Abstract
Aim
We hypothesized that platelet inactivation induced by drugs might interfere with periodontal repair in experimental periodontitis by suppressing the release of biological mediators from platelets at the site of injury.
Material and Methods
60 rats were randomly assigned to 6 groups (n=10) and ligatures were placed around lower first molars of three groups. The other three groups were used as negative controls. Ligatures were removed after 10 days of periodontitis induction and all groups were submitted to treatment with aspirin (Asp) (30 mg/kg), clopidogrel (Clop) (75 mg/kg) or NaCl 0.9% intragastrically once daily for 3 days. Periodontal tissue was assessed by the measurement of CXCL12, CXCL4, CCL5 and PDGF by ELISA; histomorphometric analysis of PMN infiltration, attachment loss, bone loss and osteoclast numbers and quantification of blood vessels by imunnohistochemistry.
Results
During periodontal repair and treatment with NaCl 0.9%, CCL5 was decreased and CXCL12 increased when compared to negative control groups. Asp and Clop did not affect CCL5 expression, decreased CXCL12 but only Clop decreased CXCL4 and PDGF content compared to saline-treated animals. Clop increased blood vessel number, reduced PMN count, and decreased attachment and bone loss, also decreased osteoclast number in animals submitted or not to periodontal repair.
Conclusion
Systemic administration of Clop during 3 days improved the repair process associated with experimental periodontal disease, suggesting that it may have therapeutic value under situations where tissues undergo a transition from inflammation to repair.
Keywords: clopidogrel, periodontal repair, inflammation, innate immunity, chemokines
INTRODUCTION
Platelet degranulation releases a plethora of biological mediators, such as chemokines, cytokines and growth factors, such as platelet derived growth factor (PDGF), that have a role in the inflammatory process and tissue regeneration. An alteration in the amount and function of platelets affects thrombotic and inflammatory events that contribute to cardiovascular diseases such as atherosclerosis (von Hundelshausen et al, 2007). C-X-C motif ligand 4 (CXCL4 or Platelet Factor 4), C-X-C motif chemokine 12 (CXCL12) and C-C motif ligand 5 (CCL5 or Regulated on Activation, Normal T cell Expressed and Secreted - RANTES) are platelet chemokines released upon activation. CXCL12 is chemotactic for lymphocytes. CXCL4, also known as platelet factor 4 promotes blood coagulation and is chemotactic for neutrophils and fibroblasts, while CCL5 is chemotactic for T cells and plays an active role in recruiting leukocytes into inflammatory sites. They act in the onset of tissue repair and establish a pro-inflammatory environment that culminates in the recruitment and activation of a variety of immune cells (Flad & Brandt, 2010).
In the context of periodontal regeneration, we have previously demonstrated that a pronounced reduction of circulating platelets (thrombocytopenia) leads to a delay in periodontal healing which is associated, at least in part, with decreased serum concentrations of platelet-derived VEGF and endostatin (Spolidorio et al, 2010). Furthermore, platelet inactivation by systemic administration of aspirin and clopidogrel attenuates the amount of destruction that occurs with periodontitis by reducing the concentration of the inflammatory mediators Interleukin-6 (IL-6), Tumor Necrosis Factor-alpha (TNF-alpha) and Thromboxane A2 (TXA2); reducing the inflammatory infiltrate and increasing the amount of collagen fibers (Coimbra et al, 2011). Moreover, platelet rich plasma (PRP) has been shown to enhance regenerative treatment (Lekovic et al, 2012; Suaid et al, 2012; Dominiak et al, 2012).
Antiplatelet treatments include aspirin and thienopyridines (such as clopidogrel and dipyridamole), abciximab or eptifibatide (Schomig et al, 1997; Bennett, 2001). Aspirin irreversibly inactivates cyclooxygenase-1 (COX-1) activity and subsequent synthesis of TXA-2. It also modifies the COX-2 enzymatic activity, induces lipoxin production that facilitates the resolution of inflammation and inhibits prostanoid production that is generally pro-inflammatory (Warner & Mitchell, 2002). Clopidogrel acts by irreversible antagonism of platelet P2Y12 receptors expressed in the platelet membrane by inhibiting binding of the endogenous ligand, adenosine diphosphate (ADP) (Bennett, 2001; Gao et al, 2009).
Because platelets contain an array of cytokines and growth factors they modulate inflammatory and repair processes. In this study, we evaluated the effects of aspirin and clopidogrel, the two most frequently prescribed antiplatelet drugs, on the transition from periodontal inflammation to periodontal repair by the measurement of growth factors and chemokines that acts in the onset of tissue repair CXCL12, CXCL4, CCL5 and PDGF, histomorphometric analysis of PMN infiltration, attachment loss, bone loss and osteoclast numbers and quantification of blood vessels by imunnohistochemistry.
MATERIAL AND METHODS
Animals
Sixty male adult Holtzman rats (Rattus norvergicus albinus) weighing 250-300 g were housed under similar conditions in cages with access to food and water ad libitum. During the whole experimental protocol, the rats were kept in a quiet room with controlled temperature (22±1°C), humidity (65-75%) and a 12-h light-dark cycle. All experimental protocols were approved by the local Ethics Committee for Animal Experimentation and performed in accordance with the guidelines of the Brazilian College for Animal Experimentation (COBEA).
Chemicals
All drugs and reagents were purchased from Sigma Aldrich (Brazil), unless otherwise stated. Ketamine and xylazine chloride (Francotar® and Virbaxil® respectively, both from Virbac do Brasil Ind. Com. Ltda, São Paulo, SP, Brazil) were used for dissociative anesthesia of the animals.
Evaluation of antiplatelet drug treatment on periodontal repair
Rats were randomly distributed into 6 groups (10 animals/group) as follows: vehicle-treated: ‘NaCl’, Aspirin-treated: ‘Asp’, Clopidogrel-treated: ‘Clop’, Repair (Rep)+vehicle-treated: ‘Rep+NaCl’, Repair+Aspirin-treated: ‘Rep+Asp’, Repair+Clopidogrel-treated: ‘Rep+Clop’. The animals in the groups subjected to experimental periodontal repair were anesthetized with 1 mg/kg body weight of ketamine and 0.4 mL/kg of xylazine for the induction of experimental periodontal disease by the insertion of a 3.0 cotton ligature in a submarginal position on the lower right and left first molars of each rat, as previously described (Holzhausen et al, 2002). After 10 days, ligatures were removed to allow the spontaneous repair of periodontal tissues over the subsequent 3 days. During these three days, animals were treated with intragastrical daily doses of aspirin (Asp; 30 mg/kg) or clopidogrel (Clop; 75 mg/kg). Control rats were treated with the same volume of vehicle (NaCl 0.9%). These doses have been previously shown to cause effective inhibition of platelet aggregation and thrombus formation in rats (Coimbra et al, 2011; Ma et al, 2001; Sasaki et al, 1996; Taka et al, 1999; Wallace et al, 1995). The animals from the other three groups were used as negative controls, had no ligature-induced periodontitis and were treated with Asp, Clop and NaCl 0.9% as described above.
Harvesting and processing of samples
At the end of the 13-day experimental period, animals were killed by anesthesia overdose and the mandibles were carefully removed and dissected. Gingivo-mucosal tissues from the right mandibular first molar of all animals were removed and total proteins extracted from gingival tissue samples using a detergent-based extraction buffer (T-PER, Tissue Protein Extraction Reagent; Pierce Biotechnology) containing a protease inhibitor cocktail (Protein Stabilizing Cocktail; Santa Cruz Biotechnology) according to manufacturer’s instructions (Pierce Biotechnology). The proteins were quantified using Lowry method (DC assay, Bio-Rad Laboratories) and used for the analysis of PDGF, CXCL12, CCL5 and CXCL4 contents by enzyme-linked immunosorbent assay (ELISA) from Life Technologies (Carlsbad, CA, USA), according to the manufacturer’s instructions.
Histomorphometric analysis
Subsequently, left hemi-mandibles were fixed in 4% paraformaldehyde at 4°C for 48h and decalcified in 4.13% EDTA solution (pH 7.2) at room temperature for approximately 3 months. Serial paraffin sections 5 μm thick were obtained from buccal–lingual aspects of the whole left first molars and the samples of 5 animals subsequently stained with hematoxylin and eosin (H&E) for histological/histometric evaluation. Bone loss was measured as the distance between the cemento-enamel-junction (CEJ) and the highest peak from the top of the alveolar bone crest (ABC). Attachment loss was measured by the distance between the CEJ and the most coronal extent of connective tissue attachment to cementum and the the bone area per total area (percent bone) by measuring the amount of bone in an area bounded occlusally by the cemento-enamel junction to a distance 1mm apical and by the root surfaces on the mesial and distal. This space included the gingiva, periodontal ligament and alveolar bone. Bone loss is represented by the relative decrease on the area occupied by bone, as described in Liu et al, 2006. The number of polymorphonuclear (PMNs) leukocytes was counted in the connective tissue 0.5 mm above the CEJ. 5 randomly chosen sections of each animal in the total of 5 animals per group were examined at 200X magnification. A blinded examiner who did not know the group to which an animal belonged analyzed all data.
Immunohistochemistry to identify osteoclasts and blood vessels
The sections from the other 5 animals were used to immunohistochemistry. An anti-tartrate-resistant acid phosphatase primary antibody (Santa Cruz Biotechnology, 1:100) was used to identify multinucleated osteoclasts adjacent to bone. In separate sections an antibody specific for Factor VIII (Abcam, Cambridge, MA) diluted 1:100 was used to identify blood vessels. Primary antibody was localized by a biotinylated secondary antibody followed by avidin-biotin complex staining (Burlingame, CA, USA). For blood vessels tyramide signal amplification was used to enhance the chromogenic signal (PerkinElmer, Waltham, MA, USA). The number of TRAP+ cells was counted as the multinucleated cells adjacent from the alveolar bone crest to an apical distance 1 mm below the CEJ and expressed as the number per mm bone length. Blood vessels were counted in the connective tissue between the CEJ-ABC. For both analyses, 5 sections were examined in 5 randomly selected fields at 600X magnification.
Statistical analysis
All data were expressed as mean ± SD. Differences among the groups were analyzed by pairwise comparisons (Student t-test). P values less than 5% were considered statistically significant.
RESULTS
All animals were alive at the end of the experimental period (13 days), and the recorded body weights were 283.7±1.8, 292.0±2.1, 275.0±1.5, 258.0±4.1, 261.0±2.3 and 277.0±2.6 g for NaCl, Asp, Clop, Rep+NaCl, Rep+Asp and Rep+Clop groups, respectively, without significant difference. Neither occult bleeding nor hematoma after gingival intervention was observed.
ELISA analysis
After induction of periodontitis and repair there was no difference in CXCL4 levels compared to negative control groups. However, treatment with Clop significantly reduced the expression of CXCL4 by 60% to 70% with (P<0.001) or without (P<0.001) the induction of periodontitis. Asp induced a 50% decrease (P<0.0001) only after the induction of periodontitis and repair (Figure 1B).
FIGURE 1.
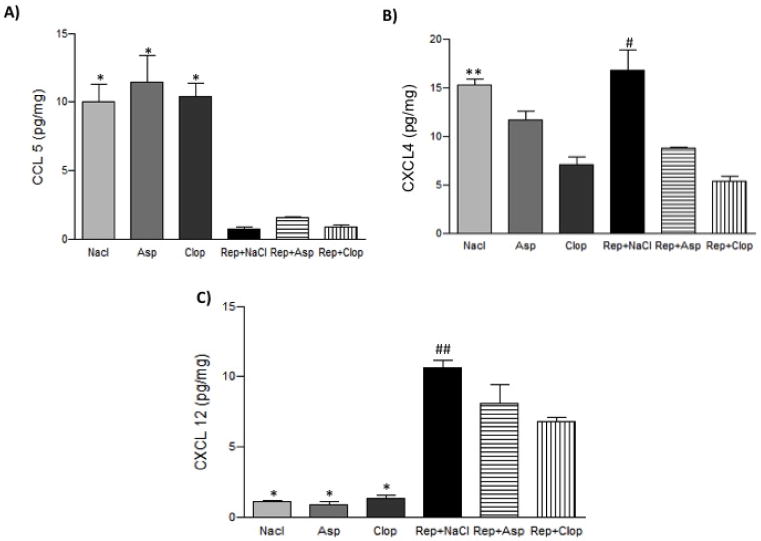
Effect of systemic treatment with Asp or Clop on the gingival contents of CCL5 (panel A), CXCL4 (panel B), CXCL12 (panel C) in rats with ligature induced periodontal disease when stimulus is removed. Results were normalized to the total protein contents in each sample and expressed as pg/mg of protein. Values are expressed as mean±SD. * P<0.0001 vs Repair (Rep)+ NaCl, Rep+ Asp, Rep+ Clop; **P<0.001 vs Clop; #P<0.0001 vs Rep+ Asp, Rep+ Clop; ##P<0.001 vs Rep+ Asp and Rep+ Clop.
The gingival level of CCL5 was significantly decreased by 80% (P<0.0001) and CXCL12 increased by 75% (p<0.0001) in rats subjected to the induction of periodontitis and repair compared to negative controls. During repair phase, treatment with Asp induced a 28% and Clop a 37% decrease (P<0.001) in CXCL12 expression compared with the saline-treated animals (Figure 1A and C) and did not affect CCL5 expression.
PDGF-AB expression was stimulated approximately 10 fold by induction of periodontitis and repair (P<0.001) compared to negative control groups. During periodontal repair, treatment with Clop but not Asp had a 75% reduction in PDGF-AB expression compared to saline-treated animals (Figure 2).
FIGURE 2.
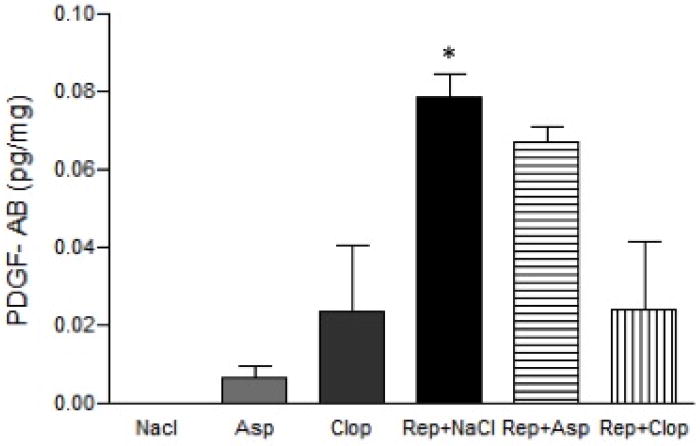
Effect of systemic treatment with Asp or Clop on the gingival contents of PDGF in rats with ligature induced periodontal disease when stimulus is removed. Results were normalized to the total protein contents in each sample and expressed as pg/mg of protein. Values are expressed as mean±SD. *P<0.001 vs Rep+ Clop.
HISTOMORPHOMETRIC analysis
To evaluate the role of antiplatelet drugs on the repair process, ligatures were removed after 10 days in the animals of three experimental groups and they were submitted to treatments with Asp, Clop and vehicle as described above.
Administration of Clop during the period of repair appears to have reduced inflammation and improved the healing process. Clop-treated animals showed a reduced cellularity (polymorphonuclear leukocytes (PMN) infiltrate by 62% (P <0.001), reduced attachment loss by 45% (P <0.001) and reduced loss of periodontal bone height (CEJ-ABC distance) by 25% (P <0.001); therefore an augmented bone percentage when compared to saline-treated animals (Figure 3 and 4 A, B, C and D).
FIGURE 3.
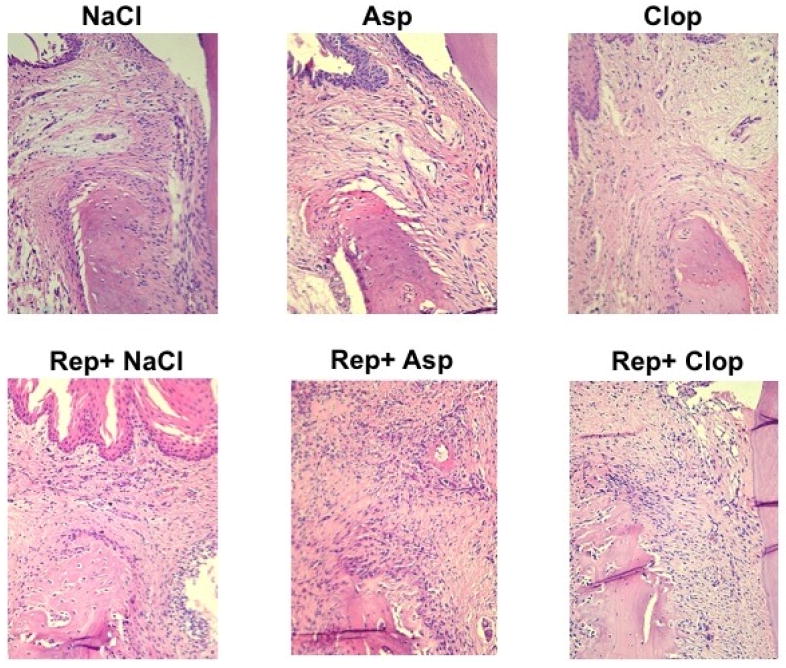
Histological analysis of the alveolar bone crest/gingival margin region of right lower first molar stained with hematoxylin and eosin (H&E). Images are representative of the results observed in 5 animals in each group. 200X magnification.
FIGURE 4.
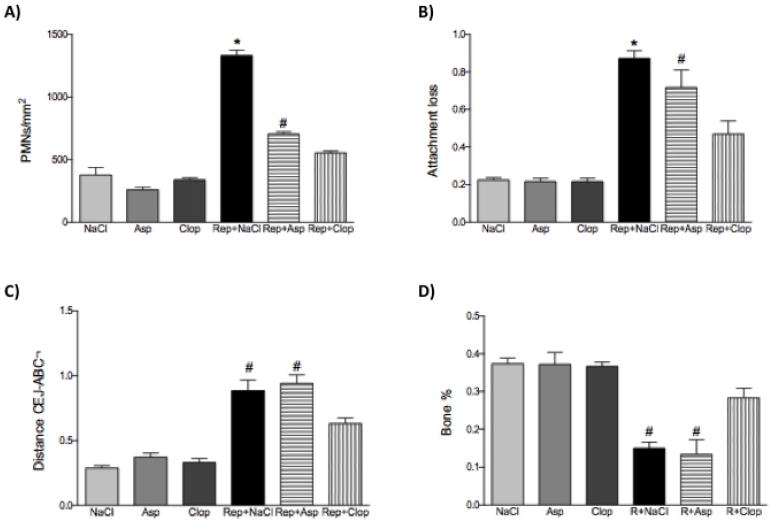
Effect of systemic treatment with Asp or Clop on the PMN count (panel A); attachment loss (panel B); alveolar bone loss (panel C) and bone percentage (panel D) in animals submitted or not to experimental periodontal disease. The results are representative from 5 animals/group. Values are expressed as mean±SD. *P<0.0001 vs Rep+Clop; #P<0.001 vs Rep+Clop.
Osteoclast numbers
To establish how antiplatelet therapy may affect bone resorption osteoclast numbers were measured. The number of osteoclasts per millimeter of bone length was increased 3 fold by induction of periodontitis and repair. Treatment of the experimental animals with Clop completely blocked this increase (P<0.0001) and also decreased levels of osteoclasts (P<0.001) in negative control group. In contrast Asp had no effect (P>0.05) (Figure 5 and 6).
FIGURE 5.
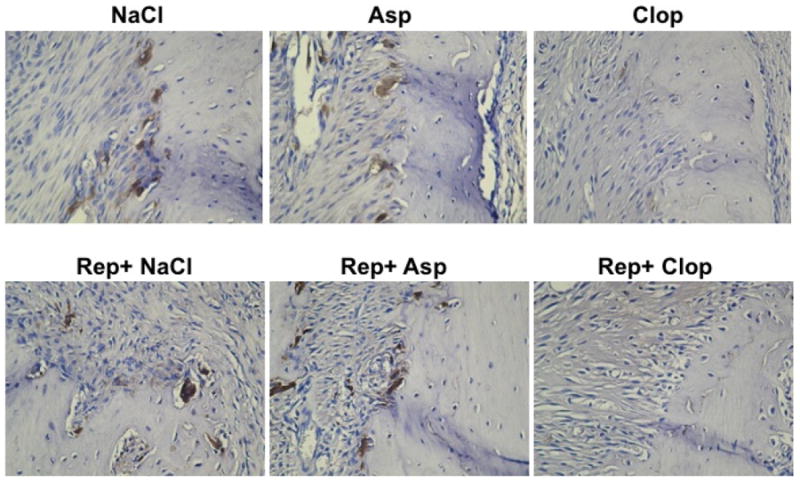
Immunohistochemistry sections of the alveolar bone crest of right lower first molar of rats stained with anti-tartrate-resistant acid phosphatase (TRAP) to measure osteoclast number. Images are representative of the results observed in 5 animals in each group. 200X magnification.
FIGURE 6.
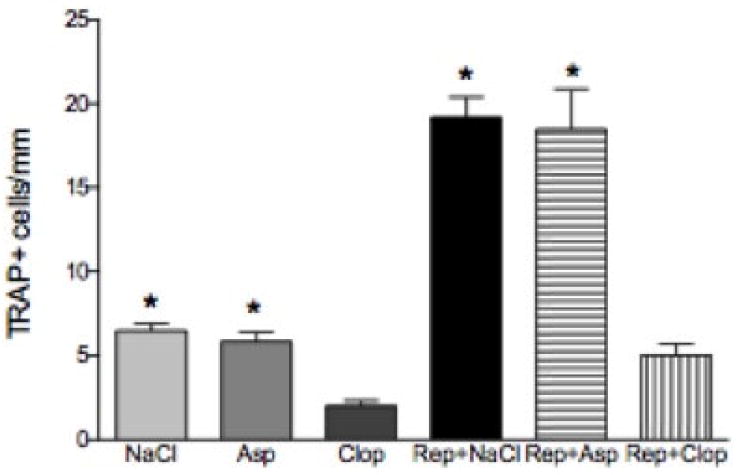
Effect of systemic treatment with Asp or Clop on the osteoclast number in rats with ligature induced periodontal disease when stimulus is removed. Values are expressed as mean±SD. *P<0.0001 vs Clop, Rep+Clop.
Blood Vessels
Treatment with Clop during periodontal repair increased the number of blood vessels by 20% (p<0.0001) while Asp had no effect (P>0.05) compared to saline treated experimental animals (Figure 7).
FIGURE 7.
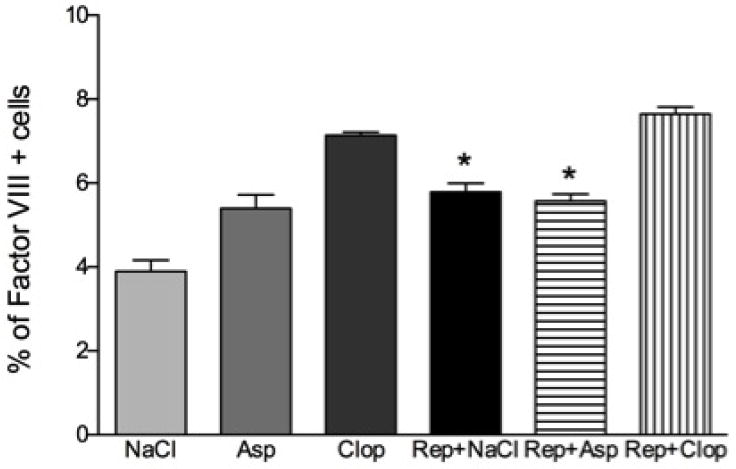
Effect of systemic treatment with Asp or Clop on the number of blood vessels in rats submitted or not to experimental periodontal repair. The results are representative from 5 animals/group. Values are expressed as mean±SD. *P<0.0001 vs Rep+Clop.
DISCUSSION
In our experimental model, removal of the disease-causing stimulus (ligature) initiates a process that involves resolution of inflammation and a transition to repair. During the proliferative phase of repair, a plethora of cells undergo dynamic phenotypic alterations that can activate the inflammasome secreting cytokines and chemokines directly involved in the inflammatory process. Some conditions such as diabetes, impairs the resolution of inflammation and may affect the transition to repair. According to Pacios et al (2012) diabetes prolongs inflammation and osteoclastogenesis in periodontitis and limits the normal reparative process by negatively modulating factors that regulate bone. On the other hand, Wada et al (2013) showed that a bone graft treatment with sustained release of salicylic acid enhances bone regeneration in diabetic rats and accelerates bone regeneration in normoglycemic animals.
In the context of periodontal repair, previously we have shown a net effect of inflammation for regeneration after 15 days, without differences between the antiinflammatory effect of aspirin and clopidogrel (Coimbra, 2011). In the early transition phase analysed in the present study, there is an increased CXCL4, CXCL12, PDGF and higher levels of PMN infiltration and osteoclasts compared to negative control animals that did not have periodontitis. Administration of clopidogrel significantly decreased levels of chemokines CXCL4 and CXCL12, PMN infiltration and osteoclast numbers suggesting that clopidogrel substantially reduced the level of inflammation during the transition phase. This decrease in inflammation is thought to be important in subsequent steps that involve repair. This is supported by an increase in the number of blood vessels observed with clopidogrel and a decrease in the loss of connective tissue attachment. Administration of aspirin had similar, but less marked effects on the production of platelet-derived chemokines and production of PDGF was barely attenuated. Also, aspirin did not attenuate loss of connective attachment and bone resorption. The latter is supported by the lack of effect on osteoclast numbers in the animals treated with aspirin. Interestingly, administration of aspirin was not as effective as clopidogrel in reducing inflammation as noted by a reduced effect on PMN infiltration or osteoclast numbers and was less effective in promoting the transition to repair as noted by relatively little effect on the number of blood vessels or connective tissue attachment levels.
The mechanisms by which clopidogrel improves the transition from inflammation to repair has not yet been fully elucidated. Our data suggests that modulation of the disease-associated inflammatory process in this early/transition phase is part of the mechanism. The differences between the results of clopidogrel- and aspirin-treated animals may reflect different biological mechanisms and potencies of these drugs. Clopidogrel is a thyenopiridine that acts by irreversibly binding to P2Y12 receptor. This binding diminishes the release of alpha and dense granules from platelets (Garcia et al, 2011) and also inhibits beta3 integrin engagement, attenuating the synthesis of IL-1beta induced upon platelet stimulation (Lindemann et al, 2001; Su et al, 2012). In the majority of studies Clopidogrel inhibits expression of inflammatory markers and reduces leukocyte activation (Ayral et al, 2007; Evangelista et al, 2005). Moreover, clopidogrel inhibits the progression of atherosclerotic lesions by reducing levels of inflammatory factors (Li et al, 2007). On the other hand, in a rat experimental arthritis model, treatment with clopidogrel had a pro-inflammatory effect measured by clinical manifestations of inflammation, elevated neutrophil blood count and the plasma levels of pro-inflammatory cytokines (Garcia et al, 2011). Aspirin inhibits the activity of cyclooxygenases and promotes the expression of lipoxins and resolvins (Chiang & Serhan, 2006). In addition to inhibiting platelets aspirin works through a number of different pathways that can reduce inflammation (Dovizio et al, 2013; Berk et al, 2013). Aspirin has also been shown to reduce PMN infiltration related to inflammation (Gil-Villa et al, 2012).
We have used a well-established model of experimental periodontal disease that presents histological and immunological characteristics similar to those of periodontal disease in humans (Holzhausen, 2005; Guimarães, 2007; Nassar, 2009; Spolidorio, 2010). The removal of the pathogenic stimulus represented by the ligatures may be considered similar to periodontal disease treatment by the removal of dental biofilm and calculus, allowing the evaluation of the repair process. In the periodontal repair process established 3 days after the removal of the stimulus, it is expected to see a slightly decrease in the inflammatory process characterized by a decrease in polymorphonuclear leucocytes migration to the site of injury. It is possible that a platelet-derived CXCL4 and CCL5 signaling mechanism is involved in this recruitment. In fact, CXCL4 is a potent inhibitor of angiogenesis and, together with CCL5, participates in the recruitment of polymorphonuclear leukocytes and monocytes into the blood vessel wall (Flad & Brandt, 2010). Our results indicate that both clopidogrel and aspirin reduced production of chemokines CXCL4 and CCL5; this result was somewhat unexpected given the prominent role of CCL5 in the inflammatory process supported by the literature. Offenbacher (2009) and Thunell (2010) showed high levels of CCL5 in periodontal disease in humans. Also, Gamonal (2000) demonstrated a marked CCL5 reduction in gingival crevicular fluid, after periodontal treatment in humans. The differences in the main source of both chemokines can partially explain our results. While platelets are not the main source of CCL5 (it can also be produced by other cell types in the gingival tissues, such as epithelial cells, fibroblasts and macrophages), CXCL4 is only released from alpha-granules of activated platelets. Our research group has previously reported an anti-inflammatory effect associated with systemic administration of aspirin and clopidogrel in a rat ligature-induced periodontal disease model, which was characterized by a reduction in the influx of inflammatory cells and consistent decrease in the production of proinflammatory cytokines (IL-6, TNF-alpha and TXA-2), even thought it did not affect periodontal repair, 15 days after the removal of the pathogenic stimulus (Coimbra, 2011). In the present study we have used a 3-day experimental time point. The major point to be considered regarding the differences in the results observed would be that growth factors and other biological mediators secreted by platelets with a prominent role in the repair process have a major importance in the beginning of the pathophysiology of the repair process
There was a marked reduction in osteoclast numbers in the tissues of clopidogrel-treated animals, which may be a direct effect of this drug on osteoclastogenesis or an indirect effect related with the modulation of the cytokine network and the pro- and anti-osteoclastic factors. P2RY2 receptors, which are inhibited by clopidogrel play a role in pathologic bone loss induced by arthritis, tumor growth in bone in osteoporosis (Su et al, 2012). In addition ADP, which is the natural ligand for P2RY2 receptors can stimulate osteoclast formation in vitro (Hoebertz et al, 2001). Gruber et al (2003) showed that treatment with aspirin had no effect in osteoclastogenesis, suggesting that the anti-inflammatory effects of aspirin are not related to this mechanism.
According to our results, clopidogrel but not aspirin enhanced angiogenesis by increasing the number of blood vessels and reducing PDGF expression during periodontal repair. Interestingly, Clop has been shown to affect platelet degranulation, reducing secretion of PDGF, which plays an important role on tissue repair and angiogenesis (Graff et al, 2002). Regarding the angiogenic process we can partially explain the link between the increase in the number of blood vessels and PDGF expression as platelets are a primary source of this growth factor. Moreover, Duelsner et al (2012) showed that aspirin, but not clopidogrel, inhibits arteriogenesis in vivo and in vitro. Another point to be raised is that this result can be due to a sampling error. Because Clop-treated animals had a reduced inflammation and thus less tissue volume, consequently had an increase in vessel density, rather than a true increase in the number of vessels.
In summary, we showed that after removal of the pathologic stimulus in the early/transition phase between disease-associated inflammation and repair both aspirin and clopidogrel decrease the production of chemokines associated with leukocyte chemotaxis in the periodontal microenvironment. Overall, the effects of clopidogrel were more potent than those of aspirin. Thus, clopidogrel had a stronger effect in reducing PMN infiltration, attachment and bone loss and osteoclast number and increasing bone percentage and blood vessels suggesting that it may have therapeutic value under situations where tissues undergo a transition from inflammation to repair.
CLINICAL RELEVANCE.
Scientific rationale
Systemic administration of antiplatelet drugs might interfere with periodontal repair by suppressing the release of biological mediators.
Principal findings
Clopidogrel, but not aspirin, appears to have improved the repair process showed by an increase in blood vessels number, reduced leukocyte count, augmented bone percentage and significant reduction in the attachment loss and CEJ-ABC distance.
Practical implications
Aspirin and Clopidogrel, the two most frequently prescribed antiplatelet drugs in patients with cardiovascular diseases may affect the inflammatory and repair process in periodontal disease.
Acknowledgments
source of funding statement: This study has been supported by The State of São Paulo Research Foundation (FAPESP; 2010/10715-9) and National Council for Scientific and Technological, Development (CNPq) and by a grant from the NIDCR, DE017332 (DTG).
Footnotes
Conflict of interest The authors declare that they have no conflict of interests.
References
- Al-Bahrani A, Taha S, Shaath H, Bakhiet M. TNF-alpha and IL-8 in acute stroke and the modulation of these cytokines by antiplatelet agents. Curr Neurovasc Res. 2007;4:31–7. doi: 10.2174/156720207779940716. [DOI] [PubMed] [Google Scholar]
- Ayral Y, Rauch U, Goldin-Lang P, Stellbaum C, Deiner C, Schwimmbeck PL, et al. Prolonged application of clopidogrel reduces inflammation after percutaneous coronary intervention in the porcine model. Cardiovasc Revasc Med. 2007;8:183–8. doi: 10.1016/j.carrev.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Bennett JS. Novel platelet inhibitors. Annu Rev Med. 2001;52:161–84. doi: 10.1146/annurev.med.52.1.161. [DOI] [PubMed] [Google Scholar]
- Berk M, Dean O, Drexhage H, McNeil JJ, Moylan S, Oneil A, et al. Aspirin: a review of its neurobiological properties and therapeutic potential for mental illness. BMC Med. 2013;11:74. doi: 10.1186/1741-7015-11-74. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo PM, Lekovic V, Weinlaender M, Vasilic N, Madzarevic M, Kenney EB. Platelet-rich plasma and bovine porous bone mineral combined with guided tissue regeneration in the treatment of intrabony defects in human. J Periodontal Res. 2002;37:300–6. doi: 10.1034/j.1600-0765.2002.01001.x. [DOI] [PubMed] [Google Scholar]
- Chiang N, Serhan CN. New mechanism for an old drug: aspirin triggers anti-inflammatory lipid mediators with gender implications. Compr Ther. 2006;32:150–7. doi: 10.1007/s12019-006-0005-6. [DOI] [PubMed] [Google Scholar]
- Coimbra LS, Rossa C, Jr, Guimarães MR, Gerlach RF, Muscará MN, Spolidorio DM, et al. Influence of antiplatelet drugs in the pathogenesis of experimental periodontitis and periodontal repair in rats. J Periodontol. 2011;82:767–77. doi: 10.1902/jop.2010.100555. [DOI] [PubMed] [Google Scholar]
- Dominiak M, Łysiak-Drwal K, Solski L, Żywicka B, Rybak Z, Gedrange T. Evaluation of healing processes of intraosseous defects with and without guided bone regeneration and platelet rich plasma. An animal study. Ann Anat. 2012;194:549–55. doi: 10.1016/j.aanat.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Dovizio M, Bruno A, Tacconelli S, Patrignani P. Mode of action of aspirin as a chemopreventive agent. Recent Results Cancer Res. 2013;191:39–65. doi: 10.1007/978-3-642-30331-9_3. [DOI] [PubMed] [Google Scholar]
- Duelsner A, Gatzke N, Glaser J, Hillmeister P, Li M, Lee EJ, et al. Acetylsalicylic acid, but not clopidogrel, inhibits therapeutically induced cerebral arteriogenesis in the hypoperfused rat brai. J Cereb Blood Flow Metab. 2012;32:105–14. doi: 10.1038/jcbfm.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista V, Manarini S, Dell’Elba G, Martelli N, Napoleone E, Di Santo A, et al. Clopidogrel inhibits platelet-leukocyte adhesion and platelet-dependent leukocyte activatio. Thromb Haemost. 2005;94:568–77. [PubMed] [Google Scholar]
- Flad HD, Brandt E. Platelet-derived chemokines: pathophysiology and therapeutic aspects. Cell Mol Life Sci. 2010 Jul;67(14):2363–86. doi: 10.1007/s00018-010-0306-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AE, Mada SR, Rico MC, Dela Cadena RA, Kunapuli SP. Clopidogrel, a P2Y12 receptor antagonist, potentiates the inflammatory response in a rat model of peptidoglycan polysaccharide-induced arthritis. PLoS One. 2011;6:e26035. doi: 10.1371/journal.pone.0026035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Ren C, Li D, Li L. Clopidogrel and aspirin versus clopidogrel alone on graft patency after coronary artery bypass grafting. Ann Thorac Surg. 2009;88:59–62. doi: 10.1016/j.athoracsur.2009.04.024. [DOI] [PubMed] [Google Scholar]
- Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–84. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Villa AM, Norling LV, Serhan CN, Cordero D, Rojas M, Cadavid A. Aspirin triggered-lipoxin A4 reduces the adhesion of human polymorphonuclear neutrophils to endothelial cells initiated by preeclamptic plasma. Prostaglandins Leukot Essent Fatty Acids. 2012;87:127–34. doi: 10.1016/j.plefa.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J, Klinkhardt U, Schini-Kerth VB, Harder S, Franz N, Bassus S, et al. Close relationship between the platelet activation marker CD62 and the granular release of platelet-derived growth factor. J Pharmacol Exp Ther. 2002;300:952–7. doi: 10.1124/jpet.300.3.952. [DOI] [PubMed] [Google Scholar]
- Graves DT, Oates T, Garlet GP. Review of osteoimmunology and the host response in endodontic and periodontal lesions. J Oral Microbiol. 2011;17:5304. doi: 10.3402/jom.v3i0.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber R, Schöfnagl M, Karreth F, Fischer MB, Watzek G. The stable analog carbocyclic TXA2 but not platelet-released TXA2 induces osteoclast-like cell formation. Prostaglandins Leukot Essent Fatty Acids. 2003;68:267–72. doi: 10.1016/s0952-3278(03)00005-x. [DOI] [PubMed] [Google Scholar]
- Guimarães MR, Nassar PO, Andia DC, Nassar CA, Spolidorio DM, Rossa C, Jr, et al. Protective effects of Tacrolimus, a calcineurin inhibitor, in experimental periodontitis in rats. Arch Oral Biol. 2007;52:882–8. doi: 10.1016/j.archoralbio.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Hoebertz A, Meghji S, Burnstock G, Arnett TR. Extracellular ADP is a powerful osteolytic agent: evidence for signaling through the P2Y(1) receptor on bone cells. FASEB J. 2001;15:1139–48. doi: 10.1096/fj.00-0395com. [DOI] [PubMed] [Google Scholar]
- Holzhausen M, Rossa Júnior C, Marcantonio Júnior E, Nassar PO, Spolidório DM, Spolidório LC. Effect of selective cyclooxygenase-2 inhibition on the development of ligature-induced periodontitis in rats. J Periodontol. 2002;73:1030–6. doi: 10.1902/jop.2002.73.9.1030. [DOI] [PubMed] [Google Scholar]
- Holzhausen M, Spolidorio DM, Muscará MN, Hebling J, Spolidorio LC. Protective effects of etoricoxib, a selective inhibitor of cyclooxygenase-2, in experimental periodontitis in rats. J Periodontal Res. 2005;40:208–11. doi: 10.1111/j.1600-0765.2005.00787.x. [DOI] [PubMed] [Google Scholar]
- Lekovic V, Milinkovic I, Aleksic Z, Jankovic S, Stankovic P, Kenney EB, et al. Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects. J Periodontal Res. 2012;47:409–17. doi: 10.1111/j.1600-0765.2011.01446.x. [DOI] [PubMed] [Google Scholar]
- Li M, Zhang Y, Ren H, Zhang Y, Zhu X. Effect of clopidogrel on the inflammatory progression of early atherosclerosis in rabbits model. Atherosclerosis. 2007;194:348–56. doi: 10.1016/j.atherosclerosis.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Liu R, Bal HS, Desta T, et al. Diabetes enhances periodontal bone loss through enhanced resorption and diminished bone formation. J Dent Res. 2006;85:510–4. doi: 10.1177/154405910608500606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann S, Tolley ND, Dixon DA, McIntyre TM, Prescott SM, Zimmerman GA, et al. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol. 2001;154:485–90. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Elliott SN, Cirino G, Buret A, Ignarro LJ, Wallace JL. Platelets modulate gastric ulcer healing: role of endostatin and vascular endothelial growth factor release. Proc Natl Acad Sci U S A. 2001;98:6470–5. doi: 10.1073/pnas.111150798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar PO, Nassar CA, Guimarães MR, Aquino SG, Andia DC, Muscara MN, et al. Simvastatin therapy in cyclosporine A-induced alveolar bone loss in rats. J Periodontal Res. 2009;44:479–88. doi: 10.1111/j.1600-0765.2008.01143.x. [DOI] [PubMed] [Google Scholar]
- Pacios S, Kang J, Galicia J, Gluck K, Patel H, Ovaydi-Mandel A, et al. Diabetes aggravates periodontitis by limiting repair through enhanced inflammation. FASEB J. 2012;26:1423–30. doi: 10.1096/fj.11-196279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Ishii I, Giddings JC, Yamamoto J. Protective effects of ticlopidine and aspirin, administered alone and in combination, on thrombus formation in rat cerebral vessels. Haemostasis. 1996;26:150–156. doi: 10.1159/000217200. [DOI] [PubMed] [Google Scholar]
- Schömig A, Neumann FJ, Walter H, Schühlen H, Hadamitzky M, Zitzmann-Roth EM, et al. Coronary stent placement in patients with acute myocardial infarction: comparison of clinical and angiographic outcome after randomization to antiplatelet or anticoagulant therapy. J Am Coll Cardiol. 1997;29:28–34. doi: 10.1016/s0735-1097(96)00450-0. [DOI] [PubMed] [Google Scholar]
- Spolidorio LC, Herrera BS, Coimbra LS, Figueiredo MN, Spolidorio DM, Muscará MN. Short-term induction of thrombocytopenia delays periodontal healing in rats with periodontal disease: participation of endostatin and vascular endothelial growth factor. J Periodontal Res. 2010;45:184–92. doi: 10.1111/j.1600-0765.2009.01216.x. [DOI] [PubMed] [Google Scholar]
- Su X, Floyd DH, Hughes A, Xiang J, Schneider JG, Uluckan O, et al. The ADP receptor P2RY12 regulates osteoclast function and pathologic bone remodeling. J Clin Invest. 2012;122:3579–92. doi: 10.1172/JCI38576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suaid FF, Carvalho MD, Ambrosano GM, Nociti FH, Jr, Casati MZ, Sallum EA. Platelet-rich plasma in the treatment of Class II furcation defects: a histometrical study in dogs. J Appl Oral Sci. 2012;20:162–9. doi: 10.1590/S1678-77572012000200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taka T, Okano E, Seki J, Yamamoto J. Effects of clopidogrel on platelet activation and coagulation of non-anticoagulated rat blood under high shear stress. Haemostasis. 1999;29:189–196. doi: 10.1159/000022501. [DOI] [PubMed] [Google Scholar]
- Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 2003;82:82–90. doi: 10.1177/154405910308200202. [DOI] [PubMed] [Google Scholar]
- von Hundelshausen P, Petersen F, Brandt E. Platelet-derived chemokines in vascular biology. Thromb Haemost. 2007;97:704–13. doi: 10.1160/th07-01-0066. [DOI] [PubMed] [Google Scholar]
- Wada K, Yu W, Elazizi M, Barakat S, Ouimet MA, Rosario-Meléndez R, et al. Locally delivered salicylic acid from a poly(anhydride-ester): Impact on diabetic bone regeneration. J Control Release. 2013;171:33–7. doi: 10.1016/j.jconrel.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JL, McKnight W, Del Soldato P, Baydoun AR, Cirino G. Anti-thrombotic effects of a nitric oxide- releasing, gastric-sparing aspirin derivative. J Clin Invest. 1995;96:2711–2718. doi: 10.1172/JCI118338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner TD, Mitchell JA. Cyclooxygenase-3 (COX-3): filling in the gaps toward a COX continuum? Proc Natl Acad Sci U S A. 2002;99:13371–3. doi: 10.1073/pnas.222543099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C. Platelets and chemokines in atherosclerosis: partners in crime. Circ Res. 2005;96:612–6. doi: 10.1161/01.RES.0000160077.17427.57. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Su Y, Ye L. Slit-Robo: a potential way to treat periodontitis. Med Hypotheses. 2012;79:186–8. doi: 10.1016/j.mehy.2012.04.030. [DOI] [PubMed] [Google Scholar]


