Abstract
Context:
Little is known of the relationships between muscle function and bone, based on the recently developed technique of jumping mechanography.
Objective:
Our objective was to determine associations between peak ground reaction force and peak power during a 1-legged hopping test and a single 2-legged jump, respectively, and cortical bone parameters.
Design and Setting:
This was a cross-sectional observational study in participants from the high bone mass cohort.
Participants:
Participants included 70 males (mean age 58 years) and 119 females (mean age 56 years); high bone mass cases and controls were pooled.
Main Outcome Measures:
Total hip bone mineral density (BMD) (measured by dual-energy x-ray absorptiometry scanning) and mid-tibial peripheral quantitative computed tomography (Stratec XCT2000L).
Results:
Jump power was positively related to hip BMD (standardized β [95% confidence interval] = 0.29 [0.07, 0.51], P = .01), but hopping force was not (0.03 [−0.16, 0.22], P = .74) (linear regression analysis adjusted for age, gender, height, and weight). In 113 participants with force and peripheral quantitative computed tomography data, both jump power and hopping force were positively associated with tibial strength strain index (0.26 [0.09, 0.44], P < .01; and 0.24 [0.07, 0.42], P = .01 respectively). Although hopping force was positively associated with bone size (total bone area 0.22 [0.03, 0.42], P = .02), jump power was not (0.10 [−0.10, 0.30], P = .33). In contrast, jump power was inversely associated with endocortical circumference adjusted for periosteal circumference (−0.24 [−0.40, −0.08], P < .01) whereas no association was seen for hopping force (−0.10 [−0.26, 0.07], P = .24).
Conclusions:
Although power and force are both positively associated with cortical bone strength, distinct mechanisms appear to be involved because power was primarily associated with reduced endocortical expansion (reflected by endocortical circumference adjusted for periosteal circumference, and hip BMD), whereas force was associated with increased periosteal expansion (reflected by total bone area).
The concept that the skeleton is able to adapt to loads imposed upon it has been termed the mechanostat theory, according to which the bone mass of an animal is proportional to its typical mechanical use (1), as bone must be strong enough to withstand peak forces in order to prevent fractures under normal use (2, 3). Regional muscle contractions are suggested to be the most significant source of strains acting upon the skeleton in day-to-day life (4–6), exceeding contributions from body weight even in weight-bearing bones (2, 5). In support of this theory, several cross-sectional studies have observed that muscle mass in humans is proportional to bone mass (4, 7–9). Furthermore, studies examining isometric/isokinetic muscle strength have found positive associations with bone mass/bone mineral density (BMD) (3, 10–12). Muscle function has also been associated with bone strength in cross-sectional studies, such as strength strain index (SSI) as measured by peripheral quantitative computed tomography (pQCT) (13, 14). Loss of muscle function is also likely to play a key role in the pathogenesis of osteoporosis, as reflected by evidence that sarcopenia contributes to bone loss and increased fracture risk in older populations (15, 16).
Jumping mechanography provides an alternative method for assessing muscle function by measurement of ground reaction forces produced while jumping on a force plate. Unlike isometric methods, this allows maximal muscle forces to be estimated, providing a more accurate estimate of peak strains thought to be key drivers of skeletal adaptation (17). Peak force is measured directly in newtons by jumping mechanography, ideally during the multiple 1-legged hopping test because peak forces are typically greater during 1-legged serial hopping than during either 2-legged serial hopping or countermovement or squat jumps (17). Jumping mechanography also allows peak power to be calculated as the product of force and velocity (18, 19), typically measured during the standing 2-legged countermovement jump. Jumping mechanography is reproducible across a broad age range from children to frail older populations (19, 20).
Relatively few studies have compared the functional consequences of peak force and power as measured by jumping mechanography. While peak power is thought to reflect the action of hip, thigh, and (to a lesser extent) calf muscles, in addition to muscle activity peak force is also likely to be influenced by joint angle kinematics, tensile properties of the Achilles tendon, and biomechanical properties of the ankle, subtalar, and midfoot joints. Therefore, whilst peak muscle power is strongly influenced by muscle conditioning, peak force may have other determinants such as Achilles tendon elasticity that are largely governed by constitutional factors and likely to be more consistent over the lifespan. In support of this, age predicts declines in peak power more strongly than declines in peak force when both are assessed using jumping mechanography (21). However, the extent to which peak muscle power and ground reaction force show distinct relationships with cortical bone strength is currently unclear. In one study of 105 prepubertal children, bone strength, as reflected by SSI obtained from tibial pQCT scans, was related to peak power as measured by jumping mechanography more strongly than to peak force (22). Whether these relationships persist into adulthood remains to be determined.
In this study, we used jumping mechanography to investigate the role of muscle function in maintaining lower limb bone strength in later life. We aimed to establish 1) whether peak jumping power and force are both related to bone strength as assessed at the midtibia by pQCT, 2) whether these relationships are greater for power as opposed to force in adults, and 3) whether any potential differences in the relationships between peak power/force and bone strength are explained by distinct associations with bone microarchitecture (cortical bone size, thickness, and density). To aid statistical power and maximize our ability to detect underlying associations, we studied a population augmented by individuals with extreme high bone mass (HBM), in whom cortical bone strength as measured by pQCT is known to be increased (23); by pooling HBM and non-HBM individuals, we ensured a wide range of values for the bone traits of interest within our study population.
Subjects and Methods
Participant recruitment
The HBM study is a United Kingdom-based multicenter observational study of adults with unexplained HBM and unaffected family/spouse controls. At 3 of our larger study centers, 338 cases of unexplained HBM were identified by screening National Health Service dual-energy x-ray absorptiometry (DXA) databases (n = 158 769); these methods have been previously reported (24). HBM in index cases was defined as 1) both L1 Z-score of ≥+3.2 and total hip Z-score of ≥+1.2 or 2) both total hip Z-score ≥+3.2 and L1 Z-score of ≥+1.2. HBM status in first-degree relatives was defined as summed L1 Z-score plus total hip Z-score of ≥+3.2; in spouses, the index definition was applied. Family controls comprised unaffected relatives and spouses. All participants were clinically assessed by one doctor using a standardized structured history questionnaire and examination, after which lumbar spine and hip DXA scans were performed for relatives and spouses, using local GE Lunar Inc DXA systems applying manufacturer's standard scan and positioning protocols. The questionnaire included smoking status, alcohol use and diabetes. Routine height measurements were recorded. Most participants later completed a postal physical activity questionnaire based on the short last 7 days self-administered International Physical Activity Questionnaire (25) (revised version August 2002) and scored using standard protocols with activity levels categorized as low, moderate, or high. Participants were excluded if under 18 years of age, pregnant, or unable to provide written informed consent for any reason.
Jumping mechanography
A Leonardo Mechanography Ground Reaction Force Platform (Leonardo software version 4.2; Novotec Medical GmbH) was used to assess lower limb muscle force, power, velocity, jumping height, and total body weight by a trained assistant using a standard operating procedure. The ground reaction platform was calibrated before each participant assessment, and participants wore standard footwear. When an individual had difficulty performing either jump type, free text details were recorded by the operator. Peak power and jump height were assessed during countermovement jumps; ie, individuals briefly squat before jumping. The initial jump was 2-footed. Individuals were asked to jump, aiming to get their head as high as possible, thus producing the maximum elevation of the center of mass. Peak force was assessed for the dominant leg, during the hopping test, with participants instructed to bounce on the ball of the foot with the knee almost straight and to keep the ankle joint as stiff as possible. Each type of jump was repeated until 3 acceptable jumps were obtained, and the jump with the greatest peak power/force was then analyzed.
pQCT methods
pQCT scans were performed in those participants attending the center at Hull, where the largest number were seen. The diaphyseal midshaft of the tibia (66% from the distal endplate) was scanned in the nondominant lower limb using a Stratec XCT2000L (Stratec Medizintechnik) with voxel size 0.5 mm, CT speed 30 mm/s (XCT software version 5.50d). A reference line at the distal endplate was determined from the initial frontal scout view. Cortical bone was defined using a threshold above 650 mg/cm3 (optimal for bone geometry) (26). Cortical thickness, periosteal circumference, and endocortical circumference were derived using a circular ring model. Other cortical parameters were measured, including cortical BMD, total bone area, cortical bone area (reflecting a combination of periosteal and endocortical expansion), and cortical bone area/total bone area (percent). SSI was calculated according to Stratec's user manual (27). Data acquisition and analysis methods were uniform for all participants.
Ethics
Recruitment ran from September 2008 until April 2010. Written informed consent was obtained for all in line with the Declaration of Helsinki (28). This study was approved by the Bath Multicenter Research Ethics Committee and at each National Health Service Local Research Ethics Committee.
Statistical methods
Descriptive statistics are presented as mean (SD) for continuous variables and counts (percent) for categorical variables for all relevant outcomes, exposures, and covariates. In summarizing the jumping mechanography data, mean values for both absolute muscle power (kilowatts) and force (kilonewtons) as well as relative muscle power (watts per kilogram) and force (newtons per kilogram) were generated. Power and force were both log-transformed. Multiple linear regression was used to examine associations between the exposure variables muscle power (standing 2-legged jump) and force (hopping test) and bone outcomes (DXA hip BMD and pQCT mid-tibial structural variables). Age, gender, and height were considered a priori confounders (model 1), and weight was an additional key confounder (model 2); we planned therefore to base our inferences primarily on model 2. The influence of other potential confounders (smoking, alcohol, diabetes, and physical activity) was additionally explored. The relationship between force/log force and weight was approximately linear, but that between power/log power and weight was better represented by adding a quadratic; therefore, absolute force (kilonewtons) and power (kilowatts) were the exposures, adjusting for weight (force)/weight and weight2 (power) in the regression model. A similar approach has been used in a previous study analyzing jumping mechanography variables in a population of adolescent girls (18). All bone outcome variables were normally distributed, although one isolated extreme outlier with total hip Z-score of +9.4 was excluded. Exposure and outcome variables were standardized for regression analyses, with standardized β-coefficients presented. Analyses of endocortical circumference were additionally adjusted for periosteal circumference (ECPC) to provide an estimate of endocortical expansion, independent of bone size. Data were managed using Microsoft Access (data entry checks; error rate <0.12%) and analyzed using Stata release 12 statistical software (StataCorp). Preplanned sensitivity analyses were as follows: 1) exclusion of individuals with difficulty performing the relevant jump and 2) restriction of analyses to individuals who successfully completed both jump types.
Results
Participant characteristics
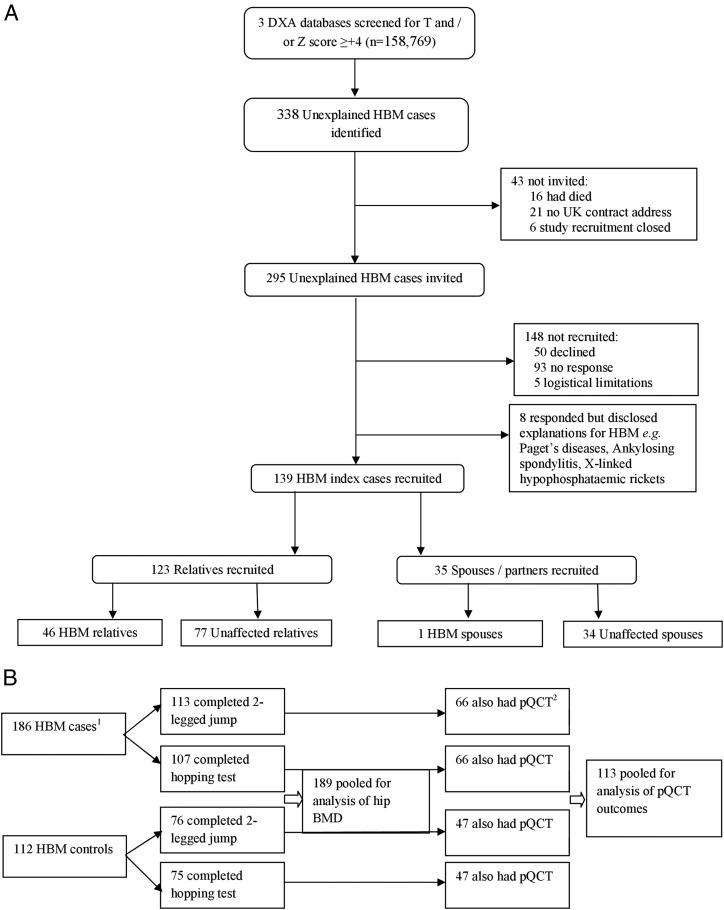
The study population comprised 189 participants who completed the 2-legged jump, of whom 113 were originally recruited as HBM cases and 76 as controls (Figure 1); 182 of the participants also completed the hopping test. The mean age of the study population was 57 years, with 63% females (of whom 68.9% were postmenopausal) (Table 1). The population was overweight with a mean body mass index (BMI) of 29.4 kg/m2. Mean total hip Z-score was +1.9 (range −1.9 to +6.4). All individuals were of white European origin. The principal characteristics of individuals who received a tibial pQCT scan (n = 113, 59%) were compared with those who did not (Supplemental Table 1, published on The Endocrine Society's Journals Online website at http://jcem.endojournals.org). There were no differences in age, absolute hopping force, or menopausal status in females according to whether a subject underwent pQCT. Weight, height, BMI, jump power, and relative hopping force were all greater in those who did not undergo pQCT, explained by the greater proportion of females in the pQCT group.
Figure 1.
Flow diagram summarizing (A) recruitment of HBM cases and controls to this study and (B) jumping mechanography and pQCT data collection.
Table 1.
Descriptive Characteristics of Overall Study Populationa
| All (n = 189) | HBM Cases (n = 113) |
HBM Controls (n = 76) |
|||
|---|---|---|---|---|---|
| Males (n = 24) | Females (n = 89) | Males (n = 46) | Females (n = 30) | ||
| Age, y | 57.03 ± 13.66 | 63.04 ± 13.74 | 58.41 ± 11.94 | 55.65 ± 14.51 | 50.23 ± 14.62 |
| Weight, kg | 84.76 ± 18.17 | 92.21 ± 14.11 | 82.99 ± 18.29 | 89.18 ± 15.23 | 77.26 ± 21.49 |
| Height, m | 169.57 ± 9.53 | 178.27 ± 7.59 | 164.08 ± 6.08 | 178.82 ± 7.01 | 164.72 ± 6.27 |
| BMI, kg/m2 | 29.43 ± 5.67 | 28.96 ± 3.65 | 30.77 ± 6.10 | 27.89 ± 4.50 | 28.22 ± 6.49 |
| Two-legged max jump power, kW | 2.49 ± 0.96 | 3.02 ± 0.95 | 2.05 ± 0.56 | 3.35 ± 1.02 | 2.01 ± 0.64 |
| Relative 2-legged max jump power, W/kg | 29.29 ± 8.92 | 32.69 ± 8.81 | 25.06 ± 5.76 | 37.61 ± 9.33 | 26.41 ± 5.99 |
| Two-legged max jump height, m (n = 188) | 0.27 ± 0.16 | 0.32 ± 0.13 | 0.24 ± 0.19 | 0.33 ± 0.11 | 0.26 ± 0.11 |
| Two-legged max jump velocity m/s | 1.81 ± 0.40 | 1.97 ± 0.40 | 1.63 ± 0.29 | 2.16 ± 0.39 | 1.68 ± 0.30 |
| One-legged max hopping force, kN (n = 182) | 2.04 ± 0.50 | 2.31 ± 0.43 | 1.85 ± 0.39 | 2.36 ± 0.53 | 1.88 ± 0.44 |
| Relative 1-legged max hopping force, N/kg (n = 182) | 24.50 ± 4.83 | 24.89 ± 4.48 | 23.10 ± 4.33 | 26.58 ± 4.90 | 25.06 ± 5.22) |
| Females | 119 (62.96) | 89 (100.00) | 30 (100.00) | ||
| Postmenopausal | 82 (68.91) | 70 (78.65) | 12 (40.00) | ||
| Physical activityb (n = 172) | |||||
| Low | 18 (10.50) | 2 (9.09) | 9 (10.98) | 3 (7.69) | 4 (13.79) |
| Moderate | 66 (38.40) | 11 (50.00) | 32 (39.02) | 11 (28.21) | 12 (41.38) |
| High | 88 (51.20) | 9 (40.91) | 41 (50.00) | 25 (64.10) | 13 (44.83) |
| Smoking status (n = 188) | |||||
| Never | 81 (43.10) | 4 (17.39) | 41 (46.07) | 21 (45.65) | 15 (50.00) |
| Ex | 83 (44.20) | 16 (69.57) | 36 (40.45) | 20 (43.48) | 11 (36.67) |
| Current | 24 (12.80) | 3 (13.04) | 12 (13.48) | 5 (10.87) | 4 (13.33) |
| Alcohol intakec | |||||
| None | 45 (23.80) | 4 (16.67) | 28 (31.46) | 4 (8.70) | 9 (30.00) |
| Occasional | 29 (15.30) | 5 (20.83) | 18 (20.22) | 2 (4.35) | 4 (13.33) |
| Regular | 85 (45.00) | 14 (58.33) | 35 (39.33) | 22 (47.83) | 14 (46.67) |
| Heavy | 30 (15.90) | 1 (4.17) | 8 (8.99) | 18 (39.13) | 3 (10.00) |
| Diabetes | 18 (9.50) | 2 (8.33) | 8 (8.99) | 5 (10.87) | 3 (10.00) |
Results are shown as mean ±SD or n (%). Unless stated otherwise, n = 189.
Physical activity categories derived from International Physical Activity Questionnaire short form last 7 days questionnaire (standard scoring used).
Occasional means <2 U/wk, regular is 3 to 21 U/wk, and heavy is >21 U/wk.
Two-legged jump power and bone outcomes
After adjustment for age, gender, and participant height, jump power was strongly associated with total hip BMD (standardized β [95% confidence interval, CI] = 0.48 [0.28, 0.67], P < .01, model 1, Table 2). Jump power was also strongly positively associated with midtibial cortical area, total bone area, cortical to total bone area ratio, and tibial SSI (model 1, Table 2). A strong inverse association between 2-legged jump power and endocortical circumference (ECPC) was also observed.
Table 2.
Regression Analysis of Logged Maximum 2-Legged Jump Power Versus Bone Outcomesa
| Outcome | Model | β-Coefficient | 95% CI | P Value |
|---|---|---|---|---|
| Hip BMD, g/cm2 | 1 | 0.48 | 0.28, 0.67 | <.01 |
| 2 | 0.29 | 0.07, 0.51 | .01 | |
| Cortical area, mm2 | 1 | 0.38 | 0.23, 0.53 | <.01 |
| 2 | 0.29 | 0.11, 0.46 | <.01 | |
| Cortical BMD, mg/cm3 | 1 | 0.23 | −0.06, 0.51 | .12 |
| 2 | 0.39 | 0.06, 0.73 | .02 | |
| ECPC, mm | 1 | −0.32 | −0.46, −0.18 | <.01 |
| 2 | −0.24 | −0.40, −0.08 | <.01 | |
| Total bone area, mm2 | 1 | 0.18 | (0.01, 0.36 | .04 |
| 2 | 0.10 | −0.10, 0.30 | .33 | |
| Cortical to total bone area ratio, % | 1 | 0.29 | 0.03, 0.56 | .03 |
| 2 | 0.26 | −0.06, 0.57 | .11 | |
| Tibial SSI, mm3 | 1 | 0.34 | 0.18, 0.49 | <.01 |
| 2 | 0.26 | 0.09, 0.44 | <.01 |
All outcome and exposure variables were standardized. Standardized β-coefficients represent SD change in outcome per SD change in exposure (log 2-legged jump power). Model 1 was adjusted for age, gender, and height. Model 2 was adjusted for age, gender, height, and weight (as quadratic term). For hip BMD, n = 189; for all other other outcomes, n = 113.
After additional weight adjustment (model 2, Table 2), jump power remained strongly associated with hip BMD (0.29 [0.07, 0.51], P = .01), cortical area of the midtibia (0.29 [0.11, 0.46], P < .01), and tibial strength (SSI 0.26 [0.09, 0.44], P < .01); however, the association with overall bone size (total bone area (0.10 [−0.10, 0.30], P = .33, model 2, Table 2) was attenuated. Interestingly, power remained strongly inversely associated with ECPC after age, gender, height, and weight adjustment (−0.24 [−0.40, −0.08], P < .01, model 2, Table 2), suggesting that jump power is more closely related to reduced endocortical expansion than periosteal expansion. Although a positive association was seen between jump power and cortical BMD (0.39 [0.06, 0.73], P = .02), examination of the distribution revealed one obvious outlier, exclusion of which attenuated this association (0.23 [−0.08, 0.54], P = .15 excluding outlier).
Hopping force and bone outcomes
After adjustment for age, gender, and height (model 1), hopping force was positively associated with hip BMD (0.21 [0.04, 0.38], P = .02). Further positive associations were seen with midtibial cortical area, total bone area, and tibial SSI, and again, an inverse association was seen with ECPC (model 1, Table 3). There was no association between hopping force and cortical to total bone area ratio (0.09 [−0.15, 0.32], P = .47) or cortical BMD (−0.11 [−0.36, 0.14], P = .38). Weight adjustment (model 2) fully attenuated the association between hopping force and hip BMD (0.03 [−0.16, 0.22], P = .74) and ECPC (−0.10 [−0.26, 0.07], P = .24). The association with midtibial cortical area was also weakened (0.17 [−0.01, 0.35], P = .06). However, a strong association persisted between hopping force and total bone area (0.22 [0.03, 0.42], P = .02) and tibial SSI (0.24 [0.07, 0.42], P = .01) after weight adjustment.
Table 3.
Regression Analysis of Logged Maximum 1-Legged Hopping Force Versus Bone Outcomesa
| Outcome | Model | β-Coefficient | 95% CI | P Value |
|---|---|---|---|---|
| Hip BMD, g/cm2 | 1 | 0.21 | 0.04, 0.38 | .02 |
| 2 | 0.03 | −0.16, 0.22 | .74 | |
| Cortical area, mm2 | 1 | 0.26 | 0.12, 0.40 | <.01 |
| 2 | 0.17 | −0.01, 0.35 | .06 | |
| Cortical BMD, mg/cm3 | 1 | −0.11 | −0.36, 0.14 | .38 |
| 2 | −0.10 | −0.42, 0.23 | .55 | |
| ECPC, mm | 1 | −0.20 | −0.33, −0.07 | <.01 |
| 2 | −0.10 | −0.26, 0.07 | .24 | |
| Total bone area, mm2 | 1 | 0.21 | 0.06, 0.36 | .01 |
| 2 | 0.22 | 0.03, 0.42 | .02 | |
| Cortical to total bone area ratio, % | 1 | 0.09 | −0.15, 0.32 | .47 |
| 2 | −0.07 | −0.38, 0.23 | .63 | |
| Tibial SSI, mm3 | 1 | 0.26 | 0.12, 0.39 | <.01 |
| 2 | 0.24 | 0.07, 0.42 | .01 |
All outcome and exposure variables were standardized. Standardized β-coefficients represent SD change in outcome per SD change in exposure (log 1-legged hopping force). Model 1 was adjusted for age, gender, and height. Model 2 was adjusted for age, gender, height, and weight. For hip BMD, n = 182; for all other other outcomes, n = 113.
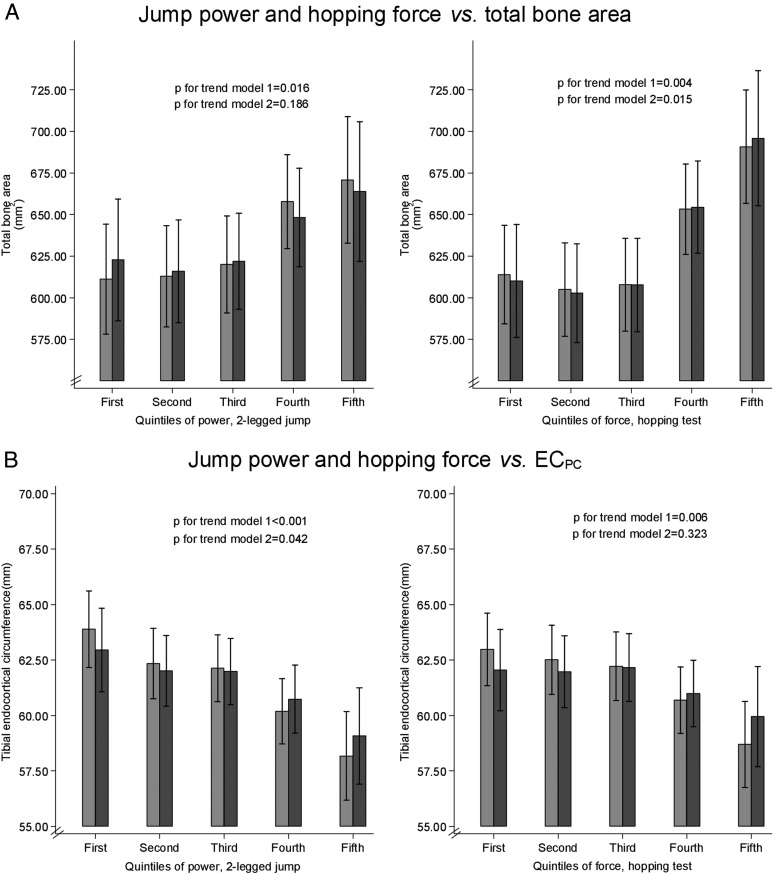
Analysis by quintiles
Further analyses were carried out subdividing jump power and force into quintiles to ascertain the dose-response relationship between the muscle variables and bone outcomes (Figure 2). After adjusting for age, gender, height, and weight (model 2), a stronger association was observed between quintile of hopping force and total bone area compared with quintile of jump power. There was a possible threshold effect, with the higher values for total bone area largely restricted to the upper 2 quintiles of power/force. Conversely, quintile of jump power was more strongly associated with ECPC compared with quintile of hopping force. The association between jump power quintile and ECPC was in keeping with a dose-response relationship, with no evidence of a threshold effect. No association was seen between quintile of jump power or hopping force and cortical BMD (not shown).
Figure 2.
Quintiles of jump power/hopping force plotted against pQCT outcomes. A, Quintiles of jump power and hopping force vs total bone area (square millimeters). B, Quintiles of jump power and hopping force vs ECPC (millimeters). Model 1 was adjusted for age, gender, and height; model 2 was adjusted for age, gender, height, and weight.
Sensitivity analyses and interactions
Physical activity data were available for 172 study participants (91%) a mean of 7.9 months after the jump test, adjustment for which did not materially alter the results (data not shown). Likewise adjusting for smoking status, alcohol intake, and diabetes did not alter the associations seen. Point estimates were unaffected by exclusion of those with difficulty in jumping (n = 6 for 2-legged jump, n = 23 for hopping test) (data not shown). Repeating the analysis in the subset of individuals who completed both jump types and therefore had both force and power data (n = 181 for hip BMD, n = 111 for pQCT variables) also did not materially alter the associations seen. In the above analyses, data from HBM cases and controls were pooled to increase statistical power. The validity of this approach was checked by examination of scatter plots for hip BMD. Although there was some evidence that the positive association between 2-legged jump power and hip BMD was stronger in controls, interaction tests showed P > .1 (Supplemental Figure 1, left panels). In further analyses stratified by gender, there was no evidence of a gender interaction (Supplemental Figure 1, right panels).
Discussion
We used jumping mechanography to determine associations between peak power and force, based on the 2-legged jump and hopping test, respectively, and skeletal parameters from hip DXA and mid-tibial pQCT scans. In our fully adjusted model, peak jump force and power showed equivalent positive associations with bone strength, as reflected by tibial SSI. However, the pathways involved appeared to differ. Peak force was primarily positively associated with total bone area of the mid-tibia, suggesting a relationship with periosteal expansion. Conversely, peak power was primarily inversely associated with ECPC, suggesting an inverse relationship with endocortical expansion. Furthermore, peak power was positively associated with hip BMD, whereas an equivalent association was not seen for peak force. In contrast, after exclusion of an outlier, there was little evidence that peak power was related to cortical BMD. Hip BMD is affected by several different parameters measured by pQCT, including overall bone size, cortical BMD, and cortical thickness. However when taken together, the present findings suggest that associations between peak power and hip BMD that we observed are predominantly related to changes in cortical thickness as reflected by ECPC.
Our findings are broadly consistent with previous studies indicating a positive relationship between measures of muscle function and bone outcomes. For example, grip strength was found to be positively related to SSI as assessed by radial pQCT in older men (13) and in a population of women aged 65 to 75 years, peak muscle power was related to bone strength as assessed by tibial pQCT (14). However, few previous studies have evaluated relationships between bone parameters and peak force/power as assessed by jumping mechanography. Bailey and Brooke-Wavell (3) measured peak ground reaction force during a maximal countermovement hop in a population of 88 sedentary premenopausal women in relation to areal lumbar spine and hip BMD. Although an association between peak force and BMD was initially observed, this did not persist after adjustment for body mass, in contrast to a positive association that persisted between isometric knee extension strength and hip BMD. Anliker et al (17) examined associations between maximum force during multiple 1-legged hopping and tibial volumetric bone mineral content (vBMC) as a measure of bone strength in a mixed-gender population aged 8 to 82 years. Peak force was strongly correlated with vBMC at the 14% tibial site; this correlation was stronger than that between vBMC and muscle area, suggesting a greater influence of muscle function compared with muscle mass on skeletal strength. Similarly, Rantalainen et al (29, 30) showed a strong correlation between peak power, measured during a maximum countermovement jump, and SSI at the tibial midshaft in a population of pre- and postmenopausal women (29), and correlation of both maximal ground reaction force and peak power with bone strength (as section modulus) in young and elderly men (30). We are aware of only one previous jumping mechanography study to have compared peak power and force in terms of skeletal outcomes. Binkley and Specker in 2008 (22) examined muscle-bone relationships in prepubertal children, measuring peak power and force during a 2-footed countermovement jump. Their observation of a stronger positive association between peak power and cortical area (assessed at the 20% tibial site), as compared with peak force, is consistent with our present findings. However, in contrast to our observations, they found peak power to be more strongly related to total bone area and SSI compared with force, raising the possibility that relationships between peak force and bone area and strength are stronger in older adults as compared with children. A limitation of this study compared with ours was the use of the 2-legged jump to assess force, which may lead to underestimation of maximal force (17).
As experimental studies have shown that most bending moments on a bone arise from muscle forces (6), it follows that muscles capable of producing greater force should result in stronger bones. However, it is not clear how maximum force and power outputs that can be generated during an activity such as jumping relate to day-to-day muscle-induced bone strains. The strains that bones are habitually subjected to may depend upon other factors such as physical activity level in addition to intrinsic muscle properties. For example, studies in the myostatin-knockout mouse have shown that the effects of increased muscle size and strength on bone may depend upon physical activity, with differences between mutant mice and controls becoming apparent only after a period of exercise (6, 31). However in the present study, adjusting for self-reported levels of physical activity did not alter our results. Other factors that may limit the effect of intrinsic muscle force/power on bone include tendon properties, which alter with age (21) and may not be readily modifiable.
A few studies in humans have suggested that physical activity has similar associations with pQCT-measured bone geometry to those seen here for jumping mechanography parameters. For example, Sayers et al (32) looked at the associations between different intensities of habitual physical activity (quantified by accelerometer) in adolescents with tibial pQCT outcomes. Vigorous physical activity equivalent to jogging was associated with increased cortical BMC, arising from both increased periosteal circumference and reduced ECPC. Vainionpää et al (33) in a study of 120 women aged 35 to 40 years randomized to an exercise or control group also found that relatively high impact activity (equivalent to running/jumping) was associated with increased cortical thickness and bone circumference measured by QCT. Interestingly, studies comparing high-performance athletes with controls have additionally suggested that sports requiring very high muscle power outputs (eg, the triple jump) are associated with relatively larger differences in cortical area/thickness compared with those observed for total cross-sectional bone area (34, 35). This would support a particular association between reduced endocortical expansion and muscle power, although it is not clear to what extent findings in elite athletes translate to our much older, more sedentary study population.
The present study is consistent with these studies of physical activity that suggest that both periosteal and endocortical envelopes are responsive to mechanical strain. As for why peak force and power appear to differ in respect of their associations with these 2 envelopes, presumably this may reflect additional determinants of peak force as compared with peak power. Whereas muscle power is strongly influenced by physical activity and strength training, peak force may additionally be affected by features such as Achilles tendon elasticity, governed by constitutive factors that are currently poorly understood. Based upon our results, it is tempting to speculate that Achilles tendon elasticity shares common constitutive influences with skeletal traits such as overall bone size. Because both traits are relatively stable over time, their relationship is likely to persist or even strengthen throughout life. If this interpretation is correct, it would imply that, through its ability to evaluate peak force, jumping mechanography provides key additional information about the strain environment responsible for maintaining skeletal integrity as compared with conventional approaches centered on assessment of physical activity and/or muscle function.
One limitation of our study is its cross-sectional design, making it difficult to assess the direction of causality. As outlined by Robling (6), the close relationship between muscle strength/mass and bone mass/strength could be interpreted in several ways, including skeletal adaptation to greater muscle forces, muscular adaptation to increased bone mass, or an underlying genetic predisposition to both high muscle and bone mass/strength independently. Temporal relationships between gain of muscle strength and increases in BMD support a pathway by which changes in muscle strength affect bone rather than vice versa (5, 13). However, although the genetic basis of HBM in the majority of our cases is currently unknown (36), it is likely to be primarily genetically determined, raising the possibility that genes contributing to increased bone mass and strength may have pleiotropic influences on muscle properties. There is therefore a need to confirm these findings in other cohorts, because it is unclear whether findings in this extreme population would also apply to other populations with a more typical BMD distribution. An additional limitation of the study is the higher proportion of females and consequently reduced values for the key muscle measures in the subgroup who underwent pQCT scanning compared with the study population as a whole; our conclusions regarding the pQCT bone outcomes therefore require replication in other populations, particularly males. Another point to note is that in this study, pQCT was performed on the nondominant leg, whereas hopping force was tested on the dominant leg; although these measurements would ideally have been made on the same limb, we feel that in this nonathletic population, leg dominance is unlikely to significantly influence local strains and therefore should not have materially affected our findings. pQCT also has some inherent technical limitations. Nondifferential partial volume effect, which has a greater impact on thinner than thicker cortices, may have increased measurement error.
In conclusion, we have studied the relationship between peak force and power, as measured by jumping mechanography, and cortical bone traits in an adult population enriched by HBM cases in addition to unaffected family controls. In common with other studies, we demonstrated an association between peak force/power and bone strength (SSI) as measured by pQCT. However, peak force and power may modify cortical bone strength through distinct mechanisms, as force and power appeared to be primarily associated with increased periosteal expansion and reduced endocortical expansion, respectively. Additional studies are required to establish the basis for these differences and, in particular, whether these reflect a previously unappreciated role of factors unrelated to muscle function, such as Achilles tendon elasticity, in determining lower limb bone strength.
Acknowledgments
We thank all of our study participants and particularly the staff at the Wellcome Trust Clinical Research Facility in Birmingham, Cambridge NIHR Biomedical Research Centre and Addenbrooke's Wellcome Trust Clinical Research Facility, and the Brocklehurst Centre for Metabolic Bone Disease in Hull. We also acknowledge other members of the United Kingdom DXA Databases to Identify Novel Anabolic Genes consortium for assistance in setting up the local study centers including Sue Steel and Victor Lazar (Hull and East Yorkshire Hospitals National Health Service [NHS] Trust), Dr John Ayuk (University Hospitals Birmingham NHS Foundation Trust), and Dr Ken Poole (Cambridge University Hospitals NHS Foundation Trust).
This study was supported by The Wellcome Trust and the National Institute for Health Research Clinical Research Network-Comprehensive Local Research Networks (portfolio number 5163); supporting CLRNs included Birmingham and the Black Country, North and East Yorkshire and Northern Lincolnshire, and West Anglia. K.A.W. is supported by the core program of the Medical Research Council (MRC) Nutrition and Bone Health group at MRC Human Nutrition Research, funded by the United Kingdom MRC (Grant U10590371). C.L.G. was funded through a Wellcome Trust Clinical Research Training Fellowship (080280/Z/06/Z). Ongoing support is being provided by Arthritis Research UK, who fund S.H. through a Clinical PhD Studentship (Grant 19580) and C.L.G. through a Clinician Scientist Fellowship (Grant 20000).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMD
- bone mineral density
- BMI
- body mass index
- CI
- confidence interval
- DXA
- dual-energy x-ray absorptiometry
- ECPC
- endocortical circumference adjusted for periosteal circumference
- HBM
- high bone mass
- pQCT
- peripheral quantitative computed tomography
- SSI
- strength strain index
- vBMC
- volumetric bone mineral content.
References
- 1. Frost HM. Bone. “mass” and the “mechanostat”: a proposal. Anatomical Rec. 1987;219(1):1–9 [DOI] [PubMed] [Google Scholar]
- 2. Frost HM. Bone's mechanostat: a 2003 update. Anat Rec. 2003;275(2):1081–1101 [DOI] [PubMed] [Google Scholar]
- 3. Bailey CA, Brooke-Wavell K. Association of body composition and muscle function with hip geometry and BMD in premenopausal women. Ann Hum Biol. 2010;37(4):524–535 [DOI] [PubMed] [Google Scholar]
- 4. Ferretti JL, Capozza RF, Cointry GR, et al. Gender-related differences in the relationship between densitometric values of whole-body bone mineral content and lean body mass in humans between 2 and 87 years of age. Bone. 1998;22(6):683–690 [DOI] [PubMed] [Google Scholar]
- 5. Burr DB. Muscle strength, bone mass, and age-related bone loss. J Bone Miner Res. 1997;12(10):1547–1551 [DOI] [PubMed] [Google Scholar]
- 6. Robling AG. Is bone's response to mechanical signals dominated by muscle forces? Med Sci Sports Exerc. 2009;41(11):2044–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Capozza RF, Cointry GR, Cure-Ramírez P, Ferretti JL, Cure-Cure C. A DXA study of muscle-bone relationships in the whole body and limbs of 2512 normal men and pre- and post-menopausal women. Bone. 2004;35(1):283–295 [DOI] [PubMed] [Google Scholar]
- 8. Compston JE, Bhambhani M, Laskey MA, Murphy S, Khaw KT. Body composition and bone mass in post-menopausal women. Clin Endocrinol (Oxf). 1992;37(5):426–431 [DOI] [PubMed] [Google Scholar]
- 9. Tsunenari T, Tsutsumi M, Ohno K, et al. Age- and gender-related changes in body composition in Japanese subjects. J Bone Miner Res. 1993;8(4):397–402 [DOI] [PubMed] [Google Scholar]
- 10. Bauer DC, Browner WS, Cauley JA, et al. Factors associated with appendicular bone mass in older women. The Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1993;118(9):657–665 [DOI] [PubMed] [Google Scholar]
- 11. Nguyen TV, Center JR, Eisman JA. Osteoporosis in elderly men and women: effects of dietary calcium, physical activity, and body mass index. J Bone Miner Res. 2000;15(2):322–331 [DOI] [PubMed] [Google Scholar]
- 12. Snow-Harter C, Bouxsein M, Lewis B, Charette S, Weinstein P, Marcus R. Muscle strength as a predictor of bone mineral density in young women. J Bone Miner Res. 1990;5(6):589–595 [DOI] [PubMed] [Google Scholar]
- 13. Cousins JM, Petit MA, Paudel ML, et al. ; Osteoporotic Fractures in Men (MrOS) Study Group. Muscle power and physical activity are associated with bone strength in older men: The osteoporotic fractures in men study. Bone. 2010;47(2):205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ashe MC, Liu-Ambrose TY, Cooper DM, Khan KM, McKay HA. Muscle power is related to tibial bone strength in older women. Osteoporos Int. 2008;19(12):1725–1732 [DOI] [PubMed] [Google Scholar]
- 15. DiGirolamo DJ, Kiel DP, Esser KA. Bone and skeletal muscle: neighbors with close ties. J Bone Miner Res. 2013;28(7):1509–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gielen E, Verschueren S, O'Neill TW, et al. Musculoskeletal frailty: a geriatric syndrome at the core of fracture occurrence in older age. Calcif Tissue Int. 2012;91(3):161–177 [DOI] [PubMed] [Google Scholar]
- 17. Anliker E, Rawer R, Boutellier U, Toigo M. Maximum ground reaction force in relation to tibial bone mass in children and adults. Med Sci Sports Exerc. 2011;43(11):2102–2109 [DOI] [PubMed] [Google Scholar]
- 18. Ward KA, Das G, Berry JL, et al. Vitamin D status and muscle function in post-menarchal adolescent girls. J Clin Endocrinol Metab. 2009;94(2):559–563 [DOI] [PubMed] [Google Scholar]
- 19. Rittweger J, Schiessl H, Felsenberg D, Runge M. Reproducibility of the jumping mechanography as a test of mechanical power output in physically competent adult and elderly subjects. J Am Geriatr Soc. 2004;52(1):128–131 [DOI] [PubMed] [Google Scholar]
- 20. Veilleux LN, Rauch F. Reproducibility of jumping mechanography in healthy children and adults. J Musculoskelet Neuronal Interact. 2010;10(4):256–266 [PubMed] [Google Scholar]
- 21. Runge M, Rittweger J, Russo CR, Schiessl H, Felsenberg D. Is muscle power output a key factor in the age-related decline in physical performance? A comparison of muscle cross section, chair-rising test and jumping power. Clin Physiol Funct Imaging. 2004;24(6):335–340 [DOI] [PubMed] [Google Scholar]
- 22. Binkley TL, Specker BL. Muscle-bone relationships in the lower leg of healthy pre-pubertal females and males. J Musculoskelet Neuronal Interact. 2008;8(3):239–243 [PubMed] [Google Scholar]
- 23. Gregson CL, Sayers A, Lazar V, Steel S, Dennison EM, Cooper C, Smith GD, Rittweger J, Tobias JH. The high bone mass phenotype is characterised by a combined cortical and trabecular bone phenotype: findings from a pQCT case-control study. Bone. 2013;52(1):380–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gregson CL, Steel SA, O'Rourke KP, et al. ‘Sink or swim’: an evaluation of the clinical characteristics of individuals with high bone mass. Osteoporos Int. 2012;23(2):643–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395 [DOI] [PubMed] [Google Scholar]
- 26. Ward KA, Adams JE, Hangartner TN. Recommendations for thresholds for cortical bone geometry and density measurement by peripheral quantitative computed tomography. Calcif Tissue Int. 2005;77(5):275–280 [DOI] [PubMed] [Google Scholar]
- 27. XCT 2000 Manual Software Version 5.50, M-pQCT550.002. Pforzheim, Germany: Stratec Medizintechnik; 2004 [Google Scholar]
- 28. World Medical Association. Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. 59th WMA General Assembly, Seoul, Korea; 2008 [Google Scholar]
- 29. Rantalainen T, Nikander R, Heinonen A, et al. Neuromuscular performance and body mass as indices of bone loading in premenopausal and postmenopausal women. Bone. 2010;46(4):964–969 [DOI] [PubMed] [Google Scholar]
- 30. Rantalainen T, Sievänen H, Linnamo V, et al. Bone rigidity to neuromuscular performance ratio in young and elderly men. Bone. 2009;45(5):956–963 [DOI] [PubMed] [Google Scholar]
- 31. Hamrick MW, Samaddar T, Pennington C, McCormick J. Increased muscle mass with myostatin deficiency improves gains in bone strength with exercise. J Bone Miner Res. 2006;21(3):477–483 [DOI] [PubMed] [Google Scholar]
- 32. Sayers A, Mattocks C, Deere K, Ness A, Riddoch C, Tobias JH. Habitual levels of vigorous, but not moderate or light, physical activity is positively related to cortical bone mass in adolescents. J Clin Endocrinol Metab. 2011;96(5):E793–E802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vainionpää A, Korpelainen R, Sievänen H, Vihriälä E, Leppäluoto J, Jämsä T. Effect of impact exercise and its intensity on bone geometry at weight-bearing tibia and femur. Bone. 2007;40(3):604–611 [DOI] [PubMed] [Google Scholar]
- 34. Heinonen A, Sievänen H, Kyröläinen H, Perttunen J, Kannus P. Mineral mass, size, and estimated mechanical strength of triple jumpers' lower limb. Bone. 2001;29(3):279–285 [DOI] [PubMed] [Google Scholar]
- 35. Nikander R, Kannus P, Rantalainen T, Uusi-Rasi K, Heinonen A, Sievänen H. Cross-sectional geometry of weight-bearing tibia in female athletes subjected to different exercise loadings. Osteoporos Int. 2010;21(10):1687–1694 [DOI] [PubMed] [Google Scholar]
- 36. Duncan EL, Gregson CL, Addison K, et al. Mutations in LRP5 and SOST are a rare cause of high bone mass in the general population. Bone. 2009;44(suppl 2):S340–S341 [Google Scholar]