Abstract
Background & Aims
Common risk factors for obstructive sleep apnea (OSA) and Barrett's esophagus (BE) include obesity and gastroesophageal reflux disease (GERD). Aims of this study were to assess the association between OSA and BE and determine if the association is independent of GERD and body mass index (BMI)
Methods
Patients who had undergone a diagnostic polysomnogram and an esophagogastroduodenoscopy were identified using Mayo Clinic (Rochester, Minnesota) databases from January 2000 through November 2011. They were randomly matched for age, sex, and BMI at time of polysomnogram into the following groups: BE but no OSA (n=36), OSA but no BE (n=78), both (n=74), or neither (n=74). Clinical and demographic variables were abstracted from medical records. The association between OSA and BE was assessed using a multiple variable logistic model that incorporated age, sex, BMI, a clinical diagnosis of GERD, and smoking history.
Results
Subjects with OSA had an 80% increased risk for BE compared to subjects without OSA (odds ratio [OR], 1.8; 95% confidence interval [CI], 1.1–3.2; P=.03). These findings were independent of age, sex, BMI, GERD, and smoking history. Increasing severity of OSA, measured using the Apnea Hypopnea Index (AHI), was associated with an increased risk of BE (OR, 1.2 per 10 units increase in AHI; 95% CI, 1.0–1.3; P=.03).
Conclusion
In this case-control study, OSA was associated with an increased risk of BE, potentially through BMI and GERD independent mechanisms. Patients with OSA may benefit from evaluation for BE.
Keywords: obesity, esophageal adenocarcinoma, sleep-related breathing disorder, screening
BACKGROUND
The prevalence of obesity and its associated conditions is rising worldwide with significant impact on morbidity and mortality.(1) Obesity is considered a risk factor for gastroesophageal reflux disease (GERD) and a direct relationship exists between increasing body mass index (BMI) and GERD severity.(2, 3) Chronic GERD may lead to metaplasia of the distal esophagus, a premalignant condition known as Barrett’s esophagus (BE). The pathogenesis of BE in the obese is felt to be at least partially driven by mechanical disruption of the anti-reflux barrier by increased intra-abdominal pressure leading to worsening GERD.(4) Obesity, in particular central obesity, may also lead to BE independent of GERD through inflammatory pathways associated with visceral adiposity.(5, 6)
Obesity is the strongest risk factor for obstructive sleep apnea (OSA), a common sleep-related breathing disorder characterized by repetitive upper airway narrowing or collapse, transient hypoxia, and arousal from sleep.(7) OSA is associated with an increased risk of GERD as evidenced by symptomatology, esophageal pH monitoring and endoscopic findings of esophagitis.(8, 9) Studies looking at GERD in the morbid obese with OSA suggest that the association of GERD and OSA is independent of BMI.(10) It is postulated that continued breathing efforts against the occluded upper airway during obstructive apnea creates more negative intrathoracic pressure, which is transmitted to the esophageal body and precipitates reflux.(11) Esophageal manometry studies in patients with OSA show that a decline in esophageal body pressure is associated with OSA events. This is followed, however, by a compensatory increase in gastroesophageal junction and upper esophageal sphincter pressures that may actually help prevent reflux.(12) In fact, the temporal association between episodes of apnea and GERD has been inconsistent in studies using concurrent polysomnography and esophageal pH monitoring.(9, 13, 14) Despite the lack of mechanical phenomena to explain GERD in patients with OSA, several studies demonstrate that GERD improves with the use of continuous positive airway pressure (CPAP), the standard therapy for OSA.(15–17)
Patients with OSA share several risk factors with patients with BE including obesity, GERD, older age, and male gender. The asymptomatic nature of BE makes it challenging to identify populations at risk for this condition that may benefit from screening. This study aims to determine the relationship between OSA and BE and to explore if this relationship is independent of BMI and GERD.
PATIENTS AND METHODS
This study was approved by the Mayo Clinic Institutional Review Board.
Subject Identification and Data Collection
We used institution-wide searchable databases that contain demographic and medical information for patients evaluated at the Mayo Clinic in Rochester, Minnesota. These databases were used to identify subjects who had undergone both a diagnostic polysomnogram (PSG) and esophagogastroduodenoscopy (EGD) from January 2000 to November 2011. International Classification of Disease, 9th version (ICD-9) codes were used to identify subjects from this cohort diagnosed with BE and/or OSA.
Demographic information including age, gender, and race were abstracted for all study participants. Lifetime history of smoking was also recorded. Body mass index (BMI) was calculated from measurement of weight and height using the formula weight in kilograms divided by the square of height in meters within one year of the diagnostic PSG. A BMI ≥30 was used as a surrogate marker for central obesity.(20) The presence of GERD was identified using the ICD-9 code 530.81. The use of acid suppression medications (e.g., proton-pump inhibitors, histamine-2 blockers) was recorded for all subjects, even for those who did not carry a diagnosis of GERD in their medical records.
BE Subjects
Patients with BE were initially identified using the ICD-9 code 530.85. EGD reports were reviewed to confirm the diagnosis of BE based on the identification of at least 1 cm of columnar mucosa. Corresponding histopathology reports were reviewed for evidence of intestinal metaplasia (with our without dysplasia) on surveillance esophageal biopsies confirmed by an expert gastrointestinal pathologist. Endoscopy reports were also reviewed for the length of the maximal extent of the BE segment and presence and size of a hiatal hernia. The highest grade of dysplasia was determined from review of histology reports for all surveillance endoscopies. Endoscopic examinations were performed by board-certified gastroenterologists with expertise in the diagnosis of BE.
OSA Subjects
Patients with OSA were identified using the ICD-9 code 327.23. Data from PSG studies were abstracted including AHI, Arousal Profile (Arousal Index, %Movement Related, %Breathing Related), Mean and Minimal Oxygen Saturation. The AHI score represents the number of apneic (complete upper-airway collapse with near complete cessation of airflow) and hypopneic (partial upper-airway collapse) episodes per hour. The presence of OSA was defined by an AHI score ≥5/hr and confirmed by review of the diagnosis in the corresponding Sleep Medicine evaluation note. Severity of OSA was defined as mild when the AHI score was 5–15/hr, moderate 15–30/hr, and severe ≥30/hr. Supportive evidence for the presence of OSA included an Arousal Index > 15 with a predominance of breathing related arousals and a mean Minimum Oxygen Saturation of <85%.
Subjects were classified into four groups based on the presence or absence of BE or OSA: with no diagnosis of BE or OSA, with a diagnosis of BE but not OSA, with no diagnosis of BE but with a diagnosis of OSA and with a diagnosis of both BE and OSA. In patients who lacked a diagnosis of BE, endoscopy reports were reviewed for presence and severity of erosive esophagitis as a possible confounder. The severity of erosive esophagitis was defined using the Los Angeles classification (grades A-D).(21) The indication and findings for each EGD were also recorded in this group. In subjects who did not meet diagnostic criteria for OSA, PSG studies and Sleep Medicine evaluations were reviewed for an alternative diagnosis. The same parameters abstracted from PSG studies in subjects with OSA were also abstracted for subjects with no OSA.
Groups were randomly matched in a 2:1 ratio for age (<60 vs. ≥60), gender and BMI (<30 vs. ≥30) at time of PSG. The group with the smallest number of subjects (i.e. group with diagnosis of BE but not of OSA) defined the number of matched subjects in the remaining groups.
Statistical Analyses
Data were summarized as mean (standard deviation) for continuous scale variables (e.g. age, BMI) and as frequency (percent) for discrete scale variables. Univariate and multiple variable logistic regression models were used to assess the associations of demographic and clinical characteristics with BE. In particular, the odds ratios (95% Confidence Intervals) for BE in those with OSA were estimated from the model coefficients (and their standard errors). A separate model using severity of OSA as a predictor of BE, and a separate model to predict the presence of a diagnosis of GERD were also examined. A separate model using BMI ≥30 (as a surrogate marker for central obesity) vs. BMI <30 explored the role of visceral adiposity in the association between BE and OSA. The analyses were done using SAS software® (Version 9.3, SAS Institute, Cary, NC). A 0.05 alpha level was considered statistically significant.
RESULTS
Baseline Characteristics
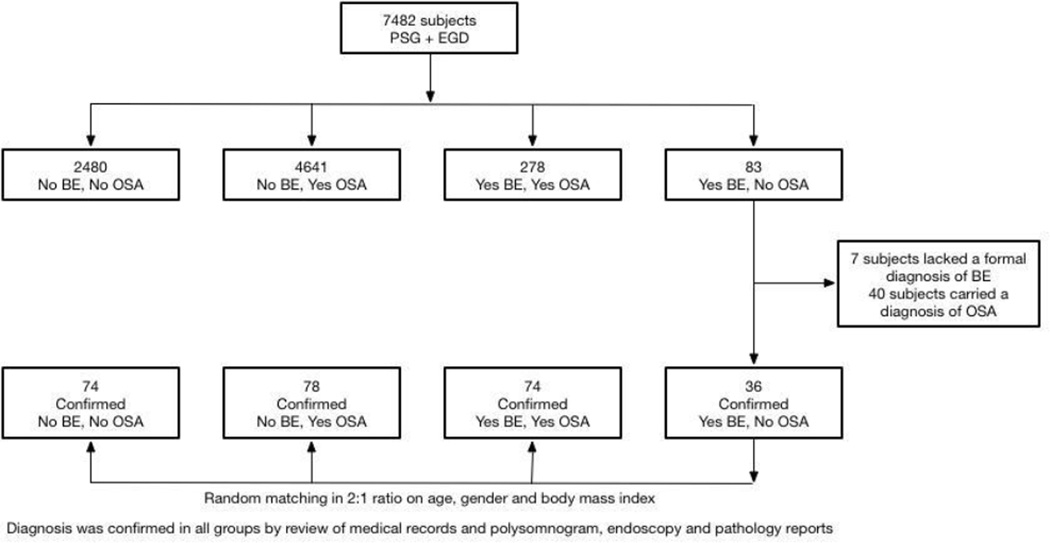
A total of 7482 subjects were identified as having undergone both a PSG and EGD in the timeframe outlined for this study (see Figure 1 for study flow). 2480 subjects had neither a diagnosis of OSA or BE, 83 subjects had a diagnosis of BE but not of OSA, 4641 subjects had a diagnosis of OSA but not of BE and 278 subjects had both a diagnosis of BE and OSA. The medical records of subjects in the smallest group (diagnosis of BE but not of OSA) were reviewed to confirm the presence and absence of BE and OSA respectively. 7 subjects lacked a formal diagnosis of BE based on endoscopic and histological criteria and 40 subjects carried a diagnosis of OSA based on PSG results. This resulted in 36 patients with a confirmed diagnosis of BE and absence of OSA. Subjects in this group were frequency matched using a 2:1 ratio on age, gender and BMI at the time of the diagnostic PSG. The presence or absence of BE and/or OSA was confirmed by review of individual medical records in each of the matched groups. A total of 74 subjects were identified as having neither OSA or BE, 74 as having both a diagnosis of OSA and BE, and 78 as having a diagnosis of OSA but not BE.
Figure 1. Flow-sheet of subject identification.
A population based patient database of 7482 subjects who underwent both a polysomnogram (PSG) and esophagogastroduodenoscopy (EGD) was screened for obstructive sleep apnea (OSA) and Barrett’s esophagus (BE) using International Classification of Disease, 9th Version (ICD-9) codes. 2480 subjects had neither a diagnosis of OSA nor BE, 4641 subjects had a diagnosis of OSA but not BE, 278 subjects had both a diagnosis of BE and OSA and 83 subjects had a diagnosis of BE but not OSA. A diagnosis of BE was confirmed by review of endoscopic records for evidence of ≥1 cm of columnar mucosa with histological evidence of intestinal metaplasia. A diagnosis of OSA was confirmed by an apnea hypopnea index ≥5/hr and review of Sleep Medicine evaluation records. Patients with a diagnosis of BE but not OSA accounted for the smallest group. In this group 36 patients had a confirmed diagnosis of BE and absence of OSA (7 subjects lacked a formal diagnosis BE and 36 subjects carried a diagnosis of OSA). These subjects were randomly matched in a 2:1 ratio on age, gender and body mass index to subjects in the remaining groups.
Baseline characteristics of participants are summarized in table 1. Overall 188 (72%) participants were male with a mean (SD) age of 61 (13) years. The majority (99%) of participants were non-Hispanic white. A total of 172 (66%) participants had a history of tobacco use with no association with BE/OSA group status. Overall 148 (56%) subjects had GERD symptoms requiring treatment with acid suppression medications. A total of 86 of 110 (78.2%) BE patients and 101 of 152 (66.4%) OSA patients had a diagnosis of GERD in their medical records. Subjects with OSA and BE as well as subjects with no OSA and a diagnosis of BE had a significantly increased odds for a diagnosis of GERD relative to subjects with neither diagnoses (OR 3.6, 95% CI 1.8–7.4, BE and OSA, and OR 3.5, 95% CI 1.4–8.7, BE only).
Table 1.
Baseline Characteristics of subjects
| BE/No OSA (N=36) |
BE/OSA (N=74) |
No BE/OSA (N=78) |
No BE/No OSA (N=74) |
p-value | |
|---|---|---|---|---|---|
| Mean(SD) Age (y) | 58(13) | 64(11) | 64(12) | 57(17) | * |
| Male gender, N (%) | 25(69%) | 58(78%) | 56(72%) | 52(70%) | * |
| Mean(SD) BMI | 30(5) | 33(8) | 32(6) | 31(7) | * |
| Caucasian race, N (%) | 32/32(100%) | 70/72(97%) | 76/77(99%) | 70/71(99%) | 0.91 |
| GERD on acid-suppression medication, N (%) | 21(58%) | 56(76%) | 39(50%) | 32(43%) | <0.001 |
| Smoking History, N (%) | 23/36(64%) | 54/73(74%) | 51/76(78%) | 44/74(59%) | 0.31 |
Subjects matched on age, gender and BMI at time of polysomnogram
In patients with BE, the mean (SD) segment length was 3.1 (2.6) cm. 74 (67.2%) had no dysplasia, 24 (21.8%) had low-grade dysplasia, 8 (7.3%) had high-grade dysplasia and 4 (3.6%) had cancer. A hiatal hernia was identified in 90 of 110 (82%) patients with BE with a mean (SD) size of 3.1 (1.8) cm. In patients with a diagnosis of both BE and OSA the diagnosis of BE preceded or was made within a year of OSA diagnosis in 92% of subjects (range: 12 years prior to 4 years after a diagnosis of OSA).
The main indication for EGD in patients without BE was dyspepsia/abdominal pain (n=37) followed by reflux symptoms (n=28), dysphagia (n=25), anemia/bleeding (n=24), diarrhea/vomiting (n=10) and other (n=28). Overall, 81 (74%) subjects in this group had normal EGDs. The most common endoscopic finding was esophagitis (n=22) followed by gastritis (n=11), Schatzki’s ring (n=12) and Cameron erosions (n=3). Ninety-one percent of cases of erosive esophagitis in patients without a diagnosis of BE were mild (grade A, n=13; grade B, n=7). Among patients having erosive esophagitis there was no significant association between the presence of OSA and the grade of esophagitis (p=0.42).
Patients with OSA had a mean (SD) AHI score of 24.7 (21.9) of which 89 (59%) had moderate to severe OSA. The mean (SD) Minimum Oxygen Saturation was 81.5% (8.0) and the mean (SD) Arousal Index was 42.6 (25.1) with 64.2% (SD=24.2%) induced by obstructive breathing, further supporting the diagnosis of OSA. Subjects with no OSA had a mean (SD) AHI score of 1.6 (1.3) and the majority were diagnosed with snoring (n=58) followed by periodic limb movement disorder (n=15) and insomnia (n=12).
Association between Obstructive Sleep Apnea and Barrett’s Esophagus
The association between OSA and BE was explored using univariate models assessing age, gender, BMI, a clinical diagnosis of GERD and history of smoking (Table 2). GERD and OSA were associated with BE (OR 3.2, 95%CI: 1.8–5.6, p<0.001 and OR 2.0, 95%CI: 1.3–3.2, p=0.01). A multiple variable model examined the association of OSA with BE; adjusting for age, gender, BMI, a clinical diagnosis of GERD and smoking history. Subjects with OSA had approximately 80% increase in odds for having BE as compared to subjects without OSA or BE (OR 1.8, 95%CI 1.1–3.2, p=0.03). (Table 2) There was no change in the association between OSA and BE in an alternative multiple variable model that included a combined variable : a diagnosis of GERD and use of acid suppressive medications (OR 1.8, 95%CI 1.0–3.1, p=0.04). (Supplemental Table 1). A model using the same variables but replacing OSA with AHI as a continuous variable examined whether the odds for BE increased with severity of OSA. An increased risk for BE with every 10 unit increase in AHI was observed (OR 1.2, 95%CI 1.0–1.3, p=0.03) suggesting a dose response association between BE and OSA. In a model including BMI ≥30 (as a surrogate marker for central obesity) vs. BMI <30; the risk of BE in OSA remained unchanged (OR=1.8, 95% CI 1.1–3.3, p=0.02). There was no significant association between increasing severity of OSA (AHI) and the length of BE (p=0.65).
Table 2.
Multiple variable logistic model for BE Risk Factors
| Univariate Models | Multiple Variable Model | |||
|---|---|---|---|---|
| Odds Ratio(95% CI) | P-value | Odds Ratio(95% CI) | P-value | |
| Age (per 5 years) | 1.0(0.9–1.1) | 0.65 | 1.0 (0.9–1.1) | 0.69 |
| Gender Male | 1.2(0.7–2.2) | 0.43 | 1.2 (0.6–2.2) | 0.62 |
| Female | 1.0(reference) | 1.0 (reference) | ||
| BMI (per 5 units) | 1.1(0.9–1.3) | 0.48 | 1.0 (0.8–1.3) | 0.73 |
| Smoking History Yes | 1.4(0.8–2.4) | 0.22 | 1.4 (0.8–2.5) | 0.23 |
| No | 1.0(reference) | 1.0(reference) | ||
| GERD Yes | 3.2(1.8–5.6) | <0.001 | 3.4 (1.9–6.0) | <0.0001 |
| No | 1.0(reference) | 1.0 (reference) | ||
| OSA Yes | 2.0(1.3–3.2) | 0.01 | 1.8 (1.1–3.2) | 0.03 |
| No | 1.0(reference) | 1.0(reference) | ||
DISCUSSION
We performed a case control study to explore the association of OSA and BE in a cohort of patients who underwent both a diagnostic PSG and EGD. In this subset of patients the presence of OSA was associated with an 80% increased risk of BE compared to subjects without OSA and BE. This association was dose-dependent, with an increase in severity of OSA (measured by AHI) being associated with an increased risk of BE.
Given that the association of BE and OSA could be confounded by gastroesophageal reflux we aimed to determine if the observed relationship between OSA and BE was independent of a clinical diagnosis of GERD. (23,12,24) Both GERD and OSA were associated with BE on univariate analysis. (Table 2) A multiple variable model including both OSA and GERD as covariates, showed that both OSA and GERD were independently associated with an increased risk of BE. (Table 2) In this study, GERD was defined as a clinical diagnosis made by a physician. The association of BE with OSA remained robust in a second multiple variable model which included a variable combining a clinical diagnosis of GERD with the use of acid suppressive medications (proton-pump inhibitors and histamine-2 blockers) as a marker for symptomatic reflux treated with medications. (Supplemental Table) From these observations, we conclude that reflux is involved in the pathogenesis of BE in patients with OSA, but likely potentiated by other mechanisms such as central obesity (increased visceral abdominal fat) and a generalized systemic inflammatory state, which have been reported to be prevalent in OSA. (11,26)
In our study cohort we found no direct association between BMI and the risk of BE in patients with OSA. Visceral fat has been implicated in the pathogenesis of BE through metabolic and pro-inflammatory pathways. (6, 25) To explore the role of visceral adiposity we used BMI≥30 as a surrogate marker for central obesity. (20) We found that the association between OSA and BE remained significant despite adjusting for central obesity, raising the possibility that OSA may increase the risk of BE through inflammatory pathways independent of visceral adiposity. Studies suggest that OSA is associated with a heightened state of systemic inflammation that is mediated in part by the generation of reactive oxygen species from repetitive hypoxia. (18,19) Increased levels of several inflammatory markers including cytokines, tumor necrosis factor-α, interleukin-6 and C-reactive protein have been reported in patients with OSA independent of BMI.(26) This state of inflammatory derangement can somewhat be reversed with treatment of OSA with CPAP.(27)
The design of our study limits our potential to further explore a specific mechanism for how OSA predisposes to BE. OSA may promote BE through pro-inflammatory pathways independent of BMI and perhaps central obesity, in addition to GERD. The lack of anthropometric measurements limits our definition of central obesity, although, previous studies have demonstrated that a BMI≥30 is an appropriate predictor of increased visceral adipose tissue in both sexes.(28) We recognize that a clinical definition of GERD that is not established through symptom questionnaires and confirmed by pH impedance is a limitation. We are consequently not able to account for the frequency and severity of gastroesophageal reflux events in our study population. This limits our ability to definitely assess the association of OSA with gastroesophageal reflux. However, the association of BE with OSA remained consistent with two definitions of reflux. Our study is also potentially limited by selection bias, given that all subjects were referred for a PSG due to suspicion of a sleep disorder. Arousals during shallow sleep are associated with transient lower sphincter relaxation and a risk of GERD. We rigorously screened PSG studies and clinical notes of patients without a diagnosis of OSA for potential confounders and found that most of these patients suffered from snoring without any evidence of other sleep disorders. To our knowledge, snoring is not associated with an increased incidence of transient lower sphincter relaxation. Finally, the use of ICD-9 codes to diagnose BE and OSA may have initially overestimated the prevalence of these conditions in our population.(29) A rigorous review of patient medical records, including endoscopy and pathology reports, helped confirm the presence or absence of these diagnoses in our final cohort of 262 subjects.
In this study, we describe that patients with untreated OSA are at an 80% increased risk of BE and that increased severity of OSA may heighten this risk. Given the asymptomatic nature of BE and the associated higher risk of esophageal adenocarcinoma, patients with OSA may benefit from screening for BE. Prospective studies are necessary to confirm this association in the general population and to explore whether this risk is reversed with OSA treatment.
Supplementary Material
Acknowledgments
FINANCIAL SUPPORT AND DISCLOSURE:
Supported in part by the American College of Gastroenterology, National Institutes of Diabetes, Digestive and Kidney Disease (grant RC4DK090413) and the Edward C. Rosenow Endowed Professorship Internal Medicine Residency Award.
ABBREVIATIONS
- AHI
Apnea Hypopnea Index
- BE
Barrett’s Esophagus
- BMI
Body Mass Index
- CI
Confidence Interval
- CPAP
Continuous positive airway pressure
- EGD
Esophagogastroduodenoscopy
- GERD
Gastroesophageal reflux disease
- ICD-9
International Classification of Disease, 9th Version
- OR
Odds Ratios
- OSA
Obstructive Sleep Apnea
- PSG
Polysomnogram
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTERETS: All authors have no conflicts of interest to declare
AUTHOR CONTRIBUTION:
Study concept and design: CLL, ECG, ADC, SC, VKS, KKW, PGI
Acquisition of data: CLL
Analysis and interpretation of data: CLL, ECG, ADC, KKW, PGI
Drafting of the manuscript: CLL, ECG, ADC, PGI
Critical revision of the manuscript for important intellectual content: SC, VKS, KKW, PGI
Statistical analysis: WSH, ARZ
Administrative, technical, or material support: LL, KD
REFERENCES
- 1.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson BC, Somers SC, Fuchs CS, et al. Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med. 2006;354:2340–2348. doi: 10.1056/NEJMoa054391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wajed SA, Streets CG, Bremner CG, et al. Elevated body mass disrupts the barrier to gastroesophageal reflux; discussion 1018–9. Arch Surg. 2001;136:1014–1018. doi: 10.1001/archsurg.136.9.1014. [DOI] [PubMed] [Google Scholar]
- 4.Corley DA, Kubo A, Levin TR, et al. Abdominal obesity and body mass index as risk factors for Barrett's esophagus. Gastroenterology. 2007;133:34–41. doi: 10.1053/j.gastro.2007.04.046. quiz 311. [DOI] [PubMed] [Google Scholar]
- 5.Edelstein ZR, Farrow DC, Bronner MP, et al. Central adiposity and risk of Barrett's esophagus. Gastroenterology. 2007;133:403–411. doi: 10.1053/j.gastro.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 6.Nelsen EM, Kirihara Y, Takahashi N, et al. Distribution of body fat and its influence on esophageal inflammation and dysplasia in patients with Barrett's esophagus. Clin Gastroenterol Hepatol. 10:728–734. doi: 10.1016/j.cgh.2012.03.007. quiz e61–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med. 2005;142:187–197. doi: 10.7326/0003-4819-142-3-200502010-00010. [DOI] [PubMed] [Google Scholar]
- 8.Demeter P, Visy KV, Magyar P. Correlation between severity of endoscopic findings and apneahypopnea index in patients with gastroesophageal reflux disease and obstructive sleep apnea. World J Gastroenterol. 2005;11:839–841. doi: 10.3748/wjg.v11.i6.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ing AJ, Ngu MC, Breslin AB. Obstructive sleep apnea and gastroesophageal reflux. Am J Med. 2000;108(Suppl 4a):120S–125S. doi: 10.1016/s0002-9343(99)00350-2. [DOI] [PubMed] [Google Scholar]
- 10.Sabate JM, Jouet P, Merrouche M, et al. Gastroesophageal reflux in patients with morbid obesity: a role of obstructive sleep apnea syndrome? Obes Surg. 2008;18:1479–1484. doi: 10.1007/s11695-008-9508-9. [DOI] [PubMed] [Google Scholar]
- 11.Romero-Corral A, Caples SM, Lopez-Jimenez F, et al. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 137:711–719. doi: 10.1378/chest.09-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuribayashi S, Massey BT, Hafeezullah M, et al. Upper esophageal sphincter and gastroesophageal junction pressure changes act to prevent gastroesophageal and esophagopharyngeal reflux during apneic episodes in patients with obstructive sleep apnea. Chest. 137:769–776. doi: 10.1378/chest.09-0913. [DOI] [PubMed] [Google Scholar]
- 13.Penzel T, Becker HF, Brandenburg U, et al. Arousal in patients with gastro-oesophageal reflux and sleep apnoea. Eur Respir J. 1999;14:1266–1270. doi: 10.1183/09031936.99.14612669. [DOI] [PubMed] [Google Scholar]
- 14.Berg S, Hoffstein V, Gislason T. Acidification of distal esophagus and sleep-related breathing disturbances. Chest. 2004;125:2101–2106. doi: 10.1378/chest.125.6.2101. [DOI] [PubMed] [Google Scholar]
- 15.Kerr P, Shoenut JP, Millar T, et al. Nasal CPAP reduces gastroesophageal reflux in obstructive sleep apnea syndrome. Chest. 1992;101:1539–1544. doi: 10.1378/chest.101.6.1539. [DOI] [PubMed] [Google Scholar]
- 16.Friedman M, Gurpinar B, Lin HC, et al. Impact of treatment of gastroesophageal reflux on obstructive sleep apnea-hypopnea syndrome. Ann Otol Rhinol Laryngol. 2007;116:805–811. doi: 10.1177/000348940711601103. [DOI] [PubMed] [Google Scholar]
- 17.Tawk M, Goodrich S, Kinasewitz G, et al. The effect of 1 week of continuous positive airway pressure treatment in obstructive sleep apnea patients with concomitant gastroesophageal reflux. Chest. 2006;130:1003–1008. doi: 10.1378/chest.130.4.1003. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki YJ, Jain V, Park AM, et al. Oxidative stress and oxidant signaling in obstructive sleep apnea and associated cardiovascular diseases. Free Radic Biol Med. 2006;40:1683–1692. doi: 10.1016/j.freeradbiomed.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulz R, Mahmoudi S, Hattar K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2000;162:566–570. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 20.Leggett CL, Nelsen EM, Tian J, et al. Metabolic syndrome as a risk factor for barrett esophagus: a population-based case-control study. Mayo Clin Proc. 88:157–165. doi: 10.1016/j.mayocp.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong D, Bennett JR, Blum AL, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996;111:85–92. doi: 10.1053/gast.1996.v111.pm8698230. [DOI] [PubMed] [Google Scholar]
- 22.Fass R. Effect of gastroesophageal reflux disease on sleep. J Gastroenterol Hepatol. 25(Suppl 1):S41–S44. doi: 10.1111/j.1440-1746.2009.06210.x. [DOI] [PubMed] [Google Scholar]
- 23.Samelson CF. Gastroesophageal reflux and obstructive sleep apnea. Sleep. 1989;12:475–476. doi: 10.1093/sleep/12.5.475. [DOI] [PubMed] [Google Scholar]
- 24.Kuribayashi S, Kusano M, Kawamura O, et al. Mechanism of gastroesophageal reflux in patients with obstructive sleep apnea syndrome. Neurogastroenterol Motil. 22 doi: 10.1111/j.1365-2982.2010.01485.x. 611-e172. [DOI] [PubMed] [Google Scholar]
- 25.El-Serag HB, Kvapil P, Hacken-Bitar J, et al. Abdominal obesity and the risk of Barrett's esophagus. Am J Gastroenterol. 2005;100:2151–2156. doi: 10.1111/j.1572-0241.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 26.Calvin AD, Albuquerque FN, Lopez-Jimenez F, et al. Obstructive sleep apnea, inflammation, and the metabolic syndrome. Metab Syndr Relat Disord. 2009;7:271–278. doi: 10.1089/met.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishida K, Kato M, Kato Y, et al. Appropriate use of nasal continuous positive airway pressure decreases elevated C-reactive protein in patients with obstructive sleep apnea. Chest. 2009;136:125–129. doi: 10.1378/chest.08-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oka R, Miura K, Sakurai M, et al. Comparison of waist circumference with body mass index for predicting abdominal adipose tissue. Diabetes Res Clin Pract. 2009;83:100–105. doi: 10.1016/j.diabres.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Corley DA, Kubo A, DeBoer J, et al. Diagnosing Barrett's esophagus: reliability of clinical and pathologic diagnoses. Gastrointest Endosc. 2009;69:1004–1010. doi: 10.1016/j.gie.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.