Abstract
The beneficial effects of chronic and early pharmacological treatment with ethosuximide on epileptogenesis were studied in a genetic absence epilepsy model comorbid for depression. It was also investigated whether there is a critical treatment period and treatment length. Cortical excitability in the form of electrical evoked potentials, but also to cortico-thalamo-cortical network activity (spike–wave discharges, SWD and afterdischarges), white matter changes representing extra corticothalamic functions and depressive-like behavior were investigated.
WAG/Rij rats received either ethosuximide for 2 months (post natal months 2–3 or 4–5), or ethosuximide for 4 months (2–5) in their drinking water, while control rats drank plain water. EEG measurements were made during treatment, and 6 days and 2 months post treatment. Behavioral test were also done 6 days post treatment. DTI was performed ex vivo post treatment.
SWD were suppressed during treatment, and 6 days and 2 months post treatment in the 4 month treated group, as well as the duration of AD elicited by cortical electrical stimulation 6 days post treatment. Increased fractional anisotropy in corpus callosum and internal capsula on DTI was found, an increased P8 evoked potential amplitude and a decreased immobility in the forced swim test. Shorter treatments with ETX had no large effects on any parameter.
Chronic ETX has widespread effects not only within but also outside the circuitry in which SWD are initiated and generated, including preventing epileptogenesis and reducing depressive-like symptoms. The treatment of patients before symptom onset might prevent many of the adverse consequences of chronic epilepsy.
Keywords: Antiepileptogenesis, Ethosuximide, Cortical focus theory, Somatosensory cortex, WAG/Rij rats, Depression, Electrical stimulation, Afterdischarges, DTI
Introduction
The gradual and slow transformation from a presymptomatic to a symptomatic phenotype in genetic epileptic rat models of absence epilepsy is a form of epileptogenesis (Giblin and Blumenfeld, 2010). Current treatments of absence epilepsy are aimed at seizure control, not to the treatment of epilepsy, or to processes that interfere with epileptogenesis.
A recent study however, demonstrated the feasibility of antiepileptogenesis in a genetic absence model, rats of the WAG/Rij strain, a well characterized and validated genetic model of absence epilepsy (Coenen and van Luijtelaar, 2003; Depaulis and van Luijtelaar, 2006; van Luijtelaar and Coenen, 1986). At 2 months of age, untreated WAG/ Rij rats are virtually devoid of SWD. From then on, the SWD start to appear, first in a primitive form, later more mature. At the age of 3 months 50% of WAG/Rij rats have SWD, and by 6 months of age SWD are abundantly present in the electrocorticogram (EEG) of all WAG/Rij rats (Akman et al., 2010; Coenen and van Luijtelaar, 1987; Kole et al., 2007; Schridde and van Luijtelaar, 2004). Antiepileptogenesis was demonstrated in a study in which early and chronic (4 months) treatment with ethosuximide (ETX) in the drinking water reduced the commonly reported age-dependent increase in SWD even after medication was stopped (Blumenfeld et al., 2008). Similar findings were reported by two other groups, again in WAG/Rij rats (Russo et al., 2010; Sarkisova et al., 2010) and in the GAERS (Genetic Absence Epileptic Rats from Strasbourg) model (Dezsi et al., 2013).
Moreover, the typical increase and decrease respectively in the expression of subtypes of Na+ channels (Nav 1.1 and 1.6) and HCN1 channels in the focal zone of the somatosensory cortex (Klein et al., 2004; Kole et al., 2007; Strauss et al., 2004), the site of origin of SWD in genetic absence models (Meeren et al., 2002; Pinault, 2003; Polack et al., 2007), no longer occurred (Blumenfeld et al., 2008). Follow-up studies, again in WAG/Rij rats, confirmed that a 4-month-long administration of ETX or levetiracetam not only prevented epileptogenesis, but also the depressive-like behavior (Russo et al., 2011; Sarkisova et al., 2010), typical for symptomatic WAG/Rij rats (Sarkisova and van Luijtelaar, 2011; Sarkisova et al., 2003; van Luijtelaar, 2011).
Focal enhanced cortical excitability is seen with electrical stimulation of the deep layers of the somatosensory cortex, which induced a larger amplitude evoked response and more and longer 8 Hz SWD-like afterdischarges (AD) in symptomatic epileptic rats than in non-epileptic control rats (Lüttjohann et al., 2011). The large-amplitude evoked potential was not found in the motor cortex, neither in WAG/Rij nor in control rats, demonstrating that the focal region in the deep layers of the somatosensory cortex in WAG/Rij rats is indeed unique and more excitable. This is in line with the ideas as proposed by the cortical focus theory of absence epilepsy (Meeren et al., 2002; Pinault, 2003; Polack and Charpier, 2009; Polack et al., 2007; van Luijtelaar and Sitnikova, 2006; van Luijtelaar et al., 2011). The increase in AD was ascribed to an increased sensitivity in the cortico-thalamo-cortical network for 8 Hz oscillations (Lüttjohann et al., 2011).
Additional evidence for chronic changes accompanying epileptogenesis comes from diffusion tensor imaging (DTI), which showed that symptomatic but not presymptomatic WAG/Rij rats had reduced fractional anisotropy (FA) and increased perpendicular diffusivity in the anterior corpus callosum (Chahboune et al., 2009). Decreased FA with increased parallel diffusivity suggests that myelin and/or axon fiber density is reduced (Beaulieu, 2002, 2006) in pathways connecting the somatosensory cortices in symptomatic rats.
The aim of the present study was to establish whether there are sensitive time windows for the drug induced antiepileptogenesis (before or during the period that SWD start to appear, at 2–3 months of age), or after they are present in almost all animals (4–5 months), whether the duration (2 vs. 4 months) of ETX treatment is a relevant factor in the process of antiepileptogenesis.
The relation between antiepileptogenesis and the depressive-like behavior in the various treatment regimes was also investigated by evaluating the behavior of the groups in the forced swim test and sucrose preference test. Further, the effects of antiepileptogenesis on fractional anisotropy of white matter were investigated with animals treated with ETX. In a second experiment the effects of 4 months of ETX treatment on electrical stimulation-induced evoked potentials (EEP) and 8 Hz AD were established in the focal region and compared with a cortical control region. It was expected that antiepileptogenesis induced by chronic ETX will indeed induce antiepileptogenesis, diminish depressive-like behavior, reduce the amplitude of the EEP in the focal region, reduce AD and enhance fractional anisotropy.
Methods
Animals
Male inbred WAG/Rij rats served as experimental subjects. The rats were all born and raised at the Donders Centre for Cognition in Nijmegen, The Netherlands. The animals were group housed in standard macrolon cages enriched with Enviro Dry, four per cage for the first two weeks after weaning at post-natal day (PND) 30, thereafter in groups of two. The rats that were used for EEG were individually housed after the implantation of the EEG electrodes. Rats were kept under a 12 h-12 h light–dark regime (light on from 7.00 am to 7.00 pm) for the first 4 months, later the cycle was reversed for all animals in order to do the behavioral testing during the active period of the rats. Standard rat chow and drinking water were always available ad libitum except when explicitly mentioned. Animal care and experimental procedures were in full compliance with approved institutional policies (RU-DEC), protocols and guidelines.
Procedures for experiment 1 and 2
For a schema of the groups including their size at the various stages of the experiments, their interventions and measures see Fig. 1.
Fig. 1.
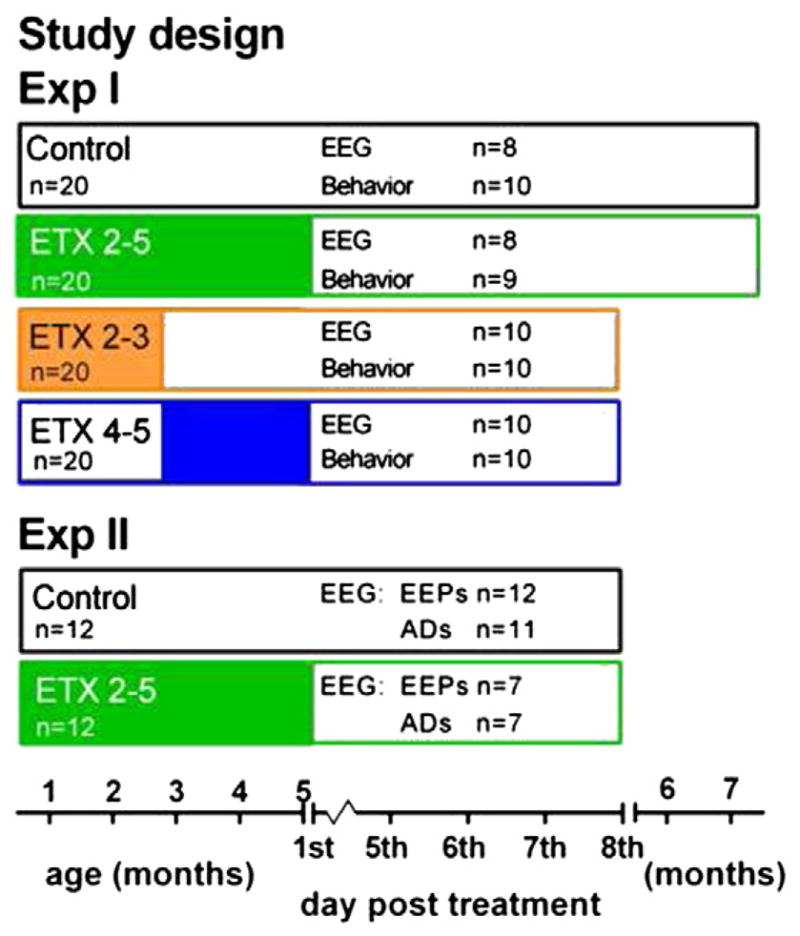
Overview of the timeline and groups for Experiment I and II. Exp I: EEG, behavior and DTI. Rats were assigned on post natal day 30 to five experimental groups. Exp II: EEP and AD testing. Rats were assigned on postnatal day 30 to 3 experimental groups. Control rats received plain water, ETX 2–5 group received ethosuximide (ETX) in their drinking water from the beginning of the 2nd month till the end of the 5th month, ETX 2–3 received ETX from the beginning of the 2nd month till the end of the 3rd month, followed by plain water, ETX 4–5 received plain water, which was changed at the beginning of the 4th month to ETX. ETX was given till the end of the 5th month. All rats were weighed weekly and drinking amounts were determined until the end of the 5th month.
Exp I
Here the effects of 2 and 4 month treatment of ETX on SWD during and after the end of treatment, on EEG, behavior (all four groups) and DTI (two groups only) were evaluated. The male offspring (n = 80) from 24 nests were randomly assigned at PND 30 to one of the five conditions (no more than 2 rats per litter were allowed in the same group). There were three ETX conditions and one control (plain water) condition. In the first ETX condition rats received ETX for four (2–5) months from PND 30 till 150, in the second condition rats received ETX for two (2–3) months, from PND 30 to 90, and in the third ETX group the rats received ETX also for two (4–5) months, but now the treatment started at PND 90 and lasted till PND 150. ETX was administered through the drinking water. The last condition was the control group, where the rats received plain tap drinking water throughout. The controls were treated exactly as all other rats, handled and weighed weekly.
Once per week the rats were weighed and the amount they drank was measured. The body weight and drinking data during the chronic drug treatment were combined to create a standard score of the amount of drinking controlled for body weight: amount of drinking in ml per 100 gram body weight per week per rat. The solutions were made fresh weekly. The drinking bottles of the ETX group were shielded from light.
Once per month and at the fifth day after the end of the treatment blood samples were collected from a sample of all rats (controls included) by means of a small incision in the tail. A drop of blood was applied to a filter paper, which was dried and posted to the laboratory of the epilepsy clinic SEIN, The Netherlands (Edelbroek et al., 2009). The amount of ETX in this bloodspot on the filter paper was determined using HPLC (Vermeij and Edelbroek, 2004).
Considering that WAG/Rij rats drink 150 ml/kg/day between PND 30 and 45 and about 80 ml/kg/day from PND 45–60 onwards, the concentrations of ETX was adapted to the quantity of daily water consumption. The ETX-treated rats were given 150 mg ETX/100 ml water from weaning onward and switched to 250 mg/100 ml from PND 45 onward. This resulted in an estimated dose of 300 mg/kg/day, chosen because it was effective in completely blocking SWD (Blumenfeld et al., 2008; Sarkisova et al., 2010), and because it was tolerated without any side effects of lethargy, ataxia, hair loss, or reduced food intake.
When the rats were 4 months of age, the rats of the four treatment condition groups were randomly divided into an EEG (n = 40) and a behavior (n = 40) group. From that moment on, rats were housed on a reversed light–dark cycle with dark onset at 7.00 a.m. This allowed us to do the experiments during the active period of the rat.
Exp II
Here the effects of 4 months of treatment with ETX on electrical evoked potentials (EEP) and AD were investigated. The male offspring (n = 24) from 8 nests were randomly assigned at PND 30 to one of the two treatment conditions (no more than 2 rats per litter were allowed to the same group). This experiment consisted of two groups (Fig. 1): 4 month ETX 2–5 group, and a plain water control group. ETX treatment was the same as in Exp I. Both groups contained 12 subjects. Rats were handled and weighed weekly, and the amount of drinking was monitored as in Exp I (these data will not be reported). Blood concentrations were not determined.
Surgical implants
Stereotactical surgery was performed under isoflurane anesthesia. All rats from the EEG group of Exp I (n = 38) and from Exp II (n = 24) were provided with permanent epidural EEG electrodes. The rats from Exp II had an additional pair of stimulation electrodes in the subgranular layers of the somatosensory cortex near bregma. (Plastics One® MS333/1-A) Paxinos and Watson (1997) rat’s atlas was used to locate the electrode positions.
Exp I
The first active EEG electrode of a threepolar EEG electrode set (Plastics One® MS2333/1-A) was placed epidurally on the frontal cortex (coordinates bregma AP +2 mm, M L −3.5 mm), the second active electrode on the parietal cortex (coordinates bregma AP-6 mm, ML −4 mm), and the reference electrode was placed above the cerebellum.
Exp II
Two identical electrode sets (Plastics One® MS333/1-A) were implanted in different locations. All three contacts of the first tripolar electrode set were aimed at the depth of the somatosensory cortex (the focal zone and initiation site of SWD) of the right hemisphere (coordinates of the middle of the 3 electrodes were: bregma AP 0 mm, ML −5 mm, DV −4.5 mm). Two of the electrodes were for stimulation and one for recording. The electrodes of the second tripolar set were implanted on the surface of the right motor cortex (active electrode, it served as a control site out of the focal zone (Lüttjohann et al., 2011)), the two others above the cerebellum. They served as reference and ground for both the depth electrode in the somatosensory cortex and surface motor cortex electrode.
Electrode assemblies were fixed to the skull with dental cement. The rats were injected preoperatively with 0.1 ml atropine concentration 0.5 mg/ml and 0.12 ml Rimadyl® (50 mg/ml, carprofen), and injected postoperatively with 0.12 ml Rimadyl® 24 and 48 hours post surgery for analgesia. Drug treatment with ETX was continued during the surgery and recovery days. There was minimally a two week interval between the implantation of the electrodes and the first recording or stimulation session.
Recording and stimulation procedures, electrophysiological data analyses
Exp I
EEG recording sessions: Three 22.5 h EEG recording sessions were scheduled (Fig. 1): 1. during one of the last days of the treatment (PND 148–149) for the 4-month-treated (ETX 2–5) and the control group to establish antiepileptic effects of the compound; 2. for all four EEG groups 6 days after the end of all treatments (treatment conditions ended at PND 150); and 3. for all four EEG groups two months later. The EEG measurements during treatment allowed us to establish the anti-absence action of ETX; the measurements after the treatment allowed us to establish the putative anti-epileptogenic effects. The rats were moved to Plexiglas transparent (20 × 35 × 25 cm) recording cages and connected to recording leads attached to a swivel-contact, which allowed them to move freely during the EEG recordings, on the day before the recording in order to habituate the animals to the experimental conditions. EEG recording sessions started after at least 16 h of habituation, and recordings always started at 9.00 a.m., the beginning of the third hour of the dark period.
The total number of spike and wave discharges (SWD), total duration of SWD, and mean duration of SWD was determined per hour over the 22.5 h recording period; SWD were identified based on a well-known criteria (Ovchinnikov et al., 2010; van Luijtelaar and Coenen, 1986).
Exp II
Electrical stimulation (ES): This took place at day 7 post treatment (PND 157). The session lasted 90 min. Testing took place during the dark period, always between 10 a.m. and 6.00 p.m. After 30 min of adapting and simultaneous base-line EEG recording, each rat received 125 pairs of stimuli at three stimulus intensities. The interstimulus interval was 300 ms, and the interval between stimulus pairs varied randomly between 7 and 11 s. Each stimulus was a monophasic square wave (width 0.4 msec) of 25, 50, or 100 microA.
The stimulation sessions took place in freely moving and fully conscious rats and lasted about 20 min per stimulus level; first 25, next 50 and then 100 microA were presented. The order of animals tested from each of the 2 treatment conditions was randomized. Stimulation was provided with the aid of a programmable stimulus generator and an isolated constant current unit (TD 10075 ERG, Radboud Electronic Research Group).
After termination of the ES study, the brains of the rats for Exp II were perfused. Half of the rats (6 ETX, 6 Water) were used for histological verification of the electrode location in the somatosensory cortex. Brains were fixed in a 30% sucrose solution, 0.1 ml TBS, cut in coronal slides of 40 μm with a microtome, and stained with cresyl violet and hematoxylin. The brains of the other 12 rats (6 ETX, 6 Water) were used for other analyses, beyond the scope of the study and will not be reported here.
All EEG and ES signals were recorded digitally with the aid of a WINDAQ-recording-system (DATAQ-Instruments, Akron, OH, USA). The sampling rate for the EEG study was 256 Hz, and for the ES study it was 2048 Hz. The signals were amplified and filtered with a physiological amplifier (TD 90087, Radboud Electronic Research Group) incorporating a band pass filter with cut-off points at 1 and 100 Hz for the EEG; 1 and 1000 Hz for the stimulation study, and a 50 Hz Notch filter.
BrainVision (Brain Products GmbH, 82205 Gilching, Germany) was used to make grand averages of electrical evoked potentials (EEP) per rat, per intensity and location (surface and depth). Only EEP occurring during passive wakefulness were used for the averaging. EEG epochs containing high-frequency low-amplitude activity intermixed with theta (typical for active wakefulness), slow wave sleep (1–5 Hz), SWD or afterdischarges (AD) were excluded. A large stimulation artifact was clearly visible. It consisted of a large sharp positive peak at .0098 s, followed by two smaller components at .0196 and 0.0293 s; they were removed from the grand averages. The inclusion criteria for the rats in the statistical analyses of the EEP were based on histological verification of the cortical depth electrode location (in the subgranular layers) of the somatosensory cortex S1 (S1FL, S1BF, S1DZ, S1HL). Amplitude and latency of the prominent components of the EEPs were analyzed per rat and per stimulation intensity.
AD, following cortical stimulation, mimicked SWD (Lüttjohann et al., 2011). They were identified based on fulfillment of the following criteria: they should occur within 0.5 s after the first pulse, have a spike amplitude of at least twice the background EEG, a frequency between 11-7 Hz, and the duration should be at least 1 s.
DTI procedures
DTI was performed using similar methods to those described previously for fixed, perfused rat brains (Chahboune et al., 2009).
Preparation of brains for imaging
Rats from the behavioral study of Exp I were used for diffusion tensor imaging, focusing on the ETX 2–5 (n = 9), and control (n = 9) groups (Fig. 1).
Rats were anesthetized with sodium pentobarbital followed by intracardiac perfusion with physiological NaCl solution and cold paraformaldehyde (PFA, 4%; obtained from Klinipath, Duiven, NL) in 0.01 M phosphate buffered saline (PBS, 8 g NaCl, 0.2 g KaCl, 1.44 g Na2PO4, 0.24 g KH2PO4 per l) (pH = 7.4).
After perfusion, brains were harvested maintaining integrity and stored in 4% PFA in PBS at 4 °C. Before MRI, the brains were washed into PBS for 24 h to remove the fixation solution and then, the brain was placed on plastic slides (Pure Slides LLC, Medford, MA, USA) and then into a custom-built MRI-compatible plastic tube in order to hold the brain in a standardized position in the volume coil. The tube was filled with Fluorinert, an MRI susceptibility-matching fluid (Sigma-Aldrich, Inc., St. Louis, MO).
MRI experiments
Imaging was conducted on a 9.4 T horizontal bore magnet (Bruker, Billerica, MA, U.S.A.) interfaced to a Varian console (Agilent Technologies, Santa Clara CA, USA) with a custom-made 1H radio-frequency Bollinger volume coil (4 cm diameter). A set of 20 contiguous coronal slices were acquired. DTI were performed using the Stejskal–Tanner spin-echo diffusion-weighted sequence (Stejskal and Tanner, 1965) with the following parameters: duration of diffusion gradient, δ = 5 ms and time elapsed between the two diffusion gradients, Δ = 15 ms; repetition time (TR)/echo time (TE) = 1500 / 25.1 ms; field of view = 25.6 × 25.6 mm; matrix size = 128 × 64, zero-filled to 256 × 256; number of averages was 24. The slice thickness was 0.5 mm, and the in-plane resolution 100 μm × 100 μm. Sixteen different images were acquired for each slice, fifteen corresponding to various noncollinear diffusion weighting directions with the same b = 1000 s/mm2 and one with no diffusion weighting.
Image processing and analysis
The six independent elements of the diffusion tensor were calculated from each series of diffusion-weighted images. The resulting tensor element maps were used to derive the eigenvalues (λ1, λ2, and λ3) and the corresponding eigenvectors (e1, e2, e3) of the diffusion tensor by matrix diagonalization (Harsan et al., 2006; Jones et al., 1999). The DTI indices fractional anisotropy (FA), parallel diffusivity (λ||), and perpendicular diffusivity (λ⊥) values were derived on a pixel-by-pixel basis using in house software written in Matlab (The MathWorks, Inc., Natick, MA) as defined by the following equations:
Image processing and region of interest (ROI) analyses were performed using BioImage Suite 2.6 (http://www.bioimagesuite.org/). FA images were rigid-body aligned and nonlinearly warped twice to a single template. The template for each group of animals was a dataset from a single animal within that group. To ensure that all slices retained the identical number of voxels for statistical analysis, any slices with missing data from the front or back of the brain following the rigid body transformation were eliminated. This step was followed by a non-linear intensity-based warping parameterized in terms of a tensor b-spline grid with uniform control point spacing (Papademetris et al., 2004). Nonlinear registration was performed twice to obtain better results (i.e. the output from the first nonlinear registration was then warped again to the same template). FA t-maps were then calculated using a two sample t-test with height threshold t = 2.00, cluster extent threshold = 30 voxels (voxel dimensions = 0.1 × 0.1 × 0.5 mm) and FA threshold of 0.30. T-maps were then overlaid on the template FA maps for identification of regional changes, by comparison with a standard rat anatomical atlas (Paxinos and Watson, 1997). The same analysis method was applied to parallel diffusivity (λ||) and perpendicular diffusivity (λ⊥) images.
ROI analyses were performed by manually drawing ROIs in each individual animal’s original image space before coregistration. Average FA, λ⊥ and λ|| values within each ROI were calculated for each animal, and group analyses were then performed across animals. The entire analysis was also confirmed using single ROIs in the common space, yielding the same results (data not shown). Two regions were identified for ROI analysis based on FA changes in the t-maps. 10 voxels were sampled across the corpus callosum near the midline at −3.6 mm posterior to bregma and 4 voxels were sampled from each side of the internal capsule (8 voxels total) at −3.6 mm posterior to bregma, just lateral and adjacent to the thalamic somatosensory relay nuclei.
Behavioral testing and analysis
The behavioral test group (n = 39) started at PND 150 with sucrose training (for 5 days), and it was followed by the sucrose-preference test and the forced swim test, both given at the 6th day post-treatment (Fig. 1). The sucrose-preference test was given first considering that it might be less stressful for the rats than the forced swim test.
Sucrose intake during training and in the sucrose-preference tests is a validated measure of hedonia (the ability to experience pleasure), which is widely used to assess the animals’ experience and degree of reward (Sarkisova et al., 2010). The behavioral tests were performed in a quiet and dimly (9 Lux) lit room. All behavioral tests were scheduled between 10.00 am and 6.00 pm. The rats were placed in a black box (30 × 30 × 40 cm). The box contained two drinking tubes, one from a bottle with plain water, the other from a bottle that contained 20% sucrose in water, in opposite corners. The placement of the sucrose and plain water bottles was counterbalanced across all rats. The bottle with sucrose water was always in the same corner for the same rat. A camera above the box attached to the ceiling recorded the position and movements of the rats. The program Ethovision® (Noldus Wageningen, NL) was used to analyze the behavior of the rats. Sucrose and plain water intake was measured by the difference between the weight of each bottle before and after every trial.
Sucrose training
Rats were mildly deprived of water by removing all drinking water for 3 to 5 h and were then allowed to drink plain water or sucrose water over a period of 15 min. This procedure was repeated daily for 5 days, and the rats learned through this procedure to locate the sucrose and plain water bottles. Plain water and sucrose intake were the dependent variables, group size n = 7–9.
Sucrose-preference test
At the 6th day rats were placed in the same box for 1 h after 16 h of water deprivation. Only animals that drank more than 0.2 g plain water or sugar water during two successive sucrose training days were included in the analyses. Dependent variables were the number of approaches to the sucrose and plain water bottle, the total motility of the rat, the entries to the water and sucrose zone, tap water and sucrose intake in this hour, and preference (%) for sucrose over water (sucrose intake, g/total fluid intake, g) × 100; group size n = 7–9.
Forced swim test
This experiment defines increased immobility and decreased active swimming as indications of depression-like behavior (Porsolt et al., 1978). A cylinder (height 60 cm, inside diameter 18 cm) was used containing 38 cm of tap water at about 22 °C. The rats were forced to swim for 5 min. A single exposure to the water cylinders was used because it prevents interference of possible memory to an earlier negative experience and it has been shown in previous research that it is sufficient to indicate depression-like features (Sarkisova and Kulikov, 2006; Sarkisova et al., 2010). The duration of passive swimming (immobility), the number of dives, headshakes, grooming and boli were observed by two trained observers, using the Observer® (Noldus Wageningen, NL, Spink et al., 2001). The observers had an inter-rater reliability of 0.86 (Pearson’s correlation coefficient) for the immobility duration. The criterion for passive swimming or immobility was the same as Sarkisova et al. (2010): floating vertically in the water making only those movements necessary to keep the head above the surface of the water; group size n = 8–10.
Statistical analyses
The effects of the treatment on body weight, standardized drinking amount and on sucrose consumption data were analyzed with a two way ANOVA with treatments as between-subject factor and time as within-subject factor. Number, mean and total duration of SWDs before the end of treatment (2 treatment groups) and MRI data per region were analyzed with Students t test for independent groups; characteristics of SWD post treatment (four treatment groups), the sucrose preference, and forced swim test were analyzed with a one-way ANOVA (treatment as between-subjects factor). ADs were analyzed with three way ANOVA with treatment as between-subject factor and location (motor vs somatosensory cortex) and intensity of stimulation as within-subject factors; also the amplitudes and latencies of EEPs were analyzed with the same ANOVA (treatment as between-subject factor and stimulation intensity and stimulus in a pair as within-subject factors). If appropriate, univariate ANOVAs and LSD tests were used as post-hoc tests.
Statistical analyses were performed with SPSS 15.0. A P value of 0.05 was chosen as the threshold level for significance.
Results
Experiment I
Liquid consumption during ontogeny
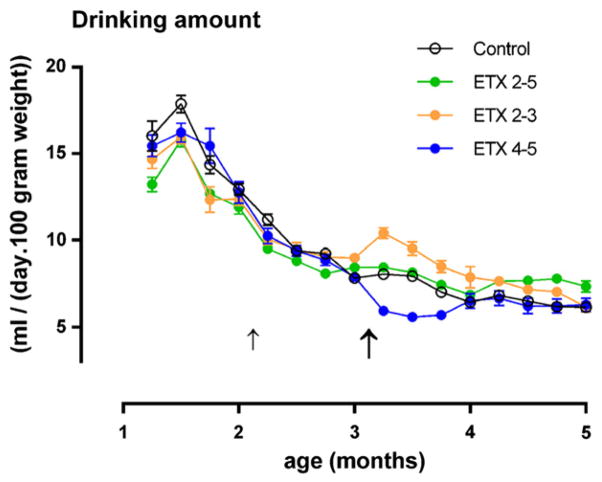
The standardized drinking scores of the four groups (n = 80), as collected during the chronic treatment are presented in Fig. 2. Their analysis showed a large effect of time (F(11.836) = 915.3, p < 0.001, η2 = 0.92), demonstrating that time has a large influence on drinking amounts: rats drank more in the first week of treatment (M = 14.8 ml/100 g in age week 5) than later (M = 6.8 ml/100 g in age week 18). There was also a small treatment (F(3.76) = 4.60, p < 0.05, η2 = 0.15) and a large treatment × time interaction (F(33.836) = 26.47, p < 0.001, η2 =0.54); the latter demonstrated that the differences between the treatments varied over time. Subsequent ANOVA for the total amount drunk over all the weeks and per week showed that ETX treated rats drank less than water controls during the weeks of drug administration, and that the ETX 2–5 group no longer differed from the water control group after 2 months of treatment. The animals that shifted from ETX to plain water (ETX 2–3 months group) drank more in week 9–12 of treatment, and so after the shift; those that shifted from plain water to ETX (ETX 4–5 months group) drank less. These differences between the groups became smaller and disappeared towards the end of treatment.
Fig. 2.
Drinking amount in ml per 100 gram body weight per day for each of the four treatments during 18 weeks (from week 5 PN (month 1) to week 22 (month 5) for the four groups (n = 20) in Experiment I. The bold arrow indicates the end of the treatment with ethosuximide of the ETX 2–3 group and the start of the ETX 4–5 group. The shift in treatment at week 9 for the ETX 2–3 month and ETX 4–5 month groups was accompanied by a respective increase and decrease in the amount of water consumption. Means and SEMs are shown.
Blood concentrations
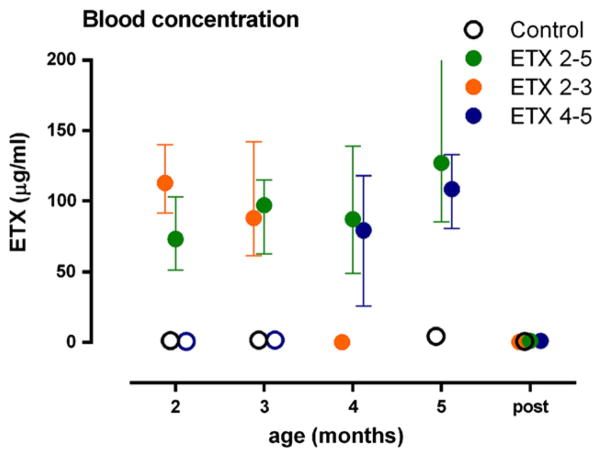
The concentration of ETX during treatment ranged from 25 to 204 μg/ml with a mean of 95. The concentration of ETX of the three treatment groups was comparable, moreover the concentration was rather stable over the treatment months (see Fig. 3), but no longer detectable six days after the end of the treatment.
Fig. 3.
Blood concentrations of ETX: on the X-axis the age of the animals, Y-axis (left) concentration of ETX in microgram per milliliter. Depicted are means and SEMs.
EEG measurement of SWD
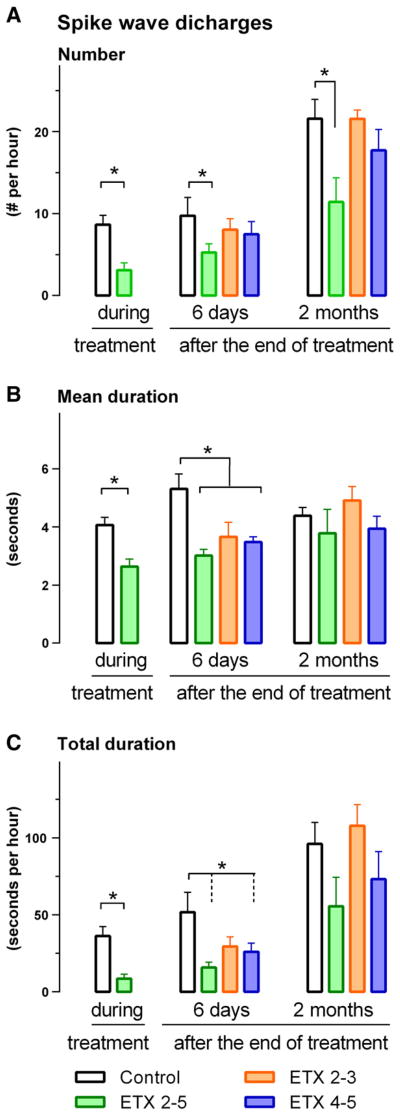
EEG was used to determine the putative anti- or proepileptic activity of ETX on SWD. For the ETX 2–5 and control group, the effects of the chronic treatment were first established during a 22.5 h recording session on the last day of the chronic treatment. All EEG’s looked normal and there were no obvious differences in the interictal EEG between the four groups. The t-test on number (t = 4.20, df 15, p < .001), mean duration (t = 4.37, df 15, p < .001) and total duration (t = 4.69, df 15, p < .001) of SWD showed less and shorter SWD for the ETX than for the control group. The results are presented in Fig. 4.
Fig. 4.
Number (top), mean duration (middle) and total duration (bottom) of SWD in a 22.5 h EEG recording session during, six days after, and 2 months after the end of the chronic treatment. ETX 2–5 means chronic treatment from beginning of 2nd till end of 5th month, ETX 2–3 means chronic treatment with ETX from begin of 2nd month till end of 3rd month; in the other periods plain water was given. ETX 4–5 means that chronic treatment with ETX started at the beginning of the 4th month till the end of the 5th month. Control rats had access to plain tap water. Means and SEMs are presented. Four months of treatment with ETX had antiepileptic and antiepileptogenic effects.
The putative anti-epileptogenic effects of the treatment were determined on the 22.5 h EEG session in the four groups at six days and two months after the end of treatment, again the results are presented in Fig. 4.
The ANOVA on the EEG data of the four groups at six days showed a significant treatment effect on number of SWD (F = 2.84, df 3.31, p < .05, η2 = .21), and post-hoc tests showed that the number of SWD was lower for the ETX 2–5 months compared to the control group. A treatment effect was also found on the mean (F = 5.60, df 3.31, p <0.01, η2 = .29) and total duration (F = 3.25, df 3.31, p <0.05, η2 =). The post hoc tests showed that the mean duration of SWD was shorter for the ETX 2–5 group compared to the control group, they showed also that the total duration of SWD was shorter in the ETX 2–5 and ETX 4–5 group compared to the control group.
The EEG study two months after the end of treatment showed the usual age dependent increase in number and duration of SWD compared to two months before (not tested). However, the group effect for the number of SWD tended to persist, it was now marginally significant (F = 3.09, df 3.15, p < .06, η2 = .38): the 4 month ETX group showed less SWD than the non-treated control group (Fig. 4A). The differences between groups for the mean and total duration had disappeared by that time, also because we lost some rats due to electrode cap loss.
Diffusion tensor imaging
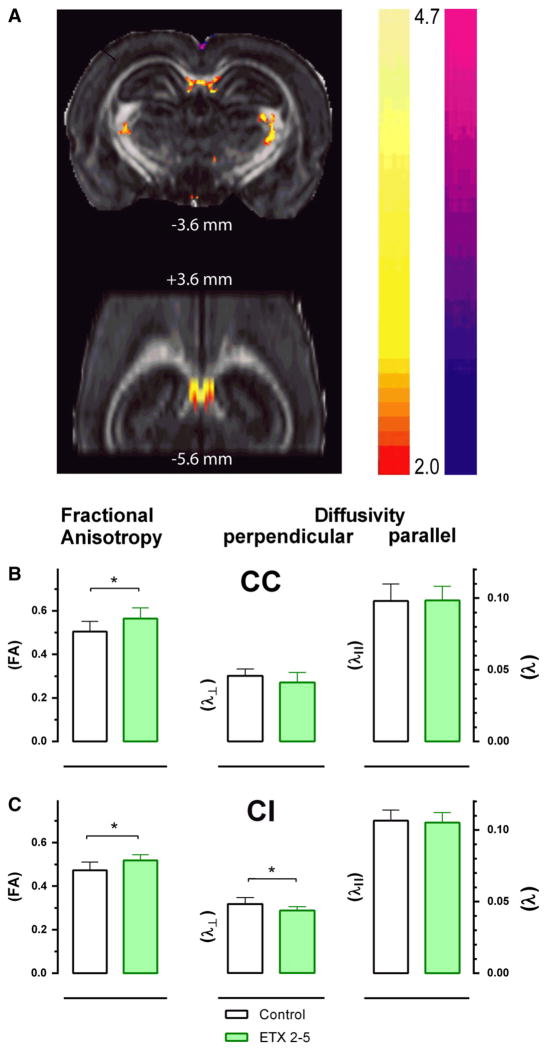
Fractional anisotropy (FA) t-maps demonstrated improvements (increases) in FA in the corpus callosum and internal capsule of 4-months-treated ETX (2–5) rats compared to untreated controls (Fig. 5A). Region of interest (ROI) analysis was performed to quantify changes in FA. A significant treatment effect (a strong localized increase) was found in FA in the midline corpus callosum (CC) (t = 2.63, df 16, p < .05, η2 = 0.30), and in the internal capsule (IC) (t = 2.93, df 16, p < .05, η2 = 0.35) of 4 months-ETX-treated group compared to age-matched untreated WAG/Rij controls (Figs. 6B, C). The increased FA was in the same region of the CC reported previously to show reduced FA in epileptic WAG/Rij rats compared to nonepileptic controls (Chahboune et al., 2009).
Fig. 5.
Chronic ETX treatment improves white matter fractional anisotropy (FA) based on DTI. A. ETX 2–5 treated WAG/Rij rats (n = 9) exhibited a strong localized increase in FA in the midline corpus callosum (CC) and in the internal capsule (IC) compared to age-matched untreated WAG/Rij controls (n = 10). Top image is coronal slice at AP −3.6 mm and bottom image is horizontal slice extending from AP +3.6 mm to −5.6 mm. B, C. Black = untreated; Green = ETX 2–5 months treated WAG/Rij rats; B. Midline corpus callosum shows significantly increased FA, and a trend in decreased perpendicular diffusivity (λ) in ETX treated WAG/Rij rats (n = 9) compared to untreated control WAG/Rij rats (n = 10), with no change in parallel diffusivity (λ||). C. Internal capsule also exhibited significantly increased FA, along with significantly decreased λ in ETX treated WAG/Rij rats (n = 9) compared to untreated controls (n = 10), with no change in λ||.
Fig. 6.
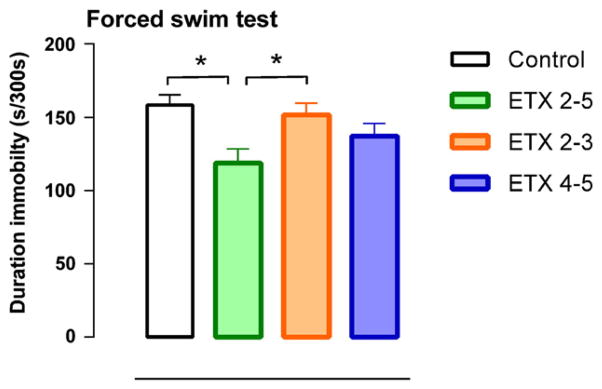
Duration of immobility response for the four groups (group size n = 9–10). Note that chronic treatment with ETX reduced the duration of immobility response. ETX (ethosuximide) 2–5 means chronic treatment from beginning of 2nd till end of 5th month, ETX 2–3 means chronic treatment with ETX from begin of 2nd month till end of 3rd month; in the other periods plain water was given. ETX 4–5 means that the chronic treatment with ETX started at the beginning of the 4th month and lasted till the end of the 5th month. Control rats had access to plain tap water. Means and SEMs are presented. Four months of treatment with E TX had antidepressant-like effects.
In order to understand mechanisms of FA changes in terms of perpendicular diffusivity (λ ) and parallel diffusivity (λ||), an analysis was performed using ROIs in the CC and in the IC that showed the strongest differences. A significant decrease was found in the IC (t = 2.61, df 16, p < .05, η2 = 0.30) and the same tendency in the CC for λ⊥, but not for λ||. In all, the increased FA values in IC and CC in 4 month-treated ETX rats were accompanied by a decreased λ⊥ in IC and to a lesser extend in CC (Figs. 5 B, C) compared to untreated controls. These findings suggest that increased FA seen in the ESX treated WAG/Rij rats at least in the IC is due to a decrease in λ⊥, possibly related to restoration in the density of axon fibers and/or myelin with treatment.
Behavior
Forced swim test: The ANOVA of the data of the forced swim test showed significant differences between the groups on the duration of immobility (F(3.35) = 4.31, p < .01, η2 = 0.27). Post-hoc tests showed that the ETX (2–5) treated rats had a lower immobility score than the water control and the ETX (2–3) treated group. Immobility scores are presented in Fig. 6. There were no differences between the groups in the number of dives, headshakes, grooming and boli.
Sucrose training and sucrose preference test: Sucrose training: although rats increased sucrose intake over the training days (F = 12.43, df 3.38, p = .000, eta2 = .59), there were no significant differences between the treatment conditions. Also the one-way ANOVA of the sucrose preference test showed no significant differences between the groups on any parameters measured (data not shown). This suggests that the groups did not differ in sucrose preference, although the results were in the expected direction (more sucrose preference, more sucrose per approach, higher number of approaches for the ETX 2–5 group).
Experiment II
Afterdischarges (AD)
Testing was performed on the 7th day afterdiscontinuing all medications. At this time, the ANOVA showed a significant treatment effect for number (F(1.17) = 9.75, p < .01, η2 = .37), mean duration (F(1.17) = 6.14, p < .05, η2 = .27) and total duration F(2.25) =6.41, p < .01, η2 = .36) of AD, the ETX 2–5 months had markedly fewer and shorter AD than the water controls (Fig. 7). The duration of the ADs was also dependent on the intensity of stimulation (F(2.16) =5.67, p < .05, η2 = .42); interestingly, the duration tended to be shorter at the highest intensity compared to the middle intensity (P < .10).
Fig. 7.
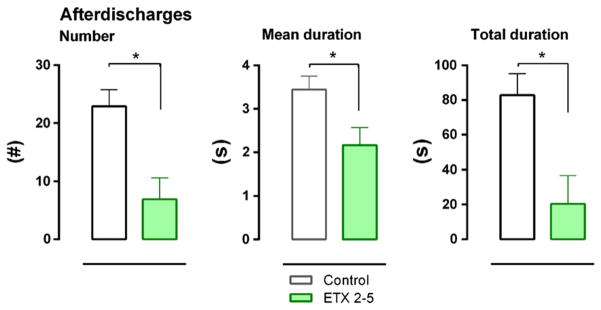
Number, mean duration and total duration of 8 Hz afterdischarges (AD), mimicking SWD. Chronic 4 months treatment with ETX reduces AD. The data were collected 7 days after the end of the chronic treatment. Means and SEMs are depicted.
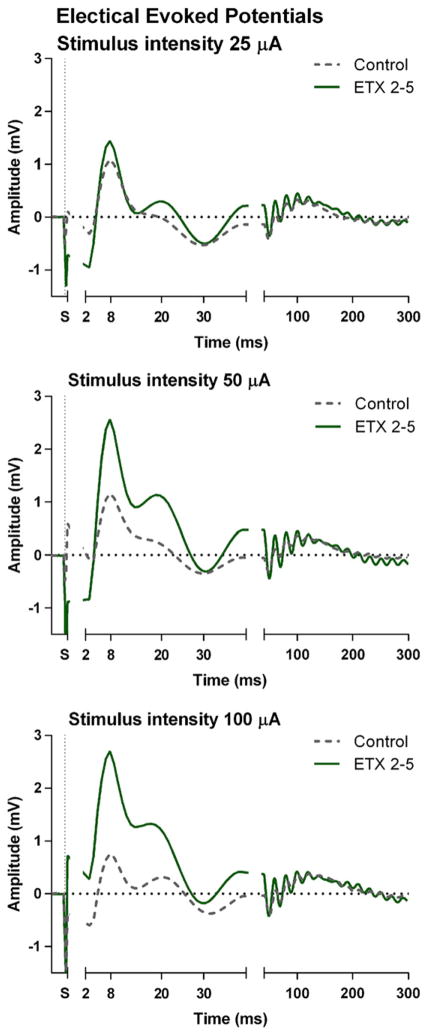
Electrical evoked potentials (EEP)
Depth EEP
EEP were recorded on the 7th day afterdiscontinuing all medications (Fig. 8). The stimulation artifact (removed from recordings) was identified by its timing (<3 msec), amplitude (>200 mV), sharpness and preciseness of its peaks. Moreover, it shifted in polarity after a change from anodal to cathodal stimulation, performed after the end of the recording session.
Fig. 8.
Grand averaged depth electrical evoked potentials (EEP) elicited by the first of the stimulus pairs measured and elicited at the subgranual layers of the somatosensory cortex for three different stimulation intensities (25, 50 and 100 microA). Chronic treatment with ETX enhances the amplitude of the inhibitory N8.
The first component of the depth EEP consisted of a negative peak at 3 msec (N3) after stimulation; it was less sharp and not a multiple of the latencies of the artifacts. It was rather small and it could be identified in 20 of the 26 animals. It is considered the primary excitatory effect of electrical stimulation (Jellema et al., 2004). The amplitude of the N3 elicited by the first stimulus of a pair was more negative than the one elicited by the second stimulus: the ANOVA revealed a significant effect for stimulation pair (F = 16.13, df 1.17, p < .001, η2 = .49). No significant effect of stimulus intensity or treatment was found on the N3.
The N3 was followed by a positive peak at 8 msec (P8). It was much larger than the N3 and was positive with a latency of 8 ms. The P8 is thought to represent inhibition following excitation (Coenen, 1995). A significant treatment effect was found for the amplitude of P8 (F = 5.24, df 1.11, p < .05, η2 = .32), it indicated that rats of the ETX group had a larger P8 amplitude compared to the control group (Fig. 8). The main effect of stimulus intensity was also significant (F = 4.41, df 2.22, p < .05, η2 = .20); the amplitude was higher for the 100 compared to the 25 and 50 microA stimuli.
The P8 was followed in some rats by a negative peak at 16 ms (N16) and a positive peak at 20 ms (P20) which was expressed as a small shoulder in the P8-N30 component. The N16 and P20 were not statistically analyzed because only a few rats showed this components. Next, a large negative peak at about 30 msec (N30) was identified in all rats. The amplitude of the first N30 in the pairs of stimuli was significantly larger than the second one (F(1.12) = 5.69, df 1.12, p < .05, η2 = .32). Also the treatment × stimulus number was significant (F = 4.47, df 1.12, p < .05, η2 = .29); post-hoc tests showed however no further treatment effects.
Finally, a positive point was defined as the mean peak between 80 and 200 ms through a few concurrent small components. Its amplitude was larger for the first compared to the second stimulus (F = 5.74, df 1.12, p < .05, η2 = .32). In addition, its amplitude increased with stimulus intensity (F = 3.77, df 2.17, p = <.05, η2 = .24) and there was an interaction between stimulus and intensity (F = 8.49, df 2.24, p < .01, η2 = .41. Post hoc tests showed that its amplitude was larger for 100 and 50 compared to 25 microA. However, there was no treatment effect for this late component.
Surface EEP
The first component of the surface EEP, recorded epidurally at the motor cortex (data are not presented since there was no treatment effect) had a negative polarity and occurred at 14 ms (N14). Its ANOVA revealed a stimulus number effect (F = 20.7, df 1.18, p < .001, η2 = .54); the amplitude of the EEP was larger (more negative) after the first compared to the second stimulus. There was also an intensity effect (F = 11.39, df 2.17, p < .001, η2 = .57). EEPs at latency 14 μs were higher for 100 and 50 compared to 25 microA.
The N14 was followed by a shoulder consisting of a small positive and negative peak which was not seen in all rats, followed by a larger peak clearly present in all subjects. It had a latency of 35 ms (P35). Again stimulation number (F = (1.18) = 15.37, df 1.18, p < .001, η2 = .46) and intensity F(2.17) = 3.65, p < .05, η2 = .30) effects were found. Post-hoc tests showed larger amplitude for the first stimulus and also showed that stronger stimuli elicited larger EEP amplitudes.
There were no treatment effects on the latencies for depth or surface components of the EEPs.
Discussion
Multiple effects of early and chronic treatment of ETX were identified. Chronic (4 month) treatment with ETX reduced the number, mean duration and total duration of SWD during treatment. When blood values demonstrated that ETX was no longer present at six days after the end of treatment, the SWD-suppressant effects of ETX were still found to be present and they persisted for at least two months after the treatment had stopped.
A reduction in FA on DTI measurements was found in the ETX 2–5 group. ETX 2–5 reduced immobility in a screening test for antidepressant drugs. Electrical stimulation of the focal region of the somatosensory cortex showed a lower probability of stimulation-induced AD, and an increased amplitude of the inhibitory P8 in the ETX group, while no changes were found in the motor cortex. Two months of treatment with ETX (ETX 2–3 and ETX 4–5) showed less convincing effects on SWD (only the mean duration of SWD was significantly reduced) and on the immobility response.
Antiabsence action of ETX
The reduction in number, total and mean duration of SWD during chronic treatment with ETX via drinking water is in line with the outcomes of other published data in the same genetic absence model (Blumenfeld et al., 2008; Russo et al., 2010; Sarkisova et al., 2010). The delivery of ETX via the drinking water is suited for obtaining constant plasma levels. The reduction in SWD was not complete (about 50%) during our treatment, while in previous studies less SWD remained present. One possible explanation is that here 22.5 h recordings were made, whereas in earlier studies shorter time windows were analyzed which may have missed some remaining SWD. Regardless, it shows that ETX does keep its potency in suppressing absence seizures chronically with the present dose, and even with a lower dose (Russo et al., 2010).
The antiepileptogenic effects of ETX and the critical period
The analyses of the EEG of all groups 6 days after the end of the chronic treatments showed that SWD were still reduced in number and duration in both of the 4 month-treated groups while the 2 month-treated groups had intermediate scores for number and mean duration of SWD. Considering that ETX was no longer detectible in the blood, the data show that ETX has suppressed or delayed the development of SWD and can be called antiepileptogenic. However, it appears that longer treatment periods (4 months) are needed for the antiepileptogenic effects to be fully achieved. The outcomes confirm the earlier results with ETX of our group (Sarkisova et al., 2010) and others in the same model (Blumenfeld et al., 2008; Russo et al., 2010). The antiepileptogenic or neuroprotective effects of the antiepileptic drugs levetiracetam and vigabatrin were also described in this model, as well as in other epilepsy models such as electrical and audiogenic kindling (Loscher et al., 1998; Shin et al., 1986; Vinogradova and van Rijn, 2008). Russo et al. (2011) described that SWD were still suppressed about one month after the end of a 17 week treatment from PND 30 onwards by vigabatrin. It is still unknown which processes in the brain are influenced by chronic treatment with vigabatrin, causing the antiepileptogenic effect. However, considering that all GABA-ergic effects in the thalamus are mediated through the reticular nucleus of the thalamus (Jones, 2007; Liu et al., 2007; Tóth et al., 2007), and that the cortex and brainstem contain a large number of GABA-ergic neurons, these structures might be good candidates.
It has been shown previously that SWD start to occur in WAG/Rij rats at age 2–3 months. Initially, SWD appear not mature and their incidence is low; from then on, there is a steady increase in the number of animals affected, the mean and number of SWD until SWD become fully established at age 4–5 months (Coenen and van Luijtelaar, 1987; Schridde and van Luijtelaar, 2004). It is proposed that the whole 4 month period from 2 to 5 months is sensitive and that 4 months of treatment is imperative for a full antiepileptogenic effect (Blumenfeld et al., 2008; Sarkisova et al., 2010). When a two month treatment period is started at age three months we found no significant benefit when animals were tested at a later age. When a two month treatment period is started at the beginning of the fourth month, a significant but smaller reduction in SWD was obtained. This seems clinically relevant since the diagnosis of SWD absence epilepsy is generally proposed after patients have had absence seizures for some time. Our data suggest that chronic ETX initiated after the symptoms are evident may still contribute to antiepileptogenesis. Indeed in most patients with childhood absence epilepsy absences tend to remit 2–5 years after onset, for which the chronic antiepileptogenic action of ETX might be responsible (Panayiotopoulos, 1999).
In a related designed study it was recently found that chronic treatment with ETX reduces REM sleep in WAG/Rij rats six days post treatment. This suggests that hypothalamic and brain stem structures known to be involved in REM sleep regulation, might also be affected by this chronic treatment (van Luijtelaar et al., 2012).
AD mimic SWD
The AD that were elicited by low intensity electrical cortical stimulation were the same as previously described in WAG/Rij rats (Lüttjohann et al., 2011). They mimic SWD as they have the same frequency and morphology, as well as the preferred occurrence during passive wake-fulness and drowsiness (Coenen et al., 1991; Drinkenburg et al., 1991; Lüttjohann et al., 2011). The number and duration of AD, as measured 6 days after the end of treatment, were reduced by early and chronic ETX treatment, effects mimicking the action of ETX on SWD. This result can be considered as additional evidence for the similarity of AD and SWD. The decrease in number of AD after chronic treatment is most likely an indication of structural changes responsible for increased inhibition or decreased excitation in the somatosensory cortex, the initiation zone of SWD in WAG/Rij rats (Meeren et al., 2002). The fact that the duration was shorter with higher than lower intensity stimulation points towards an optimal intensity for eliciting long ADs. The effects on duration of AD can be ascribed to the changes within the cortico-thalamo-cortical network, where due to the chronic treatment the cortical-thalamo-cortical networks are better able to inhibit the duration of an ongoing AD or SWD (Liu et al., 2007; Lüttjohann et al., 2011). ETX sensitive targets (T-type Ca2+ channels and Na+ channels) are abundantly present in cortex and thalamus. Therefore, it is likely that anti epileptogenesis by chronic treatment with ETX affects channel opening chronically with long lasting consequences on receptors and other molecules within the cortico-thalamo-cortical circuitry.
DTI
We found, using DTI, that chronic ETX treatment produces potentially beneficial effects on white matter microstructure in WAG/Rij rats. We observed increases in FA in the midline corpus callosum and in the internal capsule in treated WAG/Rij rats compared to untreated control WAG/Rij rats. The increased FA was related to a decrease in perpendicular diffusivity in internal capsula, and a non significant decrease in the corpus callosum, with no changes in parallel diffusivity. The area of corpus callosum exhibiting these changes has been shown through tractography to interconnect (Chahboune et al., 2009; Kim et al., 2012) the bilateral facial somatosensory cortices, regions demonstrated previously to have the most intense seizure discharges in the two hemispheres (Meeren et al., 2002; Mishra et al., 2011; Polack et al., 2007). In addition, prior work has shown that abnormally reduced FA develops in the corpus callosum of WAG/Rij rats along with the emergence of SWD (Chahboune et al., 2009). The internal capsule region showing changes is adjacent to the somatosensory thalamus, also intensely involved in SWD.
Our findings suggest that chronic ETX treatment of SWD in the cortex in absence epilepsy may protect or lead to recovery of microstructural changes in white matter pathways damaged during repeated transmission of seizure discharges. The functional consequences of these white matter changes are not known, and it is also not clear whether this protection or recovery in microstructures is a consequence of SWD treatment, or caused by some other aspect of the treatment/ underlying disorder.
It has been shown clearly that myelination and axonal changes are correlated with the degree of diffusion anisotropy, because myelin reparation during brain postnatal development induced a decrease in the λ⊥ and an increase in FA (Harsan et al., 2006). Conversely, decreased FA can be caused by decreased diffusivity parallel to axonal fibers or by increased perpendicular diffusivity (Beaulieu, 2002, 2006). Electron microscopy studies of increased FA and decreased λ⊥ attributed these changes to an enlarged oligodendrocyte population, newly formed myelin sheaths, and a gradual increase in axonal caliber (Harsan et al., 2006). Increased axonal density could also contribute to increased FA and decreased λ⊥. Clearly, further studies with electron microscopy will be necessary to determine the ultrastructural basis of the recovery in DTI that we observed in this genetic rodent absence model. The protection or recovery in DTI metrics suggests that pathological white matter changes in absence epilepsy may be prevented or reversed by treatment. Hopefully, with further investigation, DTI changes can become a useful noninvasive biomarker of absence epilepsy progression, and its amelioration by early therapy.
Electrical evoked potentials
Effects of stimulation intensity were found for most of the components of the EEP, elicited in the somatosensory cortex and recorded locally in the focal region and in the motor cortex. This is in agreement with common sense and many experimental results in many different stimulation paradigms. Stimulation and recording in the infragranular layer of the somatosensory cortex elicited a primary response consisting of the N3 followed by the P8. This mimicked the well known somatosensory evoked potential elicited by electrical stimulation of the ventro-posterior lateral nucleus of the thalamus (Kandel and Buzsáki, 1997) or of the peripheral median nerve (Jellema et al., 2004). The latter authors describe a P1-N1 that occurs epicortically 8.3 and 12.7 msec after stimulation. The differences (5 msec) in latency between their and our primary components occurring at 3 and 8 msec post stimulation are due to the conduction time from the periphery to the spinal cord, thalamus, the synaptic delay of the thalamic relay cells, and the conduction time of afferent volleys along the thalamocortical fibers. The polarity reversal of the local field potentials and event related potentials above and below the granular layer is well known (Coenen, 1995; Jellema et al., 2004; Kandel and Buzsáki, 1997). In agreement with this, the polarity of our EEP epicortical components (N14 and P35) was also reversed and 5–6 msec delayed compared to the infragranular counterparts (P8 and N30).
The latencies were very reproducible within and across subjects and not sensitive to experimental manipulations, while the amplitudes were more variable across rats. The analyses of the amplitude of the P8 in the depth recordings showed that it was increased after chronic ETX. Interestingly, this effect was restricted to the infragranular recordings in the focal region, the layers in which the pyramidal neurons show extensive bursting behavior before and during SWD (Pollack et al., 2007, 2009), since no such treatment induced changes were found in the motor cortex.
The infragranular N3 is thought to be the primary excitation to electrical local stimulation, which is followed by local cortical inhibition, the P8 (Coenen, 1995). We found that ETX enhances this inhibitory component. This interpretation of the local effect of chronic ETX is in line with the finding of Blumenfeld et al. (2008), who described that chronic ETX reduced the increased expression of Na+ channels and increased the reduced expression of HCN channels in the focal region. The changes in channel expression after chronic treatment may underlie the increase in the inhibitory P8, and in the decrease in number of SWD and also in AD elicited by electrical stimulation of the focal region.
The slower (latency >10 and <20 msec) components of the depth and all components of surface EEP did not differ between the three treatment groups. It is likely that these slower EEP components reflect cortico-cortical activity since it takes about 20/40 msec before activity has traveled to the thalamus and back (Meeren et al., 2002). Apparently, treatment with ETX has no direct impact on the functional intrahemispheric cortico-cortical connectivity. It may be that ETX has local effects in the focal area characterized by a down regulation of Na+ and upregulation of HCN channels. Since chronic ETX treatment decreases number and duration of AD, it can be speculated that this antiepileptic drug has an effect on epileptogenesis and suggests the involvement of the cortico-thalamocortical network considering that the 8 Hz AD, which can be recorded in cortex and thalamus, were reduced.
The effects of ETX on behavior
A relation between depression and presence of SWD was proposed by Sarkisova, in symptomatic WAG/Rij rats; GAERS (Jones et al., 2008) and Long-Evans rats endowed with SWD (Shaw et al., 2009) show depressive-like behavior (Sarkisova and van Luijtelaar, 2011; van Luijtelaar, 2011). The relationship between depression and absence epilepsy was underlined by the finding that antiepileptogenesis, as induced by chronic administration of ETX reduces depressive-like behavior as well (Sarkisova et al., 2010). Interestingly, also chronic administration of vigabatrin has both antiepileptogenic and antidepressant effects, as measured with the forced swim test (Russo et al., 2011). Here we confirmed that 4 (and to a smaller amount also 2) months of treatment with ETX indeed reduces the immobility duration in the forced swim test. WAG/Rij rats have a passive coping strategy (van Luijtelaar et al., 2007), while chronically treated rats are less passive and less inclined to give up. It is thought that the limbic striatum plays a crucial role in the control of the immobility response since C-fos studies demonstrated activation in the ventral striatum in WAG/Rij rats after animals were tested in the forced swim test (Sarkisova et al., 2003).
In a second behavioral paradigm with the sucrose preference test as the most critical readout parameter (sucrose preference measures hedonia), no significant differences among the groups were obtained although rats drank more sucrose over their training days. The lack of significant group differences is in contrast to what has been reported (Sarkisova et al., 2010). These authors reported that sucrose preference was increased after chronic ETX. The difference in outcomes might be due to a methodological issue: in the Sarkisova et al. study the bottle of sucrose was placed next to the bottle of plain water in the home cage. These rats could easily choose which substance they liked most without having to move to another corner, in our study the bottles were placed in opposite corners of a test cage. In addition, the rats in the Sarkisova et al. study had no SWD at all after chronic treatment with ETX, whereas we found that chronic treated rats still have some SWD, albeit significantly less than untreated rats. Because of the occurrence of SWD in the present study, the effect of the treatment might not be strong enough to completely suppress all depressive symptoms. Although the sucrose preference test has been often used, it can be questioned whether the choice between sucrose and plain water is appropriate considering the experimental history of the animals in this particular experiment. ETX treated rats faced two new flavors (plain water and sucrose) during the training and test, whereas the control rats only face one new taste (sucrose). A more equivalent situation would be if the ETX group could make a choice between ETX and sucrose. Therefore, we propose that methodological issues may have masked the effects of the chronic treatment with ETX. ETX is less affecting (an)hedonia than what is measured in the forced swim test. The latter suggests that the effects of the chronic treatment might be slightly different for the different aspects of depressive-like behavior.
In all, the results as described here demonstrate that many of the changes in brain and behavior, that can be seen in these genetic absence rats, are counteracted by a treatment, resulting in antiepileptogenesis. The changes accompanying absence epilepsy can be found in the SWD generating system, the cortico-thalamo-cortical circuit (van Luijtelaar and Sitnikova, 2006). The increased inhibition in the focal region and the reduction in AD and SWD, as found here after chronic and early drug treatment, reveal changes within this primary SWD generating circuit as a consequence of chronic treatment. The changes accompanying absence epilepsy were also found in the white matter, more specifically in parts of the corpus callosum interconnecting the bilateral focal areas and here a reduction in FA was found. It should be kept in mind that the changes in white matter were not restricted to the corpus callosum.
A number of studies in the genetic absence models point to an important link between absence seizure mechanisms and limbic structures (Onat et al., 2012). Also behavioral and sleep studies point towards the involvement of limbic and hypothalamic-brainstem changes in WAG/Rij rats accompanying epileptogenesis (van Luijtelaar, 2011; van Luijtelaar et al., 2012). Here the changes in depressive-like behavior pointing towards a role of the nucleus accumbens, part of the limbic circuitry, were counteracted by the early and chronic drug treatment. All in all a picture emerges that epileptogenesis and antiepileptogenesis are not restricted to local changes or to changes restricted to the SWD generating circuitry, but that they affect large parts of the brain and that both processes can operate via a variety of mechanisms at different sites within the brain.
Conclusions: we found through electrophysiology, diffusion tensor imaging and behavioral testing that treatment with ETX throughout the four month period of SWD development has a beneficial effect in reducing epileptogenesis in WAG/Rij rats both within and outside the networks in which SWDs emerge. Two month treatment, irrespective whether it occurs during month 2–3 or 4–5, does not seem to have large effects. Antiepileptogenesis provide new hope that as genetic prediction of epilepsy becomes more feasible, early treatment of patients before symptom onset may prevent many of the adverse consequences of chronic epilepsy.
Acknowledgments
The authors would like to thank Anna Pitas and Matthias Wilde for their contribution in the collection and analyses of the data, Hans Krijnen and Saskia Hermeling for their biotechnical assistance, Gerard van Oijen for the technical assistance, and Elly Willemsvan Bree for the preparations of the solutions and the preparations for the transport of the brains. This work was supported by the NIH R01 NS066974, R01 NS049307, P30 NS052519, the Betsy and Jonathan Blattmachr Family, and the Loughridge Foundation (to HB); and by the NIH R01 MH67528 and P30 NS052519 (to FH).
References
- Akman O, Demiralp T, Ates N, Onat FY. Electroencephalographic differences between WAG/Rij and GAERS rat models of absence epilepsy. Epilepsy Res. 2010;89:185–193. doi: 10.1016/j.eplepsyres.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system — a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The biological basis of diffusion tractography. 2006 IEEE International Symposium on Biomedical Imaging: From Nano to Macro; Arlington, Virginia, USA. 2006. pp. 347–350. [Google Scholar]
- Blumenfeld H, Klein JP, Schridde U, Vestal M, Rice T, Khera DS, Bashyal C, Giblin K, Paul-Laughinghouse C, Wang F, Phadke A, Mission J, Agarwal RK, Englot DJ, Motelow J, Nersesyan H, Waxman SG, Levin AR. Early treatment suppresses the development of spike-wave epilepsy in a rat model. Epilepsia. 2008;49:400–409. doi: 10.1111/j.1528-1167.2007.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahboune H, Mishra AM, DeSalvo MN, Staib LH, Purcaro M, Scheinost D, Papademetris X, Fyson SJ, Lorincz ML, Crunelli V, Hyder F, Blumenfeld H. DTI abnormalities in anterior corpus callosum of rats with spike-wave epilepsy. Neuroimage. 2009;47:459–466. doi: 10.1016/j.neuroimage.2009.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen AM. Neuronal activities underlying the electroencephalogram and evoked potentials of sleeping and waking: implications for information processing. Neurosci Biobehav Rev. 1995;19:447–463. doi: 10.1016/0149-7634(95)00010-c. [DOI] [PubMed] [Google Scholar]
- Coenen AML, van Luijtelaar ELJM. The WAG/Rij rat model for absence epilepsy: age and sex factors. Epilepsy Res. 1987;1:297–301. doi: 10.1016/0920-1211(87)90005-2. [DOI] [PubMed] [Google Scholar]
- Coenen AML, van Luijtelaar ELJM. Genetic animal models for absence epilepsy: a review of the WAG/Rij strain of rats. Behav Genet. 2003;33:635–655. doi: 10.1023/a:1026179013847. [DOI] [PubMed] [Google Scholar]
- Coenen AM, Drinkenburg WH, Peeters BW, Vossen JM, van Luijtelaar EL. Absence epilepsy and the level of vigilance in rats of the WAG/Rij strain. Neurosci Biobehav Rev. 1991;15:259–263. doi: 10.1016/s0149-7634(05)80005-3. [DOI] [PubMed] [Google Scholar]
- Depaulis A, van Luijtelaar G. Genetic models of absence epilepsy in the rat. In: Pitkanen A, Schwartzkroin PA, Moshe SL, editors. Models of Seizures and Epilepsy. Elsevier Academic Press; San Diego, CA: 2006. [Google Scholar]
- Dezsi G, Ozturk E, Stanic D, Powell KL, Blumenfeld H, O’Brien TJ, Jones NC. Ethosuximide reduces epileptogenesis and behavioral comorbidity in the GAERS model of genetic generalized epilepsy. Epilepsia. 2013;54:635–643. doi: 10.1111/epi.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinkenburg WH, Coenen AM, Vossen JM, Van Luijtelaar EL. Spike-wave discharges and sleep-wake states in rats with absence epilepsy. Epilepsy Res. 1991;9:218–224. doi: 10.1016/0920-1211(91)90055-k. [DOI] [PubMed] [Google Scholar]
- Edelbroek PM, van der Heijden J, Stolk LM. Dried blood spot methods in therapeutic drug monitoring: methods, assays, and pitfalls. Ther Drug Monit. 2009;231:327–336. doi: 10.1097/FTD.0b013e31819e91ce. [DOI] [PubMed] [Google Scholar]
- Giblin KA, Blumenfeld H. Is epilepsy a preventable disorder? New evidence from animal models. Neuroscientist. 2010;16:253–275. doi: 10.1177/1073858409354385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsan LA, Poulet P, Guignard B, Steibel J, Parizel N, de Sousa PL, Boehm N, Grucker D, Ghandour MS. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res. 2006;83:392–402. doi: 10.1002/jnr.20742. [DOI] [PubMed] [Google Scholar]
- Jellema T, Brunia CH, Wadman WJ. Sequential activation of microcircuits underlying somatosensory-evoked potentials in rat neocortex. Neuroscience. 2004;129:283–295. doi: 10.1016/j.neuroscience.2004.07.046. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. 2. Cambridge University Press; 2007. [Google Scholar]
- Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med. 1999;42 (3):515–525. [PubMed] [Google Scholar]
- Jones NC, Salzberg MR, Kumar G, Couper A, Morris MJ, O’Brien TJ. Elevated anxiety and depressive-like behavior in a rat model of genetic generalized epilepsy suggesting common causation. Exp Neurol. 2008;209:254–260. doi: 10.1016/j.expneurol.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Kandel A, Buzsáki G. Cellular-synaptic generation of sleep spindles, spike-and-wave discharges, and evoked thalamocortical responses in the neocortex of the rat. J Neurosci. 1997;17:6783–6797. doi: 10.1523/JNEUROSCI.17-17-06783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YB, Kalthoff D, Po C, Wiedermann D, Hoehn M. Connectivity of thalamocortical pathway in rat brain: combined diffusion spectrum imaging and functional MRI at 11.7 T. NMR Biomed. 2012 doi: 10.1002/nbm.1815. http://dx.doi.org/10.1002/nbm.1815. [DOI] [PubMed]
- Klein JP, Khera DS, Nersesyan H, Kimchi EY, Waxman SG, Blumenfeld H. Dysregulation of sodium channel expression in cortical neurons in a rodent model of absence epilepsy. Brain Res. 2004;1000:102–109. doi: 10.1016/j.brainres.2003.11.051. [DOI] [PubMed] [Google Scholar]
- Kole MH, Bräuer AU, Stuart GJ. Inherited cortical HCN1 channel loss amplifies dendritic calcium electrogenesis and burst firing in a rat absence epilepsy model. J Physiol. 2007;578 (Pt 2):507–525. doi: 10.1113/jphysiol.2006.122028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XB, Cobble J, van Luijtelaar G, Jones EG. Reticular nucleus-specific changes in α3 subunit protein at GABA synapses in genetically epilepsy-prone rats. Proc Natl Acad Sci U S A. 2007;104:12512–12517. doi: 10.1073/pnas.0705320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W, Honack D, Rundfeldt C. Antiepileptogenic effects of the novel anticonvulsant levetiracetam (ucb L059) in the kindling model of temporal lobe epilepsy. J Pharmacol Exp Ther. 1998;284:474–479. [PubMed] [Google Scholar]
- Lüttjohann A, Zhang S, de Peijper R, van Luijtelaar G. Electrical stimulation of the epileptic focus in absence epileptic WAG/Rij rats: assessment of local and network excitability. Neuroscience. 2011;188:125–134. doi: 10.1016/j.neuroscience.2011.04.038. [DOI] [PubMed] [Google Scholar]
- Meeren HK, Pijn JP, Van Luijtelaar EL, Coenen AM, Lopes da Silva FH. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci. 2002;22:1480–1495. doi: 10.1523/JNEUROSCI.22-04-01480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra AM, Ellens DJ, Schridde U, Motelow JE, Purcaro MJ, DeSalvo MN, Enev M, Sanganahalli BG, Hyder F, Blumenfeld H. Where fMRI and electrophysiology agree to disagree: corticothalamic and striatal activity patterns in the WAG/Rij rat. J Neurosci. 2011;31:15053–15064. doi: 10.1523/JNEUROSCI.0101-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochinnikov A, Lüttjohann A, Hramov A, van Luijtelaar G. An algorithm for real-time detection of spike-wave discharges in rodents. J Neurosci Methods. 2010;194:172–178. doi: 10.1016/j.jneumeth.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Onat FY, van Luijtelaar G, Nehlig A, Snead OC., III The involvement of limbic structures in typical and atypical absence epilepsy. Epilepsy Res. 2012;103 (2–3):111–123. doi: 10.1016/j.eplepsyres.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Panayiotopoulos CP. Typical absence seizures and their treatment. Arch Dis Child. 1999;81:351–355. doi: 10.1136/adc.81.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papademetris X, Staib LH, Jackowski AP, Win LY, Schultz RT, Duncan JS. Medical Image Computing and Computer-Assisted Intervention (MICCAI) 2004. Integrating intensity and feature nonrigid registration; pp. 763–770. LNCS, 3216, I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1997. [Google Scholar]
- Pinault D. Cellular interactions in the rat somatosensory thalamocortical system during normal and epileptic 5–9 Hz oscillations. J Physiol. 2003;552 (Pt 3):881–905. doi: 10.1113/jphysiol.2003.046573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack PO, Charpier S. Ethosuximide converts ictogenic neurons initiating absence seizures into normal neurons in a genetic model. Epilepsia. 2009;50:1816–1820. doi: 10.1111/j.1528-1167.2009.02047.x. [DOI] [PubMed] [Google Scholar]
- Polack P, Guillemain I, Hu E, Deransart C, Depaulis A, Charpier S. Deep layer somatosensory cortical neurons initiate spike-and-wave discharges in a genetic model of absence epilepsy. J Neurosci. 2007;27:6590–6599. doi: 10.1523/JNEUROSCI.0753-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Russo E, Citraro R, Scicchitano F, De Fazio S, Di Paola ED, Constanti A, De Sarro G. Comparison of the antiepileptogenic effects of an early long-term treatment with ethosuximide or levetiracetam in a genetic animal model of absence epilepsy. Epilepsia. 2010;51:1560–1569. doi: 10.1111/j.1528-1167.2009.02400.x. [DOI] [PubMed] [Google Scholar]
- Russo E, Citraro R, Scicchitano F, Urzono A, Marra R, Rispoli V, De Sarro G. Vigabatrin has antiepileptogenic and antidepressant effects in an animal model of epilepsy and depression comorbidity. Behav Brain Res. 2011;225:373–376. doi: 10.1016/j.bbr.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Sarkisova KY, Kulikov MA. Behavioral characteristics of WAG/Rij rats susceptible and non-susceptible to audiogenic seizures. Behav Brain Res. 2006;166:9–18. doi: 10.1016/j.bbr.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Sarkisova K, van Luijtelaar G. The WAG/Rij strain: a genetic animal model of absence epilepsy with comorbidity of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:854–876. doi: 10.1016/j.pnpbp.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Sarkisova KY, Midzianovskaia IS, Kulikov MA. Depressive-like behavioral alterations and c-fos expression in the dopaminergic brain regions in WAG/Rij rats with genetic absence epilepsy. Behav Brain Res. 2003;144:211–226. doi: 10.1016/s0166-4328(03)00090-1. [DOI] [PubMed] [Google Scholar]
- Sarkisova KY, Kuznetsova GD, Kulikov MA, van Luijtelaar G. Spike-wave discharges are necessary for the expression of behavioral depression-like symptoms. Epilepsia. 2010;51:146–160. doi: 10.1111/j.1528-1167.2009.02260.x. [DOI] [PubMed] [Google Scholar]
- Schridde U, van Luijtelaar G. The influence of strain and housing on two types of spike-wave discharges in rats. Genes Brain Behav. 2004;3:1–7. doi: 10.1111/j.1601-1848.2004.00034.x. [DOI] [PubMed] [Google Scholar]
- Shaw FZ, Chuang SH, Shieh KR, Wang YJ. Depression- and anxiety-like behaviors of a rat model with absence epileptic discharges. Neuroscience. 2009;160:382–393. doi: 10.1016/j.neuroscience.2009.02.053. [DOI] [PubMed] [Google Scholar]
- Shin C, Rigsbee LC, McNamara JO. Anti-seizure and antiepileptogenic effects of vigabatrin. Brain Res. 1986;398:370–374. doi: 10.1016/0006-8993(86)91498-8. [DOI] [PubMed] [Google Scholar]
- Spink AJ, Tegelenbosch RA, Buma MO, Noldus LP. The EthoVision video tracking system — a tool for behavioral phenotyping of transgenic mice. Physiol Behav. 2001;73:731–744. doi: 10.1016/s0031-9384(01)00530-3. [DOI] [PubMed] [Google Scholar]
- Stejskal E, Tanner J. Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J Chem Phys. 1965;42:288–292. [Google Scholar]
- Strauss U, Kole MH, Brauer AU, Pahnke J, Bajorat R, Rolfs A, Nitsch R, Deiss RA. An impaired neocortical Ih is associated with enhanced excitability and absence epilepsy. Eur J Neurosci. 2004;19:3048–3058. doi: 10.1111/j.0953-816X.2004.03392.x. [DOI] [PubMed] [Google Scholar]
- Tóth TI, Bessaïh T, Leresche N, Crunelli V. The properties of reticular thalamic neuron GABA(A) IPSCs of absence epilepsy rats lead to enhanced network excitability. Eur J Neurosci. 2007;26:1832–1844. doi: 10.1111/j.1460-9568.2007.05800.x. [DOI] [PubMed] [Google Scholar]
- van Luijtelaar G. The prevention of behavioral consequences of idiopathic generalized epilepsy: evidence from rodent models. Neurosci Lett. 2011;497:177–184. doi: 10.1016/j.neulet.2011.02.034. [DOI] [PubMed] [Google Scholar]
- van Luijtelaar EL, Coenen AM. Two types of electrocortical paroxysms in an inbred strain of rats. Neurosci Lett. 1986;70:393–397. doi: 10.1016/0304-3940(86)90586-0. [DOI] [PubMed] [Google Scholar]
- van Luijtelaar G, Sitnikova E. Global and focal aspects of absence epilepsy: the contribution of genetic models. Neurosci Biobehav Rev. 2006;30 (7):983–1003. doi: 10.1016/j.neubiorev.2006.03.002. [DOI] [PubMed] [Google Scholar]
- van Luijtelaar G, Sarkisova K, Midzyanovskaya IS, Tolmacheva EA. Stress vulnerability and depressive symptoms in genetic absence epileptic rats. In: Hollaway K, editor. New Research in Epilepsy and Behavior. Nova Science Publishers; 2007. pp. 211–278. [Google Scholar]
- van Luijtelaar G, Sitnikova E, Littjohann A. On the origin and suddenness of absences in genetic absence models. Clin EEG Neurosci. 2011;42:83–97. doi: 10.1177/155005941104200209. [DOI] [PubMed] [Google Scholar]
- van Luijtelaar G, Wilde M, Citraro R, Scicchitano F, van Rijn C. Does antiepileptogenesis affects sleep in genetic epileptic rats? Int J Psychophysiol. 2012;85:49–54. doi: 10.1016/j.ijpsycho.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Vermeij TA, Edelbroek PM. Simultaneous high-performance liquid chromatographic analysis of pregabalin, gabapentin and vigabatrin in human serum by precolumn derivatization with o-phtaldialdehyde and fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;810:297–303. doi: 10.1016/j.jchromb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Vinogradova LV, van Rijn CM. Anticonvulsive and antiepileptogenic effects of levetiracetam in the audiogenic kindling model. Epilepsia. 2008;49:1160–1168. doi: 10.1111/j.1528-1167.2008.01594.x. [DOI] [PubMed] [Google Scholar]