Abstract
Attention to internal bodily sensations is a core feature of mindfulness meditation. Previous studies have not detected differences in interoceptive accuracy between meditators and nonmeditators on heartbeat detection and perception tasks. We compared differences in respiratory interoceptive accuracy between meditators and nonmeditators in the ability to detect and discriminate respiratory resistive loads and sustain accurate perception of respiratory tidal volume during nondistracted and distracted conditions. Groups did not differ in overall performance on the detection and discrimination tasks; however, meditators were more accurate in discriminating the resistive load with the lowest ceiling effect. Meditators were also more accurate during the nondistracted tracking task at a lag time of 1 s following the breath. Results provide initial support for the notion that meditators have greater respiratory interoceptive accuracy compared to nonmeditators.
Keywords: Meditation, Interoceptive awareness, Interoceptive accuracy, Respiration, Anxiety, Mindfulness
One of the biggest problems in the world is that people don't feel themselves properly.
—Chögyam Trungpa Rinpoche, meditation master
Interoceptive awareness relies on well-defined physiological afferent pathways involving interconnected cortical networks (Craig, 2009). This sensory input into the central nervous system is essential for homeostatic regulation of physical processes and influences cognition, emotion, and behavior (Cameron, 2001). Growing research indicates that dysregulated interoceptive awareness and its neural substrates are associated with adverse health conditions, including mood disorders, chronic pain, disordered eating, and addiction (Fassino, Piero, Gramaglia, & Abbate-Daga, 2004; Flor, 2012; Paulus & Stein, 2010; Verdejo-Garcia, Clark, & Dunn, 2012). Interestingly, mindfulness interventions have been shown to improve symptoms associated with each of these conditions (Bowen et al., 2009; Daubenmier et al., 2011; Grossman, Niemann, Schmidt, & Walach, 2004; Hofmann, Sawyer, Witt, & Oh, 2010; Keng, Smoski, & Robins, 2011; Ma & Teasdale, 2004). Mindfulness is a key element of many meditation practices in the West, and 9.4% of Americans report practicing some form of meditation (Barnes, Bloom, & Nahin, 2008). Whether mindfulness training enhances interoceptive awareness and thereby improves these health conditions is an important question for research.
Mindfulness is defined as a process of regulating attention in order to bring a quality of nonelaborative awareness to current experience and to relate to one's experience with an attitude of curiosity, openness, and acceptance (Bishop et al., 2004). A common form of mindfulness meditation involves sitting in an upright, relaxed position and paying full attention to sensations of breathing. Practitioners are instructed to attend to physical sensations in a nonevaluative manner and to notice the occurrence of thoughts, emotions, sounds, and other stimuli as they arise. Once practitioners become distracted or lost in thought, attention is directed back to the breath. The repeated practice of bringing attention back to an internal sensory stimulus trains the practitioner's ability to (a) regulate attention, (b) distinguish between thinking about physical sensations versus experiencing them directly, and (c) observe passing sensations, thoughts, and emotions more clearly (Williams, 2010). The goal of mindfulness meditation is not to transcend personal experience but to observe internal sensations, thoughts, and feelings without overly identifying with them and without reacting to them in an automatic, habitual pattern of reactivity. Mindfulness allows a greater “space” between perception and response to occur so situations can be responded to more reflectively and less reflexively (Bishop et al., 2004).
As mindfulness meditation cultivates nonevaluative awareness of interoceptive sensations, such repeated training over time may result in more accurate perceptions of internal sensations, even though direct feedback regarding accuracy is not provided as in biofeedback training. For the purposes of clarity, a recent distinction has been made between interoceptive awareness and interoceptive accuracy (Ceunen, Van Diest, & Vlaeyen, 2013). Interoceptive awareness is the cognizant perception of bodily sensations, while interoceptive accuracy refers to the ability to perceive accurate changes in bodily sensations. It may be presumed that accurate perceptions depend on awareness, but awareness alone is not sufficient for accurate perceptions. Individuals may have high levels of awareness but not necessarily high levels of accuracy (see Ceunen et al., 2013; Mirams, Poliakoff, Brown, & Lloyd, 2012; Parkin et al., 2013; Silvia & Gendolla, 2001, for discussions). Hence, while mindfulness meditation may enhance awareness of interoceptive sensations, such training does not necessarily lead to more accurate perceptions (Parkin et al., 2013).
However, three lines of evidence taken together do lend support to the view that mindfulness may enhance interoceptive accuracy. First, self-reported interoceptive awareness has been found to increase following mindfulness training using a measure of responsiveness to bodily sensations (Daubenmier et al., 2011) and the Observe scale of mindfulness questionnaires, which contains items assessing interoceptive awareness in uncontrolled (Carmody & Baer, 2008; Deyo, Wilson, Ong, & Koopman, 2009) and controlled trials (Daubenmier et al., 2011; Nyklicek & Kuijpers, 2008). Second, changes in neural pathways associated with interoceptive awareness have been found in relation to mindfulness training. Specifically, increased activation of the somatosensory and insular cortices have been found among mindfulness meditators compared to controls when attending to present-moment experience (Farb et al., 2007) and respiratory sensations (Farb, Segal, & Anderson, 2013b). Mindfulness meditation practice time has been positively correlated with activation in the posterior insula, a primary interoceptive cortex region, during respiratory awareness tasks (Farb, Segal, & Anderson, 2013a, 2013b). In addition, meditation experience has been associated with increased gray matter volume in the insula and temporoparietal junction, regions both associated with interoceptive awareness, suggesting that meditation practice may have enduring trait-like effects on the processing of interoceptive information (Holzel et al., 2008, 2011; Lazar et al., 2005). A third line of evidence is that mindfulness is associated with increased accuracy of exteroceptive perceptions. Mindfulness training led to decreased perceptual thresholds in a visual discrimination task (MacLean et al., 2010), was associated with increased detection of visual stimuli in an “attentional blink task” in which a stimulus that is presented closely following an initial stimulus often goes undetected (Slagter et al., 2007), and reduced misperception of external touch and increased sensitivity on a somatic signal detection task (Mirams, Poliakoff, Brown, & Lloyd, 2013). Furthermore, in a related practice of Tai Chi Chuan, which includes mindful exercises that cultivate body-focused attention, practitioners demonstrated superior touch acuity compared to age-matched controls (Kerr et al., 2008). Altogether, this evidence supports the hypothesis that mindfulness training enhances perceptual processing of sensory stimuli, including interoceptive sensations.
Increased nonevaluative awareness and accuracy of interoceptive sensations may be important mechanisms of action to account for the therapeutic effects of mindfulness interventions on emotion regulation. First, awareness of internal sensations plays a crucial role in theories of emotion and decision making such as the James-Lange theory of emotion (James, 1884), Damasio's somatic marker hypothesis (Damasio, 2003), and theories of embodied cognition (Niedenthal, 2007). Overlapping activity in the anterior insula has been observed during interoceptive awareness and emotional awareness tasks (Terasawa, Fukushima, & Umeda, 2013; Zaki, Davis, & Ochsner, 2012), suggesting that attention to bodily states underlies awareness of one's emotional state. Thus, greater awareness of interoceptive sensations may improve the accurate identification of emotional states and enhance the adaptive regulation of emotions (Barrett & Gross, 2001; Fustos, Gramann, Herbert, & Pollatos, 2012).
Second, sustained nonevaluative attention to interoceptive sensations may serve to disengage individuals from dysfunctional cognitive patterns that perpetuate negative moods. Affective disorders, such as depression and anxiety, are characterized by repetitive thinking, which is passive, uncontrolled, and focused on negative content, such as worry and depressive rumination (Ehring & Watkins, 2008; McLaughlin & Nolen-Hoeksema, 2011). In the case of rumination, individuals are unable to successfully reappraise negative feelings, initiate active problem solving, or engage in distracting activities that uplift mood (McLaughlin & Nolen-Hoeksema, 2011). Farb and colleagues (2012, 2013b) propose that as individuals turn attention towards momentary internal sensory experience in a nonevaluative manner, they disengage from negative rumination and self-appraisal processes that increase risk for mood disorders. The cultivation of interoceptive awareness of neutral sensations under resting conditions, such as the breath, in mindfulness meditation may enhance the ability to attend to internal sensations during distressing experiences. In turn, this disruption of automatic cognitive and emotional processes allows the opportunity for more adaptive coping responses to be invoked. They argue that non-evaluative awareness of interoceptive sensations activates stable recruitment of sensory pathways in the brain, which in turn reduces habitual, evaluative, and self-referential processing supported by midline structures of the prefrontal cortex, as these networks are typically negatively correlated.
In support of this theory, Farb et al. (2007) showed that, when attending to present-moment experience, mindfulness training is associated with increased activation of viscerosomatic regions, including the insula and somatosensory cortex, and reduced involuntary activation of the medial prefrontal cortex. In another study, greater recruitment of the right insula in response to a sadness induction was related to less depressive symptoms among both mindfulness practitioners and controls (Farb et al., 2010). Behavioral research points in the same direction: a greater ability to sustain mindful attention on the breath during an 18-min period was associated with less rumination and depressive symptoms (Burg & Michalak, 2011).
Studies related to other health conditions show a similar pattern of results. Mindful attention to an acute pain stimulus decreased pain unpleasantness and anticipatory anxiety and was associated with greater activation of the right posterior insula and decreased activation of the lateral prefrontal cortex among mindfulness practitioners compared to controls (Gard et al., 2012). Mindful attention to craving sensations among smokers reduced the self-reported urge to smoke and attenuated neural activity in craving-related brain regions in response to images of smoking cues (Westbrook et al., 2013). Finally, mindfulness interventions focused on attention to bodily sensations reduced food cravings and eating in response to external food cues among overweight and obese adults (Alberts, Mulkens, Smeets, & Thewissen, 2010; Daubenmier et al., 2011).
In summary, studies suggest that mindful, nonevaluative attention to distressing interoceptive sensations and experiences may reduce reactive responses to aversive experiences and enhance self-regulation. Specifically, mindful interoceptive awareness may limit negative repetitive thinking (in the case of depression and anxiety), anticipatory anxiety (in the case of pain conditions), and impulsive health-related behaviors (in the case of addiction and disordered eating). The neural mechanisms underlying these effects may involve increased activation of regions that modulate interoceptive awareness (e.g., somatososensory and insular cortices) and correspondingly decreased activation of regions that modulate conceptual, self-referential processing (e.g., the midline structures of the prefrontal cortex). However, reverse inference conclusions about actual mental activities from neuroimaging findings should be interpreted with caution and need to be confirmed with behavioral measures (Poldrack, 2008).
In seeming contradiction to the findings on mindfulness and interoceptive awareness, other research has found that greater interoceptive accuracy as assessed by heartbeat perception tasks is associated with greater anxiety symptoms (Domschke, Stevens, Pfleiderer, & Gerlach, 2010; Pollatos, Traut-Mattausch, Schroeder, & Schandry, 2007). Increased interoceptive accuracy has been viewed as a risk factor for developing heightened state or trait anxiety by increasing the probability of catastrophic appraisals of bodily sensations (Domschke et al., 2010). Negative belief-based thoughts about bodily sensations may amplify their emotional valence and contribute to enhanced anxiety symptoms (Paulus & Stein, 2010). Accordingly, when interoceptive awareness is associated with negative, or evaluative, thoughts about interoceptive sensations, anxiety symptoms increase. Rather than providing evidence to the contrary, this perspective is consistent with data from mindfulness research. Among individuals without training in mindfulness, brain regions that recruit sensory and conceptual self-referential processing show a high degree of interconnectivity when attending to present-moment experience, but after mindfulness training, these areas are uncoupled (Farb et al., 2007). These findings suggest that untrained individuals have greater difficulty processing interoceptive sensations without conceptual interference and may be less able to distinguish nonevaluative sensory perception from narrative, conceptual thinking about sensations (Farb et al., 2007). Thus, when interoceptive sensations are attended to in an evaluative context, anxiety or somatic misperceptions may occur; however, when interoceptive awareness is nonevaluative, anxiety and other negative affective states may decrease. As one example to illustrate the effects of these two forms of interoceptive awareness, a study by Mirams and colleagues found that a heartbeat perception task, which involved counting pulse sensations at the fingertip, led to greater misperceptions on a somatic signal detection task among participants untrained in mindfulness, as participants erroneously reported feeling vibrations presented to their fingertip in the absence of a stimulus (Mirams et al., 2012). However, training in mindful interoceptive attention led to decreases in these misperceptions (Mirams et al., 2013). Altogether, these findings highlight the importance of distinguishing between evaluative and nonevaluative interoceptive awareness and the potential therapeu tic role of a nonevaluative stance towards interoceptive experience on self-regulation as a result of mindfulness training.
Although research appears to indicate that mindfulness meditation may enhance interoceptive accuracy, which, in turn, may be an important therapeutic component of mindfulness training, evidence using behavioral measures is lacking. Studies assessing interoceptive accuracy found no differences between meditators and controls on heartbeat detection tasks (Khalsa et al., 2008; Nielsen & Kaszniak, 2006) or improvement in heartbeat perception following mindfulness training (Parkin et al., 2013). However, the reliability and validity of heartbeat detection and perception tasks remains controversial, and none have emerged as the “gold standard” for measuring interoceptive accuracy (Knapp-Kline & Kline, 2005). These tasks have been criticized because they do not adequately assess conscious awareness of interoceptive sensations, as the majority of participants do not perform better than chance, participants frequently report guessing, and actual performance is un-correlated or negatively correlated with self-rated performance (Khalsa, Rudrauf, Sandesara, Olshansky, & Tranel, 2009; Wiens, 2005). Additionally, meditators do not commonly use the heartbeat as a direct target of observation during meditation. To our knowledge, no studies have tested whether mindfulness meditation is associated with greater respiratory interoceptive accuracy, a common sensory focus in mindfulness meditation.
To assess respiratory interoceptive accuracy, the respiratory load task has been used in physiological studies since the 1950s Zechman & Davenport, 1978; (Zechman, Hall, & Hull, 1957). It is technologically less involved than the standard rebreathing test, which uses a closed bag that gradually produces excess carbon dioxide in the blood (Bogaerts et al., 2008). One type of respiratory load task requires participants to detect when a resistance has been introduced into a tube through which they are breathing (Davenport, Chan, Zhang, & Chou, 2007; Zhao, Martin, & Davenport, 2002). A second type of resistive load task requires participants to discriminate among degrees of resistive loads introduced into the airway (Webster & Colrain, 2000). Asthma patients have shown a decreased sensitivity compared to healthy controls on the discrimination task (Dahme, Richter, & Mass, 1996; Kifle, Seng, & Davenport, 1997). Another study reported that a yoga intervention enhanced the ability to discriminate among resistive loads compared to matched controls (Villien, Yu, Barthelemy, & Jammes, 2005).
Both the detection and the discrimination tasks involve momentary assessments of respiratory interoceptive accuracy. In contrast, formal mindfulness meditation practice involves sustained attention to respiratory sensations over time. A task that could correlate estimations of real-time tracking of changes in respiration patterns with actual changes over a sustained period of time would approximate the formal mindfulness meditative experience and increase the ecological validity of the task. If mindfulness meditation improves respiratory interoceptive accuracy, meditators would be expected to show a stronger affinity between actual and estimated changes in respiratory patterns compared to nonmeditators.
In the current study, we adapted the detection and discrimination tasks and developed a novel measure of sustained attention to respiratory sensations to examine whether mindfulness meditators are more accurate than nonmeditators in perceiving respiratory sensations over time. Second, to test the validity of these tasks, we examined whether task performances correlated with one another, self-report measures of interoceptive awareness, and self-evaluation of the tasks. Finally, as discussed above, the relationship between anxiety and interoceptive awareness and accuracy may depend on the type of attentional focus. When respiratory sensations are attended to with nonevaluative attention, greater interoceptive awareness and accuracy may be associated with less anxiety; however, if interoceptive sensations are attended to in an evaluative context, greater interoceptive awareness and accuracy may be associated with greater anxiety. Hence, we explored the pattern of associations between anxiety and interoceptive awareness and accuracy in meditators and nonmeditators.
Method
Participants
Meditators were recruited through meditation centers in the San Francisco Bay Area. Nonmeditators were recruited by flyers posted at universities and surrounding community establishments. Potentially interested respondents called a dedicated study line and were screened for eligibility by trained research staff. If eligible, potential participants were sent the consent form in the mail and scheduled for the experiment.
Inclusion criteria
Individuals for both groups of the study were eligible if they were nonsmokers; did not have a current respiratory infection, a chronic bronchial or pulmonary condition, or sleep apnea; were not currently pregnant; had not been diagnosed with ankylosing spondylitis or systemic lupus; were not suffering from chronic abdominal pain or a chronic liver or kidney disease; were nonobese; had not been diagnosed with AIDS, diabetes, multiple sclerosis, chronic fatigue syndrome, metabolic syndrome, cardiovascular disease, cancer, neurological diseases, brain injury, or panic disorder; were not taking narcotic pain medications; did not report using recreational drugs; and did not have more than 50 h experience with deep water diving or a regular singing practice.
Meditators were eligible for the study if they reported practicing Vipassana meditation, which focuses attention on the breath, for at least 5 years and a current practice of 5 days/week for at least 30 min/day. Nonmeditators were eligible for the study if they had not done any mind-body practice within the past 6 months, including meditation, yoga, Tai Chi Chuan, or other mind-body practice, and did not report being a serious student of meditation or other mind-body practice earlier in life. Meditators and nonmeditators were matched on sex, body mass index, education level, and age. All study procedures were approved by the Committee on Human Research of the University of California, San Francisco.
Procedures
Participants were asked not to drink caffeine for 6 h or exercise for 1 h before the measures and to bring with them the signed informed consent. To ensure participants were at a resting state before starting study procedures, participants sat comfortably in a chair for a 15-min rest period and read magazines with neutral content (e.g., National Geographic). Afterwards, they completed computer-based self-report measures, and height and weight was assessed. Participants first performed the detection task, followed by the discrimination and tracking tasks. They had 5-min rest periods between each task. The tasks were followed by completion of post-task self-report measures.
Respiratory load detection task
Participants were seated in a chair, a mouthpiece was applied, and their noses were closed with a nose clip (Hans Rudolph, Kansas City, KS). Participants breathed through the mouthpiece, which was connected to a large-gauge, low-resistance plastic tubing connected to a pneumotachygraph and pressure transducer module (Biopac/Rudolph). The pneumotachygraph was connected to the Biopac data acquisition system, which integrated flow data recorded every 1/6 of a second into tidal volume data. The end of the plastic tubing and all other measurement equipment were hidden from participant view behind a screen.
Participants breathed normally without any added resistance for 5 min while reading a magazine with affect-neutral content to become accustomed to the breathing device and to establish baseline respiratory measures of air flow, respiration rate, and depth (tidal volume). Subsequently, five resistive loads were presented. The resistors were made out of nylon at resistances of 0.4, 0.8, 1.6, 2.4, and 3.2 cmH2O/l/s (custom made by Hans Rudolph) and referred to as resistor numbers 1–5, respectively. These resistor sizes include and extend below the detection threshold of healthy individuals found in prior research (2.6 ± .9 cmH2O/l/s, range = 1.2–4.4 cmH2O/l/s (Davenport et al., 2007). We expected that resistors in the range of 0.4 to 3.2 and differences of at least 0.3 cmH2O)/l/s between loads would be appropriate for both the detection and discrimination tasks in healthy individuals.
Each resistive load was presented seven times in random fashion for a single breath at the beginning of inhalation for a full breath cycle (inspiration and expiration), for a total of 35 presentations. The pneumotachygraph measures the air flow between its two openings, one connected with the plastic tubing ending in the mouthpiece, and the other open to the room. The resistors were placed in the open end of the pneumotachygraph. Participants wore noise-reduction headsets that played nature sounds and were unable to hear any manipulations related to the unannounced resistor placements. Between 1 and 4 no-resistance breath cycles in a random fashion were allowed between each of these presentations. Participants pressed a hand-held push button when they detected a resistive load. Participant's detection signal and load magnitude were recorded by the Biopac data acquisition system on a lab computer in the adjacent room.
The primary measure was the resistive load detection threshold as used in previous research (Davenport et al., 2007). The resistive load detection threshold is the lowest resistive load that was detected in at least 4 out of 7 presentations of a specific resistor (i.e., more than 50% of the time). To take advantage of the continuous data, secondary measures included a detection accuracy score: the number of correct detections was summed and divided by the number of presentations (35) and multiplied by 100 to determine percent accuracy for the entire task. Percent accuracy was also assessed for each resistor by dividing the number of correct detections (ranging from 0 to 7) by 7 and multiplying by 100. The number of false positives was also assessed in which participants reported detecting a resistor when no resistor was presented.
Respiratory load discrimination task
Following a brief practice period in which each of the five different resistors was presented twice together with information about its size, participants used a keyboard to enter ratings from “0” for no resistor to “5” for the largest resistive load. A set of random presentations of the six resistive load conditions (including no load) were presented in blocks of 6, with each load presented once in a block. Presentation of the resistor was announced at the onset of a new inspiration for the duration of one full breath cycle, which was then followed by regular intervals of five normal breaths. A total of 36 load trials were presented without the use of noise-reduction headsets.
For all 36 load presentations, the absolute differences between a participant's rating of “0” to “5” and the actual resistor size of 0–5 were summed for a possible maximum error score of 144. The primary measure was a percent accuracy score: one minus the sum of all absolute differences divided by the maximum error score of possible differences (144), and multiplied by 100. Secondary outcomes were percent accuracy scores for each resistor, which were calculated as follows: one minus the sum of the absolute differences divided by the maximum error score for each resistor (30, 24, 18, 18, 24, and 30 for resistors 0–5, respectively) and multiplied by 100 for a maximum score of 100 for each resistor. Higher scores indicate greater percent accuracy.
Respiratory tracking task
The respiratory tracking task was a new procedure developed for this study. The same equipment was used as in the detection and discrimination tasks; however, instead of a push button, participants used a Biopac slider box. No resistive loads were presented during this task.
Initially, inspiratory vital capacity was assessed as the maximum value of three attempts at the maximum inhaled air volume after maximal exhalation. Following a brief 2-min practice period, participants were asked to track their breath rhythm for 5 min by manually moving a slider indicating both phase and depth of breath relative to their perceived vital capacity. The movement on the sliding scale box was recorded continuously in line with the actual tidal volume. The computer display of the recording was in the neighboring laboratory room and not visible to the participant. Participants were instructed to indicate a maximum breath by maximum movement of the lever (from 0–10). With normal inhalation, the lever was to be moved upward to a point corresponding to the estimated depth of each individual breath (actual estimated tidal volume) relative to the initially established maximum capacity (actual tidal volume as the proportion of inspiratory vital capacity). At the end of inhalation, the slider was to be at its high point on the scale (peak), and with initiation of exhalation the slider was to be moved towards “0”. At the end of expiration, participants were instructed to bring the slider to the relative lowest point towards “0” on the scale for each breath cycle (trough).The Biopac data acquisition system produced simultaneous curves for tidal volume and slider movements collected at six data points per second.
In order to increase task difficulty and approximate the experience of mindfulness meditation in which attention to respiratory sensations is challenged by competing internal and external stimuli, we introduced an audio-visual distraction condition. After the 5-min nondistraction period, participants were presented with brief standardized video clips from the University of California, Berkeley Psychophysiology Lab film archive with four emotional contents at 2 min each in the following order: contentment (Blue Planet movie), sadness (21 Grams movie), disgust (Fear Factor television show), and amusement (I Love Lucy television show). All film clips were taken either from televised productions or popular films. To ensure that study participants did not simply turn their attention away from the video clips, a manipulation check was performed with a computer-based questionnaire, in which participants were asked to identify the emotions elicited by the videos immediately after the task. All participants were 100% accurate in recalling the order of the video sequence. Given that order of video clip presentation was confounded with emotional content, we did not examine Group ¥ Video Clip interactions on tracking accuracy.
As the recording provided data at six time points per second, we calculated the maximum cross-correlation between actual tidal volume and estimated values occurring anywhere between positive lags 1–6, with each lag representing 1/6 of a second following the breath, in 1-min intervals across the nondistracted and distracted periods. We also computed average cross-correlations across the 5-min nondistraction and 8-min distraction periods separately. In exploratory analyses, because accuracy may vary across lags, we also examined differences between groups at each lag period during the nondistracted and distracted periods. Respiration rate during the track task was determined manually based on objective criteria. A breath cycle was defined as a peak of at least 0.1 volume units (according to Biopac AcqKnowledge 4.0 output) greater than a trough preceding it and a trough following it.
Self-Report Measures
Interoceptive awareness
First, the 12-item Body Awareness sub-scale of the Body Connection Scale (BCS–Body Awareness) was used to assess conscious attention to internal sensations indicating bodily and emotional states, including states of anger, stress, and peacefulness (Price & Thompson, 2007). In the present study, responses to the items were given on a 7-point scale ranging from 1 = never to 7 = always.
Second, the 7-item Body Responsiveness Questionnaire (BRQ) assesses the tendency to integrate body sensations into conscious awareness to guide decision making and behavior and not suppress or react impulsively to them (Daubenmier, 2005). A factor analysis indicates the presence of two factors (Daubenmier, unpublished analyses); and, in the present study, a principal components factor analysis also revealed two factors with factor loadings greater than .50 explaining 70% of the variance. The Importance of Interoceptive Awareness subscale (BRQ–Importance) assesses the importance of using interoceptive information to regulate behavior and self-awareness (items include “It is important for me to know how my body is feeling throughout the day,” “I am confident that my body will let me know what is good for me”) and the Perceived Disconnection subscale (BRQ–Perceived Disconnection) measures the extent of perceived disconnection between psychological and bodily states, including suppressing and reacting impulsively to them (items include “My mind and my body often want to do different things,” “I suppress my bodily feelings and sensations,” “My bodily desires lead me to do things that I end up regretting”). Responses were measured on a 7-point scale ranging from 1 = not at all true about me to 7 = very true about me.
Third, a Breath Awareness Scale was created with six items. Two of the items were taken from the final version of the Multidimensional Assessment of Interoceptive Awareness (Mehling et al., 2012), and the remaining items were taken from the initial pool of the item development stage. Responses were made on a 7-point scale ranging from 1 = never to 7 = always. The items were as follows: “I notice when my breathing is shallow,” “I can be aware of my breath without changing it,” “I am aware of the movements of my breath,” “I can pay attention to my breath without being distracted by things happening around me,” “I notice that my breathing becomes free and easy when I feel comfortable,” “I notice my breathing slows down when I am deeply relaxed.” The internal consistency of the Breath Awareness Scale was high (Cron-bach's alpha = .88). The BRQ–Importance, SBC–Body Awareness, and the Breath Awareness Scales were used to assess the convergent validity of the respiratory interoceptive awareness tasks, and the BRQ–Perceived Disconnection scale was used to assess the tasks’ discriminant validity.
Anxiety
The State-Trait Anxiety Scale (state and trait forms) were used to assess general feelings of anxiety (Spielberger, Corsuch, & Lushene, 1970). Participants rated statements along a 4-point scale ranging from 1 = almost never to 4 = almost always for trait anxiety and from not at all to very much for state anxiety.
Evaluation of tasks
After completion of all of the tasks, participants evaluated their experience of each task along 6-point scales. They rated their performance level (1 = I don't think I performed well to 6 = I think I performed very well); amount of effort (1 = I did not try very hard to 6 = I tried my best); level of discomfort (1 = no discomfort at all to 6 = very uncomfortable); and willingness to do the task again (1 = absolutely not to 6 = absolutely yes).
Statistical Analysis
We used t tests for continuous variables and chi-square tests for categorical variables to assess group differences in demographic and self-report measures. Independent samples t tests were used to compare group differences on the detection and discrimination tasks. One-way analyses of variance (ANOVAs) and analyses of covariance (ANCOVAs) were used to assess differences between groups on mean cross-correlations during the nondistracted and distracted tracking tasks. Repeated measures ANOVA tests were used to assess Time × Group effects in the nondistracted and distracted conditions. These tests were assessed for violations of the sphericity assumption and corrected with the Huynh-Feldt method when necessary. In these cases, the uncorrected degrees of freedom, corrected p value, and the Huynh-Feldt correction are reported. Pearson correlations were used to assess relations between self-report interoceptive awareness and respiratory interoceptive accuracy measures.
One nonmeditator did not complete the self-report measures of interoceptive awareness, and, for the detection task, one meditator completed only 25 of 35 presentations, and one nonmeditator had two responses missing due to technical error. Adjustments were made in the calculation of percent accuracy to include available data. Two meditators had partial missing data during the distraction period due to technical error and were excluded from the Time × Group repeated measures ANOVAS for the distracted period, but available data were used to compute overall mean cross-correlations during the distraction period for the participant with missing data for the last minute.
Results
Eighteen meditators and 16 nonmeditators enrolled in the study. The groups did not differ significantly in age, sex, education, body mass index, state anxiety, or trait anxiety (Table 1). Meditators reported significantly higher levels of body awareness, the importance of listening to bodily sensations to guide behavior, and breath awareness compared to nonmeditators. The meditators reported practicing meditation at least 5 days/week for 30 min/session for 9.0 ± 7.7 years, completed 25.1 ± 27.4 days of retreat over the past year, practiced 26.0 ± 6.7 days over the past month, at an average of 43.1 ± 11.0 min/session, for a total of 18.2 ± 6.7 h of practice over the past month.
Table 1.
Demographic and Self-Report Variables for Meditators and Nonmeditators (Mean ± SD)
| Meditators (N = 18) | Nonmeditators (N = 16) | P value | |
|---|---|---|---|
| Age | 41.9 ± 11.0 | 35.4 ± 11.4 | .10 |
| Sex (% male) | 61 | 50 | .76 |
| Body mass index | 23.7 ± 3.4 | 23.2 ± 3.4 | .67 |
| Education (% college degree) | 78 | 94 | .41 |
| Meditation experience | |||
| Years | 9.0 ± 7.7 | – | |
| Retreat days/past year | 25.1 ± 27.4 | – | |
| Days/past month | 26.0 ± 6.7 | – | |
| Average minutes/session/past month | 43.1 ± 11.0 | – | |
| Total hours/past month | 18.2 ± 6.7 | – | |
| State anxiety | 1.4 ± 0.4 | 1.3 ± 0.3 | .58 |
| Trait anxiety | 1.9 ± 0.4 | 2.0 ± 0.4 | .55 |
| BCS-Body Awareness | 6.0 ± 0.5 | 4.6 ± 1.0 | < .0001 |
| BRQ-Importance | 6.1 ± 0.7 | 5.0 ± 1.2 | < .0001 |
| BRQ-Perceived Disconnection | 2.7 ± 1.0 | 2.8 ± 1.1 | .71 |
| Breath awareness | 5.6 ± 0.6 | 3.9 ± 1.1 | < .0001 |
BCS = Body Connection Scale; BRQ = Body Responsiveness Questionnaire.
Respiratory Detection Task
To assess group differences in the detection threshold, we examined the lowest resistive load that was detected more than 50% of the time and found no differences between groups, 1.7 ± 1.0 and 1.8 ± 0.9, for meditators and nonmeditators, respectively, t(32) = -0.09, p = .93. Groups also did not differ in number of false positives, 0.61 ± 0.85 vs. 0.75 ± 1.53, for meditators and nonmeditators, respectively, t(32) = -0.33, p = .74.
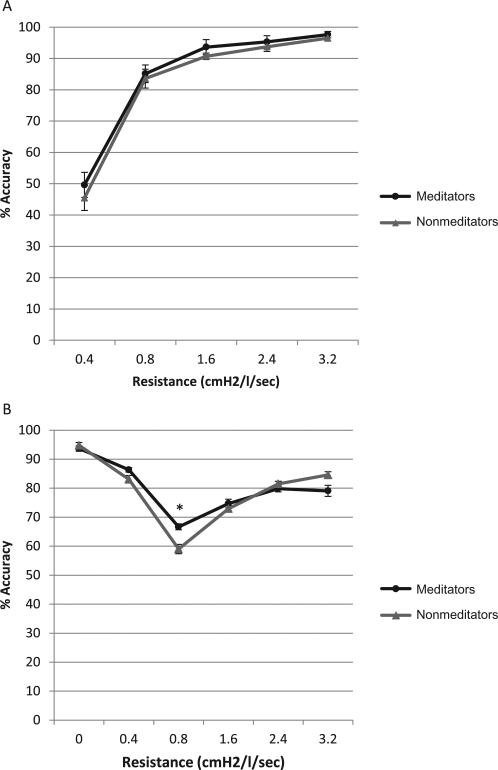
Overall, the mean percent detection accuracy across resistors was 83.2 ± 16.2. For each resistor separately, the means were as follows: resistor 1 = 47.7 ± 32.6; resistor 2 = 84.4 ± 23.1; resistor 3 = 92.2 ± 17.9; resistor 4 = 94.6 ± 14.9; and resistor 5 = 97.1 ± 0.7. Groups did not differ significantly in mean percent detection accuracy across all resistors, 84.2 ± 17.9 vs. 82.2 ± 14.6, for meditators and nonmeditators, respectively; t(32) = 0.35, p = .73 (see Figure 1A). Given the high detection rate across the entire task, the ability to detect a group difference may have been constrained by a ceiling effect. To circumvent this potential limitation, we examined group differences in the resistor that had the lowest ceiling effect, resistor 1. However, groups did not differ significantly in detection of resistor 1, 49.6 ± 34.3 vs. 45.4 ± 31.4, for meditators and nonmeditators, respectively, t(32) = 0.37, p = .72.
Figure 1.
A: Detection task. B: Discrimination task. Error bars indicate standard error of the mean. *group differences significant at p < .05.
Respiratory Discrimination Task
The mean percent accuracy across resistors for the discrimination task was 82.1 ± 6.5 and for each resistor separately: resistor 0 (no resistor) = 94.2 ± 7.8; resistor 1 = 84.8 ± 8.8; resistor 2 = 63.1 ± 11.4; resistor 3 = 73.9 ± 11.0; resistor 4 = 80.6 ± 8.2; and resistor 5 = 81.7 ± 13.0. Overall, no significant group differences in mean percent accuracy across resistors were observed, 82.0 ± 7.4 and 82.2 ± 5.6, for meditators and nonmeditators, respectively, t(32) = -0.08, p = .94 (see Figure 1B). Given the high rate of accuracy across the entire task, the ability to detect a group difference may have been constrained by a ceiling effect. To circumvent this limitation, we examined group differences in the resistor with the lowest ceiling effect, resistor 2. Meditators showed greater accuracy in discriminating resistor 2 compared to nonmeditators, 66.7 ± 8.5 and 59.0 ± 13.1, for meditators and nonmeditators, respectively; t(32) = 2.0, p = .05.
Respiratory Tracking Task
Nondistracted period
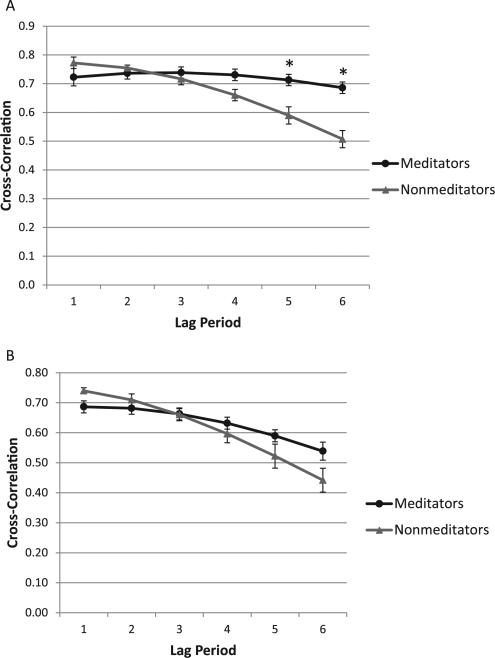
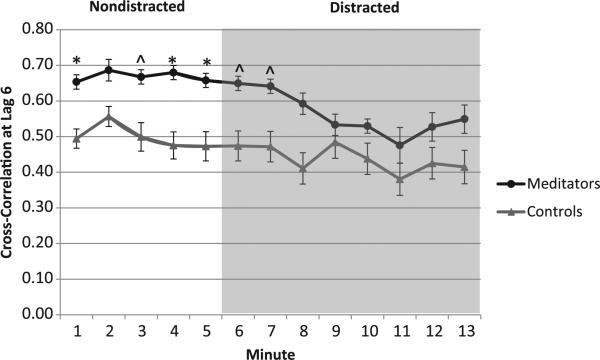
A one-way ANOVA revealed no signifi-cant difference in the overall maximum cross-correlation occurring anywhere between lags 1 to 6 (1/6–1 s following the breath) during the nondistracted period between meditators (0.78 ± 0.15) and nonmeditators (0.78 ± 0.11), F(1,32) = 0.10, p = .92. However, the maximum cross-correlation occurred at a significantly later lag period for meditators compared to nonmeditators, 3.22 ± 2.2 and 1.69 ± 1.1, for meditators and nonmeditators, respectively, F(1,32) = 6.22, p = .02, partial η2 = .16. In exploratory analyses, we found no significant differences between groups at each lag period from 1–4 over the nondistraction period (ps > .05). However, meditators had significantly higher cross-correlations compared to nonmeditators at lag 5, 0.71 ± 0.1 vs. 0.59 ± 0.2, for meditators and nonmeditators, respectively, F(1,32) = 4.5, p = .04, partial η2 = .12, and lag 6, 0.69 ± 0.1 vs. 0.50 ± 0.3, for meditators and nonmeditators, respectively, F(1,32) = 7.0, p = .01, partial η2 = .18 ( see Figure 2A). Cross-correlations at lag 6 of estimated and actual tidal volume by group and distraction period over time are presented in Figure 3.
Figure 2.
A: Nondistraction period. B: Distraction period. Error bars indicate standard error of the mean. *group differences significant at p < .05.
Figure 3.
Cross-correlations at lag 6 of estimated and actual tidal volume by group and distraction period over time. ∧group differences marginally significant at p < .10. *group differences significant at p < .05.
In order to explore whether accuracy over time differed between groups, a 5 (Minute) × 2 (Group) repeated measures ANOVA with the maximum cross-correlation from lag 1–6 during the nondistracted tracking task as the dependent variable was conducted. The repeated measures ANOVA revealed no significant Minute × Group interaction, F(4,128) = 0.28, p = .85, partial η2 = .01. No significant Minute × Group effects were observed at each lag period from 1–6 (ps > .05).
The meditators had significantly slower respiration rates during the nondistracted period compared to nonmeditators, (7.0 ± 2.0 vs. 9.6 ± 3.5 breaths/minute for meditators and nonmeditators, respectively, t(1,32) = −2.8, p = .01. When controlling for respiration rate, the cross-correlation differences between meditators and nonmeditators at lags 5 and 6 were no longer statistically signifi-cant, F(2,31) = 0.33, p = .57, partial η2 = .01, for lag 5; and F(2,31) = 0.74 p = .40, partial η2 = .02, for lag 6.
Distraction period
A one-way ANOVA revealed no significant differences in the overall maximum cross-correlation between lags 1 to 6 during the distraction period between meditators (0.73 ± .12) and nonmeditators (0.74 ± .11), F(1,31) = 0.04, p = .85, partial h2 = .001. However, the maximum cross-correlation occurred at a significantly later lag period for meditators compared to nonmeditators, 2.59 ± 2.1 and 1.20 ± 0.4, for meditators and nonmeditators, respectively, F(1,31) = 6.91, p = .01, partial η2 = .18. In exploratory analyses, we found no significant differences between groups at each lag period from 1–6 during the distraction period, (ps > .05; see Figure 2B).
An 8 (Minute) × 2 (Group) repeated measures ANOVA with the maximum cross-correlation during the distraction period of the tracking task as the dependent variable revealed no significant Minute × Group interaction, F(7,210) = .38, p = .90, partial η2 = .01. No significant Minute × Group effects were observed for cross-correlations at each lag from 1–6 (ps > .05).
Meditators and nonmeditators did not differ significantly in respiration rate during the distraction period, 8.2 ± 2.8 vs. 9.8 ± 4.5 breaths/minute, for meditators and nonmeditators, respectively, t(1,32) = -1.3, p = .21.
Correlations Among Self-Report Interoceptive Awareness and Respiratory Interoceptive Accuracy Measures
No significant correlations were found between self-report measures of interoceptive awareness and the overall measures of the respiration tasks, although higher scores on the BRQ–Perceived Disconnection scale tended to be related to less total accuracy on the detection task and less total accuracy during the distracted period of the tracking task (see Table 2). However, both the SBC–Body Awareness and Breath Awareness scales were correlated positively with performance on resistor 2 of the discrimination task and accuracy on the tracking task at lags 5 and 6 during the nondistracted periods, and tended to be correlated positively with tracking accuracy at lag 6 during the distracted period.
Table 2.
Pearson Correlations Among Measures of Self-Report Body Awareness and Respiratory Interoceptive Accuracy
| Measure | SBC-Body Awareness | BRQ-Importance | BRQ-Perceived Disconnection | Breath Awareness |
|---|---|---|---|---|
| Detection (threshold)a | -.02 | .03 | .22 | -.01 |
| Detection (total) | .08 | -.02 | -.34^ | .08 |
| Detection (resistor 1) | .11 | .15 | -.31^ | .13 |
| Discrimination (total) | .03 | -.07 | -.17 | .05 |
| Discrimination (resistor 2) | .50** | .30^ | .01 | .49** |
| Tracking—Nondistraction | ||||
| Across lags 1-6 | -.15 | .04 | -.22 | -.11 |
| Lag 1 | -.26 | -.12 | -.04 | -.22 |
| Lag 2 | -.16 | -.06 | -.11 | -.12 |
| Lag 3 | .00 | .03 | -.20 | .04 |
| Lag 4 | .21 | .13 | -.27 | .23 |
| Lag 5 | .38* | .21 | -.30^ | .39* |
| Lag 6 | .49** | .25 | -.30^ | .49** |
| Tracking—Distraction | ||||
| Across lags 1-6 | -.10 | .15 | -.34^ | -.11 |
| Lag 1 | -.23 | .03 | -.17 | -.21 |
| Lag 2 | -.09 | -.06 | -.23 | -.09 |
| Lag 3 | .08 | -.02 | -.25 | .07 |
| Lag 4 | .21 | -.07 | -.23 | .19 |
| Lag 5 | .29 | .11 | -.21 | .26 |
| Lag 6 | .33^ | .13 | -.19 | .31^ |
Lower scores indicate a lower detection threshold reflecting greater interoceptive awareness.
p < .10.
p < .05.
p < .01.
Intercorrelations between measures of the three respiratory interoceptive accuracy tasks are presented in Table 3. Accuracies on the tracking task at lags 5 and 6 were positively associated with accuracies on the detection and discrimination tasks for the resistors with the lowest ceiling effects (i.e., resistor 1 for the detection task and resistor 2 for the discrimination task). Tracking accuracy at each lag during the distracted period was related to the detection task for resistor 1 but was not related to the discrimination task. Overall accuracy on the discrimination task was related to each measure of the detection task, but performance with resistor 2 on the discrimination task was not significantly related to the detection task.
Table 3.
Pearson Correlations Among Respiratory Interoceptive Accuracy Measures
| Measure | Detection (threshold)a | Detection (total)a | Detection (resistor 1) | Discrimination (total) | Discrimination (resistor 2) |
|---|---|---|---|---|---|
| Detection (threshold)a | - | ||||
| Detection (total) | -.92*** | - | |||
| Detection (resistor 1) | -. 74*** | .79*** | - | ||
| Discrimination (total) | -.57*** | .67*** | .41** | - | |
| Discrimination (resistor 2) | .02 | .07 | .17 | .36* | - |
| Tracking—Nondistraction | |||||
| Across lags 1-6b | -.04 | .08 | .17 | -.17 | .03 |
| Lag 1 | -.04 | .01 | .12 | -.27 | -.11 |
| Lag 2 | -.05 | .05 | .19 | -.24 | -.02 |
| Lag 3 | -.07 | .11 | .27 | -.19 | .10 |
| Lag 4 | -.10 | .17 | .34* | -.12 | .24 |
| Lag 5 | -.12 | .21 | .37* | -.06 | .34* |
| Lag 6 | -.13 | .23 | .41* | -.01 | .40* |
| Tracking—Distraction | |||||
| Across lags 1-6 | .38* | -.34^ | .43* | .09 | .01 |
| Lag 1 | .22 | -.27 | .32^ | -.09 | -.13 |
| Lag 2 | .27 | -.27 | .41* | -.01 | -.03 |
| Lag 3 | .29^ | -.25 | .46** | .06 | .08 |
| Lag 4 | .28 | -.22 | .45** | .11 | .16 |
| Lag 5 | .27 | -.19 | .43* | .14 | .21 |
| Lag 6 | .26 | -.17 | .42* | .15 | .25 |
Lower scores indicate a lower detection threshold, reflecting greater interoceptive accuracy.
Indicates the maximum cross-correlation occurring anywhere between lag periods from 1-6.
p < .10.
p < .05,
p < .01,
p < .001.
Correlations Between State and Trait Anxiety and Interoceptive Awareness and Accuracy Measures
For the detection task, performance for resistor 1 was negatively associated with state anxiety among meditators (r = −.55, p = .02) but not among nonmeditators (r = .06, p = .82). State and trait anxiety was not correlated with measures of discrimination accuracy (ps > .05). For the tracking task, the cross-correlation at lag 6 during the nondistraction task tended to be negatively related to trait anxiety among meditators (r = −.43, p = .07) but not among nonmeditators (r = −.21, p = .43).
For the self-report measures of interoceptive awareness, the BRQ–Perceived Disconnection scale was positively correlated with trait anxiety among meditators (r = −.49, p = .04) but not among nonmeditators (r = −.06, p = .84). The BCS–Body Awareness Scale was not significantly related to state or trait anxiety among meditators or nonmeditators. The Breath Awareness Scale tended to be negatively related to state anxiety (r = −.45, p = .095) and trait anxiety (r = −.50, p = .055) among nonmeditators.
Evaluation of Tasks
Meditators and nonmeditators did not significantly differ in self-evaluation of level of performance or discomfort on any of the tasks (see Table 4). The willingness to do the three tasks again was generally higher in nonmeditators. The groups did not differ significantly in amount of self-reported effort on the detection and discrimination tasks, although the nonmeditators reported greater effort on the tracking task at a marginally significant level (p = .09).
Table 4.
Means and Standard Deviations of Evaluations of Respiratory Interoceptive Accuracy Tasks by Group
| Task | Meditators | Nonmeditators | P value |
|---|---|---|---|
| Detection | |||
| Performance | 4.33 ± 0.8 | 4.38 ± 1.0 | .89 |
| Effort | 5.67 ± 0.6 | 5.69 ± 0.5 | .91 |
| Discomfort | 3.00 ± 1.2 | 3.00 ± 1.2 | 1.0 |
| Willingness to do again | 4.89 ± 1.0 | 5.56 ± 0.6 | .02 |
| Discrimination | |||
| Performance | 4.33 ± 1.2 | 4.19 ± 1.1 | .72 |
| Effort | 5.33 ± 1.1 | 5.63 ± 0.6 | .37 |
| Discomfort | 2.56 ± 0.8 | 2.63 ± 1.1 | .83 |
| Willingness to do again | 4.89 ± 1.1 | 5.56 ± 0.6 | .04 |
| Tracking | |||
| Performance | 4.41 ± 1.1 | 4.19 ± 1.0 | .55 |
| Effort | 5.22 ± 1.0 | 5.69 ± 0.5 | .09 |
| Discomfort | 2.67 ± 1.1 | 2.50 ± 1.0 | .66 |
| Willingness to do again | 4.94 ± 1.3 | 5.63 ± 0.6 | .06 |
Across groups, participant evaluations were not significantly correlated with accuracy on the detection, discrimination, or tracking tasks (ps > .05), except that greater willingness to do the discrimination task again was related to poorer accuracy on resistor 2 (r = −.35, p = .04). We also found no significant correlations within groups, with one exception: self-evaluation of performance on the tracking task was positively related to actual performance during the distraction period in meditators, with the maximum cross-correlation occurring anywhere between lags 1–6 (r = .53, p = .04) but not among nonmeditators (r = −.08, p = .76).
Discussion
We examined whether experienced mindfulness meditators have greater respiratory interoceptive accuracy compared to nonmeditators. We used two adapted versions of previously validated tasks and explored group differences on one novel task of respiratory interoceptive accuracy. Contrary to prediction, groups did not differ in overall performance on the tasks; however, meditators were more accurate than nonmeditators on two of the three tasks under specific task conditions. Performances under these conditions were correlated with one another and with self-report measures of interoceptive awareness in contrast to the overall measures, providing initial support for the validity of these specific measures. To our knowledge, this is the first study to provide preliminary evidence that mindfulness meditation practice may be associated with enhanced respiratory interoceptive accuracy. However, given the pilot nature of this study, future work is needed to refine these measures and replicate results.
Both the meditators and nonmeditators had unexpectedly high accuracy on the detection and discrimination tasks. Ceiling effects may have limited the opportunity to detect robust differences between groups. However, meditators were better at discriminating the resistive load with the lowest ceiling effect compared to nonmeditators. Although the resistance loads were smaller than those used in prior studies involving healthy participants (Davenport et al., 2007), the equipment and protocol employed in the present study may have contributed to overall enhanced detection and discrimination. Future research should use smaller resistive loads than the ones used in the present study and consider inserting the resistor into the tube during only one phase of the respiratory cycle, thereby shortening load presentation time, increasing detection difficulty, and potentially avoiding ceiling effects.
A novel task to assess sustained attention to respiratory sensations was developed to approximate the experience of attending to the breath during mindfulness meditation. Surprisingly, meditators did not differ from nonmeditators when accuracy was assessed anywhere from 1/6–1 s following a moment of respiration. However, when cross-correlations were computed 5/6 and 1 s following the breath, meditators were more accurate than nonmeditators. During testing, we realized the tendency of some participants to control respiration rate and synchronize it with slider box hand movements rather than passively attend to respiratory sensations and move the slider box in response to changes in tidal volume. Thus, cross-correlations at earlier lags (e.g, 1/6–1/3 s following the breath) may reflect the tendency to synchronize respiratory and hand movements, whereas cross-correlations at later lags (e.g., lags 5 and 6) may reflect greater interoceptive awareness processes related to attending to the breath receptively without manipulating it and moving the slider box in response to respiratory sensations. This interpretation is supported by the pattern of correlations observed between the tracking task across lag periods and self-report measures of interoceptive awareness. Accuracy on the tracking task was positively correlated with self-report measures of interoceptive awareness at lags 5 and 6 but tended to be negatively correlated at earlier lags. Similarly, correlations with the detection and discrimination of the resistors with the lowest ceiling effects were significantly positively correlated with tracking accuracy only at lags 5 and 6. Thus, these results suggest that the tracking task may be a more valid measure of respiratory interoceptive accuracy when assessed approximately 1 s following the breath. Nonmeditators may be less familiar with the difference between synchronizing and following the breath, as following the breath requires the ability to disengage volitional control over respiration to observe the body breathing passively. In support of this notion, 56% of meditators and 69% of nonmeditators had maximum cross-correlations occurring at the early lags (1–2), whereas 39% of meditators and 0% of nonmeditators had maximum cross-correlations occurring at later lags (5–6; χ2 test p < .05). In future use of the tracking task, we suggest instructing participants explicitly about these two ways of performing the task and testing these methods separately with clear instructions.
Meditators had slower respiration rates than nonmeditators during the nondistraction task, confirming previous research showing that conscious awareness of respiration (Western & Patrick, 1988) and mindfulness meditation are associated with slower respiration rates (Farb et al., 2013b). Thus, a core feature of mindfulness meditation practice may be that it slows respiration. To statistically control for respiration during this task may therefore remove meaningful variance representing enhanced accuracy of respiratory sensations. When controlling for respiration rate, the effect between groups was no longer significant. It could be argued that the task may be easier for meditators because the object of attention moves at a slower rate and is therefore easier to track. Similar confounds have been observed with the heartbeat detection task, as a slower heart rate and decreased heart rate variability are associated with better performance (Knapp-Kline & Kline, 2005). It will be important for future research to control for respiration rate, for example, by using targeted rates, volumes, or airflows displayed to participants (Kifle et al., 1997) or by engaging in Ujjayi breathing prior to the task (Khalsa et al., 2008).
A distraction condition, in which participants were asked to attend to video clips, which elicited various emotions while tracking their respiration patterns, was developed to increase task difficulty. The task was successful in eliciting equal respiration rates between the two groups. The pattern of means suggested that meditators were more accurate than nonmeditators on average during the distracted condition at lag 6; however, the effect was not substantial enough to reach statistical significance. A larger sample may be needed to detect effects in the distracted condition given its increased difficulty. The task may also have been unduly complicated given the instructions to attend to both internal and external stimuli simultaneously. A future variant of this task may be to present the emotion-inducing video clips immediately before the tracking task to assess the impact of emotion-related memories on respiratory interoceptive accuracy. In addition, our manipulation check did not capture how well people paid attention to the movies. It could be argued that one group paid more attention to the movies than the other, and this difference could have affected performance on the interoceptive accuracy task. Future work could better assess attention to the movie clips, as well as quality of attention, to determine whether quantity or quality of attention to the distraction differs between groups and affects performance on the task.
An alternative interpretation of these results is that once individuals without prior training in mindfulness meditation are instructed to pay attention, they can do so rather easily and accurately; thus, prior attentional training may not confer additional ability to attend to respiratory sensations if people are sufficiently motivated. In our study, we found that the nonmeditators reported more willingness to do this task again compared to meditators. Although groups reported similar amounts of effort, nonmeditators may have enjoyed the task more, which may have affected their performance as some aspects of attention are determined by motivation rather than mindfulness experience (Jensen, Vangkilde, Frokjaer, & Hasselbalch, 2012). Although we did not find strong correlations between task evaluations and performance, future research that manipulates motivation to understand its impact on performance on these tasks is encouraged. In addition, similar to the findings of Khalsa and colleagues (2008), who found that estimated and actual performance on the heartbeat detection task tended to be positively correlated among meditators but not controls, we found a significant positive correlation between estimated and actual performance on the tracking task among meditators but not nonmeditators, consistent with mindfulness theory.
A second alternative interpretation of the results may be that, overall, nonmeditators and meditators have comparable levels of respiratory interoceptive accuracy, but the key variable of interest concerns not interoceptive accuracy per se but the quality of interoceptive awareness; that is, whether interoceptive awareness is evaluative or nonevaluative in nature. Future work would need to devise methods to assess these two forms of awareness. It would be interesting to examine whether nonevaluative awareness of bodily sensations, even if somewhat inaccurate, leads to beneficial effects on emotion regulation and other health-related outcomes.
Interestingly, we did find that greater respiratory interoceptive accuracy was related to less anxiety among meditators but not nonmeditators. A similar pattern was found with the self-report measures of interoceptive awareness. These findings support the notion that the quality of interoceptive awareness (i.e., evaluative or nonevaluative) may have important implications for emotion regulation. They also shed light on contradictory findings in the literature on interoceptive awareness and anxiety, as more anxious individuals have been shown to have greater interoceptive accuracy (Domschke et al., 2010), as assessed by heartbeat perception tasks, and increased neural processing of the perception of possibly affectively charged respiratory sensations than less-anxious individuals (Chan, von Leupoldt, Bradley, Lang, & Davenport, 2012). However, the relationship between interoceptive accuracy and anxiety may depend on whether the awareness is evaluative or nonevaluative in nature (Janssens, Verleden, De Peuter, Van Diest, & Van den Bergh, 2009). The current findings are consistent with the theory of mindfulness, which posits that open, nonevaluative attention to interoceptive sensations decreases anxiety by disengaging negative, repetitive cognitive and emotional processes that perpetuate psychological distress (Farb, Anderson, & Segal, 2012). Future research should examine whether nonevaluative interoceptive awareness and/or accuracy are mechanisms of action by which mind-body interventions reduce anxiety.
Interoceptive awareness is considered to be a critical component of emotional awareness (Zaki et al., 2012). Future research should examine the extent to which respiratory interoceptive accuracy correlates with other modalities of interoceptive accuracy, including cardiac and gastric sensations (Herbert, Muth, Pollatos, & Herbert, 2012), and whether these differentially relate to emotional awareness and regulation. Other areas for future research include assessing interoceptive awareness and accuracy during daily activities and examining whether changes in respiratory interoceptive accuracy in individuals undergoing mindfulness training mediate clinical outcomes.
Limitations
We standardized each individual's tidal volume output to his/her own vital capacity, defined as the largest volume detected during three initial maximal inhalations and exhalations. We did not use any absolute tidal volume measures for which we would have had to calibrate the pneumotachygraph flow measures output against a larger pulmonary function equipment setup. Airway resistance is dependent on flow velocity, and the resistance data provided by the manufacturer for each individual airway resistor are standardized for flow velocity with normal breathing. Only extremely high air flow velocity may have changed the absolute value for the true resistive load. However, this would not substantially influence the participants’ performance on the tasks, as these tasks assessed the relative performance comparing different resistor sizes rather than absolute measures.
Furthermore, the ability to detect a resistive load is related to the resistance of the background condition, namely, the breathing circuit tubing and the participants’ intrinsic lung airway resistance. The magnitude of the background resistance is directly proportional to the detection threshold but not strongly related to the discrimination of detected loads (Davenport et al., 2007). With the equipment used in this study, we were not able to measure the background resistance, which may explain in part why we found lower thresholds in our participants than previous studies.
Second, we abstained from using the nonrebreather two-way valve (Hans Rudolph), which would facilitate resistor presentations limited to inspiration only rather than to an entire breath cycle. The mechanics of the membrane within the two-way valve itself created subtle turbulences, and this additional system-intrinsic resistive load was detectable during pretesting. To avoid confounding the threshold detection, modifications to these valves would need to be discussed with the manufacturer.
Third, the equipment we used for the tracking task included a slider box that worked best when watching the manual movements against the scale next to the slider. This may have required multitasking with visual attention in addition to attention to respiratory sensations. A hand grip may be more appropriate as it can be used without visual attention (Zechman, Wiley, & Davenport, 1981).
Fourth, many analyses were conducted, particularly for the tracking task, and many findings were not statistically significant. However, given the adaptation and early development of these tasks, we feel that the number of analyses conducted was justified to understand the measures and results. After examination of the overall results, we conclude that the findings are not spurious but that a coherent pattern of findings emerged. First, three measures showed that meditators had statistically significant greater respiratory interoceptive accuracy than nonmeditators (resistor 2 of the discrimination task and the nondistracted tracking task at lags 5 and 6). These measures were significantly correlated with one another, and they were not significantly correlated with measures failing to distinguish meditators from nonmeditators. Secondly, these three measures were significantly correlated with self-report measures of interoceptive awareness in the expected direction. No other task measures were significantly related to the self-report measures. Given this conceptually coherent pattern of intercorrelations, the likelihood that each of the significant group differences between meditators and nonmeditators was due to randomness is extremely small. However, given the pilot nature of this study and recommendations for improving the measures, we highly encourage independent replication of these results to draw more firm conclusions.
Finally, we did not include any means of confirming level of meditation experience and therefore cannot be certain whether we recruited an optimal sample of experienced meditators to compare differences with nonmeditators. A meditator's classification as experienced could be improved by confirmation from a third party, such as asking leaders of meditation centers for referrals.
Conclusion
To our knowledge, this is the first study to assess respiratory interoceptive accuracy between meditators and nonmeditators. Overall, although inferences from our results have to be viewed with caution, results of this study provide preliminary support for the hypothesis that mindfulness meditators have greater respiratory interoceptive accuracy compared to nonmeditators, at least under specific task conditions. We provide detailed suggestions for further development and refinement of these tasks for inclusion into future studies of respiratory interoceptive accuracy. Once refined and if findings are replicated, these tasks could be incorporated into studies of mind-body interventions to determine whether respiratory interoceptive accuracy is a mechanism of action for health-related outcomes.
Acknowledgments
We are grateful to David Goldman, Anthony Maes, Derek Ramsey, Kevin Chan, Viranjini Gopisetty, and Elizabeth Bartmess for their work on this project. We thank Cynthia Price, Paul Davenport, David Anderson, and two anonymous reviewers for valuable comments on earlier versions of this manuscript, and Paul Davenport and Paul Grossman for initial consultations on the task design. This research was supported by the Mt. Zion Health Fund; and National Institutes of Health (NIH) grants K01AT004199 awarded to JD, K01AT003459 awarded to CK, and K23-AT002298 awarded to WM from the National Center for Complementary & Alternative Medicine (NCCAM). The content is solely the responsibility of the authors and does not necessarily represent the official views of NCCAM or NIH.
References
- Alberts HJ, Mulkens S, Smeets M, Thewissen R. Coping with food cravings. Investigating the potential of a mindfulness-based intervention. Appetite. 2010;55:160–163. doi: 10.1016/j.appet.2010.05.044. doi: 10.1016/j.appet.2010.05.044. [DOI] [PubMed] [Google Scholar]
- Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. National Health Statistics Report. 2008;12:1–23. [PubMed] [Google Scholar]
- Barrett LD, Gross J. Knowing what you're feeling and knowing what to do about it: Mapping the relation beween emotion differentiation and emotion regulation. Cognition and Emotion. 2001;15:713–724. [Google Scholar]
- Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, Devins G. Mindfulness: A proposed operational definition. Clinical Psychology: Science and Practice. 2004;11:230–241. [Google Scholar]
- Bogaerts K, Millen A, Li W, De Peuter S, Van Diest I, Vlemincx E, Van den Bergh O. High symptom reporters are less interoceptively accurate in a symptom-related context. Journal of Psychosomatic Research. 2008;65:417–424. doi: 10.1016/j.jpsychores.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Bowen S, Chawla N, Collins SE, Witkiewitz K, Hsu S, Grow J, Marlatt A. Mindfulness-based relapse prevention for substance use disorders: A pilot efficacy trial. Substance Abuse. 2009;30:295–305. doi: 10.1080/08897070903250084. doi: 10.1080/08897070903250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg JM, Michalak J. The healthy quality of mindful breathing: Associations with rumination and depression. Cognitive Therapy and Research. 2011;35:179–185. [Google Scholar]
- Cameron OG. Interoception: The inside story—a model for psychosomatic processes. Psychosomatic Medicine. 2001;63:697–710. doi: 10.1097/00006842-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Carmody J, Baer RA. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. Journal of Behavioral Medicine. 2008;31:23–33. doi: 10.1007/s10865-007-9130-7. doi: 10.1007/s10865-007-9130-7. [DOI] [PubMed] [Google Scholar]
- Ceunen E, Van Diest I, Vlaeyen JW. Accuracy and awareness of perception: Related, yet distinct (Commentary on). Biological Psychology. 2013;92:426–427. doi: 10.1016/j.biopsycho.2012.09.012. doi: 10.1016/j.biopsycho.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Chan PY, von Leupoldt A, Bradley MM, Lang PJ, Davenport PW. The effect of anxiety on respiratory sensory gating measured by respiratory-related evoked potentials. Biological Psychology. 2012;91:185–189. doi: 10.1016/j.biopsycho.2012.07.001. doi: 10.1016/j.biopsycho.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Dahme B, Richter R, Mass R. Interoception of respiratory resistance in asthmatic patients. Biological Psychology. 1996;42:215–229. doi: 10.1016/0301-0511(95)05156-2. [DOI] [PubMed] [Google Scholar]
- Damasio A. Feelings of emotion and the self. Annals of the New York Academy of Sciences. 2003;1001:253–261. doi: 10.1196/annals.1279.014. [DOI] [PubMed] [Google Scholar]
- Daubenmier J, Kristeller J, Hecht FM, Maninger N, Kuwata M, Jhaveri K, Epel E. Mindfulness intervention for stress eating to reduce cortisol and abdominal fat among overweight and obese women: An exploratory randomized controlled study. Journal of Obesity. 2011;2011:651936. doi: 10.1155/2011/651936. doi: 10.1155/2011/651936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenmier JJ. The relationship of yoga, body awareness, and body responsiveness to self-objectification and disordered eating. Psychology of Women Quarterly. 2005;29:207–219. [Google Scholar]
- Davenport PW, Chan PY, Zhang W, Chou YL. Detection threshold for inspiratory resistive loads and respiratory-related evoked potentials. Journal of Applied Physiology. 2007;102:276–285. doi: 10.1152/japplphysiol.01436.2005. [DOI] [PubMed] [Google Scholar]
- Deyo M, Wilson KA, Ong J, Koopman C. Mindfulness and rumination: Does mindfulness training lead to reductions in the rumi-native thinking associated with depression? Explore New York. 2009;5:265–271. doi: 10.1016/j.explore.2009.06.005. doi: 10.1016/j.explore.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Domschke K, Stevens S, Pfleiderer B, Gerlach AL. Interoceptive sensitivity in anxiety and anxiety disorders: An overview and integration of neurobiological findings. Clinical Psychology Review. 2010;30:1–11. doi: 10.1016/j.cpr.2009.08.008. doi: 10.1016/j.cpr.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Ehring T, Watkins E, R. Repetitive negative thinking as a transdiagnostic process. International Journal of Cognitive Therapy. 2008;1:192–205. [Google Scholar]
- Farb NA, Anderson AK, Mayberg H, Bean J, McKeon D, Segal ZV. Minding one's emotions: Mindfulness training alters the neural expression of sadness. Emotion. 2010;10:25–33. doi: 10.1037/a0017151. doi: 10.1037/a0017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Anderson AK, Segal ZV. The mindful brain and emotion regulation in mood disorders. Canadian Journal of Psychiatry. 2012;57:70–77. doi: 10.1177/070674371205700203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Anderson AK. Attentional modulation of primary interoceptive and exteroceptive cortices. Cerebral Cortex. 2013a;23:114–126. doi: 10.1093/cercor/bhr385. doi: 10.1093/cercor/bhr385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Anderson AK. Mindfulness meditation training alters cortical representations of interoceptive attention. Social Cognitive & Affective Neuroscience. 2013b;8:15–26. doi: 10.1093/scan/nss066. doi: 10.1093/scan/nss066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, Anderson AK. Attending to the present: Mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive & Affective Neuroscience. 2007;2:313–322. doi: 10.1093/scan/nsm030. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassino S, Piero A, Gramaglia C, Abbate-Daga G. Clinical, psychopathological and personality correlates of interoceptive awareness in anorexia nervosa, bulimia nervosa and obesity. Psychopathology. 2004;37:168–174. doi: 10.1159/000079420. doi: 10.1159/000079420. [DOI] [PubMed] [Google Scholar]
- Flor H. New developments in the understanding and management of persistent pain. Current Opinion in Psychiatry. 2012;25:109–113. doi: 10.1097/YCO.0b013e3283503510. doi: 10.1097/YCO.0b013e3283503510. [DOI] [PubMed] [Google Scholar]
- Fustos J, Gramann K, Herbert BM, Pollatos O. On the embodiment of emotion regulation: Interoceptive awareness facilitates reappraisal. Social Cognitive & Affective Neuroscience. Advance online publication. 2012 doi: 10.1093/scan/nss089. doi: 10.1093/scan/nss089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard T, Holzel BK, Sack AT, Hempel H, Lazar SW, Vaitl D, Ott U. Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cerebral Cortex. 2012;22:2692–2702. doi: 10.1093/cercor/bhr352. doi: 10.1093/cercor/bhr352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. Journal of Psychosomatic Research. 2004;57:35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- Herbert BM, Muth ER, Pollatos O, Herbert C. Interoception across modalities: On the relationship between cardiac awareness and the sensitivity for gastric functions. PLoS ONE. 2012;7:e36646. doi: 10.1371/journal.pone.0036646. doi: 10.1371/journal.pone.0036646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78:169–183. doi: 10.1037/a0018555. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Vangel M, Congleton C, Yerramsetti SM, Gard T, Lazar SW. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Research. 2011;191:36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Ott U, Gard T, Hempel H, Weygandt M, Morgen K, Vaitl D. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Social Cognitive & Affective Neuro-science. 2008;3:55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. What is an emotion? Mind. 1884;9:185–205. [Google Scholar]
- Janssens T, Verleden G, De Peuter S, Van Diest I, Van den Bergh O. Inaccurate perception of asthma symptoms: A cognitive-affective framework and implications for asthma treatment. Clinical Psychology Review. 2009;29:317–327. doi: 10.1016/j.cpr.2009.02.006. doi: 10.1016/j.cpr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Jensen CG, Vangkilde S, Frokjaer V, Hasselbalch SG. Mindfulness training affects attention—or is it attentional effort? Journal of Experimental Psychology: General. 2012;141:106–123. doi: 10.1037/a0024931. doi: 10.1037/a0024931. [DOI] [PubMed] [Google Scholar]
- Keng SL, Smoski MJ, Robins CJ. Effects of mindfulness on psychological health: A review of empirical studies. Clinical Psychology Review. 2011;31:1041–1056. doi: 10.1016/j.cpr.2011.04.006. doi: 10.1016/j.cpr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr CE, Shaw JR, Wasserman RH, Chen VW, Kanojia A, Bayer T, Kelley JM. Tactile acuity in experienced Tai Chi practitioners: Evidence for use dependent plasticity as an effect of sensory-attentional training. Experimental Brain Research. 2008;188:317–322. doi: 10.1007/s00221-008-1409-6. doi: 10.1007/s00221-008-1409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Damasio AR, Davidson RJ, Lutz A, Tranel D. Interoceptive awareness in experienced meditators. Psychophysiology. 2008;45:671–677. doi: 10.1111/j.1469-8986.2008.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Sandesara C, Olshansky B, Tranel D. Bolus isoproterenol infusions provide a reliable method for assessing interoceptive awareness. International Journal of Psycho-physiology. 2009;72:34–45. doi: 10.1016/j.ijpsycho.2008.08.010. doi: 10.1016/j.ijpsycho.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kifle Y, Seng V, Davenport PW. Magnitude estimation of inspiratory resistive loads in children with life-threatening asthma. American Journal of Respiratory Critical Care Medicine. 1997;156:1530–1535. doi: 10.1164/ajrccm.156.5.9703011. [DOI] [PubMed] [Google Scholar]
- Knapp-Kline K, Kline JP. Heart rate, heart rate variability, and heartbeat detection with the method of constant stimuli: Slow and steady wins the race. Biological Psychology. 2005;69:387–396. doi: 10.1016/j.biopsycho.2004.09.002. doi: 10.1016/ j.biopsycho.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, Fischl B. Meditation experience is associated with increased cortical thickness. NeuroReport. 2005;16:1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: Replication and exploration of differential relapse prevention effects. Journal of Consulting and Clinical Psychology. 2004;72:31–40. doi: 10.1037/0022-006X.72.1.31. doi: 10.1037/0022-006X.72.1.31. [DOI] [PubMed] [Google Scholar]
- MacLean KA, Ferrer E, Aichele SR, Bridwell DA, Zanesco AP, Jacobs TL, Saron CD. Intensive meditation training improves perceptual discrimination and sustained attention. Psychological Science. 2010;21:829–839. doi: 10.1177/0956797610371339. doi: 10.1177/0956797610371339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Nolen-Hoeksema S. Rumination as a transdiagnostic factor in depression and anxiety. Behavioral Research Therapy. 2011;49:186–193. doi: 10.1016/j.brat.2010.12.006. doi: 10.1016/j.brat.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehling WE, Price C, Daubenmier JJ, Acree M, Bartmess E, Stewart A. The Multidimensional Assessment of Interoceptive Awareness (MAIA). PLoS ONE. 2012;7:e48230. doi: 10.1371/journal.pone.0048230. doi: 10.1371/journal. pone.0048230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirams L, Poliakoff E, Brown RJ, Lloyd DM. Brief body-scan meditation practice improves somatosensory perceptual decision making. Conscious Cognition. 2013;22:348–350. doi: 10.1016/j.concog.2012.07.009. doi: 10.1016/ j.concog.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Mirams L, Poliakoff E, Brown RJ, Lloyd DM. Interoceptive and exteroceptive attention have opposite effects on subsequent somatosensory perceptual decision making. Quarterly Journal of Experimental Psychology (Hove) 2012;65:926–938. doi: 10.1080/17470218.2011.636823. doi: 10.1080/17470218.2011.636823. [DOI] [PubMed] [Google Scholar]
- Niedenthal PM. Embodying emotion. Science. 2007;316:1002–1005. doi: 10.1126/science.1136930. doi: 10.1126/science.1136930. [DOI] [PubMed] [Google Scholar]
- Nielsen L, Kaszniak AW. Awareness of subtle emotional feelings: A comparison of long-term meditators and nonmeditators. Emotion. 2006;6:392–405. doi: 10.1037/1528-3542.6.3.392. doi: 10.1037/1528-3542.6.3.392. [DOI] [PubMed] [Google Scholar]
- Nyklicek I, Kuijpers KF. Effects of mindfulness-based stress reduction intervention on psychological well-being and quality of life: Is increased mindfulness indeed the mechanism? Annals of Behavioral Medicine. 2008;35:331–340. doi: 10.1007/s12160-008-9030-2. doi: 10.1007/s12160-008-9030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin L, Morgan R, Rosselli A, Howard M, Sheppard A, Evans D, Dunn B. Mindfulness, 3. Advance online publication; 2013. Exploring the relationship between mindfulness and cardiac perception. doi: 10.1007/s12671-012-0181-7. [Google Scholar]
- Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Structure and Function. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. doi: 10.1007/ s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. The role of fMRI in cognitive neuroscience: Where do we stand? Current Opinion in Neurobiology. 2008;18:223–227. doi: 10.1016/j.conb.2008.07.006. doi: 10.1016/j.conb.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Traut-Mattausch E, Schroeder H, Schandry R. Interoceptive awareness mediates the relationship between anxiety and the intensity of unpleasant feelings. Journal of Anxiety Disorders. 2007;21:931–943. doi: 10.1016/j.janxdis.2006.12.004. doi: 10.1016/j.janxdis.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Price CJ, Thompson EA. Measuring dimensions of body connection: Body awareness and bodily dissociation. Journal of Alternative and Complementary Medicine. 2007;13:945–953. doi: 10.1089/acm.2007.0537. doi: 10.1089/acm.2007.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvia PJ, Gendolla GHE. On interospection and self-perception: Does self-focused attention enable accurate self-knowledge? Review of General Psychology. 2001;5:241–269. [Google Scholar]
- Slagter HA, Lutz A, Greischar LL, Francis AD, Nieuwenhuis S, Davis JM, Davidson RJ. Mental training affects distribution of limited brain resources. PLoS Biology. 2007;5:e138. doi: 10.1371/journal.pbio.0050138. doi: 10.1371/journal.pbio.0050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Corsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. CA: Consulting Psychologists Press; Palo Alto: 1970. [Google Scholar]
- Terasawa Y, Fukushima H, Umeda S. How does interoceptive awareness interact with the subjective experience of emotion? An fMRI study. Human Brain Mapping. 2013;34:598–612. doi: 10.1002/hbm.21458. doi: 10.1002/hbm.21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: A critical review. Neuroscience & Biobehavioral Reviews. 2012;36:1857–1869. doi: 10.1016/j.neubiorev.2012.05.007. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Villien F, Yu M, Barthelemy P, Jammes Y. Training to yoga respiration selectively increases respiratory sensation in healthy man. Respiratory Physiology and Neurobiology. 2005;146:85–96. doi: 10.1016/j.resp.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Webster KE, Colrain IM. The relationship between respiratory-related evoked potentials and the perception of inspiratory resistive loads. Psychophysiology. 2000;37:831–841. [PubMed] [Google Scholar]
- Westbrook C, Creswell JD, Tabibnia G, Julson E, Kober H, Tindle HA. Mindful attention reduces neural and self-reported cue-induced craving in smokers. Social Cognitive and Affective Neuro-science. 2013;8:73–84. doi: 10.1093/scan/nsr076. doi: 10.1093/scan/nsr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Western PJ, Patrick JM. Effects of focusing attention on breathing with and without apparatus on the face. Respiration Physiology. 1988;72:123–130. doi: 10.1016/0034-5687(88)90084-9. [DOI] [PubMed] [Google Scholar]
- Wiens S. Interoception in emotional experience. Current Opinions in Neurology. 2005;18:442–447. doi: 10.1097/01.wco.0000168079.92106.99. [DOI] [PubMed] [Google Scholar]
- Williams JM. Mindfulness and psychological process. Emotion. 2010;10:1–7. doi: 10.1037/a0018360. doi: 10.1037/a0018360. [DOI] [PubMed] [Google Scholar]
- Zaki J, Davis JI, Ochsner KN. Overlapping activity in anterior insula during interoception and emotional experience. NeuroImage. 2012;62:493–499. doi: 10.1016/j.neuroimage.2012.05.012. doi: 10.1016/j.neuroimage.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechman F, Hall FG, Hull WE. Effects of graded resistance to tracheal air flow in man. Journal of Applied Physiology. 1957;10:356–362. doi: 10.1152/jappl.1957.10.3.356. [DOI] [PubMed] [Google Scholar]
- Zechman FW, Davenport PW. Temporal differences in the detection of resistive and elastic loads to breathing. Respiration Physiology. 1978;34:267–277. doi: 10.1016/0034-5687(78)90033-6. [DOI] [PubMed] [Google Scholar]
- Zechman FW, Wiley RL, Davenport PW. Ability of healthy men to discriminate between added inspiratory resistive and elastic loads. Respiration Physiology. 1981;45:111–120. doi: 10.1016/0034-5687(81)90053-0. [DOI] [PubMed] [Google Scholar]
- Zhao W, Martin AD, Davenport PW. Detection of inspira-tory resistive loads in double-lung transplant recipients. Journal of Applied Physiology. 2002;93:1779–1785. doi: 10.1152/japplphysiol.00210.2002. doi: 10.1152/japplphysiol.00210.2002. [DOI] [PubMed] [Google Scholar]