Abstract
Recent cognitive, genetic, and histological studies have highlighted significant overlap between psychotic bipolar disorder and schizophrenia. Specifically, both bipolar disorder and schizophrenia are characterized by interneuron dysfunction within the hippocampus, an essential structure for relational memory. Relational memory impairments are a common feature of schizophrenia, but have yet to be investigated in psychotic bipolar disorder. Here, we tested the hypothesis that psychotic bipolar disorder is characterized by relational memory deficits. We used a transitive inference (TI) paradigm, previously employed to quantify relational memory deficits in schizophrenia, to assess relational memory performance in 17 patients with psychotic bipolar disorder and 22 demographically matched control participants. Functional magnetic resonance imaging was used to examine hippocampal activity during recognition memory in patients and controls. Hippocampal volumes were assessed by manual segmentation. In contrast to our hypothesis, we found similar TI performance, hippocampal volume, and hippocampal recruitment during recognition memory in both groups. Both psychotic bipolar disorder patients and controls exhibited a positive correlation between hippocampal volume and relational memory performance. These data indicate that relational memory impairments are not a shared feature of non-affective and affective psychosis.
Keywords: Psychosis, Bipolar disorder, Schizophrenia, Relational memory, Transitive inference, Hippocampus
Introduction
More than 50 % of all bipolar disorder patients experience psychosis in their lifetime [1, 2]. Preliminary evidence suggests that psychotic bipolar disorder may represent a distinct subtype of the illness [3], with a different course [4-6] and more severe cognitive impairments than non-psychotic bipolar disorder [7, 8]. Importantly, cognitive impairments in psychotic bipolar disorder have been shown to persist during euthymia [9-11] and are comparable to cognitive impairments in other psychotic disorders, such as schizophrenia, although they are often less severe [12, 13].
A growing literature points toward shared genetic and neural substrates of psychosis that are inconsistent with a strict schizophrenia/bipolar disorder distinction [14-22]. Specifically, the cellular and genetic profile of the hippocampus shows abnormalities in both psychotic bipolar disorder and schizophrenia [21], including a significant reduction in inhibitory interneurons [23, 24], which may disrupt the synchronized oscillatory activity of the hippocampus during memory function [7, 25-28].
The hippocampus plays an essential role in the consolidation and retrieval of declarative memory [29]. This form of memory is impaired in schizophrenia [30] and bipolar disorder [31-35], with more severe deficits in psychotic than non-psychotic bipolar patients [27, 35]. Functional neuroimaging studies have shown decreased hippocampal activity during learning and memory tasks in both schizophrenia and bipolar disorder [30, 36, 37]. However, no functional neuroimaging study has specifically investigated declarative memory impairments in psychotic bipolar disorder. Here, we report the study of one form of declarative memory, i.e., relational memory, in psychotic bipolar disorder.
Relational memory refers to the ability to learn and remember relationships between items [38, 39]. In this study, we used a transitive inference (TI) paradigm to quantify relational memory performance. TI tasks require participants to make inferential judgments on novel stimulus pairings based on previously learned relationships (e.g., if one learns that A > B and B > C, then A > C can be inferred). In a previous study, we showed that TI performance is impaired in chronic schizophrenia [40-42], and these deficits are linked to decreased hippocampal activity [40]. Here, using the same TI paradigm, we tested the hypothesis that patients with psychotic bipolar disorder also demonstrate impaired relational memory performance due to abnormal recruitment of the hippocampus during recognition memory, as well as smaller hippocampal volumes relative to healthy controls. If a relational memory deficit is present across multiple psychotic disorders, it could serve as a distinct neurocognitive marker of psychosis.
Methods
Participants
We studied 17 participants with a diagnosis of Bipolar disorder, type I, with psychotic features (referred to as psychotic bipolar disorder from now on) (7 males and 10 females; mean age = 37, SD = 10) and 22 healthy control subjects (11 males and 11 females; mean age = 34, SD = 8). Groups were matched with respect to age, gender, race, and parental education (see Table 1 for a detailed description of participant demographics). Patients were recruited from Vanderbilt Psychiatric Hospital and affiliated outpatient clinics. Participants were assessed by trained study personnel with the Structured Clinical Interview for DSM-IV (SCID I-P; [43]), which was supplemented by information from treating physicians and patient medical records when available. All psychiatric diagnoses were reviewed and confirmed by a study psychiatrist. Healthy participants were excluded for a history of major psychiatric disorders and psychoactive medication use. All participants were excluded for significant medical or neurological illness, significant head injury, or a history of drug dependence. All participants were administered the National Adult Reading Test (NART) to assess verbal IQ [44] and were assessed for years of education. Groups were not matched for IQ or years of education, as these may be confounded by illness, but for parental education [45-47]. As part of the clinical interview, patients were assessed for duration of illness and current antipsychotic dosage (Table 1); chlorpromazine equivalent doses were calculated according to Gardner [48]. The study protocol was approved by the Vanderbilt University Medical Center Institutional Review Board, Nashville, TN. All participants gave written informed consent prior to study procedures.
Table 1.
Demographic data and clinical characteristics
| Control group
|
Bipolar group
|
Statistics | |||
|---|---|---|---|---|---|
| (n = 22)
|
(n = 17)
|
||||
| Mean | SD | Mean | SD | ||
| Age (years) | 34.00 | 8.04 | 37.41 | 10.37 | t = 1.34 |
| Education (years) | 15.50 | 2.63 | 13.41 | 2.18 | t = 6.98* |
| Parental education (years) | 13.33 | 2.90 | 12.65 | 2.57 | t = .787 |
| Estimated verbal IQ (NART) | 116.73 | 4.84 | 111.35 | 6.75 | t = 8.39* |
| Hamilton Depression Rating Scale | – | – | 5.03 | 3.80 | – |
| Young Mania Rating Scale | – | – | 4.38 | 4.06 | – |
| Duration of illness (years) | – | – | 15.90 | 8.69 | – |
| Chlorpromazine equivalents | – | – | 276.67 | 253.66 | – |
| Gender (M/F) | (11/11) | (10/7) | χ2 = .30 | ||
| Race (C/AA/A) | (16/4/2) | (17/0/0) | χ2 = 5.48 | ||
| Handedness (right/left/ambidextrous) | (20/1/1) | (14/0/3) | χ2 = .293 | ||
M male, F female, C Caucasian, AA African American, A Asian
p < .05
Lifetime and current psychosis were assessed in bipolar disorder patients using the SCID I-P. Of the 17 patients, twelve patients had a history of both hallucinations and delusions during mood episodes, 2 patients had a history of only hallucinations during mood episodes, and 3 patients had a history of only delusions during mood episodes. At the time of the interview, 4 patients met criteria for a major mood episode with psychotic features within the previous month.
Because psychotropic medication use may affect hippocampal volume [49, 50], we assessed psychotropic medication use at the time of the MRI scan. Ten patients were taking antipsychotic, antidepressant, and mood stabilizing medication (n = 3 patients taking lithium), four patients were taking only antipsychotic medication, three patients were taking only antidepressant medication, and one patient was medication-free at the time of the MRI scan (for chlorpromazine equivalents, see Table 1). We studied the effect of antipsychotic dosage in our morphometric analysis.
We also assessed affective symptoms at the time of the MRI scan using the Hamilton Depression Rating Scale (HDRS; [51]) and the Young Mania Rating Scale (YMRS; 52) (Table 1), as current mood state has been linked to the degree of cognitive deficit in bipolar disorder [53]. Five patients had mild depressive symptoms at the time of the MRI scan, indicated by a HDRS score ≥8 [54] (scores ranged between 8 and 11). All patients scored below the YMRS mean score for minimal severity (YMRS minimal severity mean = 13) [52]. The remaining 12 patients were euthymic at the time of the interview. We studied the effect of current mood state on memory performance.
Experimental paradigm
We used a TI paradigm [40, 55] to test relational memory ability (Fig. 1). Prior to the fMRI test session, participants were trained to identify the “winning” stimulus in two sets of visual stimulus pairs: four pairs (A > B, B > C, C > D, and D > E) that create a sequence of overlapping stimuli (S condition) and four pairs (a > b, c > d, e > f, and g > h) that create a non-overlapping set of stimuli (P condition). During the fMRI scanning session, participants were asked to remember the winner from the previously seen pairs (P and S) and to make inferences about the winner in novel stimuli pairings (IS condition: AC, AD, BD, BE, and CE; IP condition: ad, af, ch, cf, and eh). This 2 × 2 factorial design (sequence × inference) allows for the study of the neural basis of transitive (IS) versus non-transitive (IP) inference. The BD pair in the IS condition is the purest test of TI ability, as it does not include either of the end items (A, E).
Fig. 1.
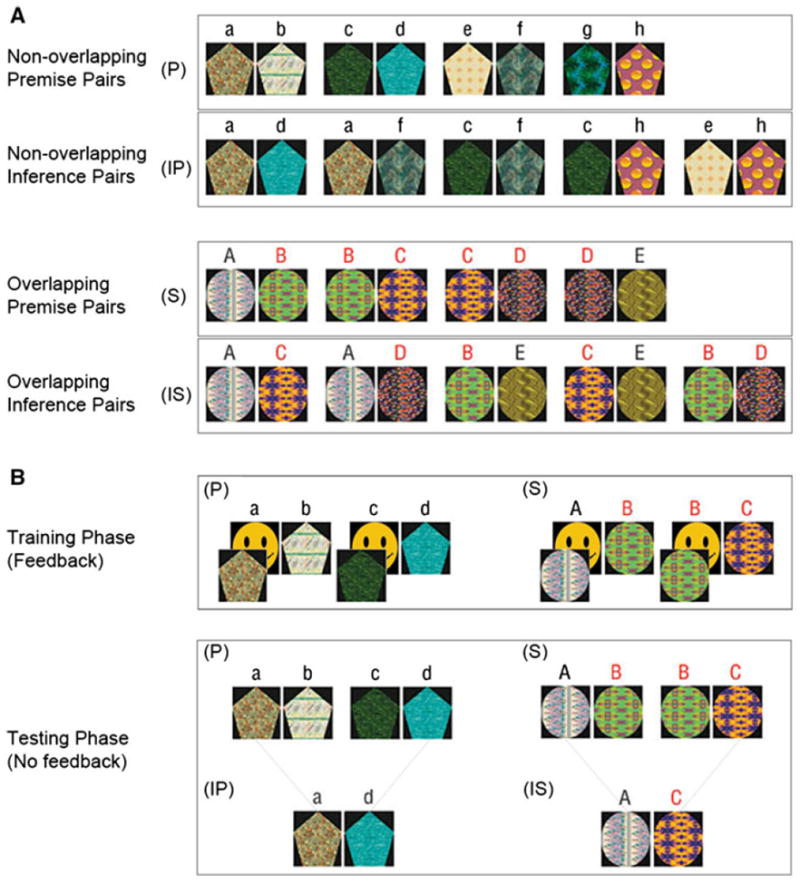
Stimulus set and experimental conditions. a The TI paradigm was comprised of four stimulus conditions: non-overlapping premise pairs (P); non-overlapping inference pairs (IP); overlapping premise pairs (S); and overlapping inference pairs (IS). The reinforced item within each pair is shown on the left (e.g., a > b, c > d, etc.). b During the training phase, premise pairs from the non-overlapping stimulus set (P) were trained first, followed by training of premise pairs from the overlapping stimulus set. Participants were presented with a stimulus pair in the middle of the screen and instructed to select the item from the pair hiding the smiling face (no letters were shown in the experiment, and items within each pair were presented an equal number of times on the left and right side of the screen). Participants received feedback during the training phase—a correct guess during training caused the item to reveal a smiling face underneath, while an incorrect guess did not. During the testing phase, trained premise pairs from the non-overlapping (P) and overlapping (S) conditions were presented and participants were asked to indicate by button press which item was hiding the smiling face. During testing, participants were also asked to make novel judgments about items which had not previously been paired together during training [non-overlapping inference pairs (IP) and overlapping inference pairs (IS)]. Participants did not receive feedback during the testing phase
Stimuli
Eight ellipsoid shapes were used in the non-overlapping task, and five pentagonal shapes were used in the overlapping task (Fig. 1). To ensure that each shape was uniquely distinguishable, shapes were filled with visually distinctive colored patterns selected from CorelDraw (Corel, www.corel.com). Pattern fills were randomly assigned across pentagonal and ellipsoid shapes. The positions of the pattern fills within both the non-overlapping and overlapping tasks were rotated across participants.
Training before scanning
All participants were shown pairs of visual items on a computer screen and asked to indicate by button press which item “hid” a smiling face [40, 55]. A correct guess during training caused the visual pattern to reveal a smiling face underneath, while an incorrect guess did not. Participants were trained first on the four premise pairs of the non-overlapping condition (P), then on the four premise pairs of the overlapping condition (S). Initial training for each condition (P and S) was completed in 3 staged blocks with short breaks offered between each block. Training blocks consisted of 60, 60, and 24 trials, for a total of 144 training trials per condition (36 presentations of each pair). Pairs were presented side-by-side centered vertically on the screen, and items within each pair were presented an equal number of times on the left and right sides of the screen. No letters were shown during the experiment. Participants who performed at less than 80 % accuracy during the last training block received extra training blocks. Three controls and one patient received one extra training block; one patient received two extra training blocks; and one patient received six extra training blocks.
After training, participants were tested on one 48-trial block of overlapping (S) and non-overlapping (P) premise pairs without feedback (one subject completed two blocks). A minimum performance criterion of >50 % average accuracy on the more difficult middle pairs in the S condition (BC and CD) was selected to ensure that participants had learned the task prior to scanning.
Task during fMRI
All participants completed one MRI scanning session approximately 20 min after completion of the training phase, which included two fMRI scans lasting 4 min (min) and 30 s (s) each. Participants were instructed to indicate by button press, using the index and middle finger of their dominant hand, which item they associated with a smiling face. Each fMRI scan started and ended with a 30-s crosshair fixation trial. Eight blocks of testing trials were presented in one of two orders: (P, IP, S, IS, P, IP, S, and IS); or (S, IS, P, IP, S, IS, P, and IP), counterbalanced across participants. Within each testing trial block (e.g., P, IP, S, or IS), 10 stimulus pairs were presented for 2.5 s each. For each of the two fMRI scans, premise pairs were presented 20 times and novel pairs were presented 4 times.
MRI/fMRI acquisition
Participants lay on the padded bed of a 3 Tesla scanner (Philips Healthcare, Inc., Best, The Netherlands) in a dimly lit room. Foam padding was used to stabilize the head. First, we acquired a structural MRI using the following parameters: 256 mm FOV, 170 slices, 1 mm slice thickness, 0 mm gap. Next, we acquired two fMRI scans. Stimuli were generated using Presentation software (Neurobehavioral Systems Inc, Albany, CA, USA) on a desktop computer. Images were projected onto a screen and viewed by the participants via a tilted mirror placed in front of their eyes. The 2 fMRI runs lasted 4 min, 46 s each. The first 16 s of each run was discarded to allow T1 signal equilibration. The remaining 4 min, 30-s acquisition consisted of 130 functional brain images [TE = 25 ms; RT = 2,000 ms; 36 axial sections, interleaved acquisition (3 mm thick, 0 mm gap); voxel size, 3 × 3 × 3 mm; field of view, 108 mm; flip angle, 79°]. The slice acquisition box was positioned to capture 1–2 slices inferior to the most ventrally visible temporal lobe. Slices were tilted 30° higher anterior than posterior in relation to the anterior commissure-posterior commissure line to minimize orbitofrontal signal dropout.
Data analysis
Behavioral data
Between-group analysis of accuracy (percentage of correct responses) and response time (ms) for correct responses was conducted using a 2 (sequence) × 2 (inference) repeated measures ANOVA with group as the between-subjects factor. Relational memory ability was tested by the TI contrast [sequence × inference interaction, ([IS − S] vs. [IP − P])], which allows for the study of the neural basis of transitive (IS) versus non-transitive (IP) inference. Relational memory ability was further analyzed by comparing performance accuracy on BD and non-BD pairs (from the IS condition) using a 2 (group) × 2 (stimulus pair) ANOVA. We initially included participant’s years of education and verbal IQ scores as covariates. Here, we present the analysis without these covariates since the results did not differ significantly. When a significant omnibus F statistic was observed, post hoc comparisons were computed. Accuracy and response time differences were considered significant at p < .05, uncorrected for multiple comparisons. Behavioral data were analyzed using SPSS (SPSS Statistics 18 for Windows, 2010, version 18.0.2).
MRI data
We calculated hippocampal volumes for each participant using a previously described segmentation protocol (adapted from [56, 57]). Each participant’s left and right hippocampi were drawn in native space by a single trained rater (AW) using 3D Slicer software (version 3.4) [58]. Intrarater reliability was assessed by repeated measurement of both left and right hippocampal volumes from 9 randomly selected participants (control = 6; psychotic bipolar disorder = 3). Intraclass correlation (ICC) analysis showed a high degree of reliability across segmentations over time (left hippocampus ICC = .98; right hippocampus ICC = .99). Each hippocampal volume was corrected for total intracranial cavity volume (ICV) using a previously detailed method [59]. ICVs were calculated using the Freesurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/). Between-group analysis of total (left + right) corrected hippocampal volume was conducted using one-way ANOVA. Hippocampal volumes were further analyzed using repeated measures 2 (hemisphere) × 2 (region) ANOVA with group as the between-subjects factor. When a significant omnibus F statistic was observed, post hoc comparisons were computed. Associations between hippocampal volumes and behavioral performance, as well as possible confounding effects of anti-psychotic dose on hippocampal volumes, were tested using Pearson’s correlation coefficient. Volume differences and correlations were considered significant at p < .05, uncorrected for multiple comparisons. Volume data were analyzed using SPSS.
fMRI data
All functional MRI data were preprocessed using SPM5 (Wellcome Department of Cognitive Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/). Data were realigned to the mean image, and acquisition artifacts were removed by regressing out the average signal per slice. fMRI data were spatially normalized into standard stereotactic space (MNI-152 brain T1 template) and high pass filtered (200 s). Data were smoothed with a 5-mm FWHM Gaussian kernel to account for individual differences in brain anatomy.
Functional images were analyzed in a mixed effects model. First, to explain the variance of blood oxygenation level-dependent (BOLD) signal change at each voxel, general linear models were created for each participant, which included the effects of session (1,2) and condition ([P], [S], [IP], and [IS]). The 4 effects of interest ([P], [S], [IP], and [IS]) were modeled as 25-s blocks with a boxcar function convolved with a canonical HRF. The general linear model was used to obtain parameter estimates corresponding to the magnitude of the BOLD signal response for each block type. We tested for the TI interaction of sequence × inference ([IS − S] − [IP − P]) as well as the main effects of sequence ([S + IS] vs. [P + IP]) across the two fMRI scans, as these two contrasts revealed significant differences in fMRI activity in schizophrenia patients [40]. At a second-level analysis, two-sample independent t tests were used to test for the main effects and interactions of within- and between-group effects. A threshold adjustment method based on Monte Carlo simulations was used to guard against identifying false positive areas of activation (AlphaSim, http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf). For the whole-brain analysis, a voxel-wise probability of p < .001 and a cluster of ≥8 voxels provided for a corrected family wise error rate of α < .05.
Given our previous findings of hippocampal abnormalities during TI in schizophrenia [40], we also performed a region of interest (ROI)-based analysis of hippocampal BOLD signal changes. For the ROI analysis, an optimized normalization procedure was used to increase hippocampal overlap in our normalized fMRI images [60]. To optimally normalize images, we created a hippocampus-weighted mask by the following procedures: first, individual native-space hippocampal ROIs from our morphometric study were normalized to the MNI-152 brain T1 template using SPM5 default normalization procedures; next, the normalized ROIs were summed together to create a single group ROI mask, which was thresholded at ≥50 % overlap and binarized; and last, using SPM’s Image Calculator, this binary group ROI mask was combined with a standard brain mask template (SPM5) to create a 20 to 1 hippocampus/brain ratio weighted mask. This hippocampus-weighted brain mask was then applied to each participant’s native-space MRI data, fMRI data, and hippocampal ROI image during spatial normalization. Following normalization, data were smoothed with a 5-mm FWHM Gaussian kernel, and the optimally normalized ROIs were summed together to create a group mask, which was then binarized for use as an explicit mask in second-level analysis. We confirmed that years of education and verbal IQ were not correlated with hippocampal activity and present the ROI analysis without these covariates. A voxel-wise probability of p < .001 and a cluster of ≥4 voxels within the hippocampal ROI provided for a corrected family wise error rate of α < .05.
Results
Behavioral data
Accuracy
We tested for accuracy differences in TI performance between the two groups with two contrasts: the interaction of sequence × inference ([IS − S] vs. [IP − P]) and the contrast of BD versus non-BD pairs. We found no differences in accuracy between the two groups (all p’s > .05) (Table 2), indicating normal TI ability in psychotic bipolar disorder patients (see Fig. 2 for task performance means by condition).
Table 2.
Accuracy and response time effects
| Accuracy (% correct responses)
|
Correct response time (ms)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Control group Mean (SD) | Bipolar group Mean (SD) | F | p value | Control group Mean (SD) | Bipolar group Mean (SD) | F | p value | |
| Main effect of sequence (IS + S) versus (IP + P) | −.25 (.05) | −.43 (.05) | 63.95 | < .001 | 695 (80) | 740 (85) | 149.03 | < .001 |
| Sequence by group | 1.36 | .25 | .15 | .70 | ||||
| Main effect of inference (IS + IP) versus (S + P) | −.08 (.04) | −.11 (.04) | 11.34 | .002 | 103 (37) | −25 (44) | 1.82 | .19 |
| Inference by group | .27 | .60 | 5.01 | .03 | ||||
| Sequence by inference Interaction (IS − S) versus (IP − P) | −.04 (.04) | −.08 (.04) | 3.99 | .05 | 59 (30) | 11 (39) | 2.07 | .16 |
| Sequence by inference by group | .43 | .52 | 1.01 | .32 | ||||
| BD versus non-BD pairs | .12 (.11) | .27 (.07) | 8.98 | .005 | 90 (45) | 153 (71) | 9.14 | .005 |
| BD versus non-BD pairs by group | 1.395 | .25 | .62 | .44 | ||||
Fig. 2.
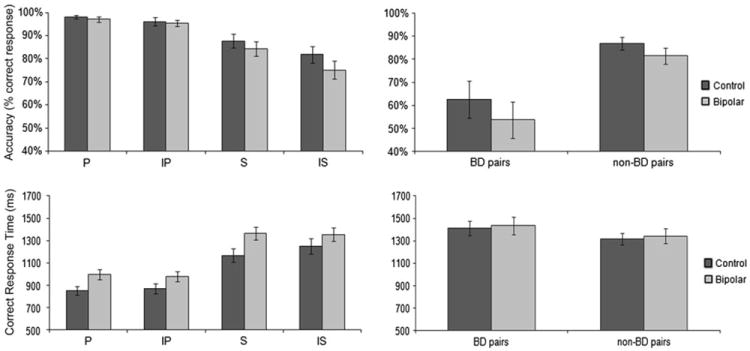
Performance accuracy (percentage of correct responses) and correct response times (ms) across psychotic bipolar disorder patients and controls. Both groups had similar accuracy across all conditions, indicating normal TI performance in psychotic bipolar disorder patients. Controls required more time to correctly identify novel pairs (IP and IS) compared to previously learned pairs (P and S), while psychotic bipolar disorder patients required a similar amount of time to correctly identify novel and previously learned pairs
Overall, subjects were less accurate on overlapping than non-overlapping pairs (main effect of sequence ([S + IS] vs. [P + IP]), F = 64.0, p < .001) and on novel than learned pairs (main effect of inference ([IS + IP] vs. [S + P]), F = 11.3, p = .002) and showed a trend toward being least accurate on novel pairs taken from the overlapping sequence (IS) (sequence × inference interaction ([IS − S] − [IP − P]), F = 4.0, p = .053). All subjects were less accurate for BD pairs (pairs that did not contain an end item) than for non-BD pairs (pairs that did include an end item) (main effect of pair type, F = 9.0, p = .005; no pair × group interaction, F = 1.4, p > .05) (Table 2; Fig. 2). There were no correlations between current mood state (YMRS and HDRS scores) or antipsychotic dose and accuracy.
Response time
As with accuracy, there were no differences between the two groups in correct response times in our two TI performance contrasts (all p’s > .05) (Table 2; Fig. 2), again indicating normal TI ability in psychotic bipolar disorder patients. Controls but not psychotic bipolar disorder patients needed more time to make inferences relative to premise pair judgments (main effect of inference by group ([IS + IP] vs. [S + P]), F = 1.82, p = .031) (Table 2; Fig. 2). Both groups needed more time to correctly identify pairs from the overlapping than the non-overlapping sequence (main effect of sequence ([IS + S] vs. [IP + P]), F = 149.0, p < .001) and needed more time to correctly evaluate BD pairs compared to non-BD pairs (main effect of pair type, F = 9.14, p = .005) (Table 2; Fig. 2). There were no correlations between current mood state (YMRS and HDRS scores) or antipsychotic dose and response time.
Volumetric MRI data
Total hippocampal volume did not differ between the two groups (mean volumes, mm3) for the left anterior/posterior and right anterior/posterior regions: 1,600/1,730 and 1,793/1,728 in control subjects and 1,759/1,569 and 1,809/1,544 in psychotic bipolar disorder patients (non-significant main effect of group, F = .7, p > .05). The right > left hippocampal volume asymmetry (main effect of hemisphere, F1,37 = 20.3, p < .001) was prominent in the controls but absent in psychotic bipolar disorder patients (hemisphere × group interaction (F = 11.9, p = .001). Hippocampal volumes were not different between lithium- and non-lithium-treated patients (all p’s > .05) and were not correlated with antipsychotic dose (all p’s > .05).
Because we hypothesized that TI ability is dependent on the hippocampus, we tested whether there was an association between task performance and hippocampal volume, irrespective of group differences. Including group as a covariate, total hippocampal volume was correlated with accuracy on the overlapping premise pairs ([S] condition; r = .34, r2 = .12, p = .037) and BD pair accuracy (r = .34, r2 = .12, p = .037).
fMRI data
Transitive inference
To investigate the TI network, we tested for a significant sequence × inference ([IS − S] vs. [IP − P]) interaction within each voxel across the whole brain. In controls, TI was associated with signal increase in the bilateral cingulate cortex, right dorsolateral prefrontal cortex, left insula, left superior temporal gyrus, right inferior parietal cortex, and right thalamus (Table 3). In contrast, there were no significant regions of activation in psychotic bipolar disorder patients. Between-group comparison revealed significantly greater activation of bilateral cingulate cortex, right dorsolateral prefrontal cortex, and left insula in the control group (Fig. 3). These findings indicated that, within the context of similar task performance, psychotic bipolar disorder patients showed less neural activity during TI judgments compared with control participants.
Table 3.
Brain regions involved in transitive inference judgments (sequence by inference interaction)
| Control group
|
Bipolar group*
|
Control−bipolar group
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K | Z score | MNI | K | Z score | MNI | K | Z score | MNI | |||||||
| Cingulate cortex (BA 31/23/24) | |||||||||||||||
| Bilateral | 341 | 4.35 | 0 | −30 | 45 | 20 | 4.11 | 0 | 12 | 33 | |||||
| 43 | 4.01 | −6 | −30 | 45 | |||||||||||
| 12 | 3.44 | 6 | −45 | 33 | |||||||||||
| Dorsolateral PFC (BA 9) | |||||||||||||||
| Right | 12 | 3.53 | 3 | 51 | 33 | 35 | 4.60 | 42 | 9 | 33 | |||||
| Insula (BA 13) | |||||||||||||||
| Left | 53 | 4.19 | −42 | −24 | 15 | 12 | 3.39 | −51 | −30 | 15 | |||||
| Superior temporal gyrus (BA 39) | |||||||||||||||
| Left | 66 | 4.29 | −51 | −57 | 27 | ||||||||||
| Inferior parietal cortex (BA 40) | |||||||||||||||
| Right | 20 | 3.82 | 51 | −51 | 36 | ||||||||||
| Thalamus | |||||||||||||||
| Right | 12 | 3.70 | 9 | −33 | 0 | ||||||||||
BA brodmann area, PFC prefrontal cortex
There were no significant activations in psychotic bipolar disorder patients
Fig. 3.
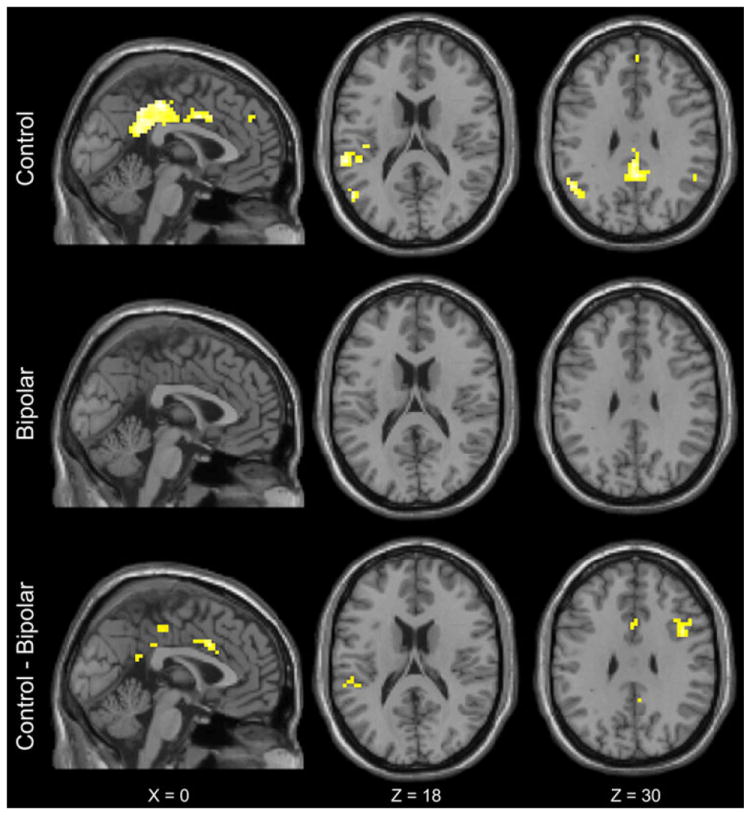
Significant activations during TI judgments (sequence × inference interaction, ([IS − S] − [IP − P])), p < .05 corrected for multiple comparisons. TI was associated with activations in the bilateral cingulate cortex, right dorsolateral prefrontal cortex, left insula, left superior temporal gyrus, right inferior parietal cortex, and right thalamus in controls (top row). There were no significant activations in psychotic bipolar disorder patients (middle row). Between-group comparison revealed significantly greater activations in the control group within bilateral cingulate cortex, right dorsolateral prefrontal cortex, and left insula (bottom row). Significant activations are shown in flame scale (p value map) where lighter-colored voxels represent the smallest p values
Hippocampal activity
The hippocampal ROI analysis did not reveal significant hippocampal activity during TI performance in either group. However, the discrimination of non-overlapping pairs ([P + IP] vs. [S + IS]) was associated with bilateral hippocampal activation in both groups (Table 4; Fig. 4).
Table 4.
Hippocampal activation during discrimination of non-overlapping pairs (P + IP) versus (S + IS)
| Control group
|
Bipolar group
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K | Z score | MNI | K | Z score | MNI | |||||
| Hippocampus | ||||||||||
| Left | 13 | 4.75 | −33 | −12 | −18 | 34 | 4.90 | −27 | −15 | −15 |
| 18 | 4.17 | −18 | −24 | −12 | ||||||
| Right | 83 | 4.21 | 27 | −27 | −12 | 28 | 4.68 | 33 | −27 | −15 |
Fig. 4.
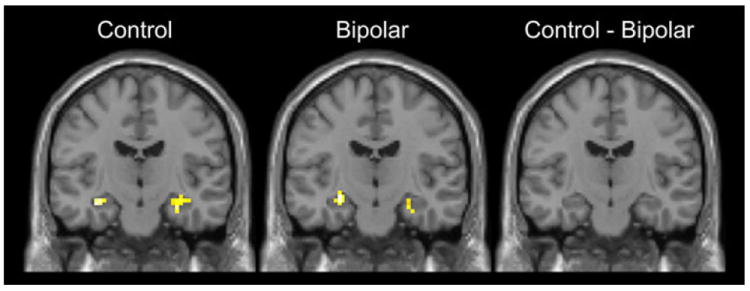
Hippocampal ROI analysis revealed bilateral hippocampal activation in healthy controls and psychotic bipolar disorder during discrimination of nonoverlapping pairs [(P + IP) − (S + IS)] (shown in coronal view, y = − 15)
Discussion
We used a TI paradigm, previously employed to show relational memory deficits in schizophrenia [40], to examine relational memory ability, hippocampal structure, and hippocampal function in psychotic bipolar disorder patients. Since relational memory deficits in schizophrenia [40-42, 61, 62] have been associated with hippocampal dysfunction [21], we hypothesized that a similar hippocampal pathology in bipolar disorder [24] is also associated with relational memory impairment. In contrast to our prediction, we found no difference in TI task performance, hippocampal volume, or hippocampal activation between psychotic bipolar disorder patients and healthy control participants, indicating that relational memory ability is not impaired across all psychotic disorders.
During the TI task, psychotic bipolar disorder patients and healthy controls had similar accuracy across all conditions. In the context of comparable memory performance, our morphometric study revealed no difference in total hippocampal volume between groups, consistent with the findings of several recent meta-analyses [63-66]. Additionally, both groups exhibited a positive correlation between hippocampal volume and performance on the difficult BD relational memory pairs within the [IS] condition. Contrary to our prediction, neither psychotic bipolar disorder patients nor healthy controls showed increased hippocampal activation during TI performance (sequence × inference interaction), possibly driven by lower task performance relative to our previous studies [40, 55]. However, both groups showed similar hippocampal recruitment when making judgments on non-overlapping pairs, as seen in our previous study [55]. This provides support for normal hippocampal function during recognition memory in psychotic bipolar disorder patients.
While memory performance did not differ between groups, healthy controls showed greater activity during TI (sequence × inference interaction) in the left posterior insula, bilateral posterior cingulate cortex, and right dorsolateral prefrontal cortex (dlPFC). Higher insula and posterior cingulate activity in the control group might reflect memory network inefficiencies in psychotic bipolar disorder patients, as the insula is recruited during TI [67, 68] and the posterior cingulate is involved in episodic memory retrieval [69]. Interestingly, while the dlPFC is typically activated during working memory tasks [70], it is also a key node of dysfunction in bipolar disorder, with evidence for cellular abnormalities in the dlPFC, smaller dlPFC volumes, and reduced dlPFC activation during affective and cognitive tasks [71-73]. Therefore, dlPFC differences, including the reduced activation found in our study, may be attributable to previously described affective and cognitive dysfunction in patients with bipolar disorder [71-73].
Relational memory deficits and associated neural dysfunction have been consistently observed in schizophrenia [40-42, 61, 62, 74], but the few studies to assess relational memory performance across the psychosis spectrum have yielded equivocal findings. In comparing paired associates learning (a measure of relational memory performance) between patients with bipolar disorder and healthy controls, one behavioral experiment found deficits in performance in bipolar patients [75], whereas two experiments have found no differences in performance [76, 77]. Neuroimaging studies of paired associates tests in bipolar disorder have also yielded conflicting results, with one study finding evidence for reduced hippocampal activation during recognition [36], while another study found no differences between bipolar disorder and control participants [78]. Relational memory studies with early psychosis samples have yielded similarly mixed findings [79-81]. In addition, we recently quantified TI ability in a sample of early psychosis patients, finding no difference in TI performance or neural activation from a sample of comparable control participants [82]. In the context of these previous findings, the results of the current study are consistent with the conclusion that relational memory deficits are not present across the psychosis spectrum.
This study has several limitations. First, we designed our study to detect a difference of hippocampal volume and activation during TI between psychotic bipolar disorder patients and healthy controls and were not powered to detect a behavioral difference in TI performance. While we cannot conclude from our study that relational memory is normal in psychotic bipolar disorder, we can compare our results with previous studies of schizophrenia, which have demonstrated significant relational memory deficits in study samples comparable to the present study [40, 61, 62, 83]. Second, a majority of our patients were treated with psychotropic medications. However, antipsychotic dose was not correlated with behavioral performance, hippocampal volume, or hippocampal function. Additionally, there were no differences in hippocampal volume between patients taking lithium and patients not on lithium, although this finding should be interpreted with caution given the small number of lithium-treated patients (n = 4). Third, the patients included in this study were mostly euthymic (n = 5 patients had mild depressive symptoms). Although previous studies in psychotic bipolar disorder have demonstrated that cognitive impairments persist during euthymia [9-11], it is possible that relational memory is only substantially impaired during periods of affective psychosis. Future studies of relational memory should include patients with current affective psychotic symptoms. Fourth, the use of a block design does not allow for an analysis of correct trials only or for analysis of the critical BD pair in isolation. An event-related fMRI version of this paradigm was developed to address this concern [67]; however, the increased temporal spacing of the task proved very difficult for healthy controls [67], making it not feasible for our study of psychotic bipolar disorder patients. Finally, while groups had comparable performance, accuracy in the IS condition was lower than in previous studies, which may have contributed to a more variable pattern of brain activation in the critical TI contrast.
The results of this study indicate that psychotic bipolar disorder patients do not show the same degree of hippocampal dysfunction during the performance of a relational memory task as seen previously in schizophrenia patients [40]. We conclude that relational memory deficits are not a neurocognitive marker of psychosis, but are more prominent in schizophrenia. While genetic, histological, and in vitro protein expression studies suggest a similar pathology of the hippocampus in psychotic bipolar disorder and schizophrenia [21], our data indicate that relational memory is differentially affected in these two psychotic disorders, possibly due to additional impairments of cortico-hippocampal function in schizophrenia.
Acknowledgments
Authors were supported by grant R01 MH070560 (SH).
Footnotes
Conflict of interest None.
Contributor Information
Suzanne N. Avery, Vanderbilt Brain Institute, Vanderbilt University, Nashville, TN, USA
Lisa E. Williams, Department of Psychiatry, University of Wisconsin, Madison, WI, USA
Austin A. Woolard, Department of Psychiatry, Vanderbilt Psychiatric Hospital, Vanderbilt University, 1601 23rd Avenue South, Room 3060, Nashville, TN 37212, USA
Stephan Heckers, Email: stephan.heckers@vanderbilt.edu, Department of Psychiatry, Vanderbilt Psychiatric Hospital, Vanderbilt University, 1601 23rd Avenue South, Room 3060, Nashville, TN 37212, USA.
References
- 1.Keck PE, Jr, McElroy SL, Havens JR, Altshuler LL, Nolen WA, Frye MA, Suppes T, Denicoff KD, Kupka R, Leverich GS, Rush AJ, Post RM. Psychosis in bipolar disorder: phenomenology and impact on morbidity and course of illness. Compr Psychiatry. 2003;44(4):263–269. doi: 10.1016/S0010-440X(03)00089-0. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin FK, Jamison KR. Manic-depressive Illness. Oxford University Press; New York, NY: 1990. [Google Scholar]
- 3.Bora E, Yucel M, Fornito A, Berk M, Pantelis C. Major psychoses with mixed psychotic and mood symptoms: are mixed psychoses associated with different neurobiological markers? Acta Psychiatr Scand. 2008;118(3):172–187. doi: 10.1111/j.1600-0447.2008.01230.x. [DOI] [PubMed] [Google Scholar]
- 4.Rosen LN, Rosenthal NE, Dunner DL, Fieve RR. Social outcome compared in psychotic and nonpsychotic bipolar I patients. J Nerv Ment Dis. 1983;171(5):272–275. doi: 10.1097/00005053-198305000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal NE, Rosenthal LN, Stallone F, Fleiss J, Dunner DL, Fieve RR. Psychosis as a predictor of response to lithium maintenance treatment in bipolar affective disorder. J Affect Disord. 1979;1(4):237–245. doi: 10.1016/0165-0327(79)90010-7. [DOI] [PubMed] [Google Scholar]
- 6.Tohen M, Waternaux CM, Tsuang MT, Hunt AT. Four-year follow-up of twenty-four first-episode manic patients. J Affect Disord. 1990;19(2):79–86. doi: 10.1016/0165-0327(90)90012-w. [DOI] [PubMed] [Google Scholar]
- 7.Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, Soares JC, Velligan DI. The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry. 2007;62(8):910–916. doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Simonsen C, Sundet K, Vaskinn A, Birkenaes AB, Engh JA, Faerden A, Jonsdottir H, Ringen PA, Opjordsmoen S, Melle I, Friis S, Andreassen OA. Neurocognitive dysfunction in bipolar and schizophrenia spectrum disorders depends on history of psychosis rather than diagnostic group. Schizophr Bull. 2011;37(1):73–83. doi: 10.1093/schbul/sbp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 2009;113(1–2):1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Arts B, Jabben N, Krabbendam L, van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol Med. 2008;38(6):771–785. doi: 10.1017/S0033291707001675. [DOI] [PubMed] [Google Scholar]
- 11.Robinson LJ, Thompson JM, Gallagher P, Goswami U, Young AH, Ferrier IN, Moore PB. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J Affect Disord. 2006;93(1–3):105–115. doi: 10.1016/j.jad.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Seidman LJ, Kremen WS, Koren D, Faraone SV, Goldstein JM, Tsuang MT. A comparative profile analysis of neuropsychological functioning in patients with schizophrenia and bipolar psychoses. Schizophr Res. 2002;53(1–2):31–44. doi: 10.1016/s0920-9964(01)00162-1. [DOI] [PubMed] [Google Scholar]
- 13.Reichenberg A, Harvey PD, Bowie CR, Mojtabai R, Rabinowitz J, Heaton RK, Bromet E. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr Bull. 2009;35(5):1022–1029. doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berrettini WH. Susceptibility loci for bipolar disorder: overlap with inherited vulnerability to schizophrenia. Biol Psychiatry. 2000;47(3):245–251. doi: 10.1016/s0006-3223(99)00226-7. [DOI] [PubMed] [Google Scholar]
- 15.Kendler KS, McGuire M, Gruenberg AM, O’Hare A, Spellman M, Walsh D. The Roscommon Family Study. I. Methods, diagnosis of probands, and risk of schizophrenia in relatives. Arch Gen Psychiatry. 1993;50(7):527–540. doi: 10.1001/archpsyc.1993.01820190029004. [DOI] [PubMed] [Google Scholar]
- 16.Erlenmeyer-Kimling L, Adamo UH, Rock D, Roberts SA, Bassett AS, Squires-Wheeler E, Cornblatt BA, Endicott J, Pape S, Gottesman II. The New York High-Risk Project. Prevalence and comorbidity of axis I disorders in offspring of schizophrenic parents at 25-year follow-up. Arch Gen Psychiatry. 1997;54(12):1096–1102. doi: 10.1001/archpsyc.1997.01830240052008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Craddock N, O’Donovan MC, Owen MJ. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull. 2006;32(1):9–16. doi: 10.1093/schbul/sbj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craddock N, O’Donovan MC, Owen MJ. Psychosis genetics: modeling the relationship between schizophrenia, bipolar disorder, and mixed (or “schizoaffective”) psychoses. Schizophr Bull. 2009;35(3):482–490. doi: 10.1093/schbul/sbp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callicott JH, Straub RE, Pezawas L, Egan MF, Mattay VS, Hariri AR, Verchinski BA, Meyer-Lindenberg A, Balkissoon R, Kolachana B, Goldberg TE, Weinberger DR. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci USA. 2005;102(24):8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goes FS, Sanders LL, Potash JB. The genetics of psychotic bipolar disorder. Curr Psychiatry Rep. 2008;10(2):178–189. doi: 10.1007/s11920-008-0030-5. [DOI] [PubMed] [Google Scholar]
- 21.Heckers S, Konradi C. Hippocampal pathology in schizophrenia. Curr Top Behav Neurosci. 2010;4:529–553. doi: 10.1007/7854_2010_43. [DOI] [PubMed] [Google Scholar]
- 22.Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44(2):88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- 23.Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res. 2011;131(1–3):165–173. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konradi C, Zimmerman EI, Yang CK, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. Hippocampal interneurons in bipolar disorder. Arch Gen Psychiatry. 2011;68(4):340–350. doi: 10.1001/archgenpsychiatry.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8(1):45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 26.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Aran A, Torrent C, Tabares-Seisdedos R, Salamero M, Daban C, Balanza-Martinez V, Sanchez-Moreno J, Manuel GJ, Benabarre A, Colom F, Vieta E. Neurocognitive impairment in bipolar patients with and without history of psychosis. J Clin Psychiatry. 2008;69(2):233–239. doi: 10.4088/jcp.v69n0209. [DOI] [PubMed] [Google Scholar]
- 28.Kurtz MM, Gerraty RT. A meta-analytic investigation of neurocognitive deficits in bipolar illness: profile and effects of clinical state. Neuropsychology. 2009;23(5):551–562. doi: 10.1037/a0016277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eichenbaum H. A cortical–hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1(1):41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- 30.Stone WS, Hsi X. Declarative memory deficits and schizophrenia: problems and prospects. Neurobiol Learn Mem. 2011;96(4):544–552. doi: 10.1016/j.nlm.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Deckersbach T, Savage CR, Reilly-Harrington N, Clark L, Sachs G, Rauch SL. Episodic memory impairment in bipolar disorder and obsessive-compulsive disorder: the role of memory strategies. Bipolar Disord. 2004;6(3):233–244. doi: 10.1111/j.1399-5618.2004.00118.x. [DOI] [PubMed] [Google Scholar]
- 32.Glahn DC, Bearden CE, Caetano S, Fonseca M, Najt P, Hunter K, Pliszka SR, Olvera RL, Soares JC. Declarative memory impairment in pediatric bipolar disorder. Bipolar Disord. 2005;7(6):546–554. doi: 10.1111/j.1399-5618.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 33.Bearden CE, Glahn DC, Monkul ES, Barrett J, Najt P, Kaur S, Sanches M, Villarreal V, Bowden C, Soares JC. Sources of declarative memory impairment in bipolar disorder: mnemonic processes and clinical features. J Psychiatr Res. 2006;40(1):47–58. doi: 10.1016/j.jpsychires.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Frey BN, Andreazza AC, Nery FG, Martins MR, Quevedo J, Soares JC, Kapczinski F. The role of hippocampus in the pathophysiology of bipolar disorder. Behav Pharmacol. 2007;18(5–6):419–430. doi: 10.1097/FBP.0b013e3282df3cde. [DOI] [PubMed] [Google Scholar]
- 35.Bora E, Yucel M, Pantelis C. Neurocognitive markers of psychosis in bipolar disorder: a meta-analytic study. J Affect Disord. 2010;127(1–3):1–9. doi: 10.1016/j.jad.2010.02.117. [DOI] [PubMed] [Google Scholar]
- 36.Glahn DC, Robinson JL, Tordesillas-Gutierrez D, Monkul ES, Holmes MK, Green MJ, Bearden CE. Fronto-temporal dysregulation in asymptomatic bipolar I patients: a paired associate functional MRI study. Hum Brain Mapp. 2010;31(7):1041–1051. doi: 10.1002/hbm.20918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deckersbach T, Dougherty DD, Savage C, McMurrich S, Fischman AJ, Nierenberg A, Sachs G, Rauch SL. Impaired recruitment of the dorsolateral prefrontal cortex and hippocampus during encoding in bipolar disorder. Biol Psychiatry. 2006;59(2):138–146. doi: 10.1016/j.biopsych.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 38.Eichenbaum H, Otto T, Cohen NJ. Two functional components of the hippocampal memory system. Behav Brain Sci. 1994;17(03):449–472. [Google Scholar]
- 39.Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44(1):109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 40.Ongur D, Cullen TJ, Wolf DH, Rohan M, Barreira P, Zalesak M, Heckers S. The neural basis of relational memory deficits in schizophrenia. Arch Gen Psychiatry. 2006;63(4):356–365. doi: 10.1001/archpsyc.63.4.356. [DOI] [PubMed] [Google Scholar]
- 41.Coleman MJ, Titone D, Krastoshevsky O, Krause V, Huang Z, Mendell NR, Eichenbaum H, Levy DL. Reinforcement ambiguity and novelty do not account for transitive inference deficits in schizophrenia. Schizophr Bull. 2010;36(6):1187–1200. doi: 10.1093/schbul/sbp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Titone D, Ditman T, Holzman PS, Eichenbaum H, Levy DL. Transitive inference in schizophrenia: impairments in relational memory organization. Schizophr Res. 2004;68(2–3):235–247. doi: 10.1016/S0920-9964(03)00152-X. [DOI] [PubMed] [Google Scholar]
- 43.First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research. New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) [Google Scholar]
- 44.Nelson HE. National Adult Reading Test (NART): test manual. NFER-Nelson; Windsor: 1982. [Google Scholar]
- 45.MacCabe JH, Lambe MP, Cnattingius S, Torrang A, Bjork C, Sham PC, David AS, Murray RM, Hultman CM. Scholastic achievement at age 16 and risk of schizophrenia and other psychoses: a national cohort study. Psychol Med. 2008;38(8):1133–1140. doi: 10.1017/S0033291707002048. [DOI] [PubMed] [Google Scholar]
- 46.Resnick SM. Matching for education in studies of schizophrenia. Arch Gen Psychiatry. 1992;49(3):246. doi: 10.1001/archpsyc.1992.01820030078011. [DOI] [PubMed] [Google Scholar]
- 47.Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, Soares JC, Velligan DI. The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry. 2007;62(8):910–916. doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167(6):686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- 49.van Erp TG, Thompson PM, Kieseppa T, Bearden CE, Marino AC, Hoftman GD, Haukka J, Partonen T, Huttunen M, Kaprio J, Lonnqvist J, Poutanen VP, Toga AW, Cannon TD. Hippocampal morphology in lithium and non-lithium-treated bipolar I disorder patients, non-bipolar co-twins, and control twins. Hum Brain Mapp. 2012;33(3):501–510. doi: 10.1002/hbm.21239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koolschijn PC, van Haren NE, Cahn W, Schnack HG, Janssen J, Klumpers F, Hulshoff Pol HE, Kahn RS. Hippocampal volume change in schizophrenia. J Clin Psychiatry. 2010;71(6):737–744. doi: 10.4088/JCP.08m04574yel. [DOI] [PubMed] [Google Scholar]
- 51.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 53.Martinez-Aran A, Vieta E, Reinares M, Colom F, Torrent C, Sanchez-Moreno J, Benabarre A, Goikolea JM, Comes M, Salamero M. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004;161(2):262–270. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- 54.Furukawa TA, Akechi T, Azuma H, Okuyama T, Higuchi T. Evidence-based guidelines for interpretation of the Hamilton Rating Scale for Depression. J Clin Psychopharmacol. 2007;27(5):531–534. doi: 10.1097/JCP.0b013e31814f30b1. [DOI] [PubMed] [Google Scholar]
- 55.Heckers S, Zalesak M, Weiss AP, Ditman T, Titone D. Hippocampal activation during transitive inference in humans. Hippocampus. 2004;14(2):153–162. doi: 10.1002/hipo.10189. [DOI] [PubMed] [Google Scholar]
- 56.Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10(4):433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- 57.Weiss AP, DeWitt I, Goff D, Ditman T, Heckers S. Anterior and posterior hippocampal volumes in schizophrenia. Schizophr Res. 2005;73(1):103–112. doi: 10.1016/j.schres.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 58.Pieper A, Halle M, Kikinis R. 3D slicer. Proceeding of IEEE international symposium on biomedical imaging: from nano to macro 2004;448 [Google Scholar]
- 59.Jack CR, Jr, Twomey CK, Zinsmeister AR, Sharbrough FW, Petersen RC, Cascino GD. Anterior temporal lobes and hippocampal formations: normative volumetric measurements from MR images in young adults. Radiology. 1989;172(2):549–554. doi: 10.1148/radiology.172.2.2748838. [DOI] [PubMed] [Google Scholar]
- 60.Kippenhan JS, Padmanabhan A, Mervis C, Morris C, Kohn P, Koch P, Meyer-Lindenberg A, Berman KF. Hippocampus-specific spatial normalization using optimized SPM parameters improves sensitivity of functional neuroimaging group analyses in Williams syndrome. Biol Psychiatry. 2008;63(7, Supplement 1):74S–75S. [Google Scholar]
- 61.Williams LE, Must A, Avery S, Woolard A, Woodward ND, Cohen NJ, Heckers S. Eye-movement behavior reveals relational memory impairment in schizophrenia. Biol Psychiatry. 2010;68(7):617–624. doi: 10.1016/j.biopsych.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Armstrong K, Kose S, Williams L, Woolard A, Heckers S. Impaired associative inference in patients with schizophrenia. Schizophr Bull. 2012;38(3):622–629. doi: 10.1093/schbul/sbq145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bora E, Fornito A, Yucel M, Pantelis C. Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder. Biol Psychiatry. 2010;67(11):1097–1105. doi: 10.1016/j.biopsych.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 64.Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res. 2010;117(1):1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 65.Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry. 2008;65(9):1017–1032. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- 66.Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatry. 2009;195(3):194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- 67.Zalesak M, Heckers S. The role of the hippocampus in transitive inference. Psychiatry Res. 2009;172(1):24–30. doi: 10.1016/j.pscychresns.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Acuna BD, Eliassen JC, Donoghue JP, Sanes JN. Frontal and parietal lobe activation during transitive inference in humans. Cereb Cortex. 2002;12(12):1312–1321. doi: 10.1093/cercor/12.12.1312. [DOI] [PubMed] [Google Scholar]
- 69.Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brambilla P, Glahn DC, Balestrieri M, Soares JC. Magnetic resonance findings in bipolar disorder. Psychiatr Clin North Am. 2005;28(2):443–467. doi: 10.1016/j.psc.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 72.Keener MT, Phillips ML. Neuroimaging in bipolar disorder: a critical review of current findings. Curr Psychiatry Rep. 2007;9(6):512–520. doi: 10.1007/s11920-007-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Strakowski SM, DelBello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10(1):105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 74.Kircher T, Whitney C, Krings T, Huber W, Weis S. Hippocampal dysfunction during free word association in male patients with schizophrenia. Schizophr Res. 2008;101(1–3):242–255. doi: 10.1016/j.schres.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 75.Thompson JM, Gallagher P, Hughes JH, Watson S, Gray JM, Ferrier IN, Young AH. Neurocognitive impairment in euthymic patients with bipolar affective disorder. Br J Psychiatry. 2005;186:32–40. doi: 10.1192/bjp.186.1.32. [DOI] [PubMed] [Google Scholar]
- 76.Sheffield JM, Williams LE, Cohen N, Heckers S. Relational memory in psychotic bipolar disorder. Bipolar Disord. 2012;14(5):537–546. doi: 10.1111/j.1399-5618.2012.01036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Killgore WD, Rosso IM, Gruber SA, Yurgelun-Todd DA. Amygdala volume and verbal memory performance in schizophrenia and bipolar disorder. Cogn Behav Neurol. 2009;22(1):28–37. doi: 10.1097/WNN.0b013e318192cc67. [DOI] [PubMed] [Google Scholar]
- 78.Hall J, Whalley HC, Marwick K, McKirdy J, Sussmann J, Romaniuk L, Johnstone EC, Wan HI, McIntosh AM, Lawrie SM. Hippocampal function in schizophrenia and bipolar disorder. Psychol Med. 2010;40(5):761–770. doi: 10.1017/S0033291709991000. [DOI] [PubMed] [Google Scholar]
- 79.Bartholomeusz CF, Proffitt TM, Savage G, Simpson L, Markulev C, Kerr M, McConchie M, McGorry PD, Pantelis C, Berger GE, Wood SJ. Relational memory in first episode psychosis: implications for progressive hippocampal dysfunction after illness onset. Aust N Z J Psychiatry. 2011;45(3):206–213. doi: 10.3109/00048674.2010.547456. [DOI] [PubMed] [Google Scholar]
- 80.Wood SJ, Proffitt T, Mahony K, Smith DJ, Buchanan JA, Brewer W, Stuart GW, Velakoulis D, McGorry PD, Pantelis C. Visuospatial memory and learning in first-episode schizophreniform psychosis and established schizophrenia: a functional correlate of hippocampal pathology? Psychol Med. 2002;32(3):429–438. doi: 10.1017/s0033291702005275. [DOI] [PubMed] [Google Scholar]
- 81.Achim AM, Bertrand MC, Sutton H, Montoya A, Czechowska Y, Malla AK, Joober R, Pruessner JC, Lepage M. Selective abnormal modulation of hippocampal activity during memory formation in first-episode psychosis. Arch Gen Psychiatry. 2007;64(9):999–1014. doi: 10.1001/archpsyc.64.9.999. [DOI] [PubMed] [Google Scholar]
- 82.Williams LE, Avery SN, Woolard AA, Heckers S. Intact relational memory and normal hippocampal structure in the early stage of psychosis. Biol Psychiatry. 2012;71(2):105–113. doi: 10.1016/j.biopsych.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Armstrong K, Williams LE, Heckers S. Revised associative inference paradigm confirms relational memory impairment in schizophrenia. Neuropsychology. 2012;26(4):451–458. doi: 10.1037/a0028667. [DOI] [PMC free article] [PubMed] [Google Scholar]


