Abstract
Mesenchymal stem cells (MSCs) have gained increasing research interest for their potential in improving healing and regeneration of injured tendon tissues. Developing functional three-dimensional (3D) scaffolds to promote MSC proliferation and differentiation is a critical requirement in tendon tissue engineering. Tendon extracellular matrix has been shown to maintain the tenogenic potential of tendon stem cells and stimulate tenogenesis of human adipose stem cells (hASCs) in 2D culture. This study aims at characterizing the biological composition of urea-extracted fraction of tendon ECM (tECM) and its tenogenic effect on hASCs cultured in a 3D collagen scaffold under uniaxial tension. The tECM obtained was cell-free and rich in ECM proteins. hASCs seeded in tECM supplemented scaffold exhibited significantly increased proliferation and tenogenic differentiation. The presence of tECM also greatly suppressed the osteogenic differentiation of hASCs triggered by uniaxial tension. In addition, tECM-supplemented constructs displayed enhanced mechanical strength, accompanied by reduced expression and activity of MMPs in the seeded hASCs, indicating a regulatory activity of tECM in cell-mediated scaffold remodeling. These findings support the utility of tECM in creating bio-functional scaffolds for tendon tissue engineering.
Keywords: decellularized matrix, adipose stem cells, collagen scaffold, tenogenesis, tendon regeneration
1. Introduction
Tendons are fibrous tissues composed of densely packed collagen fiber bundles that connect muscle to bone and function in transmitting force from muscle to bone [1]. These tissues bear high tensile force and are therefore prone to injuries such as rupture and laceration. In the U.S. alone, tendon, ligament, and joint capsular injuries account for 45% of the 32 million musculoskeletal injuries each year [2]. It was estimated that about 200,000 tendon and ligament repair surgeries are performed annually in the U.S [3]. Unfortunately, the natural healing process of tendons is slow and the resulting tissue is inferior in structure and function due to their hypocellular and hypovascular nature [4]. In cases of severe tendon injury, surgical treatments may be used to repair or replace the damaged tendon with autografts, allografts, xenografts, or prosthetic devices [5]. However, the clinical outcomes remain unsatisfactory due to limitations including donor site morbidity, high failure rates, risk of injury recurrence and limited long-term function recovery [6–8]. These limitations have spurred the development of tissue engineering strategies, which use a combination of cells, scaffolds and bioactive molecules, to treat tendon injuries [9–12].
The use of adult mesenchymal stem cells (MSCs) as the cellular component for tendon tissue engineering has been of particular research interest in recent years [5]. While the most common approach to direct MSCs to differentiate into a specific lineage in vitro is through growth factors stimulation, differentiation of MSCs into tendon cells (tenogenesis) can be induced through uniaxial tension in three-dimensional (3D) culture [11, 13, 14]. For this purpose, a variety of biomaterials have been used as the scaffold to convey desired mechanical loads to the seeded cells. For example, when cultured under cyclic stretch, MSCs seeded on polyester fibrous scaffolds demonstrated enhanced tenogenesis [15]. Similarly, collagen gel has been used as a scaffold for MSC culture under uniaxial tension to create engineered tissues mimicking native tendons and ligaments [14, 16, 17]. However, none of these load-transmitting scaffolds possesses ideal bio-functionality, which refers to the ability to support cell proliferation, differentiation into tendon-forming cells without ossification, and matrix remodeling to generate a functional replacement at the wound site [1, 18, 19], necessary for successful tendon tissue engineering. Therefore, the need remains in tendon tissue engineering to develop supportive scaffolds with desired bio-functionality.
To date, a number of extracellular matrices have been used in various approaches to address the lack of bio-functionality in tissue engineering scaffolds [20]. In native tissues, the extracellular matrix (ECM), a complex network of interacting macromolecules that occupies the space between cells, is principally responsible for both biomechanical and microenvironmental signaling functions [21]. While there are many common ECM components, each tissue and organ possesses a unique ECM composition tailored to tissue-specific physiologic and mechanical requirements in order to maintain a particular cell phenotype and functionality [20]. Tendon tissues are rich in ECM components and contain fewer cells than most tissues, and many of the tendon ECM components as well as growth factors embedded in the ECM appear to be retained in decellularized tendons [22]. The influence of ECM on tendon cell behavior is many-fold, including regulation of proliferation and differentiation, as depletion of key ECM components impairs the self-renewal and tendon-specific gene expression of tendon progenitor cells [23]. Thus, the use of native tissue matrices in scaffold synthesis may be very beneficial in tissue engineering approaches to tendon repair. For example, both human adipose derived stem cells (hASCs) and rabbit tendon-derived stem cells (rTDSCs) seeded on decellularized tendon/ligament ECM showed improved proliferation and differentiation in a 2D format [24, 25]. These findings, taken together, suggest that tendon ECM may be included in 3D scaffold to enhance tenogenic differentiation of encapsulated MSCs, particularly in constructs cultured under tensile conditions, to produce a more functional neo-tendinous tissue.
In this study, we hypothesize that incorporation of native tendon ECM components will enhance the bio-functionality of 3D tension-bearing scaffold. To test this hypothesis, tendon ECM was extracted with urea and incorporated into a 3D collagen scaffold into which hASCs were seeded, and the construct was placed in culture under uniaxial tension for over 7 days. Cell proliferation, differentiation, and matrix remodeling capacity within these tECM-enhanced constructs were analyzed, and the influence of tECM on the mechanical properties of the constructs was assessed.
2. Materials and methods
2.1 Tissue Harvest
Bovine Achilles tendons were harvested from eight 2–3 week old bovine hind legs (Research 87, Boyleston, MA). The midsubstance of the tendinous portion between the distal end of gastrocnemius muscle and the calcaneal insertion was dissected and immediately immersed in phosphate buffered saline (PBS; Gibco, Grand Island, NY) supplemented with 5 mM ethylenediaminetetraacetic acid (EDTA; Sigma, St. Louis, MO), 0.5 mM phenylmethylsufonyl fluoride (PMSF; Sigma) and 1 × Penicillin-Streptomycin (P/S; Gibco), and stored at −20°C until processing. EDTA, PMSF, and P/S were added for their metalloproteinase inhibition, serine protease inhibition and anti-bacterial effect, respectively.
2.2 Extraction of tECM
To obtain tECM powder, Achilles tendons were frozen in PBS at −80 °C and then thinly sliced at 40 μm thickness using a cryotome (Leica CM 1850, Buffalo Grove, IL). The tendon sections were pulverized in liquid nitrogen with a mortar pestle, and the tissue powder was then decellularized by incubation in 1% Triton X-100 (Sigma-Aldrich) in PBS under continuous agitation for 24 h at 4°C, followed by three washes, 30 min each in PBS. The decellularized material was then treated with 200 U/ml DNase and 50 U/ml RNase (Worthington, Lakewood, NJ) solution at 37°C for 12 h and then washed in PBS 6 times, 30 min for each wash. After nuclease digestion, the preparation was extracted with 3 M urea (Sigma-Aldrich) in water with gentle agitation for 3 days at 4°C. The suspension was then centrifuged for 20 min at 1,500 g to collect the extract supernatant. Urea was removed by dialysis of the extract contained in dialysis cassettes (3,500 MWCO, Thermo Scientific, Rockford, IL) against deionized water for 2 days at 4°C. Water was changed every 4 h. The dialyzed tECM extract was transferred into centrifugal filter tubes (3,000 MWCO, Millipore, Carrigtwohill, Ireland) and spin-concentrated by 10 folds for 30 min at 1,500 g. The final ECM solution was filter-sterilized through PVDF syringe filter units (0.22 μm, Millipore), protein concentration determined using the BCA assay (Thermo Scientific), and then stored at 4°C until further use. Total proteins were extracted from homogenized original tendon tissues by TM buffer (Total Protein Extraction Kit, Millipore) and served as a control.
2.3 SDS-PAGE and Western Blot Assay
For SDS-PAGE and Western blotting, protein samples of the same concentration was mixed with loading buffer and reducing agent (NuPAGE Bis-tris Mini Gel System, Life Technology, Grand Island, NY), and heated at 70°C for 10 min. Protein samples were then loaded into wells of pre-cast NuPAGE gels (NuPAGE Bis-tris Mini Gel, Life Technology) and separated by electrophoresis in MOPS SDS running buffer for 50 min at constant 200 V. Proteins were transferred onto PVDF membranes using the iBlot dry blotting system (Invitrogen). After incubating in 5% powder milk in TBST for blocking, membranes were incubated at 4°C overnight with the following primary antibodies: mouse anti-fibronectin, sheep anti-decorin, goat anti-biglycan or rabbit anti-fibromodulin (Abcam, Cambridge, MA). The membranes were then washed in TBST 6 times and incubated with corresponding horseradish perioxidase conjugated secondary antibodies for 1 h at room temperature. Blots were allowed to react with chemiluminescence substrate (West Dura Extended Duration Substrate, Thermo Scientific) for 5 min and imaged with a CCD camera gel imaging system. (FOTODYNE, Hartland, WI)
2.4 Cell Isolation and Culture
hASCs were isolated from the lipoaspirate-derived fat tissue from three female donors (28, 32, and 36 years old) with Institutional Review Board approval (University of Pittsburgh) using an automated cell isolation system (Tissue Genesis Inc, Honolulu, HI). hASCs were cultured in growth medium (DMEM-high glucose, 10% fetal bovine serum (FBS), 100 units/ml penicillin and 100 mg/ml streptomycin, Gibco). At 80% confluence, cells were detached with 0.25% trypsin in 1 mM EDTA (Gibco) and passaged. All experiments were performed with passage 3 (p3) hASCs. To reduce donor to donor variation, cells from the three donors were pooled in equal number.
2.5 3D Culture of Cells under Static Tension
To study the combined effect of mechanical stimulation and tECM on hASCs, the Flexcell Tissue Train Culture System (Flexcell, Hillsborough, NC) was employed. Briefly, hACSs at p3 were trypsinized from cell culture flasks and mixed at 1 × 106 cells/ml with 0.5% collagen type I solution at neutral pH (Purecol EZ, Advanced Biomatrix, San Diego, CA) containing either 10% (v/v) 800 μg/ml collagen type I solution (Purecol EZ, Advanced Biomatrix) or 10% (v/v) 800 μg/ml tECM solution. To set up 3D gel constructs, linear Trough Loaders™ were placed in baseplates beneath the flexible membrane of Tissue Train™ culture plate so that the anchor stems were aligned with the long axis of the Trough Loader. A vacuum was applied to deform the membrane into the conformation of the trough. 300 μl of cell-gel suspension was then pipetted into each well and allowed to gel at 37°C for 2 h. After the gel set, the vacuum was released and the culture plates were dissociated from the base plates. Gels appeared as linear bands attached at each anchor end in the well (see Fig. 3A), and uniaxial tension was generated as a result of cell traction. hASCs were also seeded into 6-well tissue culture plates as 2D controls at 1 × 105 cells/well. Growth medium was added to each well and changed every 3 days. Contraction rate was determined from digital images taken on days 1, 3 and 7 of culture analyzed in Image J (NIH). Total construct area between the anchors divided by the length between anchors was defined as the average width. Constructs were also harvested at the same time points for other analyses.
Figure 3.
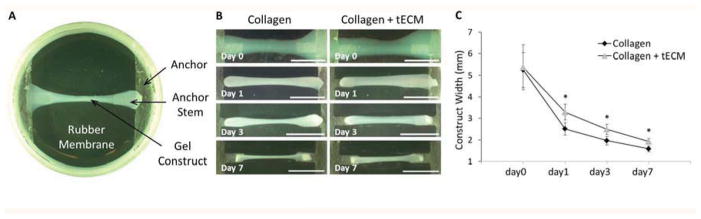
Contraction of constructs. (A) Top view of Tissue Train culture plate together with gel construct shows that the cell-collagen construct was fixed at each end by bonding to the nylon anchor stem. (B) Photos were taken to record the change of construct width. Reduced contraction is seen in the tECM-supplemented collagen constructs (right panels) compared to the collagen gel contructs (left panels). (C) The average width of the tECM-supplemented constructs is significantly higher than that of pure collagen constructs at days 1, 3 and 7. Scale bar=10 mm; * indicates p<0.05, n=6.
2.6 Scanning Electron Microscopy (SEM)
hASC-seeded 3D constructs were collected at day 1, 3 and 7, washed twice in PBS, fixed with 2.5% glutaraldehyde in PBS for 2.5 h at room temperature, and then rinsed in PBS and dehydrated in a graded ethanol series. After critical point drying, samples were mounted on aluminum stubs, sputter-coated with 3.5 nm gold, and examined by SEM (field emission, JEOL JSM6335F, Peabody, MA), operated at 3 kV accelerating voltage and 8 mm working distance. The external surface of the central part of the constructs was selected for imaging.
2.7 Cell Viability Analysis
On days 1, 3 and 7, MTS assay (CellTiter 96 Aqueous One Solution Cell Proliferation Assay, Promega, Madison, WI) was performed to determine cell viability. 3 ml working solution was added into each flexcell well, incubated for 3 h at 37°C and A490 determined spectrophotometrically using a microplate reader (BioTek, Winooski, VT). For Picogreen (Quant-iT PicoGreen dsDNA Reagent, Invitrogen, Grand Island, NY) assay, samples were first washed twice in PBS for 5 min and then digested in 500 μg/ml papain (Sigma-Aldrich) at 60°C for 2 h. 100 μl 1× Picogreen working solution in TE buffer was added into 100 μl papain solution containing one digested gel construct, incubated for 2 min protected from light, and measured using a fluorescence microplate reader (excitation 480 nm, emission 520 nm, BioTek). All readings were normalized to the pure collagen group at each time point. The calcein acetoxymethyl (calcein-AM)/ethidium homodimer-1 (EthD-1) based Live/Dead assay (Invitrogen) was performed to confirm the cell viability. At days 1 and 7, constructs from each group were incubated in calcein-AM and EthD-1 (diluted 1:1000 in growth medium) for 1 h, and then visualized by fluorescence microscopy. For nuclear staining, tendon tissues were placed on glass slides and incubated with 500 μl 300 nM diamidino-2-phenylindole, dilactate (DAPI, D3571 Invitrogen) in PBS for 1 min. The samples were then washed 3 times in PBS and viewed using fluorescence microscopy.
2.8 Histology
Tendon tissues and Flexcell constructs were washed 3 times in PBS, fixed for 12 h in 10% phosphate buffered formalin (Fisher Scientific, Pittsburgh, PA) at room temperature, dehydrated in serial ethanol solutions followed by xylene, and embedded in paraffin. The embedded samples were sectioned at 10 μm thickness, deparaffinized in xylene, rehydrated, and stained with hematoxylin and eosin (H&E), or Picosirius red for visualization.
2.9 Real-Time PCR Analysis
At each time point, total RNA was isolated using an RNA extraction Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. First-strand cDNA was synthesized with oligo(dT) primers using a cDNA synthesis kit (Invitrogen). Quantitative real-time PCR was performed using SYBR green Supermix in a Step One Plus real-time PCR system (Applied Biosystem, Life Techonology) and then analyzed by comparative Ct quantification (delta-delta Ct method). Primers were used for tenogensis marker genes including scleraxis (SCX) and tenomodulin (TNMD), osteogenesis marker genes including runt-related transcription factor 2 (RUNX2), alkaline phosphatase (ALP) and osteocalcein (OCN), ECM protein genes including collagen I (COL I), collagen III (COL III), decorin (DCR) and tenascin-C (TNC), and matrix metalloproteinase (MMP) genes including MMP-1, -2, -8, -9 and -13. The targets and sequences of primers are shown in Table 1 except tenomodulin, which was purchased from Qiagen (Hs TNMD, QuantiTect Primer Assay, Qiagen). Primer specificity and product sizes were confirmed by melting curve analysis and agarose gel electrophoresis. The expression level of each gene was normalized to GAPDH.
Table1.
Primer sequences used in assessment of gene expression by real time PCR analysis
| Gene | Primer sequence (5’-3’) | Product size (bp) | |
|---|---|---|---|
| GAPDH | Forward | CAAGGCTGAGAACGGGAAGC | 194 |
| Reverse | AGGGGGCAGAGATGATGACC | ||
| SCX | Forward | ACACCCAGCCCAAACAGA | 65 |
| Reverse | GCGGTCCTTGCTCAACTTTC | ||
| COL I | Forward | GGCTCCTGCTCCTCTTAGCG | 123 |
| Reverse | CATGGTACCTGAGGCCGTTC | ||
| COL III | Forward | CAGCGGTTCTCCAGGCAAGG | 179 |
| Reverse | CTCCAGTGATCCCAGCAATCC | ||
| DCR | Forward | CGCCTCATCTGAGGGAGCTT | 205 |
| Reverse | TACTGGACCGGGTTGCTGAA | ||
| TNC | Forward | GGTGGATGGATTGTGTTCCTGAGA | 328 |
| Reverse | CTGTGTCCTTGTCAAAGGTGGAGA | ||
| RUNX2 | Forward | CAACCACAGAACCACAAGTGC | 196 |
| Reverse | TGTTTGATGCCATAGTCCCTCC | ||
| ALP | Forward | TGGAGCTTCAGAAGCTCAACACCA | 454 |
| Reverse | ATCTCGTTGTCTGAGTACCAGTCC | ||
| OCN | Forward | ATGAGAGCCCTCACACTCCTC | 294 |
| Reverse | GCCGTAGAAGCGCCGATAGGC | ||
| MMP-1 | Forward | GGGGCTTTGATGTACCCTAGC | 142 |
| Reverse | TGTCACACGCTTTTGGGGTTT | ||
| MMP-2 | Forward | GATACCCCTTTGACGGTAAGGA | 112 |
| Reverse | CCTTCTCCCAAGGTCCATAGC | ||
| MMP-8 | Forward | TTTTGATGCCGAAGAAACATGGA | 97 |
| Reverse | GTGAGCGAGCCCCAAAGAA | ||
| MMP-9 | Forward | TGTACCGCTATGGTTACACTCG | 97 |
| Reverse | GGCAGGGACAGTTGCTTCT | ||
| MMP-13 | Forward | ACTGAGAGGCTCCGAGAAATG | 103 |
| Reverse | GAACCCCGCATCTTGGCTT |
2.10 Mechanical Test
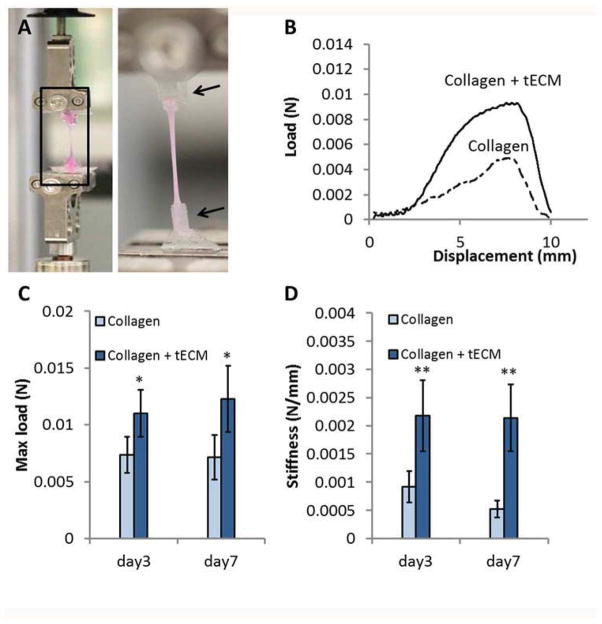
Mechanical testing was performed using a mechanical tester (Bose 3230, Eden Prairie, MN) consisting of a 2.45 N (250 g) load cell with accuracy of 0.004 N. At days 3 and 7, constructs were washed twice in PBS for 5 min, and then separated from the rubber membrane, while remaining attached to the anchors. The construct was then fixed between two clamps through the anchors (Fig. 7A, black arrows), so that the integrity of the construct was not compromised by clamping. Samples were pre-loaded to remove slack and then stretched 10 mm at an elongation rate of 0.02 mm/sec. The load/displacement curve was recorded. For each construct, maximum force recorded at the peak of the curve was defined as maximum load (N), and the slope of the linear region in load/displacement curve as stiffness (N/mm).
Figure 7.
Mechanical properties of constructs. (A) At days 3 and 7, constructs were excised from the rubber membrane together with the anchors and fixed between two clamps (black arrows). All constructs then underwent 10 mm displacement at an elongation rate of 0.02 mm/sec. (B) A representative load/displacement curve for day 3 samples shows steeper linear slope and higher failure force of the tECM-supplemented scaffold (Collagen + tECM) compared to the pure collagen scaffold (Collagen). (C, D) Quantitative analysis demonstrates significantly higher maximum load (C) and stiffness (D) of tECM-supplemented scaffolds (Collagen+ECM) than that of cell seeded pure collagen scaffolds (Collagen) at each time point. * indicates p<0.05, ** indicates p<0.01, n=5.
2.11 MMP Activity Assay
MMP activity in conditioned medium was assayed. Briefly, hASC-seeded constructs were cultured in serum- and Phenol Red-free medium (DMEM + 1% ITS (Gibco) + penicillin/ streptomycin) for up to 7 days. At day 1, 3 and 7, conditioned media were collected and incubated with an equal volume of 1 mM 520 MMP FRET substrate XI working solution (AnaSpec, Fremont, CA) in a black 96-well plate at 37 °C for 1 h. Fluorescence was measured every 5 min (excitation 485 nm, emission 528 nm). The slope of the linear portion in the kinetics curve represented the activity level of MMPs.
2.12 Statistical Analysis
Data are presented as mean ± standard deviation (SD). Each experiment was performed independently at least three times. Two-tailed Student’s t-test and one-way ANOVA followed by Bonferroni post hoc test were used for determining statistical significance of two-group comparisons and multiple-group comparisons, respectively. Results with p<0.05 were considered statistically significant.
3. Results
3.1 Characteristics of tendon-derived ECM
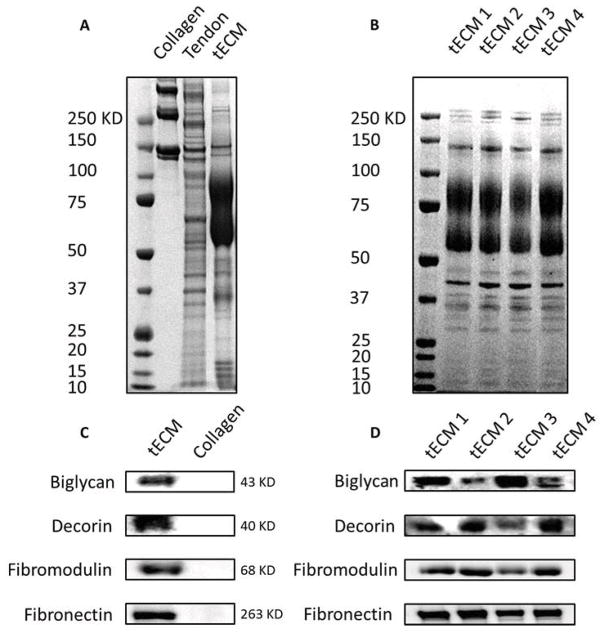
Decellularization was verified by the lack of cell nuclei using H&E and DAPI staining (Fig. 1A–D) and by the reduction in DNA content after Triton X-100 and subsequent nuclease treatment compared to that of original tendon tissues (Fig. 1E, n=4, p<0.01). SDS-PAGE analysis revealed that tECM contained a number of low molecular weight (<100 KD) protein components that were absent in commercial bovine collagen type I solution (Fig. 2A), but present in total tendon extract. This observation indicated the preservation of biochemical composition of tendon tissue after decellularization and protein extraction. Western blot assay showed that at least four types of critical tendon ECM protein components were present in this tECM extract: decorin, biglycan, fibromodulin and fibronectin (Fig. 2 C). In contrast, none of these proteins was detected in the collagen type I sample. To confirm the consistency of the extraction method, the compositions of tECM extracts harvested independently from 4 calves were analyzed by SDS-PAGE and Western blot assay (Fig. 2 B, D). A consistent protein pattern was seen in all 4 extracts. Western blotting confirmed the presence of the 4 representative tendon ECM protein components in all 4 extracts. The yield, expressed as the final protein content in the extract solution divided by the wet weight of starting tissue, was 0.125% ± 0.019%.
Figure 1.
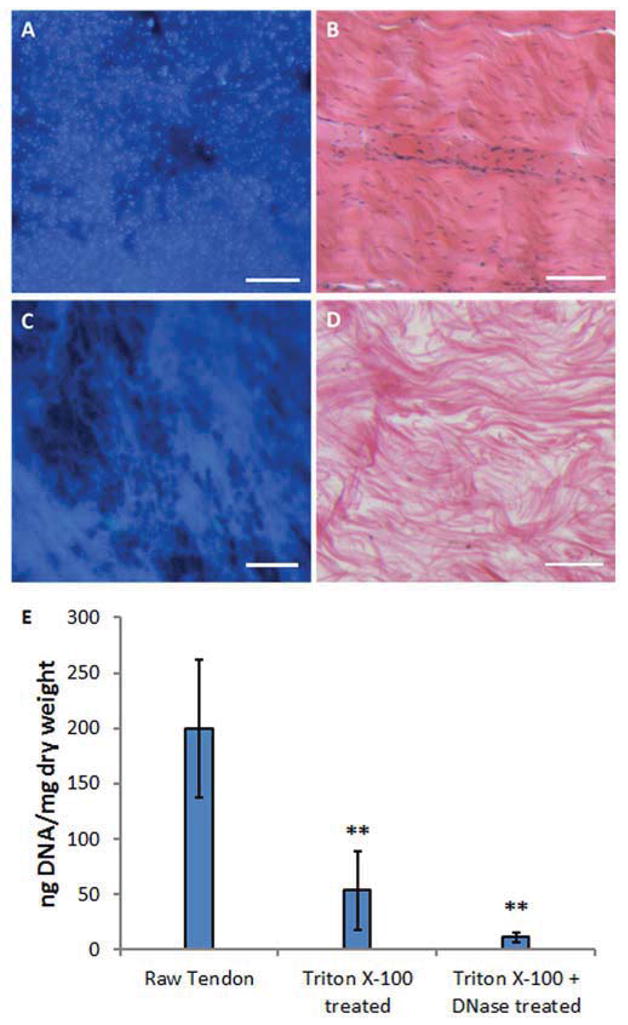
Decellularization of tendon tissue. Tendon tissue was stained with DAPI (A, C) or H&E (B, D). Prior to decellularization (A, B), abundant cell nuclei are visible. After decellularization (C, D), nuclei are rarely seen in the tissue, whereas ECM remains. The efficacy of decellularization was quantified by measuring DNA content. (E) Triton X-100 treatment alone (Triton X-100 treated) and with subsequent nuclease treatment (Triton X-100 + DNase treated) both significantly reduce the double stranded DNA content compared to untreated tendon tissues (Raw Tendon). Scale bar=100 μm; ** indicates p<0.01 compared to Raw Tendon group, n=4.
Figure 2.
Characterization of tECM. tECM proteins were subjected to SDS-PAGE (A, B) and Western blot assay (C, D). (A) Both original tendon tissue (Tendon) and tECM contain abundant low molecular weight proteins (<100 KD) that are absent in collagen type I solution (Collagen). (C) As shown by Western blotting, at least four types of matrix proteins, biglycan, decorin, fibromodulin and fibronectin, are preserved in ECM solution. (B, D) The consistency of the extraction procedure is confirmed by a nearly identical protein profile and immunoreactivity between four independent extracts of tECM (tECM 1–4) from 4 different calves.
3.2 Constructs and cell morphology
Under static tension, all hASC-gel complexes gradually formed cylindrical 3D constructs with each end attached to an anchor stem (Fig. 3A). With the passage of time, all constructs contracted to form narrower bands, presumably due to the combined effect of cell traction and matrix remodeling (Fig. 3B). The width of the tECM supplemented constructs was significantly greater than that of pure collagen constructs at days 1, 3 and 7 (Fig. 3C, n=6, p<0.05). Three days after gel setting, the pure collagen constructs contracted their diameter by 62.6% and tECM-supplemented constructs by 53.6% on average. Over a 7-day period, the final diameters were 69.9% and 64.4% smaller than the starting values for pure collagen constructs and tECM-supplemented constructs, respectively.
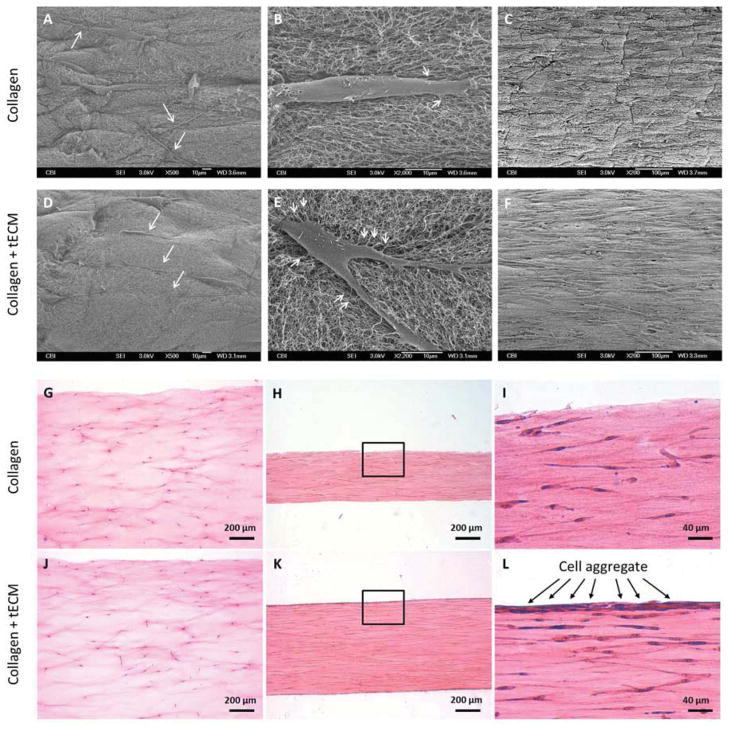
Cell morphology within the constructs was analyzed by SEM and histological staining. SEM revealed the presence of wavy, randomly oriented collagen fibrils in both collagen and tECM-supplemented scaffolds. At day 1, cells in the tECM-supplemented scaffold demonstrated well aligned cell bodies and extensions along the longitudinal axis of the scaffold (Fig. 4D, white arrows), whereas cells in the pure collagen scaffold were more randomly oriented (Fig. 4A, white arrows). Higher magnification images showed that cells within tECM-supplemented scaffolds projected more fine extensions attached to collagen fibrils (Fig. 4E) than that of pure collagen scaffolds, where extensions were fewer and only appeared at the extremities of cells (Fig. 4B). The difference in cell orientation between scaffold types was no longer evident at day 7, when most cells were longitudinally aligned, and cell density was dramatically higher than that of day 1 in each group (Fig. 4C, F). H&E staining of longitudinally sectioned samples revealed uniform cell distribution within the constructs on day 1 (Fig. 4G, J). At day 7, the difference in construct width was apparent between the pure collagen construct (Fig. 4H) and the tECM-supplemented construct (Fig. 4K), in agreement with the results of gross width measurement. Longitudinally elongated cytoplasm and nuclei were apparent in cells in both construct types. More intense hematoxylin nuclear staining, indicating higher cell number, was found in the peripheral region of the tECM-supplemented constructs, resembling an epitendinous sheath surrounding native tendon fibers (Fig. 4 L). With Picosirius red staining and under polarized light, no observable difference in birefringence of collagen fibers was found between two groups (not shown).
Figure 4.
Examination of scaffold structure, cell morphology and distribution by SEM (A–F) and H&E staining (G–L). (A, B, D, E) hASCs attached to both types of scaffold 1 day after seeding. Cells in the tECM-supplemented scaffolds exhibit aligned cell bodies and extensions along the longitudinal axis of the scaffold (D, white arrows), whereas cells in the pure collagen scaffold are more randomly oriented (A, white arrows). (B, E) Higher magnification images show more extensions from the cell body attached to the collagen fibrils in tECM-supplemented scaffolds (E, white arrows) compared to pure collagen scaffolds (B, white arrows). (C, F) After 7 days of culture, cell density in each group is dramatically higher than that of day 1, and most cells assume an elongated spindle shape along the longitudinal axis of the scaffold. (G, J) H&E staining demonstrates uniform cell distribution along the depth of the constructs 1 day after gel setting. (H, I, K, L) At day 7, cells in each group are longitudinally aligned. (I, L) Interestingly, intense hematoxylin nuclear staining is evident at the peripheral region of tECM-supplemented scaffolds (L, black arrows).
3.3 Cell viability and proliferation
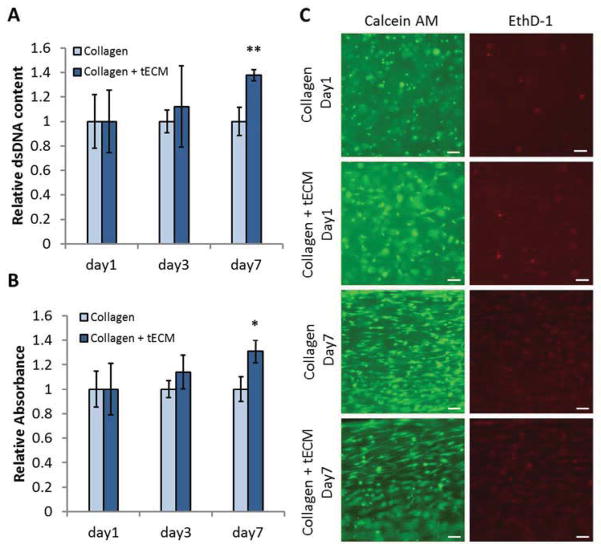
At days 1, 3 and 7, cell metabolic activity and cell number were determined using MTS assay and Picogreen staining, respectively (n=3). Cells seeded on tECM-supplemented scaffolds demonstrated significantly increased metabolic activity (p<0.05) and cell number (p<0.01) compared to that of pure collagen scaffolds at day 7, suggesting that the presence of tECM greatly promoted cell proliferation and cell activity in a 3D culture under uniaxial tension (Fig. 5A, B). A high percentage of viable cells were seen by Live/Dead assay in both pure collagen and tECM-supplemented scaffolds 24 h after gel setting, indicating that the tECM solution was non-toxic to cells. At day 1, most cells were round in shape, and evenly distributed inside the scaffolds. After 7 days of culture, cells retained a high rate of viability and exhibited longitudinal elongation, in agreement with histological analysis (Fig. 5C).
Figure 5.
Cell viability assays. Quantification of cell number and metabolic activity in scaffolds by Picogreen staining (A) and MTS assay (B). (A) After 7 days of culture, cell number, represented by DNA content, is significantly higher in the tECM group. (B) Similarly, cells in the tECM group demonstrate increased metabolic activity, as evidenced by elevated level of reduced MTS reagent. (C) Live/Dead cell viability staining (green, live cells; red, dead cells) showed a high percentage of viable cells, round shaped and evenly distributed, in each group 1 day after seeding. After 7 days of culture, cells retain a high rate of viability and exhibit longitudinally elongated cell bodies.* indicates p<0.05, ** indicates p<0.01, scale bar=100 μm, n=3.
3.4 Cell differentiation
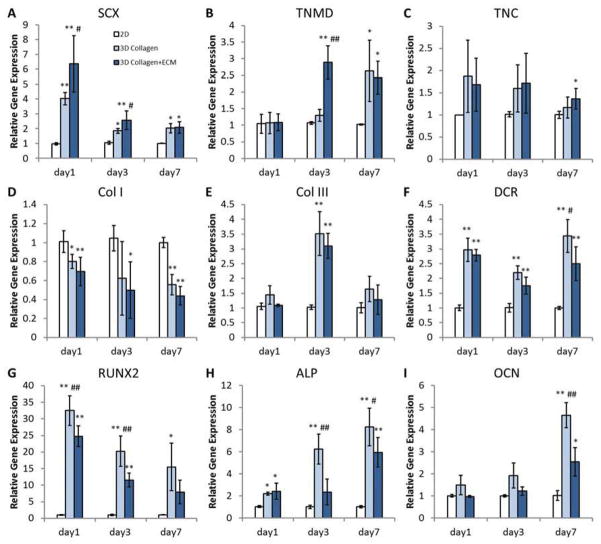
Gene expression of hASCs in both 2D and 3D culture (n=5) were quantified at the mRNA level by real-time PCR. The results showed that the 3D scaffolds under uniaxial tension promoted tenogenic differentiation of hASCs, as compared to 2D controls, and that tenogenesis was further enhanced by tECM supplementation (Fig. 6A–C). The expression of SCX was significantly higher in the tECM group than the pure collagen group at days 1 and 3. Both 3D groups demonstrated an up-regulation of SCX at all three time points compared to 2D culture. The expression of TNMD was found significantly higher in the tECM group than the 2D and the 3D collagen group at day 3, but the difference between the two 3D groups was negligible at day 7, when they were both higher than the 2D group. The expression pattern of TNC was similar among all three groups at the first two time points, but was significantly higher in the tECM group at day 7. Taken together, these findings demonstrated that hASCs underwent tenogenic differentiation in 3D culture under tension, and that exposure to tECM improved this lineage-specific differentiation. In contrast, the gene expression of other matrix proteins remained similar (Fig. 6D–F). There was no significant difference for COL I or COL III between the tECM and the pure collagen group. The expression of COL I in 3D culture was lower than that in 2D, which could be attributed to the collagen rich environment. DCR was up-regulated in 3D cultures at all time points, with expression in the tECM group lower than the collagen group at day 7.
Figure 6.
Real time PCR analysis of gene expression. (A–C) hASCs seeded in tECM-supplemented scaffolds (3D Collagen+ECM) show higher expression levels of tendon-specific genes (SCX, TNMD, and TNC) compared to both pure collagen scaffolds (3D Collagen) and 2D culture group (2D). (D–F) The expression levels of ECM genes (Col I, Col III, and DCR). (G–I) Osteogenesis-related genes (RUNX2, ALP, and OCN) are expressed at lower levels in hASCs seeded in tECM-supplemented scaffolds compared to the pure collagen scaffold group, although some of them remain still higher than that in 2D culture. * indicates p<0.05 compared to 2D; ** indicates p<0.01 compared to 2D; # indicates p<0.05 compared to the other 3D group; and ## indicates p<0.01 compared to the other 3D group; n=5.
To evaluate the influence of tECM on osteogenic differentiation, the expression of osteogenesis markers, including RUNX 2, ALP and OCN (Fig. 6G–I), was analyzed. All these markers were expressed at lower levels in hASCs seeded in tECM-supplemented scaffolds, although some of them were still higher than those seen in 2D culture. At days 1 and 3, the level of RUNX2 gene expression in hASCs in the tECM group was significantly lower than that of the pure collagen group. hASCs in the tECM group also expressed lower amount of ALP and OCN than in pure collagen at days 3 and 7. Taken together, these results suggested that the exposure to tECM in 3D scaffold promoted tenogenesis of hASCs and partially suppressed osteogenesis.
3.5 Mechanical properties of the scaffold
A representative load/displacement curve for day 3 samples showed a steeper linear slope of the tECM-supplemented scaffold when compared with the pure collagen scaffold, indicating better resistance to deformation under stretch (Fig. 7B). Quantitative analysis demonstrated significantly higher maximum load of tECM-supplemented scaffold than that of pure collagen scaffold at both time points (Fig. 7C, n=5, p<0.05). The stiffness of tECM-supplemented scaffolds was 2.37 and 4.11 times that of the pure collagen scaffolds at days 3 and 7, respectively (Fig. 7 D, p<0.01). In summary, these results showed that the addition of tECM enhanced the mechanical properties of hASC-seeded scaffolds under static tension.
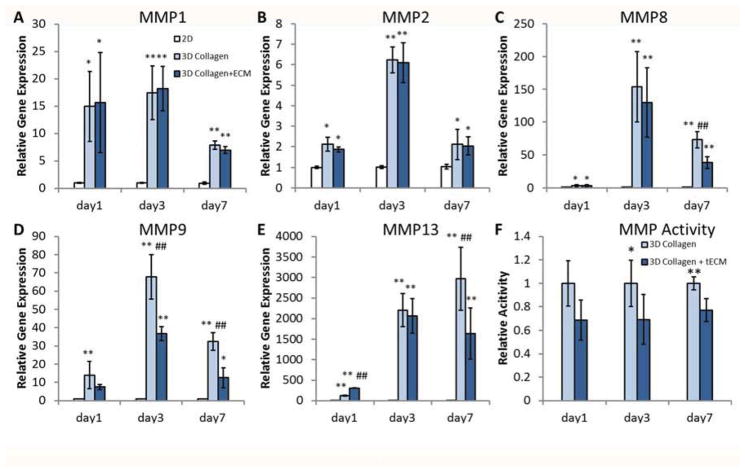
3.6 Gene expression and activity of MMPs
Interestingly, none of the cell-seeded scaffolds demonstrated improved mechanical strength compared to cell free controls (data not shown). Moreover, we found little difference in ECM protein gene expression patterns between 3D groups (see results 3.4). Combined, these results implied that the difference in mechanical properties between the cellular scaffold groups might be due to remodeling of the matrix rather than a net increase in matrix deposition. To test this hypothesis, we investigated the expression and activity of matrix metalloproteinases (MMPs), an endopeptidase family responsible for degradation and remodeling the ECM. Real-time PCR analysis revealed that gene expression level of MMPs significantly increased in 3D groups compared to 2D, indicating highly active matrix remodeling occurred in 3D culture under tension (Fig. 8A–E, n=5). MMP-8, -9 and -13 were down-regulated in the tECM-supplemented group by day 3 or 7 compared to the pure collagen group (Fig. 8C–E, p<0.01). Finally, the total MMP activities in conditioned medium collected from the tECM group was found to be significantly lower than that of the collagen group at days 3 and 7 (Fig. 8F, n=5).
Figure 8.
Matrix metalloproteinase (MMP) expression and activity. (A–E) hASCs seeded in 3D scaffolds express significantly higher amounts of various MMPs compared to 2D culture group (2D). (C–E) Expression levels of MMP-8, -9 and -13 are down-regulated in the tECM group (3D Collagen+ECM) at various time points compared to the collagen group (3D Collagen). (F) At days 3 and 7, the total MMP activity in conditioned medium collected from the tECM group (3D Collagen+ECM) is significantly lower than that of the collagen group (3D Collagen). * indicates p<0.05 compared to 2D; ** indicates p<0.01 compared to 2D; # indicates p<0.05 compared to the other 3D group; and ## indicates p<0.01 compared to the other 3D group; n=5.
4. Discussion
In this study, we have investigated the influence of a soluble ECM extract from bovine tendon tissue, used as a supplement to collagen scaffold, on hASCs cultured in 3D collagen gel under uniaxial tension. We hypothesize that incorporation of tECM will enhance the bio-functionality of 3D tension-bearing scaffolds. Our results show that the tECM contains a variety of tendon matrix proteins, and is substantially different from a commercially available, pure collagen type I solution. The inclusion of tECM within collagen scaffolds promotes hASC proliferation and tenogenic differentiation, as compared with pure collagen scaffold. Moreover, hASCs in tECM-containing scaffolds demonstrate reduced osteogenesis compared to hASCs in pure collagen scaffolds. The addition of tECM also results in changes of the mechanical properties of the constructs, including contraction rate, maximum load, and stiffness, partially by altering the expression level and activity of MMPs of the seeded hASCs. Collectively, these findings reveal the bio-functionality of tECM on hASC behaviors in 3D culture under tension, and strongly suggest the feasibility of using tECM to facilitate hASC-based tendon healing and regeneration.
ASCs, a multipotent mesenchymal stem cell population derived from adipose tissues, possess the potential to differentiate into a variety of cell lineages [26]. As far as the regeneration of mesodermal tissues are concerned, ASCs can undergo adipogenic, osteogenic, chondrogenic [27], myogenic [28], and tenogenic differentiation [29–31]. Compared to other types of adult stem cells, such as bone marrow MSCs (BMSC) and tendon stem cells (TDSC), ASCs are more readily available, since human adipose tissue is ubiquitous and can be isolated in large quantities with little donor site morbidity or patient discomfort. Given their availability and tenogenic capacity, the use of ASCs thus expands potential approaches to tendon tissue engineering and tendon repair. Tenogenesis of MSCs can be induced through uniaxial tension in 3D culture [14, 16, 32]. In this study, we have used the Flexcell Strain Unit with the Tissue Train culture plates to generate 3D cylindrical constructs under tension [17]. Collagen type I gel was used as the material to cast the scaffold for two reasons. First, collagen type I is the primary component of tendon tissue, and has been widely used as a scaffold in tendon tissue engineering. Second, cells could be easily suspended in collagen solution before setting, ensuring uniform distribution within the constructs. In contrast, previous studies have shown that cells re-seeded on acellular tendon tissue attached only to the surface but did not penetrate the matrix easily. In these studies, few cells were observed to migrate into the center of acellular flexor tendons even after 8 weeks in culture [33, 34].
For the purpose of facilitating tendon healing and regeneration, MSCs need to proliferate and then undergo appropriate differentiation and matrix remodeling on the scaffold in order to develop into a functional replacement tissue at sites of injury [1], [5]. The ECM plays important roles in regulating cell behavior. Tissue-derived ECMs have been found to induce corresponding tissue-specific differentiation of MSCs, and are therefore considered as attractive candidate biomaterials to support stem cell-based regeneration of the tissue from which it is derived. For example, soluble ECM derived from skeletal muscle tissue induced myogenesis of muscle progenitor cells [35]. Similarly, acellular cartilage sheets were found to stimulate BMSCs to undergo chondrogenesis [36]. As for tendon, BMSC seeded on acellular tendon scaffold expressed TNMD, a tendon phenotype marker [37]. TDSCs were also found to express more TNMD when seeded on tendon ECM film than on tissue culture plastic [25]. In this study, we have used urea, a nonionic chaotropic reagent that disrupts lipid-lipid, lipid-protein, and protein-protein interactions, for tECM extraction, producing a soluble extract that is easily miscible in aqueous solutions. SDS-PAGE and western blot assay revealed that soluble tECM isolated from tendons contains not only collagen but also numerous non-collagenous proteins, including fibronectin, fibromodulin, biglycan and decorin, all of which are known to regulate MSC behavior. For example, fibronectin is a multi-domain protein that contains binding sites for integrins, collagen and other ECM proteins, glycosaminoglycans, as well as self-association sites, thereby influencing cell shape and adhesion to the matrix [38]. Moreover, fibronectin has also been reported to induce chemotaxis and mitogenic activity for human MSCs [39]. These characteristics of fibronectin may contribute to the improved interaction between cells and tECM-containing scaffold, as evidenced by increased extensions from the cell body connected to the collagen fibrils, and increased retention of the seeded cells (data not shown). Decorin greatly affects the assembly of collagen fibrils during tendon development [40], and also prevents aberrant lateral fusion of collagen fibrils [41]. Biglycan and fibromodulin appear to have substantial impacts on cell fate [42], [43]. They were also identified as critical components of the tendon stem cell niche controlling the fate of tendon stem cells [23]. It is thus reasonable to hypothesize that the known activities of these ECM molecules, and of other yet-to-be-determined components in the tECM, are responsible for the observed bioactivity of the tECM on the hASCs seeded in the composite 3D scaffold.
Our hypothesis is supported by the results of the cell proliferation assay, real-time PCR analysis, and mechanical testing. DNA content and cell metabolic activity are significantly higher in the tECM-supplemented constructs than pure collagen constructs after 7 days of culture, indicating more rapid proliferation in the tECM group. It is noteworthy that DNA content measured immediately after gel setting (defined as day 0) showed no significant difference between the two groups (data not shown), indicating minimal residual, tissue-derived nuclear remnants. Consistent with our results, rabbit TDSCs have been shown to grow faster in tECM than on plastic surfaces, as indicated by the population doubling times (PDTs) [25]. Furthermore, hASC cultures seeded in constructs containing ligament-derived matrix were also found to contain more dsDNA than in collagen constructs [24].
Real-time PCR analysis revealed enhanced tenogenesis of hASCs in the tECM group based on tendon-specific gene expression (Fig. 6). The expression of SCX, a transcription factor specifically detected in tendon precursor cells [44], is significantly higher in the tECM group than the pure collagen group at days 1 and 3. Higher expression of TNMD, a transmembrane glycoprotein predominantly expressed in tendons and ligaments [45], is observed in the tECM group, compared to the 2D and 3D collagen groups at day 3. The level of TNC, an ECM glycoprotein involved in tendon development, is also significantly higher in tECM group at day 7. As enhanced expression of these markers are specific to the tECM group, tECM likely acts to promote tenogenic differentiation of hASCs in 3D culture under tension.
Despite the efficacy of 3D culture under tension to promote tenogenesis, simultaneous osteogenesis had been reported for hASCs cultured under mechanical stimulation, an undesirable effect that compromises the application of hASCs for tendon repair. For example, 6 hour cyclic mechanical loading was found to increase RUNX2 expression of rat ASCs by 7-fold [46, 47]. In another study, up-regulation of ALP and OCN expression in hASCs was achieved by 1 and 2 hour cyclic strain [48]. It has been suggested that such tension-induced osteogenesis is the cause of pathological ossification seen in mechanically overused tendon, such as in tendinosis [49]. Therefore, we asked whether hASCs cultured under uniaxial tension undergo osteogenesis and if this differentiation can be attenuated by incorporation of tECM. Our findings show that tECM significantly reduces osteogenic differentiation of hASCs when cultured in 3D under tension (Fig. 6 G–I). Based on the results reported here, we speculate that the reduced osteogenesis is the result of exposure to a more tenogenic niche provided by the tECM incorporated into the 3D scaffold, implying a tendon tissue-specificity of the pro-differentation effect of the tECM on hASCs.
Since tendons are primarily load-bearing tissues, the mechanical properties of engineered tendons need careful consideration. In this study, we investigated the mechanical properties of the constructs from two perspectives: contraction rate and mechanical strength. As the cells reorganized and remodeled the collagen matrix, macroscopic radial contraction of the construct was evident (see Figure 3). Similar to our results, constructs made from collagen type I gel seeded by BMSCs at 1 million cells/mL were found to contract by 61% of its initial diameter after 3 days [16]. In another study, the contraction rate peaked 1 day after gel setting with 82% reduction of the cross-sectional area of the construct after 8 days [17]. Interestingly, we observed that the presence of tECM significantly reduced the ASC-mediated contraction of the scaffold. To our knowledge, this study is the first to provide quantitative data for the effect of tECM on the contraction rate of cell-seeded collagen gels. In addition, tECM-supplemented constructs demonstrates improved mechanical strength, evidenced by significantly higher maximum load and stiffness of the constructs. Greater scaffold stiffness may result in greater tactile forces placed upon the encapsulated cells during contraction, causing tenogenic differentiation through the induction of SCX expression, which is known to be responsive to change in mechanical stimulus [50, 51].
Interestingly, we have observed no increase in mechanical properties of each type of cell-seeded scaffold compared to cell-free scaffolds. This observation, combined with the finding that there was little difference in ECM protein gene expression between 3D groups, ruled out the contribution of neo-matrix deposition. Therefore, we hypothesized that the change in mechanical properties was due to cell-mediated remodeling of the scaffold. To test this hypothesis, we investigated the expression and activity of MMPs, endopeptidases capable of degrading and remodeling extracellular matrix. MMP-1, -8 and -13 are able to cleave collagen fibrils in their triple helical domains. The denatured collagen can be further degraded by gelatinases, such as MMP-2 and -9. These MMPs also have the capacity to degrade a variety of non-collagenous ECM components [52]. Due to their matrix degrading capability, MMPs are involved in the degradation of a number of collagenous scaffolds. Real-time PCR analysis revealed a dramatic up-regulation of the expression of all five types of MMPs in hASCs seeded in the 3D culture under tension compared to 2D culture. Similar to our results, a significant up-regulation of MMP-1 and -13 expression was observed after 14 days of culture, when BMSCs were seeded on collagen type I gels [53]. In the same study, MMP-2 was found only activated in cells cultivated in collagen gels. In addition to studies of in vitro models, the alteration of MMP expression has been widely observed in a variety of tendon injuries in vivo [54]. For example, increased expression of MMP-1 and -9 was found in supraspinatus tendons in the defect group versus the healed group in a patient study [55]. MMP-2, -9 and -13 were up-regulated in ruptured human Achilles tendons [56, 57]. Similarly, there was a significant increase in MMP-13 mRNA in rotator cuff tendon tissue obtained from patients with rotator cuff tears [58]. MMP-9 and 13 were also found to participate in collagen degradation but not remodeling in a rat flexor tendon laceration model [59]. In our study, MMP-8, -9 and -13 are down-regulated by nearly 50% in the tECM group compared to the pure collagen group at day 7. The total MMP activity in the culture-conditioned medium was also significantly lower at day 3 and day 7 in the tECM group. These findings therefore suggest the possible influence of tECM on suppressing MMP-mediated scaffold degradation, which could contribute to the improvement of tendon healing.
5. Conclusions
A bioactive, soluble fraction of tendon-derived extracellular matrix (tECM) was prepared. Exposure to tECM greatly promoted human adipose stem cell (hASC) proliferation and tenogenic differentiation in 3D collagen gels under static tension. hASCs in tECM-containing scaffolds also demonstrated suppressed osteogenesis compared to hASCs in pure collagen scaffolds. Furthermore, the addition of tECM improved the mechanical properties of the constructs, in part by altering the amount of MMPs expressed by seeded hASCs and their activity in culture medium. These findings suggest a new approach to inducing hASC tenogenesis that may improve tendon tissue engineering to facilitate tendon healing and regeneration.
Acknowledgments
We gratefully acknowledge Jian Tan M.D, for hASC isolation, and Jonathan Franks (Center of Biological Imaging, University of Pittsburgh) for technical support for SEM. This work is supported in part by the Commonwealth of Pennsylvania Dept. of Health (SAP4100062224, SAP4100050913), NIH (1R01AR062947,1U18TR000532), U.S. Dept. of Defense (W81XWH-10-1-0850, W81XWH-11-2-0143), and the RiMed Foundation (Italy).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008;33:102–12. doi: 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Butler DL, Juncosa N, Dressler MR. Functional efficacy of tendon repair processes. Annu Rev Biomed Eng. 2004;6:303–29. doi: 10.1146/annurev.bioeng.6.040803.140240. [DOI] [PubMed] [Google Scholar]
- 3.Pennisi E. Tending tender tendons. Science. 2002;295:1011. doi: 10.1126/science.295.5557.1011. [DOI] [PubMed] [Google Scholar]
- 4.Liu CF, Aschbacher-Smith L, Barthelery NJ, Dyment N, Butler D, Wylie C. What we should know before using tissue engineering techniques to repair injured tendons: a developmental biology perspective. Tissue Eng Part B Rev. 2011;17:165–76. doi: 10.1089/ten.teb.2010.0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lui PP, Rui YF, Ni M, Chan KM. Tenogenic differentiation of stem cells for tendon repair-what is the current evidence? J Tissue Eng Regen Med. 2011;5:e144–63. doi: 10.1002/term.424. [DOI] [PubMed] [Google Scholar]
- 6.Krueger-Franke M, Siebert CH, Scherzer S. Surgical treatment of ruptures of the Achilles tendon: a review of long-term results. Br J Sports Med. 1995;29:121–5. doi: 10.1136/bjsm.29.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klepps S, Bishop J, Lin J, Cahlon O, Strauss A, Hayes P, et al. Prospective evaluation of the effect of rotator cuff integrity on the outcome of open rotator cuff repairs. Am J Sports Med. 2004;32:1716–22. doi: 10.1177/0363546504265262. [DOI] [PubMed] [Google Scholar]
- 8.Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annu Rev Biomed Eng. 2012;14:47–71. doi: 10.1146/annurev-bioeng-071811-150122. [DOI] [PubMed] [Google Scholar]
- 9.James R, Kumbar SG, Laurencin CT, Balian G, Chhabra AB. Tendon tissue engineering: adipose-derived stem cell and GDF-5 mediated regeneration using electrospun matrix systems. Biomed Mater. 2011;6:025011. doi: 10.1088/1748-6041/6/2/025011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahoo S, Ang LT, Cho-Hong Goh J, Toh SL. Bioactive nanofibers for fibroblastic differentiation of mesenchymal precursor cells for ligament/tendon tissue engineering applications. Differentiation. 2010;79:102–10. doi: 10.1016/j.diff.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Ramanath HS, Wang DA. Tendon tissue engineering using scaffold enhancing strategies. Trends Biotechnol. 2008;26:201–9. doi: 10.1016/j.tibtech.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Kuo CK, Marturano JE, Tuan RS. Novel strategies in tendon and ligament tissue engineering: Advanced biomaterials and regeneration motifs. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:20. doi: 10.1186/1758-2555-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riehl BD, Park JH, Kwon IK, Lim JY. Mechanical stretching for tissue engineering: two-dimensional and three-dimensional constructs. Tissue Eng Part B Rev. 2012;18:288–300. doi: 10.1089/ten.teb.2011.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo CK, Tuan RS. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2008;14:1615–27. doi: 10.1089/ten.tea.2006.0415. [DOI] [PubMed] [Google Scholar]
- 15.Subramony SD, Dargis BR, Castillo M, Azeloglu EU, Tracey MS, Su A, et al. The guidance of stem cell differentiation by substrate alignment and mechanical stimulation. Biomaterials. 2013;34:1942–53. doi: 10.1016/j.biomaterials.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awad HA, Butler DL, Harris MT, Ibrahim RE, Wu Y, Young RG, et al. In vitro characterization of mesenchymal stem cell-seeded collagen scaffolds for tendon repair: effects of initial seeding density on contraction kinetics. J Biomed Mater Res. 2000;51:233–40. doi: 10.1002/(sici)1097-4636(200008)51:2<233::aid-jbm12>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Garvin J, Qi J, Maloney M, Banes AJ. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng. 2003;9:967–79. doi: 10.1089/107632703322495619. [DOI] [PubMed] [Google Scholar]
- 18.Harris MT, Butler DL, Boivin GP, Florer JB, Schantz EJ, Wenstrup RJ. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 2004;22:998–1003. doi: 10.1016/j.orthres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Lin H, Yang G, Tan J, Tuan RS. Influence of decellularized matrix derived from human mesenchymal stem cells on their proliferation, migration and multi-lineage differentiation potential. Biomaterials. 2012;33:4480–9. doi: 10.1016/j.biomaterials.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–7. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ning LJ, Zhang Y, Chen XH, Luo JC, Li XQ, Yang ZM, et al. Preparation and characterization of decellularized tendon slices for tendon tissue engineering. J Biomed Mater Res A. 2012;100:1448–56. doi: 10.1002/jbm.a.34083. [DOI] [PubMed] [Google Scholar]
- 23.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–27. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 24.Little D, Guilak F, Ruch DS. Ligament-derived matrix stimulates a ligamentous phenotype in human adipose-derived stem cells. Tissue Eng Part A. 2010;16:2307–19. doi: 10.1089/ten.tea.2009.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Li B, Wang JH. The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like tissue formation in vivo. Biomaterials. 2011;32:6972–81. doi: 10.1016/j.biomaterials.2011.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizuno H. Adipose-derived stem and stromal cells for cell-based therapy: current status of preclinical studies and clinical trials. Curr Opin Mol Ther. 2010;12:442–9. [PubMed] [Google Scholar]
- 27.Mizuno H, Tobita M, Uysal AC. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30:804–10. doi: 10.1002/stem.1076. [DOI] [PubMed] [Google Scholar]
- 28.Mizuno H, Zuk PA, Zhu M, Lorenz HP, Benhaim P, Hedrick MH. Myogenic differentiation by human processed lipoaspirate cells. Plast Reconstr Surg. 2002;109:199–209. doi: 10.1097/00006534-200201000-00030. [DOI] [PubMed] [Google Scholar]
- 29.Uysal AC, Mizuno H. Tendon regeneration and repair with adipose derived stem cells. Curr Stem Cell Res Ther. 2010;5:161–7. doi: 10.2174/157488810791268609. [DOI] [PubMed] [Google Scholar]
- 30.Park A, Hogan MV, Kesturu GS, James R, Balian G, Chhabra AB. Adipose-derived mesenchymal stem cells treated with growth differentiation factor-5 express tendon-specific markers. Tissue Eng Part A. 2010;16:2941–51. doi: 10.1089/ten.tea.2009.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–60. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raabe O, Shell K, Fietz D, Freitag C, Ohrndorf A, Christ HJ, et al. Tenogenic differentiation of equine adipose-tissue-derived stem cells under the influence of tensile strain, growth differentiation factors and various oxygen tensions. Cell Tissue Res. 2013;352:509–21. doi: 10.1007/s00441-013-1574-1. [DOI] [PubMed] [Google Scholar]
- 33.Kryger GS, Chong AK, Costa M, Pham H, Bates SJ, Chang J. A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. J Hand Surg Am. 2007;32:597–605. doi: 10.1016/j.jhsa.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 34.Pridgen BC, Woon CY, Kim M, Thorfinn J, Lindsey D, Pham H, et al. Flexor tendon tissue engineering: acellularization of human flexor tendons with preservation of biomechanical properties and biocompatibility. Tissue Eng Part C Methods. 2011;17:819–28. doi: 10.1089/ten.tec.2010.0457. [DOI] [PubMed] [Google Scholar]
- 35.Stern MM, Myers RL, Hammam N, Stern KA, Eberli D, Kritchevsky SB, et al. The influence of extracellular matrix derived from skeletal muscle tissue on the proliferation and differentiation of myogenic progenitor cells ex vivo. Biomaterials. 2009;30:2393–9. doi: 10.1016/j.biomaterials.2008.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xue JX, Gong YY, Zhou GD, Liu W, Cao Y, Zhang WJ. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells induced by acellular cartilage sheets. Biomaterials. 2012;33:5832–40. doi: 10.1016/j.biomaterials.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 37.Omae H, Zhao C, Sun YL, An KN, Amadio PC. Multilayer tendon slices seeded with bone marrow stromal cells: a novel composite for tendon engineering. J Orthop Res. 2009;27:937–42. doi: 10.1002/jor.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh P, Schwarzbauer JE. Fibronectin and stem cell differentiation - lessons from chondrogenesis. J Sci. 2012;125:3703–12. doi: 10.1242/jcs.095786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thibault MM, Hoemann CD, Buschmann MD. Fibronectin, vitronectin, and collagen I induce chemotaxis and haptotaxis of human and rabbit mesenchymal stem cells in a standardized transmembrane assay. Stem Cells Dev. 2007;16:489–502. doi: 10.1089/scd.2006.0100. [DOI] [PubMed] [Google Scholar]
- 40.Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, et al. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436–49. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- 41.Chen B, Wang B, Zhang WJ, Zhou G, Cao Y, Liu W. In vivo tendon engineering with skeletal muscle derived cells in a mouse model. Biomaterials. 2012;33:6086–97. doi: 10.1016/j.biomaterials.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 42.Bi Y, Stuelten CH, Kilts T, Wadhwa S, Iozzo RV, Robey PG, et al. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J Biol Chem. 2005;280:30481–9. doi: 10.1074/jbc.M500573200. [DOI] [PubMed] [Google Scholar]
- 43.Zheng Z, Jian J, Zhang X, Zara JN, Yin W, Chiang M, et al. Reprogramming of human fibroblasts into multipotent cells with a single ECM proteoglycan, fibromodulin. Biomaterials. 2012;33:5821–31. doi: 10.1016/j.biomaterials.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 44.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–66. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 45.Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298:234–47. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 46.Yang X, Cai X, Wang J, Tang H, Yuan Q, Gong P, et al. Mechanical stretch inhibits adipogenesis and stimulates osteogenesis of adipose stem cells. Cell Prolif. 2012;45:158–66. doi: 10.1111/j.1365-2184.2011.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang X, Gong P, Lin Y, Zhang L, Li X, Yuan Q, et al. Cyclic tensile stretch modulates osteogenic differentiation of adipose-derived stem cells via the BMP-2 pathway. Arch Med Sci. 2010;6:152–9. doi: 10.5114/aoms.2010.13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diederichs S, Bohm S, Peterbauer A, Kasper C, Scheper T, van Griensven M. Application of different strain regimes in two-dimensional and three-dimensional adipose tissue-derived stem cell cultures induces osteogenesis: implications for bone tissue engineering. J Biomed Mater Res A. 2010;94:927–36. doi: 10.1002/jbm.a.32772. [DOI] [PubMed] [Google Scholar]
- 49.Shi Y, Fu Y, Tong W, Geng Y, Lui PP, Tang T, et al. Uniaxial mechanical tension promoted osteogenic differentiation of rat tendon-derived stem cells (rTDSCs) via the Wnt5a-RhoA pathway. J Cell Biochem. 2012;113:3133–42. doi: 10.1002/jcb.24190. [DOI] [PubMed] [Google Scholar]
- 50.Scott A, Danielson P, Abraham T, Fong G, Sampaio AV, Underhill TM. Mechanical force modulates scleraxis expression in bioartificial tendons. J Musculoskelet Neuronal Interact. 2011;11:124–32. [PubMed] [Google Scholar]
- 51.Mendias CL, Gumucio JP, Bakhurin KI, Lynch EB, Brooks SV. Physiological loading of tendons induces scleraxis expression in epitenon fibroblasts. J Orthop Res. 2012;30:606–12. doi: 10.1002/jor.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Somerville RP, Oblander SA, Apte SS. Matrix metalloproteinases: old dogs with new tricks. Genome Biol. 2003;4:216. doi: 10.1186/gb-2003-4-6-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heckmann L, Fiedler J, Mattes T, Dauner M, Brenner RE. Interactive effects of growth factors and three-dimensional scaffolds on multipotent mesenchymal stromal cells. Biotechnol Appl Biochem. 2008;49:185–94. doi: 10.1042/BA20070071. [DOI] [PubMed] [Google Scholar]
- 54.Garofalo R, Cesari E, Vinci E, Castagna A. Role of metalloproteinases in rotator cuff tear. Sports Med Arthrosc. 2011;19:207–12. doi: 10.1097/JSA.0b013e318227b07b. [DOI] [PubMed] [Google Scholar]
- 55.Robertson CM, Chen CT, Shindle MK, Cordasco FA, Rodeo SA, Warren RF. Failed healing of rotator cuff repair correlates with altered collagenase and gelatinase in supraspinatus and subscapularis tendons. Am J Sports Med. 2012;40:1993–2001. doi: 10.1177/0363546512456519. [DOI] [PubMed] [Google Scholar]
- 56.Karousou E, Ronga M, Vigetti D, Passi A, Maffulli N. Collagens, proteoglycans, MMP-2, MMP-9 and TIMPs in human achilles tendon rupture. Clin Orthop Relat Res. 2008;466:1577–82. doi: 10.1007/s11999-008-0255-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Mos M, van El B, DeGroot J, Jahr H, van Schie HT, van Arkel ER, et al. Achilles tendinosis: changes in biochemical composition and collagen turnover rate. Am J Sports Med. 2007;35:1549–56. doi: 10.1177/0363546507301885. [DOI] [PubMed] [Google Scholar]
- 58.Lo IK, Marchuk LL, Hollinshead R, Hart DA, Frank CB. Matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase mRNA levels are specifically altered in torn rotator cuff tendons. Am J Sports Med. 2004;32:1223–9. doi: 10.1177/0363546503262200. [DOI] [PubMed] [Google Scholar]
- 59.Oshiro W, Lou J, Xing X, Tu Y, Manske PR. Flexor tendon healing in the rat: a histologic and gene expression study. J Hand Surg Am. 2003;28:814–23. doi: 10.1016/s0363-5023(03)00366-6. [DOI] [PubMed] [Google Scholar]