] [Google Scholar]
- 41.Cosgrove D, Piscaglia F, Bamber J, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography.Part 2: clinical applications. Ultraschall Med. 2013;34(3):238–253. doi: 10.1055/s-0033-1335375. [DOI] [PubMed] [Google Scholar]
- 42.Russ G, Bienvenu-Perrard M, Royer B, et al. Prospective evaluation of thyroid imaging reporting and data system on 4550 nodules with and without elastography. Eur J Endocrinol. 2011;168(5):649–655. doi: 10.1530/EJE-12-0936. [DOI] [PubMed] [Google Scholar]
- 43.Leenhardt L, Borson-Chazot F, Calzada M, et al. Good practice guide for cervical ultrasound scan and echo-guided techniques in treating differentiated thyroid cancer of vesicular origin. Ann Endocri-nol (Paris) 2011;72(3):173–197. doi: 10.1016/j.ando.2011.04.001. [DOI] [PubMed] [Google Scholar]