Abstract
Expansion of the extracellular matrix is a prominent but poorly characterized feature of tendinosis. The present study aimed to characterize the extent and distribution of the large aggregating proteoglycan versican in patients with patellar tendinosis. We obtained tendon from tendinopathy patients undergoing debridement of the patellar tendon and from controls undergoing intramedullary tibial nailing. Versican content was investigated by Western blotting and immunohistochemistry. Microvessel thickness and density were determined using computer-assisted image analysis. Markers for smooth muscle (α-SMA), endothelial cells (CD31) and proliferating cells (Ki67) were examined immunohistochemically. Western blot analysis and immunohistochemical staining revealed elevated versican content in the proximal patellar tendon of tendinosis patients (p=0.042). Versican content was enriched in regions of fibrocartilage metaplasia and fibroblast proliferation, as well as in the perivascular matrix of proliferating microvessels and within the media and intima of arterioles. Microvessel density was higher in tendinosis tissue compared to control tissue. Versican deposition is a prominent feature of patellar tendinosis. Because this molecule is not only a component of normal fibrocartilagenous matrices, but is also implicated in a variety of soft tissue pathologies, future studies should further detail both pathological and adaptive roles of versican in tendons.
INTRODUCTION
Tendinopathy is a common source of chronic musculoskeletal pain (Hazleman et al. 2004). The histology of tendinosis – the underlying pathology in many cases of tendinopathy - is well described and certain histopathological features of tendinosis appear to be consistent across tendons (Khan et al. 1999), but the molecular changes in tendon composition have been investigated less extensively. Imaging abnormalities include hypoechoic areas on greyscale ultrasound and high signal on magnetic resonance imaging (MRI), corresponding to an increase in glycosaminoglycans (Cook et al. 2000). Cross-sectional studies of tendon from competitive athletes have demonstrated that such abnormal tendon morphology on imaging – even when not associated with symptoms -- is associated with a 4.2 times greater risk of developing into symptomatic tendinopathy compared with a sonographically normal tendon (Cook et al. 2000). Thus, changes in the extracellular matrix may represent an early step in the pathogenesis of tendinosis.
Unlike the pathology of osteoarthritis, which is characterized by a loss of glycosaminoglycan and decreased DNA content, biochemical assays of tendinosis have demonstrated an increased presence of glycosaminoglycan and DNA. Chondroitin sulphate is the major glycosaminoglycan in tendinosis tissue and the accompanying increase in DNA content has been primarily attributed to fibroblasts and vascular cells (endothelial and smooth muscle cells) (Khan et al. 1996; Fenwick et al. 2002). Therefore, the excessive chondroitin sulphate found in biochemical assays of tendinosis could be produced by resident fibroblasts (tenocytes) or by vascular cells, or both (Riley 2005). Furthermore, the core proteins to which the glycosaminoglycan is likely bound have not yet been examined to our knowledge.
Versican is the most abundant of the large chondroitin sulphate proteoglycans in tendon (Yoon and Halper 2005). Immunohistochemical studies of normal tendon and of collagenase-digested tenocyte arrays have found versican in the endotendon around vessels, and in the pericellular matrix around tenocytes (Bode-Lesniewska et al. 1996; Ritty et al. 2003). In an experimental model of subfailure ligament injury, versican mRNA in resident fibroblasts was reported to be acutely upregulated (Provenzano et al. 2005). This finding may be consistent with versican’s role in creating a hydrated, anti-adhesive matrix that promotes cellular proliferation and migration following injury (Kinsella et al. 2004). Versican expression is also upregulated in bovine tendon by in vitro compressive loading (Robbins et al. 1997), a mechanical stimulus which has been hypothesized to occur at the inferior patellar pole during loaded knee flexion (Almekinders et al. 2003; Hamilton and Purdam 2004). Thus, both injury or altered mechanical loading could lead to distinct patterns of increased versican synthesis in tendon, and the distribution of versican in tendons may give insight into the underlying mechanisms.
Therefore, we aimed to characterize the quantity and distribution of versican in the patellar tendon of athletes presenting with tendinosis. We hypothesized that versican would be present in excess in patellar tendinosis, and that it would be distributed in the extracellular matrix surrounding tenocytes, endothelial cells, and/or smooth muscle-containing cells.
METHODS
Subjects
Tendon tissue was obtained from patients with documented patellar tendinosis (n=21) and from control participants (n=10). The patients (18 males and 3 females) were athletes who had experienced pain for longer than 3 months and were unable to participate in sport at their pre-injury level. The average Victorian Institute of Sports Assessment (VISA) score for patients was 42 (15–65) indicating significant functional limitation (0=very poor function and 100=perfect function and no pain) (Visentini et al. 1998). All patients in the present study also had MRI abnormalities at the proximal aspect of the patellar tendon confirmed by a board-certified musculoskeletal radiologist and a specialist orthopaedic surgeon. The control group (7 males and 3 females) was selected from patients undergoing intramedullary nailing for low-energy tibia fractures and who had no current or previous knee pain. None of the patients or controls had previous surgical treatment in or around the knee, corticosteroid injections in or around the knee, serious traumatic injury affecting the knee, or any rheumatic or degenerative knee condition. The average age of subjects was 30 years (24–34 years, n=21) in the patient group and 29 years (19–43 years, n=10) in controls. All subjects gave their written, informed consent. The study was approved by the local Committees for Research Ethics in Australia, Norway and Canada.
Biopsy procedure
The surgical technique and biopsy handling was identical in the two groups. Biopsies were taken from the proximal bone-tendon junction, and the tendon tissue was excised using a full thickness wedge-shaped incision, widest at the patellar pole (1 cm) and narrowing distally (2–3 cm in length). Biopsies were either immediately frozen in liquid nitrogen and stored at −80°, or chemically fixed in Zamboni’s solution for 4 to 24 h and then washed in 0.1 M phosphate-buffered NaCl, pH 7.2, with 15% sucrose (weight/volume) (PBS) and 0.1% natriumazide. After fixation, the biopsies were stored in PBS at 4°C, dehydrated, paraffin embedded and serially sectioned at 5 μm thickness.
We applied Bonar’s classification system (Khan et al. 1999) to confirm the presence of tendinosis in patients, and its absence in controls, using tendon sections stained with Alcian blue and haematoxylin & eosin (H&E). Distinct features of tendinosis (fibrocartilage metaplasia, neovascularization, fibroblastic hypercellularity) were independently identified by two investigators (AS, JLC) blinded to group. The inter-rater reliability for identifying these features was 100%. Patient biopsies that yielded tissue sections at least 1cm2 in size (10 of 21 biopsies) were used for more extensive qualitative observations regarding the relation of versican expression to features of tendinosis.
Detection and quantification of versican in tissue sections
Western Blot methodology
Tendon samples were extracted from samples of tendon with 4M guanidium chloride and 50 mM sodium acetate buffer (50 mM sodium acetate, 100 mM disodium sulphate; pH 5.8) containing proteinase inhibitors for 72 hours at 4°C. The protease inhibitor cocktail was prepared as a 10X concentrate and added to the buffered guanidine hydrochloride solution to give final concentration of 8 mg/l soybean trypsin inhibitor (SBTI), 0.1 M aminohexanoic acid, 0.01 M ethylenediaminetetra-acetic acid (EDTA), 1.0 mM benzamidine hydrochloride, 0.1 mM phenylmethylsulfonyl fluoride (PMSF), 2.0 mM iodoacetic acid and 0.02 % (w/v) sodium azide. The proteoglycans present in the extract were isolated by ion-exchange chromatography on Q-Sepharose, deglycosylated with chondroitinase ABC and keratanase, and separated by SDS-polyacrylamide electrophoresis as previously described (Samiric et al. 2004). Handling and processing of patient and control biopsy material was identical. The gels were then analysed by Western blotting using the LF99 antibody (a kind gift of Dr Larry Fisher, NIH) and 2B1 (Seikagaku, Japan) to versican. The LF99 antibody was raised against a peptide corresponding to the N-terminus of versican (LHKVGKSPPVRC) (Bernstein et al. 1995). This epitope is cleaved during versican maturation, and therefore represents newly synthesized proteoglycan. The 2B1 antibody was raised against proteoglycans extracted from a human yolk sac tumour, and later identified as specifically recognizing an epitope close to the C-terminal domain in the versican core protein (Isogai et al. 1996). Samples equivalent to 2μg wet weight of tissue were applied to each well.
Quantitative immunohistochemistry
The distribution of versican was examined in tissue sections using the 2B1 antibody followed by a commercially available avidin-biotin-HRP visualization system (Vector Labs). Stained tissue sections were systematically scanned at 20x magnification by a technician who was unaware of the labeling code using the BLISS microscope workstation (Bacus Laboratories, Lombard, U.S.A.). Image Pro Plus (version 4.5.1.22 from Media Cybernetics, San Diego, California, USA) was used to highlight positive DAB end-reaction product using the same RGB threshold file for all sections. Values are expressed as the average number of positive pixels per field.
Labeling of proliferating cells and vascular cell types
Proliferation of endothelial and smooth muscle cells are key events in angiogenesis -- an important feature of tendinosis (Kristoffersen et al. 2005). Therefore, we used monoclonal antibodies against markers for endothelial cells (CD31; DAKO JC70A), smooth muscle actin (α-SMA; Biomeda 1A4), and proliferating cells (Ki67; BD Pharmingen B56) to label cell types in normal and patellar tendinosis tissue. CD31 (platelet endothelial cell adhesion molecule) has been commonly used to identify microvascular endothelial cells (Biberthaler et al. 2003). α-SMA has been shown to identify the contractile cytoskeleton in the media of arterioles, as well as pericytes and myofibroblasts (Skalli et al. 1986, 1989; Darby et al. 1990). Ki67 is a nuclear protein expressed in proliferating cells, with peak expression during M phase (Landberg et al. 1990).
Determination of microvessel parameters
Microvessels (defined here as vessels with diameter <50 μm or smaller) from the 10 most proximal viewing fields (40x objective, 0.35mm2 total area) were outlined using digitized, systematically scanned micrographs of H&E sections. All individual vessel profiles were manually traced in Image Pro Plus and counted with the digital counting tool. Vessel density was defined as the number of profiles per mm2. Vessel thickness and lumen were determined by measuring vessel profiles in the transverse plane. Thickness was defined by the external borders of the adventitia, whereas the lumen diameter was defined by the internal borders of the intima. To obtain a sufficient number of vessels for analysis of thickness and lumen (n=303), both circular and oblique vessel profiles were included as long as the plane of section passed completely through the vessel; diameters were thus obtained using the least diameter method, which minimizes measurement error due to oblique sectioning (Clarkson et al. 1980).
Statistical analysis
To compare the amount of versican immunostaining in control and patient samples, the average number of positive pixels was subjected to a t-test with significance predetermined at α=0.05, after ensuring the data were normally distributed and did not violate the assumption of equal variance (Levene’s test). Results are expressed as mean ± SD. Vessel density was compared in the same way. Vessel diameters were not normally distributed, and the distribution appeared to differ between patients and controls; therefore the non-parametric Kolmogorov-Smirnov test was used to test the hypothesis that the distributions were drawn from different populations. Analysis was carried out using SPSS version 13.0 (SPSS Inc., Chicago, U.S.A.).
RESULTS
Comparison of versican expression in normal tendon and tendinosis
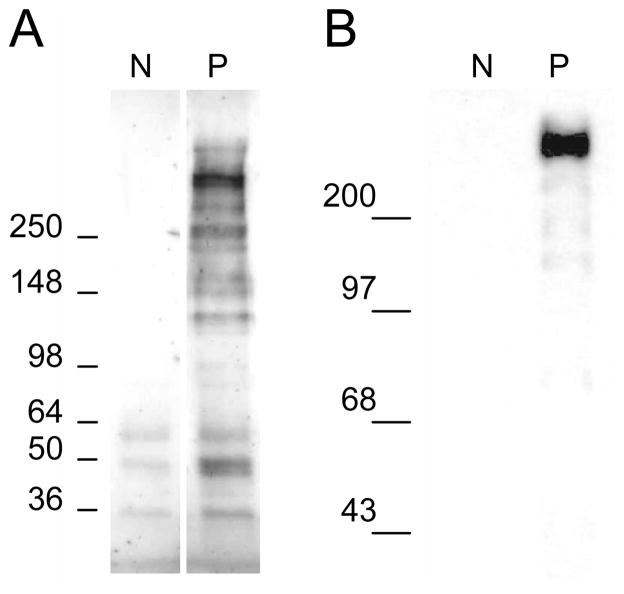
Western blot analysis of proteoglycan samples extracted from patellar tendon samples demonstrated that in pathological tissue there were clearly increased levels of versican present in pathological tissue (Figure 1). Most of the versican labeled by the 2B1 antibody appeared to be catabolic products of the proteoglycan (Samiric et al. 2004). Labeling with the LF99 antibody was prominent in gels obtained from patient samples but was barely detectable in normal tendon, and appeared to preferentially identify a high molecular weight form of versican.
Figure 1.
Detection of versican in normal and pathological tendon. Western blots of purified tendon proteoglycan extracts (~2ug/lane) were probed with 2B1 (versican C-terminal domain) or LF-99 (N-terminal domain). Lanes represent normal (N) and pathological (P) human patellar tendon samples. Molecular weights are shown in kDa. Similar results were obtained in gels from 5 different patients.
Semi-quantitative immunohistochemistry independently confirmed that versican was present in both normal and pathological tendon, but was significantly increased in patients (2.63×105 ± 9.0 × 104) compared to controls (1.97×105 ± 6.0×104, p=0.042).
Versican distribution in patellar tendinosis – qualitative observations
Tendons from patients with patellar tendinosis demonstrated three main patterns of versican expression (Figure 2). A proximal-distal pattern of these tendinosis features could be detected in 9 of the 10 patient biopsies which had sufficient tissue for extensive qualitative observation (as described in Methods).
Figure 2.
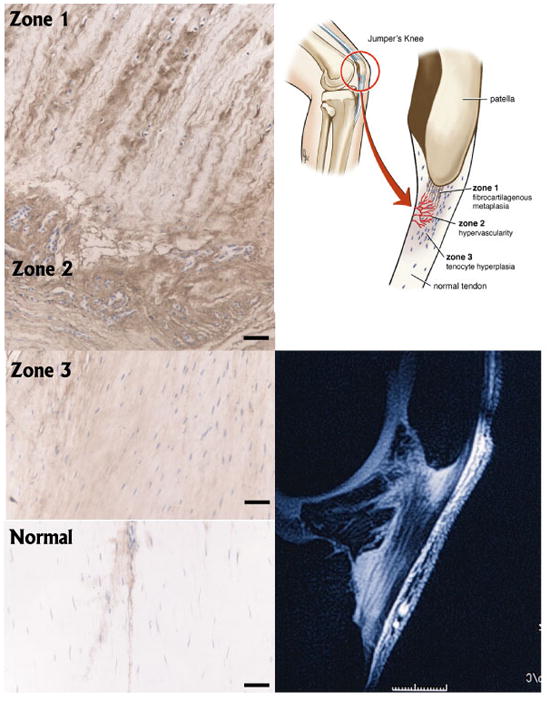
Representative micrographs demonstrating zones of versican deposition in association with specific features of tendinosis pathology; MRI from a jumper’s knee patient is shown for orientation. Zone 1 contains banded distribution of versican (brown staining) in association with chondrocytic cells (Haematoxylin counterstain), bordered distally by microvascular hyperplasia (Zone 2). Zone 2 is interspersed and bordered by tenocyte hypercellularity and a generalized increase in versican content (Zone 3). In surrounding normal tendon versican staining is fainter and is localized to the endotendon. Scale bar on micrographs = 50 microns, on MRI = 1cm.
In the most proximal part of the tendon (Zone 1), there were prominent regions of fibrocartilage. These regions extended into to the tendon proper up to several millimeters in length and width. In Zone 1, versican was more prominent in tendinosis tissue than in control tissue in the vicinity of more rounded, chondrocytic-appearing tenocytes, sometimes in alternating layers consistent with classic descriptions of fibrocartilage.
Bordering this zone distally in all cases was a region of vascular hyperplasia and endothelial proliferation (Zone 2), in which versican was also enriched (Figure 2).
Moving further distally, Zone 3 was characterized primarily by increased tenocyte density but otherwise normal tendon structure (Figure 2). In these regions, a greater quantity of versican was detected which was distributed evenly throughout the extracellular matrix of the tendon proper, i.e, there was no focal increase in versican in Zone 3.
Finally, large regions of the anterior and distal patellar tendon in patients with tendinosis were frequently normal and demonstrated no general or focal increase in versican (Figure 2). As in uninjured tendon from control samples, tendon from patellar tendinosis patients contained versican predominantly around microvessels in the endotendon running longitudinally with the principal line of action of the tendon (Bode-Lesniewska;Dours-Zimmermann et al. 1996).
Association of versican with vascular hyperplasia
In the hypervascular region (Zone 2), versican formed an expanded glycosaminoglycan-rich matrix penetrated by CD31+ microvessels exhibiting positive Ki67 nuclear labeling in the intima (Figure 3A–C). In microvessels from both normal and tendinosis samples, versican expression was typically complementary to α-SMA (i.e. absent from the tunica media) (Figure 3D,E). However, in some larger arterioles of tendinosis biopsies, versican was present in association with medial and/or intimal hyperplasia (Figure 4), giving the vessel walls an irregular, thickened appearance. In patellar tendinosis tissue samples, α-SMA-positive fibroblasts (i.e. myofibroblasts) were present in areas of versican-rich tissue, often adjacent to proliferating microvessels (Figure 5), but this was not the case in normal tendon.
Figure 3.
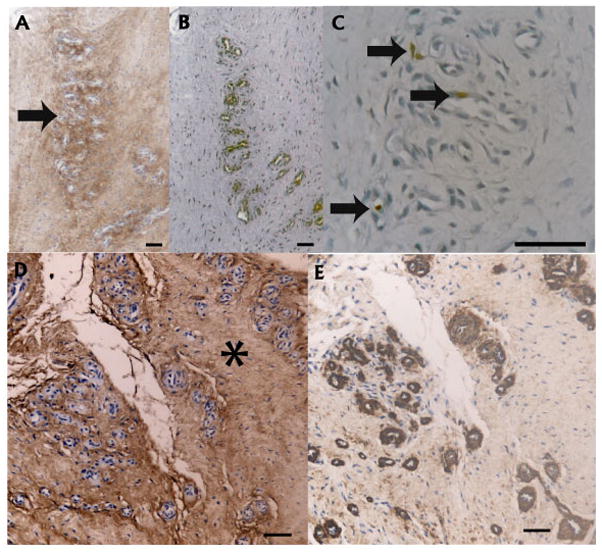
Association of versican with vascular proliferation A: Diffuse versican labeling (brown) around an abnormally dense capillary bed within the tendon proper, with more intense labeling directly adjacent to the vessels (arrow). B: CD31 labeling endothelial cells serial to A. C: Higher power view of the same region showing Ki67 labeling of endothelial cells in the microvascular walls. D: Intense versican labeling (asterisk) in a region of arteriolar proliferation. E: Serial to D, confirming the presence of α-SMA (brown) in the intima media. Scale bars = 50 microns
Figure 4.
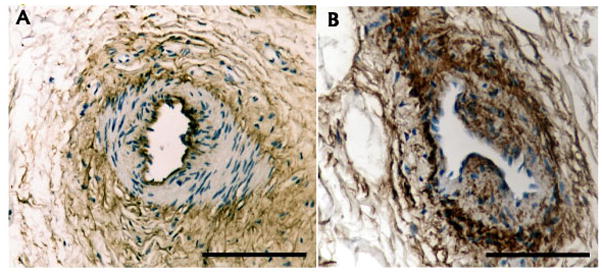
Versican distribution in arterioles from normal tendon and tendinosis tissue. A: Normal arteriole – versican is localized to the surrounding connective tissue, and to a single layer of endothelial cells (tunica intima). B: Abnormal arteriole – versican surrounds an irregularly thickened endothelial layer and extends into the smooth muscle layer (tunica media), and is also markedly increased in the outer layers. Scale bar = 50 microns.
Figure 5.
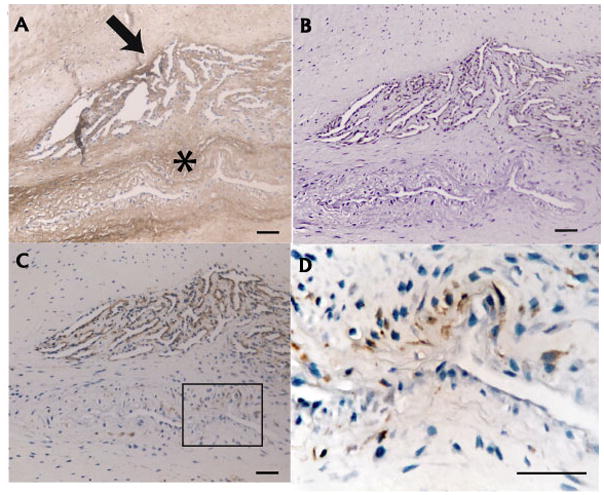
Association of versican with microvascular hyperplasia and myofibroblasts. A: Versican in association with an abnormal microvascular bed (arrow), and remodeling tissue (asterisk). B: CD31 confirms that the upper structure is vascular, whereas the lower structure is not. C: Intensely labeling of α-SMA in the vascular structure indicating arterioles. α-SMA is also positive in scattered myofibroblasts in the adjacent remodeling area (rectangle). D: Higher power view of rectangular area in C. Scale bars = 50 microns.
Quantitation of microvessel density and thickness
Vascular density was 5.6 ± 9.7 vessels per mm2 in controls, and 15.6 ± 16.7 vessels per mm2 in patients (p=0.026). The distribution of vessel diameters was significantly different in patients (p=0.035) (Figure 6) due to an increased presence of small diameter microvessels (capillaries, venules and arterioles). The distribution of lumen diameters was not different in patient and control samples.
Figure 6.
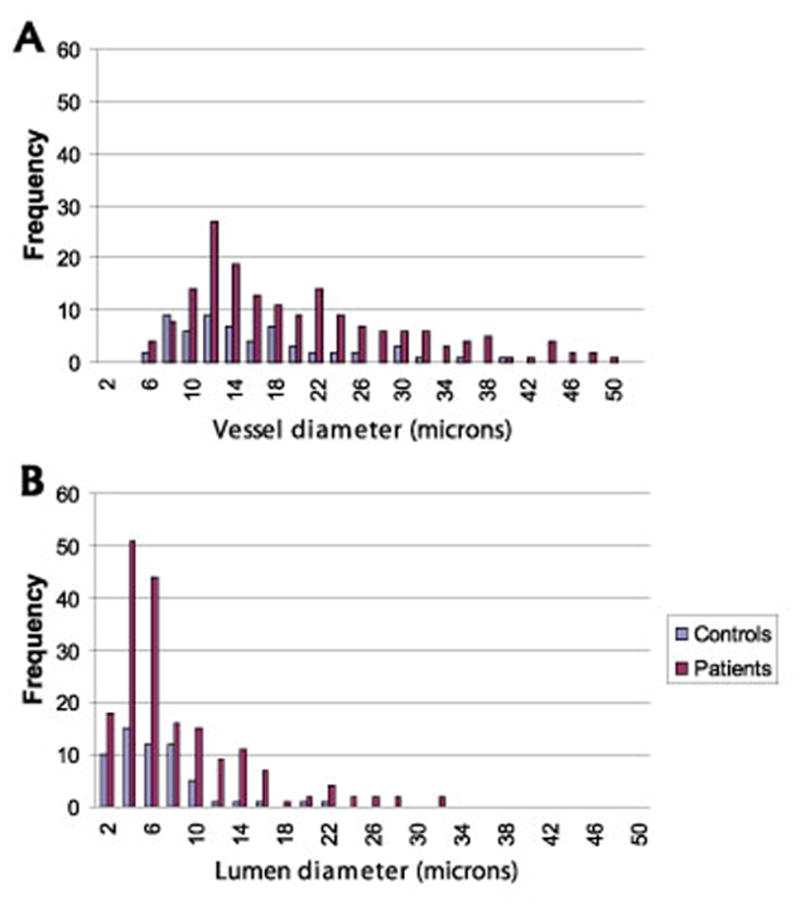
Quantitation of vessel diameters in the proximal 0.35mm2 of the patellar tendon. Morphometric analysis demonstrates increased presence of microvessels in patellar tendinosis.
DISCUSSION
The major novel finding of this study is that elevations in versican levels contribute to the increased extracellular matrix volume in symptomatic patellar tendinosis. Versican expression was associated with distinct regions of fibrocartilage, hypervascularity, hypercellularity, as well as morphologically normal tendon. Thus, several distinct cell populations and processes appear to contribute to the expression of versican in the patellar tendon with features of tendinosis. This finding extends previous work which demonstrates increased expression of aggrecan, biglycan and type II collagen in tendinosis lesions (Corps et al. 2006, Maffulli et al. 2006).
Turning first to the zone of fibrocartilage at the inferior pole of the patellar tendon in patient samples (Zone 1), versican was more prominent in the matrix surrounding rounded, chondrocytic cells. Directly adjacent to the region of chondroid metaplasia, a zone of hypervascularity (Zone 2) was consistently observed in patient samples. This was characterized by increased versican expression both in the perivascular matrix and within the vessel walls. This proximal-distal pattern of fibrocartilage bordered by vascular tissue has been noted previously in the supraspinatus tendon (Archambault et al. 2007). Fukuda and co-workers examined en-bloc sections of full thickness rotator cuff tears and reported chondroid metaplasia in the proximal remnant of the tendon, whereas the distal torn end was characterized by hypervascular granulation tissue (Fukuda et al. 1990). This is consistent with a hypothesis that fibrocartilage metaplasia may initially develop as an adaptive response to compression under the acromion, but that this adaptation weakens the tendon’s ability to withstand tensile loads, thereby leading to microtears adjacent to the site of fibrocartilage formation. A similar process has been postulated for the tibialis posterior where it is compressed by the medial malleolus (Petersen et al. 2004). These data will therefore be of interest to those who have hypothesized that patellar tendinopathy may have a compressive etiology (Almekinders et al. 2003). Fibrocartilage tends to develop within areas of tendon (distinct from the enthesis) that are subjected to compression or shear, for example at the deep surface of wrap-around tendons such as the supraspinatus or tibialis posterior. However, fibrocartilage metaplasia is also sometimes observed as a pathological dysregulation of cell phenotype, for example in scar tissue (Boor and Ferrans 1985).
Versican was increased in association with microvessels in two overlapping patterns. First, and most commonly, there were often dense beds of capillaries and/or arterioles, and the entire region demonstrated excess staining for versican. Proliferating endothelial and smooth muscle cells could be identified in these regions. This pattern is consistent with versican’s role in facilitating proliferation and migration in angiogenesis. The second abnormality was the increased presence of versican in association with intimal and medial hyperplasia of arterioles. We have recently documented increased vascular endothelial growth factor (VEGF) expression by endothelial cells in patellar tendinosis tissue (AS, OL, RB, VD, DAH, KK, unpublished observations). In addition to causing endothelial cells to proliferate, VEGF has been reported to upregulate their expression of versican (Cattaruzza et al. 2002). Taken together, these findings suggest a possible causal link between the two findings and a potential role of VEGF in causing or perpetuating the increased versican expression in Zone 2.
Neovessels were occasionally seen in proximity to versican-rich tissue populated by myofibroblasts (α-SMA positive fibroblasts). The presence of myofibroblasts is in keeping with prior studies of tendinosis (Khan et al. 1996) and of other healing soft tissues (Schmitt-Graff et al. 1994), and their association with versican confirms the importance of this proteoglycan in soft-tissue repair and remodeling (Roberts 1995). The factors leading to increased versican expression in healing wounds may include hypoxia (leading to hypoxia-induced-factor-1-α and VEGF expression), cytokines including transforming growth factor-β and PDGF-A and –B (Evanko et al. 1998, 2001; Nikitovic et al. 2006), and compressive or tensile loads (Mao et al. 1998; Grande-Allen et al. 2004; Milz et al. 2005).
A third zone of versican expression was typically seen distal and/or superficial to Zones 1 and 2, and this was characterized by a relatively even elevation in versican levels in association with increased tenocyte density. This could be interpreted as evidence of adaptation. In animals and in humans, exercise can induce adaptive improvements in the material properties of the tendon, including increased stiffness and cross-sectional area (Woo et al. 1980; Kjaer 2004; Yoon and Halper 2005). Chondroitin sulphate content is correspondingly increased in animals subjected to treadmill running vs. sedentary controls (Woo et al. 1980; Banes et al. 1999; Kjaer 2004; Yoon and Halper 2005). Conversely, the increased versican in Zone 3 could represent a secondary effect due to diffusion of cytokines from adjacent areas.
In the current study, Western blot analysis demonstrated that versican content in patient samples consisted both of newly synthesized, high molecular weight forms, as well as lower weight catabolic products. The regulation of versican expression and turnover is complex and includes alternate splicing of mRNA from the versican gene to generate at least three possible isoforms (Rahmani et al. 2006). Versican degradation involves proteolytic cleavage into distinct entities, and regulation of its catabolism by aggrecanases and matrix metalloproteases, and by their endogenous inhibitors (Kinsella et al. 2004; Rahmani et al. 2006). Future studies should therefore examine these regulatory processes in relation to the development of tendinosis pathology.
In this study, we did not examine the distribution of other potentially relevant components of the extracellular matrix (e.g. aggrecan, biglycan, decorin, lumican, collagens, fibronectin, hyaluronan). In particular, aggrecan is likely to contribute to the regions of fibrocartilagenous metaplasia observed here (Milz et al. 2005). Aggrecan mRNA levels were increased in Achilles tendinosis (Corps et al. 2006) and in the ruptured supraspinatus (Lo et al. 2005) whereas versican levels were unchanged compared to controls (Corps et al. 2006). The discrepancy between findings at the protein and mRNA levels in these studies is consistent with a report from cartilage showing that versican protein can remain in the matrix long after mRNA levels have declined (Matsumoto et al. 2006). The complex regulation of versican and other matrix molecules in the development and progression of tendinosis clearly requires further research.
In summary, we found that versican is present at elevated levels in human patellar tendinopathy. Versican was enriched in distinct regions of the tendon tissue, specificially in areas of fibrocartilage, those with fibroblast proliferation, as well as in the perivascular matrix of proliferating microvessels and within the media and intima of arterioles. We conclude that future studies should address both pathological and adaptive mechanisms underlying the upregulation of versican in the tendon proper and the vascular matrix to better understand the basis and consequences of the elevated protein levels detected in the present studies.
PERSPECTIVES
The current study deepens our understanding of patellar tendinosis and the clinical rationale for its treatment. Formerly known as “tendinitis,” a lack of knowledge regarding the underlying pathological processes has hampered the development of effective treatments (Maffulli et al. 1998; Kjaer 2004; Riley 2004). In laboratory studies, the transformation of normal, tensile tendon to fibrocartilage, and vice versa, is related to the dynamic balance of compressive, shear, and tensile forces experienced by tenocytes at specific regions in the tendon (Robbins et al. 1997; Malaviya et al. 2000; Milz et al. 2005). Tensile (eccentric) loading of the patellar tendon using a decline board gives superior results to standard knee flexion exercises in the treatment of patellar tendinosis (Young et al. 2005) and this clinical success has been suggested to result from increased tensile vs. compressive loading of tenocytes in the posterior, proximal tendon with the decline board (Young et al. 2005). Therefore, appropriate tensile loads delivered in such a way as to miminize compression may therefore be able to reverse the process of fibrocartilage metaplasia in the patellar tendon. Future developments in imaging technology may allow the dynamic visualization of versican and other matrix components in tendinopathy patients, and thus allow a direct assessment of the molecular response to rehabilitation.
Acknowledgments
This work was funded by grants from the Canadian Institutes of Health Research (CIHR; MOP-77551) and the Worker’s Compensation Board of British Columbia (grant RS0203-DG-13). The Oslo Sports Trauma Research Center has been established at the Norwegian University of Sport & Physical Education through generous grants from the Royal Norwegian Ministry of Culture, the Norwegian Olympic Committee & Confederation of Sport, Norsk Tipping, and Pfizer. Mr Scott is recipient of a CIHR Post-Doctoral Fellowship. Dr Hart is the Calgary Foundation-Grace Glaum Professor in Arthritis Research and supported by IGH of CIHR. Dr Duronio is a Michael Smith Foundation for Health Research Senior Scholar. Dr Khan is a CIHR New Investigator.
References
- Almekinders LC, Weinhold PS, Maffulli N. Compression etiology in tendinopathy. Clin Sports Med. 2003;22:703–10. doi: 10.1016/s0278-5919(03)00067-x. [DOI] [PubMed] [Google Scholar]
- Archambault JM, Jelinsky SA, Lake SP, Hill AA, Glaser DL, Soslowsky LJ. Rat supraspinatus tendon expresses cartilage markers with overuse. J Orthop Res. 2007;25:617–24. doi: 10.1002/jor.20347. [DOI] [PubMed] [Google Scholar]
- Banes AJ, Horesovsky G, Larson C, Tsuzaki M, Judex S, Archambault J, Zernicke R, Herzog W, Kelley S, Miller L. Mechanical load stimulates expression of novel genes in vivo and in vitro in avian flexor tendon cells. Osteoarthritis Cartilage. 1999;7:141–53. doi: 10.1053/joca.1998.0169. [DOI] [PubMed] [Google Scholar]
- Bernstein EF, Fisher LW, Li K, LeBaron RG, Tan EM, Uitto J. Differential expression of the versican and decorin genes in photoaged and sun-protected skin. Comparison by immunohistochemical and northern analyses. Lab Invest. 1995;72:662–9. [PubMed] [Google Scholar]
- Biberthaler P, Wiedemann E, Nerlich A, Kettler M, Mussack T, Deckelmann S, Mutschler W. Microcirculation associated with degenerative rotator cuff lesions. In vivo assessment with orthogonal polarization spectral imaging during arthroscopy of the shoulder. J Bone Joint Surg Am. 2003;85-A:475–80. [PubMed] [Google Scholar]
- Bode-Lesniewska B, Dours-Zimmermann MT, Odermatt BF, Briner J, Heitz PU, Zimmermann DR. Distribution of the large aggregating proteoglycan versican in adult human tissues. J Histochem Cytochem. 1996;44:303–12. doi: 10.1177/44.4.8601689. [DOI] [PubMed] [Google Scholar]
- Boor PJ, Ferrans VJ. Ultrastructural alterations in allylamine cardiovascular toxicity. Late myocardial and vascular lesions. Am J Pathol. 1985;121:39–54. [PMC free article] [PubMed] [Google Scholar]
- Cattaruzza S, Schiappacassi M, Ljungberg-Rose A, Spessotto P, Perissinotto D, Morgelin M, Mucignat MT, Colombatti A, Perris R. Distribution of PG-M/versican variants in human tissues and de novo expression of isoform V3 upon endothelial cell activation, migration, and neoangiogenesis in vitro. J Biol Chem. 2002;277:47626–35. doi: 10.1074/jbc.M206521200. [DOI] [PubMed] [Google Scholar]
- Clarkson PM, Kroll W, McBride TC. Maximal isometric strength and fiber type composition in power and endurance athletes. Eur J Appl Physiol Occup Physiol. 1980;44:35–42. doi: 10.1007/BF00421761. [DOI] [PubMed] [Google Scholar]
- Cook JL, Khan KM, Kiss ZS, Purdam CR, Griffiths L. Prospective imaging study of asymptomatic patellar tendinopathy in elite junior basketball players. J Ultrasound Med. 2000;19:473–9. doi: 10.7863/jum.2000.19.7.473. [DOI] [PubMed] [Google Scholar]
- Corps AN, Robinson AH, Movin T, Costa ML, Hazleman BL, Riley GP. Increased expression of aggrecan and biglycan mRNA in Achilles tendinopathy. Rheumatology (Oxford) 2006;45:291–4. doi: 10.1093/rheumatology/kei152. [DOI] [PubMed] [Google Scholar]
- Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63:21–9. [PubMed] [Google Scholar]
- Evanko SP, Johnson PY, Braun KR, Underhill CB, Dudhia J, Wight TN. Platelet-derived growth factor stimulates the formation of versican-hyaluronan aggregates and pericellular matrix expansion in arterial smooth muscle cells. Arch Biochem Biophys. 2001;394:29–38. doi: 10.1006/abbi.2001.2507. [DOI] [PubMed] [Google Scholar]
- Evanko SP, Raines EW, Ross R, Gold LI, Wight TN. Proteoglycan distribution in lesions of atherosclerosis depends on lesion severity, structural characteristics, and the proximity of platelet-derived growth factor and transforming growth factor-beta. Am J Pathol. 1998;152:533–46. [PMC free article] [PubMed] [Google Scholar]
- Fenwick S, Harrall R, Hackney R, Bord S, Horner A, Hazleman B, Riley G. Endochondral ossification in Achilles and patella tendinopathy. Rheumatology (Oxford) 2002;41:474–6. doi: 10.1093/rheumatology/41.4.474. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Hamada K, Yamanaka K. Pathology and pathogenesis of bursal-side rotator cuff tears viewed from en bloc histologic sections. Clin Orthop Relat Res. 1990:75–80. [PubMed] [Google Scholar]
- Grande-Allen KJ, Calabro A, Gupta V, Wight TN, Hascall VC, Vesely I. Glycosaminoglycans and proteoglycans in normal mitral valve leaflets and chordae: association with regions of tensile and compressive loading. Glycobiology. 2004;14:621–33. doi: 10.1093/glycob/cwh076. [DOI] [PubMed] [Google Scholar]
- Hamilton B, Purdam C. Patellar tendinosis as an adaptive process: a new hypothesis. Br J Sports Med. 2004;38:758–61. doi: 10.1136/bjsm.2003.005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazleman B, Riley G, Speed CA. Soft Tissue Rheumatology. Oxford University Press; 2004. [Google Scholar]
- Isogai Z, Shinomura T, Yamakawa N, Takeuchi J, Tsuji T, Heinegard D, Kimata K. 2B1 antigen characteristically expressed on extracellular matrices of human malignant tumors is a large chondroitin sulfate proteoglycan, PG-M/versican. Cancer Res. 1996;56:3902–8. [PubMed] [Google Scholar]
- Khan KM, Bonar F, Desmond PM, Cook JL, Young DA, Visentini PJ, Fehrmann MW, Kiss ZS, O’Brien PA, Harcourt PR, Dowling RJ, O’Sullivan RM, Crichton KJ, Tress BM, Wark JD. Patellar tendinosis (jumper’s knee): findings at histopathologic examination, US, and MR imaging. Victorian Institute of Sport Tendon Study Group. Radiology. 1996;200:821–7. doi: 10.1148/radiology.200.3.8756939. [DOI] [PubMed] [Google Scholar]
- Khan KM, Cook JL, Bonar F, Harcourt P, Astrom M. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393–408. doi: 10.2165/00007256-199927060-00004. [DOI] [PubMed] [Google Scholar]
- Kinsella MG, Bressler SL, Wight TN. The regulated synthesis of versican, decorin, and biglycan: extracellular matrix proteoglycans that influence cellular phenotype. Crit Rev Eukaryot Gene Expr. 2004;14:203–34. doi: 10.1615/critreveukaryotgeneexpr.v14.i3.40. [DOI] [PubMed] [Google Scholar]
- Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–98. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Kristoffersen M, Ohberg L, Johnston C, Alfredson H. Neovascularisation in chronic tendon injuries detected with colour Doppler ultrasound in horse and man: implications for research and treatment. Knee Surg Sports Traumatol Arthrosc. 2005 doi: 10.1007/s00167-005-0648-3. [DOI] [PubMed] [Google Scholar]
- Landberg G, Tan EM, Roos G. Flow cytometric multiparameter analysis of proliferating cell nuclear antigen/cyclin and Ki-67 antigen: a new view of the cell cycle. Exp Cell Res. 1990;187:111–8. doi: 10.1016/0014-4827(90)90124-s. [DOI] [PubMed] [Google Scholar]
- Lo IK, Boorman R, Marchuk L, Hollinshead R, Hart DA, Frank CB. Matrix molecule mRNA levels in the bursa and rotator cuff of patients with full-thickness rotator cuff tears. Arthroscopy. 2005;21:645–51. doi: 10.1016/j.arthro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14:840–3. doi: 10.1016/s0749-8063(98)70021-0. [DOI] [PubMed] [Google Scholar]
- Malaviya P, Butler DL, Boivin GP, Smith FN, Barry FP, Murphy JM, Vogel KG. An in vivo model for load-modulated remodeling in the rabbit flexor tendon. J Orthop Res. 2000;18:116–25. doi: 10.1002/jor.1100180117. [DOI] [PubMed] [Google Scholar]
- Mao JJ, Rahemtulla F, Scott PG. Proteoglycan expression in the rat temporomandibular joint in response to unilateral bite raise. J Dent Res. 1998;77:1520–8. doi: 10.1177/00220345980770070701. [DOI] [PubMed] [Google Scholar]
- Maffulli N, Reaper J, Ewen SW, Waterston SW, Barrass V. Chondral metaplasia in calcific insertional tendinopathy of the Achilles tendon. Clin J Sports Med. 2006;16:329–34. doi: 10.1097/00042752-200607000-00008. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Kamiya N, Suwan K, Atsumi F, Shimizu K, Shinomura T, Yamada Y, Kimata K, Watanabe H. Identification and characterization of versican/PG-M aggregates in cartilage. J Biol Chem. 2006;281:18257–63. doi: 10.1074/jbc.M510330200. [DOI] [PubMed] [Google Scholar]
- Milz S, Benjamin M, Putz R. Molecular parameters indicating adaptation to mechanical stress in fibrous connective tissue. Adv Anat Embryol Cell Biol. 2005;178:1–71. [PubMed] [Google Scholar]
- Nikitovic D, Zafiropoulos A, Katonis P, Tsatsakis A, Theocharis AD, Karamanos NK, Tzanakakis GN. Transforming growth factor-beta as a key molecule triggering the expression of versican isoforms v0 and v1, hyaluronan synthase-2 and synthesis of hyaluronan in malignant osteosarcoma cells. IUBMB Life. 2006;58:47–53. doi: 10.1080/15216540500531713. [DOI] [PubMed] [Google Scholar]
- Petersen W, Hohmann G, Pufe T, Tsokos M, Zantop T, Paulsen F, Tillmann B. Structure of the human tibialis posterior tendon. Arch Orthop Trauma Surg. 2004;124:237–42. doi: 10.1007/s00402-003-0500-5. [DOI] [PubMed] [Google Scholar]
- Provenzano PP, Alejandro-Osorio AL, Valhmu WB, Jensen KT, Vanderby R., Jr Intrinsic fibroblast-mediated remodeling of damaged collagenous matrices in vivo. Matrix Biol. 2005;23:543–55. doi: 10.1016/j.matbio.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Rahmani M, Wong BW, Ang L, Cheung CC, Carthy JM, Walinski H, McManus BM. Versican: signaling to transcriptional control pathways. Can J Physiol Pharmacol. 2006;84:77–92. doi: 10.1139/y05-154. [DOI] [PubMed] [Google Scholar]
- Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology (Oxford) 2004;43:131–42. doi: 10.1093/rheumatology/keg448. [DOI] [PubMed] [Google Scholar]
- Riley GP. Gene expression and matrix turnover in overused and damaged tendons. Scand J Med Sci Sports. 2005;15:241–51. doi: 10.1111/j.1600-0838.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- Ritty TM, Roth R, Heuser JE. Tendon cell array isolation reveals a previously unknown fibrillin-2-containing macromolecular assembly. Structure. 2003;11:1179–88. doi: 10.1016/s0969-2126(03)00181-3. [DOI] [PubMed] [Google Scholar]
- Robbins JR, Evanko SP, Vogel KG. Mechanical loading and TGF-beta regulate proteoglycan synthesis in tendon. Arch Biochem Biophys. 1997;342:203–11. doi: 10.1006/abbi.1997.0102. [DOI] [PubMed] [Google Scholar]
- Roberts CR. Is asthma a fibrotic disease? Chest. 1995;107:111S–117S. doi: 10.1378/chest.107.3_supplement.111s. [DOI] [PubMed] [Google Scholar]
- Samiric T, Ilic MZ, Handley CJ. Characterisation of proteoglycans and their catabolic products in tendon and explant cultures of tendon. Matrix Biol. 2004;23:127–40. doi: 10.1016/j.matbio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Schmitt-Graff A, Desmouliere A, Gabbiani G. Heterogeneity of myofibroblast phenotypic features: an example of fibroblastic cell plasticity. Virchows Arch. 1994;425:3–24. doi: 10.1007/BF00193944. [DOI] [PubMed] [Google Scholar]
- Skalli O, Pelte MF, Peclet MC, Gabbiani G, Gugliotta P, Bussolati G, Ravazzola M, Orci L. Alpha-smooth muscle actin, a differentiation marker of smooth muscle cells, is present in microfilamentous bundles of pericytes. J Histochem Cytochem. 1989;37:315–21. doi: 10.1177/37.3.2918221. [DOI] [PubMed] [Google Scholar]
- Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986;103:2787–96. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visentini PJ, Khan KM, Cook JL, Kiss ZS, Harcourt PR, Wark JD. The VISA score: an index of severity of symptoms in patients with jumper’s knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. Journal of science and medicine in sport/Sports Medicine Australia. 1998;1:22–28. doi: 10.1016/s1440-2440(98)80005-4. [DOI] [PubMed] [Google Scholar]
- Woo SL, Ritter MA, Amiel D, Sanders TM, Gomez MA, Kuei SC, Garfin SR, Akeson WH. The biomechanical and biochemical properties of swine tendons--long term effects of exercise on the digital extensors. Connect Tissue Res. 1980;7:177–83. doi: 10.3109/03008208009152109. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Halper J. Tendon proteoglycans: biochemistry and function. J Musculoskelet Neuronal Interact. 2005;5:22–34. [PubMed] [Google Scholar]
- Young MA, Cook JL, Purdam CR, Kiss ZS, Alfredson H. Eccentric decline squat protocol offers superior results at 12 months compared with traditional eccentric protocol for patellar tendinopathy in volleyball players. Br J Sports Med. 2005;39:102–5. doi: 10.1136/bjsm.2003.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]