Abstract
Background:
Neurokinin B (NKB) is a member of the tachykinin family of peptides. Inactivating mutations in the tachykinin 3 or tachykinin 3 receptor gene are associated with pubertal failure and congenital hypogonadotrophic hypogonadism in humans. This suggests that NKB may have a critical role in human reproduction. The effects of NKB administration have not been investigated previously in humans.
Aim:
The aim of this study was to determine the effects of iv administration of NKB on gonadotrophin secretion in healthy male and female volunteers.
Methods:
A total of 23 healthy men and 11 healthy women participated in the study. After an initial dose-finding study (study 1), men received a 4-hour infusion of vehicle (gelofusin) followed by a 4-hour infusion of NKB (2.56 or 5.12 nmol/kg/h) (study 2), and an 8-hour infusion of vehicle or NKB during different visits (study 3). Healthy women underwent a dose-finding study consisting of a 3-hour NKB administration during the follicular phase of the menstrual cycle, and the maximum dose of NKB was also tested during the preovulatory and midluteal phases of menstrual cycle (study 4).
Results:
Mean LH, FSH, and T secretion were not significantly altered during a 90-minute infusion of NKB (0.4–5.12 nmol/kg/h), or a 4-hour infusion of NKB (5.12 nmol/kg/h). No alterations in gonadotrophin secretion or LH pulsatility were observed during an 8-hour infusion of NKB when compared with vehicle. Doses of 0.64–5.12 nmol/kg/h NKB did not significantly alter LH, FSH, or estradiol secretion in healthy women during the follicular phase of the menstrual cycle. Finally, 5.12 nmol/kg/h did not significantly alter reproductive hormone secretion during the preovulatory or midluteal phases of the menstrual cycle.
Conclusions:
This is the first clinical study of NKB administration. None of the doses of NKB tested were associated with significant alterations in reproductive hormone secretion in healthy male or female volunteers. These novel data add to our understanding of the physiological actions of NKB in human reproduction.
The decapeptide neurokinin B (NKB) is a member of the tachykinin family of peptides, which share a common C-terminal amino acid motif (Phe-X-Gly-Leu-Met-NH2) (1). In humans, NKB is encoded by the TAC3 gene and binds preferentially to the neurokinin 3 receptor (NK3R; encoded by the TAC3R gene) (2). Several lines of evidence suggest that NKB signaling has a critical role in human reproduction. Inactivating mutations in tachykinin 3 (TAC3) or TAC3 receptor (TAC3R) are associated with pubertal failure and congenital hypogonadotrophic hypogonadism in humans (3–7). Pulsatile GnRH administration reverses the gonadotropin deficiency observed in patients with TAC3 and TAC3R mutations; this suggests that affected patients have endogenous GnRH deficiency (5). NKB is coexpressed with two other neuropeptides, kisspeptin and dynorphin, in specialized neurones within the hypothalamic infundibular nucleus (equivalent to the rodent arcuate nucleus) of humans and monkeys, which project to GnRH neurones (8–10). TAC3 expression is increased, with associated cellular hypertrophy within the infundibular nucleus of postmenopausal women and ovariectomized monkeys when compared with controls with intact ovarian function; furthermore, these changes within the infundibular nucleus are reversed after estrogen replacement in monkeys (11, 12). These data suggest that NKB signaling is necessary for reproductive maturation and is a novel regulator of fertility in humans.
Intravenous administration of 100 μg (approximately 30 nmol/kg) NKB has been shown to stimulate LH secretion in agonadal juvenile male monkeys (13). However, NKB has been suggested to have both excitatory and inhibitory effects on gonadotrophin secretion in rodents, depending on the precise model studied (9, 10, 13–20). It is important to determine whether NKB plays a physiological role in regulating reproductive hormone secretion in humans. However, the effects of NKB administration in human subjects have not been investigated previously. We aimed to determine in healthy male and female volunteers, the effects of iv administration of NKB on gonadotrophin secretion.
Materials and Methods
For subject and peptide characteristics and protocol for blood collection and analysis, see Supplemental Methods, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org (see Table 1 for subject baseline hormone levels).
Table 1.
Baseline Hormone Levels for Each of the Four Studies at Time 0
| Male |
Female |
|||||
|---|---|---|---|---|---|---|
| Study 1 (n = 5–6) | Study 2 (n = 5) | Study 3 (n = 6) | Study 4: Follicular (n = 5–8) | Study 4: Preovulatory (n = 5) | Study 4: Midluteal (n = 5) | |
| LH, IU/L | 2.9 ± 0.2 | 2.1 ± 0.2 | 3.5 ± 0.5 | 3.9 ± 0.2 | 12.0 ± 2.8 | 5.8 ± 2.0 |
| FSH, IU/L | 2.7 ± 0.2 | 2.4 ± 0.6 | 3.3 ± 0.6 | 5.8 ± 0.7 | 5.2 ± 1.1 | 3.2 ± 0.4 |
| T, nmol/L | 18.1 ± 1.0 | 15.8 ± 2.1 | 16.8 ± 1.2 | |||
| Estradiol, pmol/L | 190 ± 18 | 592 ± 161 | 557 ± 122 | |||
LH, FSH, T (males), and estradiol (females) were measured at time 0 before vehicle or NKB administration. Studies 1–3 were performed in healthy men. Study 4 was performed in healthy women in the follicular, preovulatory or midluteal phases of the menstrual cycle. Data are mean ± SEM.
Protocol
Studies were performed in the Clinical Investigation Unit, during which subjects were asked to lay supine. This study was blinded to subjects but not investigators. NKB was dissolved in a vehicle consisting of saline containing Gelofusin [5% (vol/vol)] (B.Braun Medical) to minimize peptide adsorption (21). Continuous cardiac monitoring was performed during all studies, and two experienced physicians were in attendance at all times. Heart rate, blood pressure (BP), and adverse symptoms were recorded at regular intervals. Some subjects participated in more than one of the four studies described below; however, study visits were scheduled at least a week from each other.
Study 1: dose-finding study of iv infusion of NKB to healthy men (protocol summary in Supplemental Figure 1A)
We performed a dose-finding study in healthy male subjects. A cannula was inserted into a large forearm vein in both arms: one for collection of blood and the second for NKB infusion. Each volunteer received a 90-minute iv infusion commencing at 0 minutes, and blood was sampled at −20, −10, 0, 15, 30, 45, 60, 75, 90, 120, 150, 180, 210, and 240 minutes. Subjects were randomized to receive vehicle or 0.04, 0.16, 0.64, 2.56, 5.12, or 10.24 nmol/kg/h NKB (n = 5–6 per group) (Supplemental Figure 1A); these doses refer to the rate of NKB administration during the first 30 minutes of infusion; the infusion rate was halved after 30 minutes to achieve a steady-state concentration of circulating peptide (22). Blood samples were collected for measurement of serum LH, FSH, and T at all time points (see Supplemental Figure 1A for protocol diagram).
Study 2: frequent blood sampling during 4-hour iv infusion of vehicle followed by 4-hour NKB to healthy men (protocol summary in Supplemental Figure 1B)
We studied in more detail the effects of prolonged infusion of the two highest tolerated doses of NKB (2.56 and 5.12 nmol/kg/h) in five healthy male volunteers. Blood was sampled every 10 minutes for measurement of serum LH and FSH over an 8-hour period (h 1–8 of the study). During hours 1–4 of the study, subjects received a 4-hour infusion of vehicle. During hours 5–8 of the study (ie, immediately after the vehicle infusion), subjects received a 4-hour infusion of NKB (2.56 or 5.12 nmol/kg/h). The infusion rate of NKB was halved 30 minutes after the commencement of NKB administration. Thus, total doses administered during 4-hour infusions of 2.56 and 5.12 nmol/kg/h NKB were 5.76 and 11.52 nmol/kg, respectively (see Supplemental Figure 1B for protocol diagram).
Study 3: frequent blood sampling during 8-hour iv infusion of vehicle or NKB to healthy men (protocol summary in Supplemental Figure 1C)
We studied in further detail the effects of prolonged infusion of the highest tolerated dose of NKB (5.12 nmol/kg/h) on reproductive hormone secretion and LH pulsatility. Six healthy male subjects each attended two study visits within 2 days of each other. During the first study visit, subjects received an 8-hour infusion of vehicle and underwent blood sampling every 10 minutes for measurement of serum LH and FSH. The second study visit was identical to the first visit, except that subjects received an 8-hour infusion of NKB (5.12 nmol/kg/h). The infusion rate of NKB was halved 30 minutes after the commencement of NKB administration, giving a total dose of 21.76 nmol/kg NKB during the second study visit (see Supplemental Figure 1C for protocol diagram).
Study 4: effects of iv infusion of vehicle or NKB in healthy women (protocol summary in Supplemental Figure 1D)
A dose-finding study was performed to investigate the effects of iv NKB in healthy women during the follicular phase of the menstrual cycle. Each volunteer underwent blood sampling every 10 minutes for 4 hours. One hour after the commencement of the study, iv infusion of vehicle or NKB (0.32, 0.64, 1.28, 2.56, or 5.12 nmol/kg/h) was administered for the remaining 3 hours of the study visit (n = 5–8/dose). The infusion rate of NKB was halved 30 minutes after the commencement of NKB administration (see Supplemental Figure 1D for protocol diagram). The highest dose of NKB (5.12 nmol/kg/h) was also administered to five healthy women during the preovulatory and midluteal phases of menstrual cycle.
Data analysis
Data are presented as mean ± SEM. Multiple means were compared using one-way ANOVA with a Dunnett's post hoc test. Linear regression slopes were tested for significance using an F test. Two-tailed t tests were used to compare two groups. One-way ANOVA with a Bonferroni post hoc analysis was used to compare three or more groups. Area under curve values for changes in reproductive hormone levels vs time were calculated and were denoted as negative if changes in reproductive hormone levels were lower than during the commencement of infusion. J.D.V. used a blinded deconvolution method with 93% sensitivity and specificity to identify LH pulses (23).
Results
Study 1: dose-finding study of iv infusion of NKB to healthy men
Safety
Doses 0.04–5.12 nmol/kg/h NKB were well tolerated without any significant changes in heart rate or BP (data not shown). At the dose of 10.24 nmol/kg/h, three subjects experienced a hot sensation and appeared flushed. A mild increase in heart rate (<100 beats/min) without any change in BP was noted in two of three of the subjects. An increase in BP to 148/90 mm Hg and sinus tachycardia were noted in the third subject. These symptoms stopped promptly after the cessation of the NKB infusion and clinical assessment including cardiorespiratory examination, electrocardiogram, and routine biochemistry were otherwise normal.
Reproductive hormones
No significant changes in LH, FSH, or T secretion were observed after commencing iv infusion of vehicle or NKB at doses 0.04–5.12 nmol/kg/h (Figure 1). LH and FSH responses became more positive at higher doses, but only FSH changes were significantly dose dependent using log-linear regression (FSH: F = 4.39, degrees of freedom numerator = 1.00, degrees of freedom denominator = 23.0, P < .05; LH: F = 1.32, degrees of freedom numerator = 1.00, degrees of freedom denominator = 23.0; P = .26) (Supplemental Figure 2).
Figure 1.
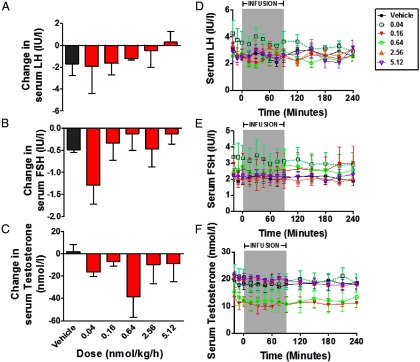
Dose-finding study with a 90-minute iv infusion of NKB or vehicle to healthy men (study 1). Subjects underwent 15 minutes of blood sampling for 4 hours during which they were administered either vehicle or NKB at an initial rate of 0.04, 0.16, 0.64, 2.56, or 5.12 nmol/kg/h (n = 5–6/group). The infusion rate was halved 30 minutes after the commencement of infusion to achieve a steady-state concentration of peptide during the infusion period (12). A–C, Data are presented as mean area under the curve increase from baseline for LH (A), FSH (B), and T (C). D–F, Time profiles are presented for mean LH (D), FSH (E), and T (F). Data are shown as mean ± SEM.
Study 2: frequent blood sampling during 4-hour iv infusion of vehicle followed by 4 hours of NKB to healthy men
We then examined the effects of the two highest tolerated doses of NKB administration (initial rate 2.56 and 5.12 nmol/kg/h) in more detail (Figure 2 and Supplemental Figures 3–5). In healthy men, no significant change in mean LH secretion was observed during 5.12 nmol/kg/h NKB infusion (h 5–8) when compared with vehicle infusion (h 1–4) (mean LH during 4 h infusion in international units per liter: 2.7 ± 0.3, vehicle; 3.3 ± 0.4, NKB, P = .3) (Figure 2, A and B, and Supplemental Figure 3).Mean LH secretion was not significantly different during 2.56 nmol/kg/h NKB infusion when compared with vehicle infusion in healthy men (mean LH during 4 h infusion in international units per liter: 3.2 ± 0.3, vehicle; 3.0 ± 0.2, 2.56 nmol/kg/h NKB, P = .7) (Supplemental Figure 4, A and B). No significant changes in serum levels of FSH or T were observed during either 2.56 nmol/kg/h NKB infusion (Supplemental Figure 4, C–F and Supplemental Figure 5) or 5.12 nmol/kg/h NKB infusion (Figure 2, C–F and Supplemental Figure 3), when compared with vehicle infusion. No significant changes in the number of LH pulses per 4 hours, pulse interval, or secretory mass were observed during NKB infusion (2.56 or 5.12 nmol/kg/h) when compared with the vehicle infusion (Table 2).
Figure 2.
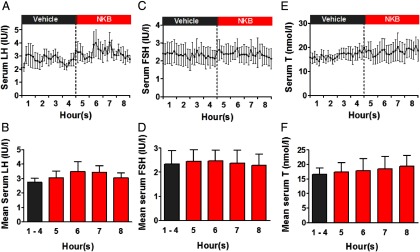
Frequent blood sampling during a 4-hour iv infusion of vehicle followed by 4 hours of NKB (at an initial rate of 5.12 nmol/kg/h) to healthy men (study 2). Subjects underwent 10 minutes of blood sampling for 8 hours (n = 5). A–C, Time profiles are presented for mean LH (A), FSH (B), and T (C). The vertical dotted line denotes the transition from vehicle to NKB administration. D–F, Mean levels of LH (D), FSH (E), and T (F) are pooled into discrete time bins. Hours 1–4 are coincident with the vehicle administration. Hours 5, 6, 7, and 8 are coincident with the NKB administration. Data are shown as mean ± SEM.
Table 2.
Summary of LH Pulse Analysis During Infusion of Vehicle or NKB in Healthy Male Subjects (Study 2)
| Dose of NKB, nmol/kg · h) | Number of LH Pulses, Pulses per 4 h | Interpulse Interval, min | Secretory Mass, IU/L |
|---|---|---|---|
| 2.56 | |||
| Vehicle | 2.83 ± 0.40 | 78.1 ± 9.2 | 12.4 ± 1.8 |
| NKB | 2.50 ± 0.43 | 82.2 ± 15.5 | 11.5 ± 1.7 |
| P = .58 | P = .82 | P = .72 | |
| 5.12 | |||
| Vehicle | 2.00 ± 0.45 | 110.0 ± 22.4 | 11.3 ± 2.2 |
| NKB | 2.67 ± 0.42 P = .30 | 85.0 ± 11.2 P = .30 | 10.9 ± 1.6 P = .90 |
Healthy male subjects underwent blood sampling every 10 minutes for 8 hours (n = 5–6 per dose). During each study visit, continuous iv infusion of vehicle was administered during hours 1–4, and continuous iv infusion of NKB was administered during hours 5–8. Data are mean ± SEM.
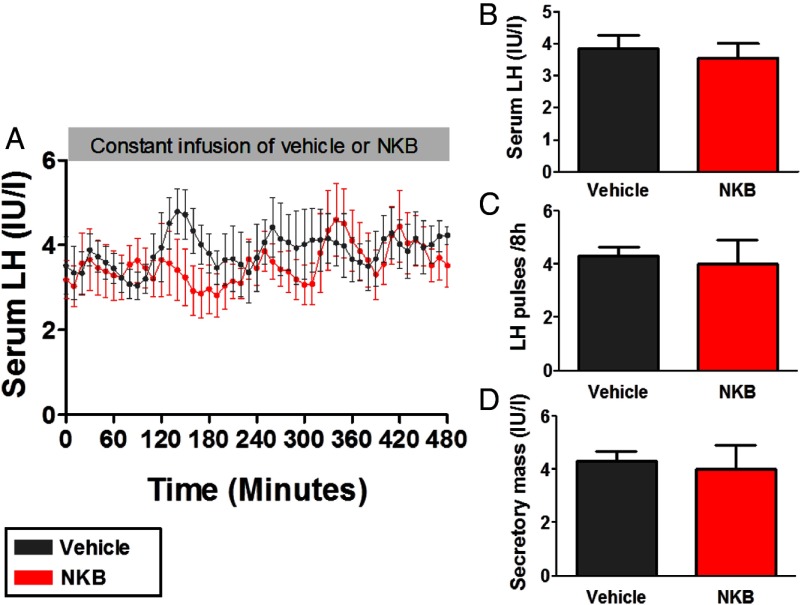
Study 3: frequent blood sampling during 8-hour iv infusion of vehicle or NKB to healthy men
We examined the effects of the highest tolerated dose of NKB administration (initial rate 5.12 nmol/kg/h) in further detail in healthy male volunteers. No significant alterations in LH secretion were observed during 8 hours of administration of 5.12 nmol/kg/h NKB when compared with 8 hours of administration of vehicle in healthy male volunteers (serum levels of LH during 8 h iv infusion in international units per liter: 3.9 ± 0.5, vehicle; 3.5 ± 0.4, NKB, P = .32 vs vehicle) (Figure 3, A and B). Furthermore, no significant changes in number of LH pulses per 8 hours or mean pulse secretory mass were observed during an 8-hour infusion of 5.12 nmol/kg · h NKB when compared with the vehicle (Figure 3, C and D).
Figure 3.
Frequent blood sampling during an 8-hour iv infusion of vehicle or NKB (at an initial rate of 5.12 nmol/kg/h) to healthy men (study 3). Subjects underwent 10 minutes of blood sampling for 8 hours (n = 5/group) during an 8-hour vehicle infusion and then an 8-hour NKB infusion within 2 days. A, Time profile of mean serum LH during vehicle or NKB infusion. B, Mean serum LH for entire vehicle or NKB study. C, Mean number of LH pulses during the entire vehicle or NKB study. D, Mean secretory mass per LH pulse during the entire vehicle or NKB study. Data are shown as mean ± SEM.
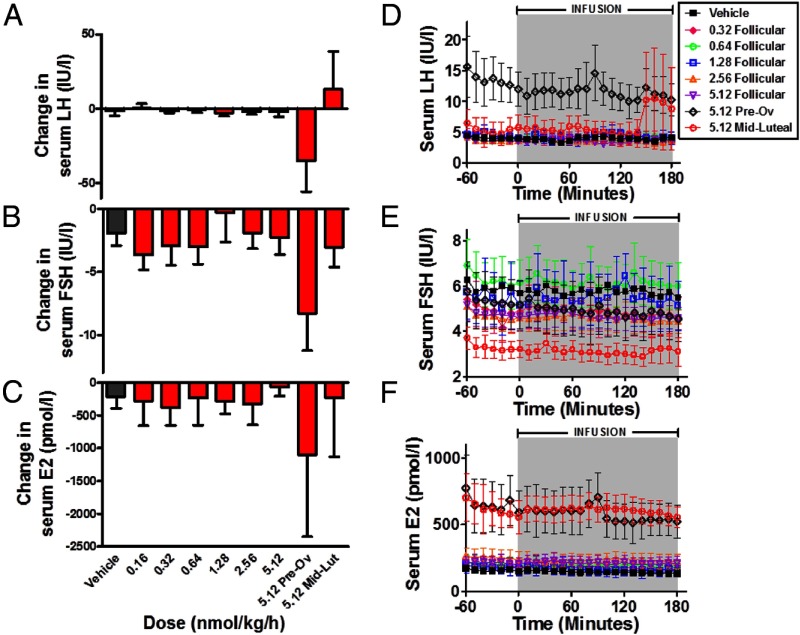
Study 4: effects of iv infusion of vehicle or NKB in healthy women
Finally, we performed a dose-finding study examining effects of 3 hours of iv administration of NKB (0.16, 0.32, 0.64, 1.28, 2.56, and 5.12 nmol/kg/h) in healthy female volunteers (Figure 4). None of the tested doses of NKB were associated with significant changes in serum LH, FSH, or estradiol secretion when compared with vehicle in women during the follicular phase of menstrual cycle (Figure 4, A–F). We also examined the effects of the highest dose of NKB (5.12 nmol/kg/h) in healthy female volunteers during the preovulatory and midfollicular phases of menstrual cycle. There was no significant change in mean LH during the preovulatory phase of menstrual cycle when compared with vehicle (area under curve LH in hours per international units per liter: −1.5 ± 3.4, vehicle; −34.8 ± 20.9, NKB, P = NS vs vehicle). Serum FSH was not changed significantly during the preovulatory phase of the menstrual cycle when compared with vehicle (area under curve serum FSH in hours per international units per liter: −1.9 ± 1.0, vehicle; −8.3 ± 3.0, NKB, P = NS vs vehicle). Serum estradiol secretion was also not changed significantly during the preovulatory phase of menstrual cycle when compared with vehicle (area under curve serum estradiol in picomoles per liter: −211 ± 177, vehicle; −1098 ± 1249, NKB, P = NS vs vehicle).
Figure 4.
Effects of iv infusion of vehicle or NKB in healthy women (study 4). Subjects in the study underwent 10 minutes blood for 4 hours during which they were administered either vehicle or NKB at an initial rate of 0.16, 0.32, 0.64, 1.28, 2.56, or 5.12 nmol/kg/h during the follicular phase or 5.12 nmol/kg/h during the preovulatory and midluteal phases (n = 5–8/dose). A–C, Data are presented as mean area under the curve increase from baseline for LH (A), FSH (B), and estradiol (C). D–F, Time profiles are presented for mean LH (D), FSH (E), and estradiol (F). Data are shown as mean ± SEM.
During the midluteal phase of the menstrual cycle, there were no significant alterations in serum LH, FSH, or estradiol during iv administration of vehicle when compared with 5.2 nmol/kg/h NKB (Figure 4, A–F).
Discussion
The hypothalamic neuropeptide NKB is strongly implicated in the physiological regulation of GnRH secretion in humans. It is therefore therapeutically important to determine the effects of NKB administration on the human reproductive axis. We report for the first time that iv infusion of NKB does not stimulate significant LH secretion in healthy men or women at the doses tested in this study.
Studies of NK3R agonist administration in nonhuman species have yielded conflicting results that appear dependent on the animal model selected and gonadal status. NKB and the neurokinin 3 antagonist, senktide, increases LH secretion in male juvenile agonadal monkeys (13). Intracerebroventricular (icv) administration of senktide stimulates LH in gonad-intact male mice; however, in gonad-intact male rats, the effects of senktide appear to be lost after pubertal development (18). Senktide stimulates LH secretion in ovary-intact rats but inhibits LH in ovariectomized rats (16, 17) and ovariectomized mice (10). In sheep, senktide stimulates LH secretion during the follicular phase of estrous cycle but has no effect during the luteal phase (15). In ovariectomized goats, icv senktide inhibits LH secretion but increases the frequency of multiunit volleys (a surrogate measure of neuronal activity) in the arcuate nucleus (9). Recent studies have attempted to explain the reasons for the discrepancies that underlie the complex effects of NKB signaling on gonadotrophin secretion. 17β-Estradiol replacement has been reported to prevent the inhibitory effects of senktide on LH secretion in gonadectomized female rats (16) and male rats (18). However, other studies report that the inhibitory effect of senktide on LH secretion (14, 17) or LH pulsatility (19) are not altered by 17β-estradiol replacement in ovariectomized rats. It has also been postulated that the inhibitory peptide dynorphin, which is coexpressed with NKB and kisspeptin in kisspeptin/neurokinin B/dynorphin (KNDy) neurones, may mediate the inhibitory effects of NKB on LH secretion; pretreatment with an antagonist to dynorphin blocks the inhibitory effects of senktide in ovariectomized rats with 17β-estradiol replacement (20).
We did not observe any significant alteration of sex hormone secretion after any dose of NKB administration to healthy men. Significantly higher numbers of NKB immunoreactive fibers and neurones are observed in females vs males in humans (24) and rats (18). We therefore performed a dose-finding study of NKB administration in women during the follicular phase of the menstrual cycle, which revealed no significant effect on reproductive hormone secretion. The effects of NKB signaling have been observed to vary during different phases of the sheep estrous cycle (15). Furthermore, we have previously demonstrated that kisspeptin-10 and kisspeptin-54 stimulate LH potently in women during the preovulatory phase of menstrual cycle when compared with the follicular phase (25, 26). By contrast, the highest tested dose of NKB did not alter gonadotrophin secretion during any phase of the menstrual cycle in healthy women. Although NKB did not alter serum reproductive hormones significantly during the current study, nonsignificant reductions in circulating reproductive hormones were observed during the dose-finding studies of NKB to men and women. It is difficult to explain such nonsignificant trends in light of animal data, which suggest that inhibitory effects of NKB predominate during the gonadectomized state (10, 16, 17). One might speculate that exogenous NKB acts as a partial agonist to inhibit endogenous hypothalamic NKB tone to reduce gonadotropin secretion. However, we would conservatively conclude that at the doses tested, iv administration of NKB has no statistically or biologically significant effect on reproductive hormone secretion or LH pulsatility in healthy men or women.
It is important to consider the possible reasons that we did not observe significant alterations in gonadotrophin secretion during NKB administration; by contrast, an iv bolus injection of NKB increased serum LH 3-fold in agonadal juvenile monkeys (13). First, we used subjects with normal levels of circulating sex steroid; by comparison, the lack of sex steroid-mediated negative feedback in agonadal monkeys may heighten sensitivity of pituitary gonadotrophs to stimulation. Second, we studied the effects of NKB in adult subjects, but rodent data suggest sensitivity to NKB agonists may be heightened in juvenile animals when compared with adult animals (18). Third, NKB stimulated LH secretion in monkeys at an iv dose of approximately 30 nmol/kg (13), whereas clinical intolerance prevented us from escalating our dose-finding study beyond the dose of 5.12 nmol/kg. We therefore cannot exclude that higher doses of NKB would have significantly altered LH secretion. However, our data strongly suggest that NKB is not a potent secretogogue of reproductive hormone secretion in healthy adult volunteers; this contrasts with its coexpressed neuropeptide kisspeptin-54, which stimulates a 4-fold increase in mean LH when administered at a dose of 0.24 nmol/kg in men (27). Fourth, it is possible that the small sample size in our study may have precluded the detection of subtle changes in reproductive hormone secretion after NKB administration. Finally, NKB is thought to stimulate GnRH/gonadotrophin secretion by directly activating KNDy neurones within the hypothalamic arcuate nucleus (28). Although a peripheral route of NKB administration stimulated LH secretion in monkeys (13), icv administration has been used to demonstrate the stimulatory effects of NKB (15) or senktide (15, 16, 18) on gonadotrophin secretion in other animal studies to date. It is therefore possible that peripherally administered NKB can access KNDy neurones more readily in monkeys when compared with humans, owing to a smaller diffusion distance.
NKB activates neurokinin 2 receptor (NK2R; and neurokinin 1 receptor (NK1R) to a lesser extent) in addition to NK3R (30–34). Most animal studies investigating effects of NKB signaling on gonadotrophin secretion have examined the effects of the specific NK3R agonist, senktide, rather than NKB itself. However, recent studies suggest that the reproductive effects of NKB may not be solely mediated by NK3R. de Croft et al (28) recently demonstrated that the effects of NKB on mouse arcuate nucleus kisspeptin neurones were not blocked by selective pharmacological NK3R antagonism; simultaneous application of NK1R, NK2R, and NK3R pharmacological antagonists was required. The investigators also observed that substance P and neurokinin A (tachykinins that bind preferentially to NK1R and NK2R) directly activated kisspeptin neurones. Furthermore, preliminary data suggest that NKB may predominantly stimulate LH pulses, whereas senktide may predominantly stimulate sustained LH secretion in sheep (35). The current study was restricted to the physiological investigation of the endogenous hormone neurokinin B; however, it would be interesting to establish in the future whether NKB and senktide have differential pharmacological effects on reproductive hormone secretion in humans.
Doses of 10.24 nmol/kg/h NKB were associated with vasoactive effects in healthy male subjects. Neurokinin B is a member of the tachykinin family of peptides. Other tachykinin peptides such as neurokinin A and substance P have been previously demonstrated to be associated with vasoactive effects [in healthy volunteers (36)]. It is therefore possible that the tachykinin peptides mediate their vasoactive effects through a similar mechanism. However, further work is required to investigate this possibility. NKB senktide acts predominantly on NK3R, mutations of which cause pubertal failure (3), whereas NKB also binds to NK2R (and NK1R to a lesser extent) (30–34). Specific stimulation of NK3R may be therefore implicated in reproductive hormone secretion.
NKB is coexpressed in the infundibular nucleus with kisspeptin, a neuropeptide that has been shown to stimulate the activity of GnRH neurones in a number of mammalian species. Ramaswamy et al (13, 37) demonstrated that tachyphylaxis to kisspeptin is sufficient to blunt the effects of NKB on LH secretion in male agonadal rhesus monkeys; however, NKB tachyphylaxis does not impair the effects of kisspeptin on LH secretion. Furthermore, Young et al (29) recently demonstrated that kisspeptin administration was sufficient to restore pulsatile LH secretion in patients with inactivating mutations in TAC3 or TAC3R. These data both suggest that NKB may signal to GnRH neurons by modulating kisspeptin secretion. It would be therefore interesting to study the effects of coadministration of NKB and kisspeptin in humans.
In summary, we have performed a first-to-man study of NKB administration. Although it is accepted that NKB signaling plays an important role in development of the human reproductive axis, iv administration of NKB has no significant effect on reproductive hormone secretion or LH pulsatility in healthy men or women.
Acknowledgments
This work was supported by a Medical Research Council Experimental Medicine project grant. The department is funded by an Integrative Mammalian Biology Capacity Building Award and the National Institute for Health Research (NIHR) Biomedical Research Centre Funding Scheme. We are grateful to the McMichael Wellcome Trust Clinical Research Facility for providing infrastructure for this study. C.N.J. is supported by an NIHR Clinical Lectureship, an Academy of Medical Sciences/Wellcome Starter Grant for Clinical Lecturers, and A Society for Endocrinology Early Career Grant. A.N.C. and A.D.S. are supported by Wellcome Trust/GlaxoSmithKline Translational Medicine Training Fellowships. G.M.K.N. and A.A. are supported by Wellcome Trust Research Training Fellowships. W.S.D. is supported by an NIHR Career Development Fellowship.
Disclosure Summary: The authors declare no conflicts of interest.
Footnotes
- BP
- blood pressure
- icv
- intracerebroventricular
- KNDy
- kisspeptin/neurokinin B/dynorphin
- NKB
- neurokinin B
- NK1R
- neurokinin 1 receptor
- NK2R
- neurokinin 2 receptor
- NK3R
- neurokinin 3 receptor
- TAC3
- tachykinin 3
- TAC3R
- TAC3 receptor.
References
- 1. Maggio JE. Tachykinins. Annu Rev Neurosci. 1988;11:13–28 [DOI] [PubMed] [Google Scholar]
- 2. Page NM. New challenges in the study of the mammalian tachykinins. Peptides. 2005;26:1356–1368 [DOI] [PubMed] [Google Scholar]
- 3. Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guran T, Tolhurst G, Bereket A, et al. Hypogonadotropic hypogonadism due to a novel missense mutation in the first extracellular loop of the neurokinin B receptor. J Clin Endocrinol Metab. 2009;94:3633–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Young J, Bouligand J, Francou B, et al. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab. 2010;95:2287–2295 [DOI] [PubMed] [Google Scholar]
- 6. Gianetti E, Tusset C, Noel SD, et al. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab. 2010;95:2857–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Francou B, Bouligand J, Voican A, et al. Normosmic congenital hypogonadotropic hypogonadism due to TAC3/TACR3 mutations: characterization of neuroendocrine phenotypes and novel mutations. PloS one. 2011;6:e25614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wakabayashi Y, Nakada T, Murata K, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30:3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abel TW, Voytko ML, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J Clin Endocrinol Metab. 1999;84:2111–2118 [DOI] [PubMed] [Google Scholar]
- 12. Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92:2744–2750 [DOI] [PubMed] [Google Scholar]
- 13. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151:4494–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sandoval-Guzman T, Rance NE. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res. 2004;1026:307–312 [DOI] [PubMed] [Google Scholar]
- 15. Billings HJ, Connors JM, Altman SN, et al. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology. 2010;151:3836–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Navarro VM, Castellano JM, McConkey SM, et al. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2011;300:E202–E210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kinsey-Jones JS, Grachev P, Li XF, et al. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology. 2012;153:307–315 [DOI] [PubMed] [Google Scholar]
- 18. Ruiz-Pino F, Navarro VM, Bentsen AH, et al. Neurokinin B and the control of the gonadotropic axis in the rat: developmental changes, sexual dimorphism, and regulation by gonadal steroids. Endocrinology. 2012;153:4818–4829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grachev P, Li XF, Elsamani L, et al. GPR54-dependent stimulation of luteinizing hormone secretion by neurokinin B in prepubertal rats. PloS One. 2012;7:e44344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grachev P, Li XF, Kinsey-Jones JS, et al. Suppression of the GnRH pulse generator by neurokinin B involves a κ-opioid receptor-dependent mechanism. Endocrinology. 2012;153:4894–4904 [DOI] [PubMed] [Google Scholar]
- 21. Kraegen EW, Lazarus L, Meler H, Campbell L, Chia YO. Carrier solutions for low-level intravenous insulin infusion. BMJ. 1975;3:464–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Edwards CM, Todd JF, Mahmoudi M, et al. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9–39. Diabetes. 1999;48:86–93 [DOI] [PubMed] [Google Scholar]
- 23. Liu PY, Keenan DM, Kok P, Padmanabhan V, O'Byrne KT, Veldhuis JD. Sensitivity and specificity of pulse detection using a new deconvolution method. Am J Physiol Endocrinol Metab. 2009;297:E538–E544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hrabovszky E, Molnar CS, Sipos MT, et al. Sexual dimorphism of kisspeptin and neurokinin B immunoreactive neurons in the infundibular nucleus of aged men and women. Front Endocrinol (Lausanne). 2011;2:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dhillo WS, Chaudhri OB, Thompson EL, et al. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab. 2007;92:3958–3966 [DOI] [PubMed] [Google Scholar]
- 26. Jayasena CN, Nijher GM, Comninos AN, et al. The effects of kisspeptin-10 on reproductive hormone release show sexual dimorphism in humans. J Clin Endocrinol Metab. 2011;96:E1963–E1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dhillo WS, Chaudhri OB, Patterson M, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90:6609–6615 [DOI] [PubMed] [Google Scholar]
- 28. de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154:2750–2760 [DOI] [PubMed] [Google Scholar]
- 29. Young J, George JT, Tello JA, Guiochon-Mantel A, et al. Kisspeptin restores pulsatile LH secretion in patients with neurokinin B signaling deficiencies: physiological, pathophysiological and therapeutic implications. Neuroendocrinology. 2013;97:193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Advenier C, Naline E, Drapeau G, Regoli D. Relative potencies of neurokinins in guinea pig trachea and human bronchus. Eur J Pharmacol. 1987;139:133–137 [DOI] [PubMed] [Google Scholar]
- 31. Drapeau G, D'Orleans-Juste P, Dion S, Rhaleb NE, Rouissi NE, Regoli D. Selective agonists for substance P and neurokinin receptors. Neuropeptides. 1987;10:43–54 [DOI] [PubMed] [Google Scholar]
- 32. Bhogal N, Donnelly D, Findlay JB. The ligand binding site of the neurokinin 2 receptor. Site-directed mutagenesis and identification of neurokinin A binding residues in the human neurokinin 2 receptor. J Biol Chem. 1994;269:27269–27274 [PubMed] [Google Scholar]
- 33. Huang RR, Huang D, Strader CD, Fong TM. Conformational compatibility as a basis of differential affinities of tachykinins for the neurokinin-1 receptor. Biochemistry. 1995;34:16467–16472 [DOI] [PubMed] [Google Scholar]
- 34. Almeida TA, Rojo J, Nieto PM, Hernandez M, et al. Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem. 2004;11:2045–2081 [DOI] [PubMed] [Google Scholar]
- 35. Goodmann RL, Porter KL, Connors JM, Hileman SM. Neurokinin B and the NKB receptor agonist, senktide, act in the ovine arcuate nucleus to produce different patterns of LH release. Program of the 95th Annual Meeting of The Endocrine Society, Expo, San Francisco, CA, 2013 (Abstract OR04–3) [Google Scholar]
- 36. Evans TW, Dixon CM, Clarke B, Conradson TB, Barnes PJ. Comparison of neurokinin A and substance P on cardiovascular and airway function in man. Br J Clin Pharmacol. 1988;25:273–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramaswamy S, Seminara SB, Plant TM. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology. 2011;94:237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]