Abstract
Rationale
The N-methyl-d-aspartate (NMDA) glutamate receptor antagonist ketamine has demonstrated rapid antidepressant effects in patients with treatment-resistant depression (TRD). Despite the promise of a novel and urgently needed treatment for refractory depression, concerns regarding potential adverse neurocognitive effects of ketamine remain.
Objectives
Although extensive research has been conducted in healthy volunteers, there is a paucity of studies examining the neurocognitive effects of ketamine in depressed patients. Therefore, the aims of the current study were to characterize the relationship between baseline neurocognition and antidepressant response to ketamine, measure the acute impact of ketamine on neurocognition, and investigate the relationship between acute neurocognitive effects of ketamine and antidepressant response.
Methods
Neurocognitive functioning was assessed in 25 patients with TRD using a comprehensive battery: estimated premorbid IQ, current IQ, and tests from the MATRICS battery (MCCB). A subset of the MCCB was repeated immediately following a 40-min intravenous infusion of ketamine (0.5 mg/kg).
Results
Patients who responded to ketamine 24 hours following treatment had poorer baseline neurocognitive performance relative to non-responders, and in particular slower processing speed (F=8.42, df=23, p=0.008). Ketamine was associated with selective impairments in memory recall and the degree of cognitive change carried negative prognostic significance (e.g. negative cognitive effects immediately after ketamine predicted lower response rate at 24 hours; Fisher’s Exact Test 2-sided p=0.027).
Conclusions
Taken together, our findings suggest a potential baseline neurocognitive predictor of ketamine response and an inverse relationship between the cognitive effects of ketamine and antidepressant efficacy.
Keywords: major depressive disorder, treatment-resistant depression, neurocognition, cognitive functioning, ketamine, antidepressant, glutamate, N-methyl-d-aspartate
Introduction
Ketamine is a high-affinity, noncompetitive N-methyl-d-aspartate (NMDA) glutamate receptor antagonist that has demonstrated a rapid antidepressant effect within hours or days in patients with treatment resistant major depression (TRD) (Zarate et al. 2006; aan het Rot et al. 2010; Mathew et al. 2010; Ibrahim et al. 2012; Mathew et al. 2012; Murrough et al. 2012). Ketamine has been used as an anesthetic agent for more than three decades (Reich and Silvay 1989; Green and Li 2000) and only recently have investigations focused on the potential antidepressant properties of ketamine administered at low doses in treatment refractory mood disorders (Mathew et al. 2012; Murrough 2012). At the sub-anesthetic doses utilized in depression studies, ketamine results in transient dissociative effects that typically peak immediately following drug administration and resolve within minutes or up to 2 hours following cessation of the drug (Berman et al. 2000; Zarate et al. 2006; aan het Rot et al. 2010; Mathew et al. 2010; Murrough et al. 2012). Despite the potential utility of ketamine as a novel and urgently needed therapy for TRD, important concerns regarding adverse effects – including the potential for adverse neurocognitive effects – remain (Morgan et al. 2009).
The short-term effects of ketamine on neurocognition in healthy volunteers have been studied extensively (Krystal et al. 1994; Krystal et al. 1999; Morgan et al. 2004; Krystal et al. 2005; Parwani et al. 2005; Rowland et al. 2005; Perry et al. 2007). Ketamine appears to disrupt information encoding that occurs during drug administration but does not impair recall for previously learned information (Morgan et al. 2004; Krystal et al. 2005; Rowland et al. 2005). Some studies have found evidence for selective impairments in aspects of executive functioning related to ketamine (Krystal et al. 1994; Krystal et al. 1999), while other studies have found no impairments (Morgan et al. 2004; Parwani et al. 2005). Importantly from the perspective of treatment safety, a large review of possible untoward or prolonged events associated with ketamine administration in healthy volunteers found ketamine to carry a very low risk of adverse events (Perry et al. 2007).
A critical initial observation regarding the antidepressant effect of ketamine was the temporal discordance between acute dissociative and neurocognitive effects on the one hand and improvements in core symptoms of depression on the other (Berman et al. 2000; Zarate et al. 2006; Mathew et al. 2010). The observed reductions in depressive symptoms develop over hours or days, in contrast to the immediate but transient dissociative and neurocognitive effects. It remains unknown, however, if there is an association between the induction of acute neurocognitive effects and antidepressant response.
Despite extensive research in healthy volunteers, there is a paucity of studies examining the neurocognitive effects of ketamine in depressed patients. Therefore, the aims of the current study were to (1) characterize the relationship between baseline neurocognition and antidepressant response to ketamine in a TRD sample, (2) measure the acute impact of ketamine on neurocognition in TRD, and (3) investigate the relationship between neurocognitive effects of ketamine and antidepressant response.
Materials and Methods
This neurocognitive study was conducted in conjunction with a clinical trial involving a single open-label intravenous (IV) administration of ketamine in participants with TRD. The primary depression outcomes were previously reported in (Mathew et al. 2010) (ClinicalTrials.gov identifier NCT00419003). The study was approved by the Mount Sinai Program for the Protection of Human Subjects and participants provided written informed consent prior to participation.
Participants
Twenty-five participants (10 females) aged 21–70 years underwent a psychiatric and medical screening following completion of the informed consent process. Study participants had a primary diagnosis of major depressive disorder (MDD), chronic or recurrent as determined by a study psychiatrist and the Structured Clinical Interview for DSM-IV – Patient Edition (First et al. 1995). Depression severity was at least moderate, determined by a score of ≥ 32 on the Inventory of Depressive Symptomatology – Clinician Rated (IDS-C30) (Rush et al. 1996). Participants had failed to respond to at least 2 adequate antidepressant trials in the current episode, according to Antidepressant Treatment History Form (ATHF) criteria (Sackeim 2001). If participants were taking psychotropic medication, a wash-out period of at least two weeks was required prior to enrollment (4 weeks for fluoxetine). Participants were excluded if they had current psychotic symptoms; had lifetime histories of bipolar disorder, schizophrenia, or schizoaffective disorder; had current anorexia or bulimia nervosa; had alcohol or drug abuse within the past 6 months; or had any unstable medical or neurological illness that increased the risks of ketamine administration. Physical examination, vital signs, weight, echocardiogram, standard blood tests, and urinalysis confirmed absence of unstable medical illnesses. Urine toxicology and blood human chorionic gonadotropin tests confirmed absence of recent illicit substances use and pregnancy, respectively.
Study Design
Eligible participants underwent a battery of neurocognitive tests (see below for detail) within one week prior to receiving a single IV infusion of ketamine hydrochloride, 0.5 mg/kg over 40 minutes, in an inpatient clinical research setting at Mount Sinai Medical Center. See (Mathew et al. 2010) for detailed ketamine infusion methods. Upon completion of the infusion (+40 min), a subset of the neurocognitive tests were repeated.
Change in depression severity was measured using the Montgomery–Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg 1979); response status was defined as ≥ 50% reduction in MADRS score at 24 hours relative to the previous day’s baseline. The primary depression outcomes included the change in MADRS score from baseline to 24 hours following the infusion and the proportion of responders (response rate) (Mathew et al. 2010). Psychotomimetic and dissociative side effects were measured using the Brief Psychiatric Rating Scale (BPRS) positive symptoms subscale (four items: conceptual disorganization, hallucinations, suspiciousness, unusual thought content) (Overall et al. 1961) and the Clinician-Administered Dissociative States Scale (CADSS) (Bremner et al. 1998), respectively.
Two hours prior to receiving ketamine, participants received a single dose of either lamotrigine 300 mg by mouth or matching placebo in order to test the influence of lamotrigine on the neuropsychiatric effects of ketamine (Anand et al. 2000; Mathew et al. 2010).
Neurocognitive Assessment
Neurocognitive functioning was assessed at baseline using a comprehensive battery (testing time approximated 1–2 hours): estimated premorbid IQ (WRAT-3 Reading), current IQ [Wechlser Adult Intelligence Scale (WAIS-III) Vocabulary and Matrix Reasoning], and tests from the MATRICS battery (MCCB) [Trails A, WMS Spatial Span, BACS Digit Symbol; Letter-Number Sequencing, Hopkins Verbal Learning Test (HVLT), Brief Visual Memory Test (BVMT), Category Fluency, and the Continuous Performance Test (CPT I/P)]. We created standardized scores (T-scores with a mean of 50 and an SD of 10) for the MCCB data using the MCCB computerized scoring program, which includes correction for age and sex based on the MCCB normative sample. For non-MCCB tests we standardized scores based upon the published normative data for the WAIS-3 (Wechsler 1999).
To create neurocognitive domain scores, we calculated mean T-scores as follows: [Processing Speed (category fluency, Trails A, BACS Digit Symbol); Attention (CPT-I/P); Working Memory (WMS-III Spatial Span; letter-number); Verbal learning (HVLT learning and delay); and Visual Learning (BVMT learning)]. Estimated current IQ was a mean score including WAIS-III Vocabulary and Matrix Reasoning. Premorbid IQ was estimated using the WRAT-3 Reading subtest. Although we did not repeat the entire battery, at 40 minutes post-infusion, we administered the HVLT and Category Fluency subtests in order to measure the acute effects of ketamine on verbal learning and executive functioning.
Statistical Analysis
The analytic approach addressed several independent questions and was conducted in a step-wise manner as follows: (1) we entered a calculated depressive symptom change score [MADRS 24-hour minus MADRS Baseline] into a linear regression including demographics, baseline symptom ratings, and neurocognitive domains to identify possible clinical and cognitive predictors of ketamine response; (2) we compared groups (responder vs. non-responder) on demographic, clinical, and cognitive data at baseline using multivariate analysis of variance (MANOVA) or Chi-square where applicable; (3) we evaluated the effect of acute ketamine on cognitive performance using paired t-tests to assess the overall effect regardless of response status and (4) to address the heterogeneity in cognitive response, we categorized subjects based on whether they evidenced cognitive impairment at 40 minutes relative to baseline performance (n=18) or showed no change/improved in performance (n=7) and compared these cognitive subgroups on demographic and clinical outcome measures. To do so, we calculated a mean composite T-score [(HVLT Learning + HVLT Delayed + Category Fluency)/3] at baseline and at 40 minutes post-infusion and then created composite change scores by subtracting baseline mean T-score from 40 minute mean T-score. Subjects who showed evidence of decline (a negative value on the calculated change score) were grouped and labeled as “cognitive decline”, while those subjects who evidenced no decline were labeled “cognitively stable“.
Results
Demographic and Clinical Characteristics
Study participants were aged 49.0 ± 11.2 years, had 15.2 ± 2.8 years of education and a current IQ of 114.6 ± 10.4. Participants had mean duration of illness of 29.6 ± 13.4 years and a high level of treatment resistance (Table 1).
Table 1.
Characteristics of Study Sample
| Demographic | Responders | Non-responders | F-value | p-value |
|---|---|---|---|---|
| Age (years) | 53.81 | 40.44 | 11.85 | 0.002 |
| Education (years) | 14.56 | 16.22 | 2.15 | 0.156 |
| Premorbid IQ | 108.81 | 107.67 | 0.08 | 0.774 |
| Number of antidepressant trials | 5.63 | 6.78 | 0.43 | 0.518 |
| Age at first major depressive episode (years) | 20.31 | 16.44 | 0.56 | 0.461 |
| Duration of illness (years) | 32.31 | 24.89 | 1.83 | 0.189 |
| MADRS baseline | 36.88 | 37.00 | 0.003 | 0.958 |
| BPRS positive baseline | 5.25 | 5.89 | 0.276 | 0.604 |
| CADSS baseline | 4.44 | 3.11 | 0.193 | 0.664 |
| BPRS 40 minutes | 5.44 | 4.67 | 0.656 | 0.426 |
| CADSS 40 minutes | 8.38 | 9.33 | 0.070 | 0.794 |
The Montgomery-Asberg Depression Rating Scale (MADRS) scores range from 0 to 60 with higher scores indicating more severe symptoms.
Baseline Neurocognitive Status and Relationship to Antidepressant Response
We conducted a backward linear regression procedure with MADRS change score (24-hour MADRS minus Baseline MADRS) as the dependent variable and included each neurocognitive domain (Processing Speed, Attention, Working Memory, Verbal Learning, and Visual Learning), age, baseline depression severity, and duration of illness as predictor variables. A 7-step backward regression revealed a best-fit model (F=5.1; p=0.017) with two independent predictors of antidepressant response: Age (t= −2.08; Standardized Beta = −0.39, p=0.051) and Processing Speed performance (t = 2.23; Standardized Beta = 0.42; p=0.038). Response to ketamine was more likely in subjects who were older and had slower processing speed at baseline. Importantly, while age is likely to contribute significantly to neurocognitive performance, particularly with regard to processing speed, the effects of age were independent of the effects of baseline processing speed performance. An exploratory linear regression was repeated including several additional variables of interest. Due to a limited sample size, we considered this as secondary to the analyses described above; however, several clinical measures were evaluated for their relevance in predicting treatment response including lamotrigine treatment, psychotomimetic side effects (as measured by change in BPRS positive and change in CADSS at 40 minutes), and cognitive side effects (as measured by change score on cognitive composite at 40 minutes). When each of these variables was added to the regression described above, the model was significant (F= 5.044; p=0.017) and the same two variables remained as the only significant predictors of treatment response: Age (t= −2.08; Standardized Beta = −0.39, p=0.051) and Processing Speed performance (t = 2.23; Standardized Beta = 0.42; p=0.038).
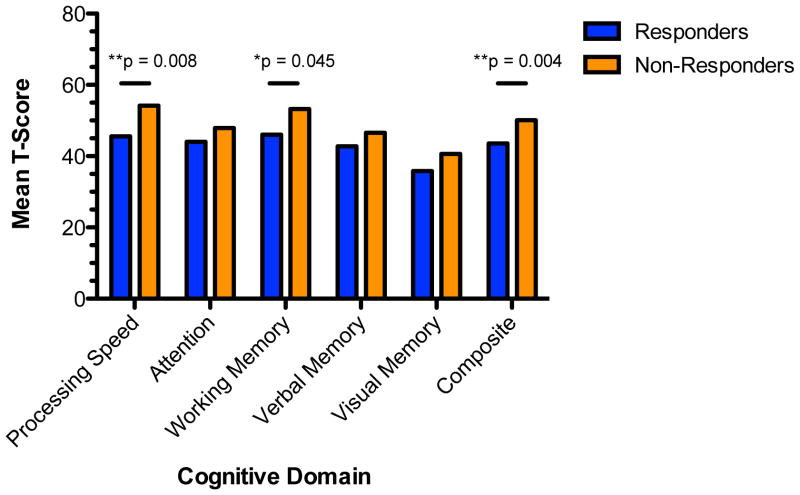
As a follow up to the linear regression approach to better understand the direction of the effects revealed, univariate analyses comparing ketamine responder (n=16) and non-responder (n=9) subgroups indicated no significant differences in terms of IQ, education, sex, depression course, level of treatment resistance, or baseline depression severity; however, consistent with regression results, treatment responders were significantly older than non-responders (F=11.85, df=23, p=0.002; Table 1). All neurocognitive domain scores are corrected for age in the MCCB calculation; therefore, age was not used as a covariate in initial cognitive analyses. When comparing groups across neurocognitive domains, analyses revealed significant baseline differences between the groups in the domains of Processing Speed (F=8.42, df=23, p=0.008); Working Memory (F=4.52; df=23; p=0.045) and MCCB Composite (F=10.77; df=23, p=0.004; Figure 1). When entering age as a covariate in the ANOVA, all significant results remained significant and there was no significant main effect of age. Individuals who showed significant clinical response to ketamine at 24 hours showed a general pattern of more severe neurocognitive impairments relative to non-responders at baseline, most notably on tasks that require speeded processing of information and working memory.
Figure 1. Baseline Neurocognitive Performance by Treatment Response Group.
Figure depicts mean T-scores in cognitive domains derived from the MCCB. Subgroups (Responder, Non-responder) are defined by response status 24 hours following a single infusion of ketamine (≥ 50% reduction in depression severity in Montgomery-Asberg Depression Rating Scale score compared to baseline).
Neurocognitive Effects of Ketamine and Relationship to Antidepressant Response
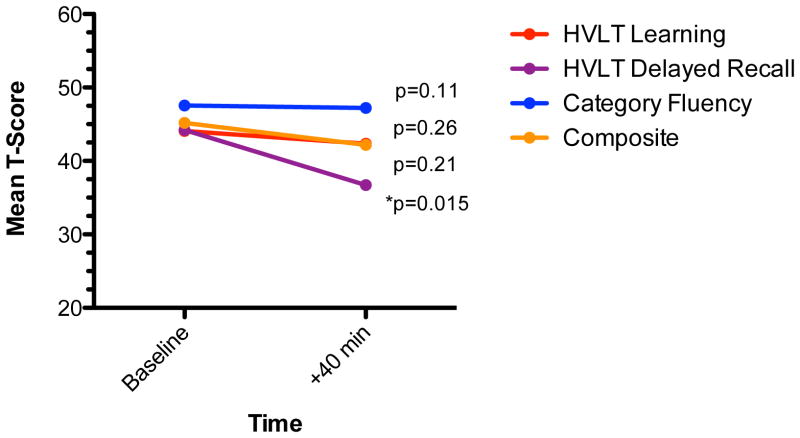
In the full sample, ketamine was associated with minimal neurocognitive change at 40 minutes post-infusion, the time-point corresponding to the peak potential for acute psychoactive effects (post-ketamine T-scores: HVLT Learning=42.4 +/− 8.8, HVLT Delayed Recall=36.7 +/− 12.5, Category Fluency=47.2 +/− 9.0). Paired t-tests comparing baseline scores and post-ketamine (+40 minutes) scores revealed a significant decline in HVLT Delayed Recall post-ketamine (t=3.08, df=24, p=0.01) but non-significant changes in HVLT Learning (t=0.81; df=24; p=0.42) or Category Fluency (t=0.15; df=23; 0.88 Figure 2).
Figure 2. Acute Effects of Ketamine on Neurocognition in Treatment-Resistant Depression.
Figure shows mean T-scores on the HVLT and Category Fluency tasks at baseline and post ketamine treatment.
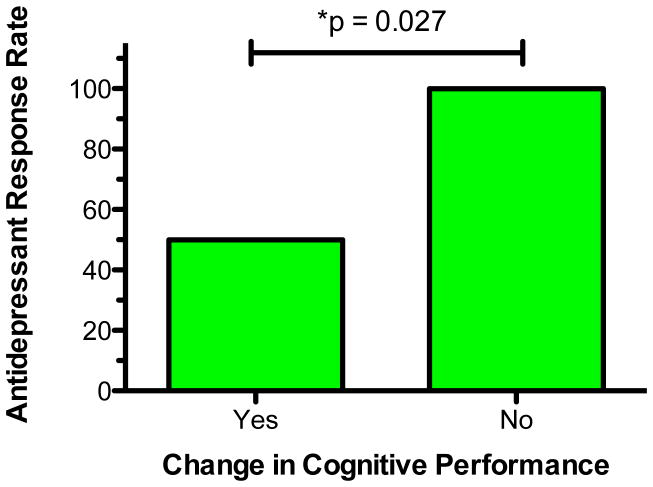
We next characterized the sample based on the presence/absence of reduced cognitive performance from baseline to 40 minutes post-infusion. This approach allowed us to account for baseline levels of cognitive performance and to define a clinically meaningful subgroup of patients who showed objective evidence of cognitive change post-ketamine infusion. Eighteen subjects showed evidence of some degree of reduced performance on a composite score of the three variables that were repeated (HVLT Learning; HVLT Delayed; and Category Fluency), while 7 subjects either remained stable or improved over time. MANOVA indicated that there were no significant group differences on demographic features, neurocognitive performance at baseline, or on clinical symptom severity at baseline (data not shown). However, we found a significant relationship between ketamine treatment response and cognitive change. Specifically, among the sub-group of patients with no change, 100% (7/7) were categorized as ketamine treatment responders. In contrast, among patients who evidenced decreased cognitive performance, only 50% (9/18) were ketamine responders (Fisher’s Exact Test 2-sided p=0.027; Figure 3).
Figure 3. Relationship Between Antidepressant Response Rate and Cognitive Performance Following Ketamine.
Figure illustrates proportion of patients meeting antidepressant response criteria 24 hours following ketamine. Response is defined as ≥ 50% reduction in depression severity in Montgomery-Asberg Depression Rating Scale score compared to baseline. Sub-groups are defined based on the presence or absence of decreased cognitive performance immediately following ketamine.
Effects of Pre-treatment with Lamotrigine
Participants in our sample received a single dose of 300 mg of lamotrigine prior to the ketamine infusion in a double-blind, placebo controlled manner (Anand et al. 2000; Mathew et al. 2010). The initial purpose of this lamotrigine administration was to test whether this could ameliorate the acute psychoactive effects of ketamine; however, we were also interested post hoc in understanding the potential effects of lamotrigine on ketamine-related cognitive change. Therefore, we conducted exploratory analyses using Chi2 to test this question. Those subjects who were treated with a single dose of 300 mg of lamotrigine prior to ketamine infusion (n=10) were less likely to experience cognitive side effects at 40 minutes (50% were in the Cognitive Decline group) than those who did not receive lamotrigine (87% were in the Cognitive Decline group); however, this difference was only significant at a trend level (Chi2 = 4.0; p=0.045; Fisher’s Exact 2-sided p=0.08).
Discussion
Three primary findings emerge from the current study of neurocognition and ketamine treatment in TRD. First, we found that lower levels of baseline neurocognitive performance (particularly processing speed) in TRD are associated with an increased antidepressant response rate to ketamine. Second, low-dose ketamine is associated with minimal acute neurocognitive effects in TRD at 40 minutes, with selective impairments confined to the delayed recall component of the HVLT while sparing HVLT learning and category fluency. Third, we found that when acute reductions in cognitive performance did occur following ketamine, it carried negative prognostic significance (e.g. cognitive impairment predicted lower response rate).
Our finding of an association between baseline neurocognitive function and antidepressant response to ketamine may suggest a practical way of identifying patients who are more likely to exhibit a favorable clinical outcome following a course of ketamine therapy. Prior studies have found an association between baseline neurocognitive function and antidepressant outcome, although by and large this association has been in the opposite direction compared to our current findings (Dunkin et al. 2000; Taylor et al. 2006; Gorlyn et al. 2008; McLennan and Mathias 2010). For example, Taylor et al reported psychomotor slowing as a predictor of fluoxetine non-response (Taylor et al. 2006). Similarly, decreased executive functioning more broadly has been associated with poor response to serotonin-selective reuptake inhibitors (SSRIs) and other antidepressant agents (McLennan and Mathias 2010). While still preliminary, our findings suggest a unique profile for patients responsive to ketamine compared to other antidepressant interventions.
In addition to potential clinical utility, the association between baseline neurocognition and ketamine response suggests the presence of specific neurobiological characteristics among treatment responders. Dopamine (DA) neurotransmission and neural circuits involving the dorsolateral prefrontal cortex (DLPFC) and the striatum have been most consistently implicated in processing speed, psychomotor and executive functioning. Ketamine has been observed to modulate DA transmission (Krystal et al. 2005; Smith et al. 1998; Vollenweider et al. 2000), although the extent to which this is related to its antidepressant activity is not known. Beyond DA, preclinical studies have implicated alterations in neuroplasticity via several mechanisms – including modulation of brain-derived neurotrophic factor (BDNF) – as critical to ketamine’s antidepressant action (Autry et al. 2011; Duman 2012). A recent study found an association between a common single nucleotide polymorphism in the gene coding for BDNF (Val66Met) and antidepressant response to ketamine (Laje et al. 2012). Since BDNF functioning has been previously linked to cognition (Swardfager et al. 2011), our results are potentially consistent with a model relating BDNF or neuroplasticity to cognition and antidepressant response to ketamine. Future studies explicitly designed to test these relationships will be required.
Our second finding concerns the acute impact of ketamine on neurocognition in TRD. Consistent with prior reports, ketamine resulted in selective disruption of delayed recall for information learned directly after administration of the drug (Morgan et al. 2004; Krystal et al. 2005; Rowland et al. 2005). In contrast, immediate recall and verbal fluency remained intact. In order to estimate a global effect of ketamine on cognition, we constructed a composite index of cognitive function and found ketamine in aggregate was not associated with a significant decline. When we categorized participants individually based on whether or not they evidenced decreased cognitive performance following ketamine, we found that negative cognitive effects were associated with non-response to the antidepressant effects of ketamine. One implication of this finding is that the antidepressant effects of ketamine do not appear to depend on acute disruptions in cognition and the neural substrates of these processes are likely distinct. As a corollary, while the NMDA receptor is widely distributed in cortical and subcortical regions, it may be theoretically possible to target only a subpopulation of NMDA receptors important for antidepressant action while sparing others and thereby decrease the incidence of cognitive side effects associated with NMDA receptor antagonists (Mony et al. 2009).
We explored the influence of the glutamate release inhibitor lamotrigine on the cognitive effects of ketamine and found a non-significant trend towards attenuation of reductions in cognitive performance. Ketamine is associated with rapid increases in extracellular glutamate (Moghaddam et al. 1997), potentially contributing to its adverse cognitive effects (Anand et al. 2000). Our findings largely replicate a previous study in healthy volunteers demonstrating a protective effect of lamotrigine on cognitive functioning (Anand et al. 2000). The potential protective effects of lamotrigine in concert with ketamine require further study.
This study has several limitations. First, ketamine was administered in an open-label fashion and therefore the specificity of the findings cannot be determined. Future randomized, controlled designs investigating the neurocognitive impact of ketamine will be required to better characterize these effects. Second, the study sample size is relatively small and was restricted to patients with TRD. Although the sample size is similar to previously published research investigating the effects of ketamine in depression, the power of the study to detect small effects is limited. For example, the trend-level association between lamotrigine and cognitive change may represent a type II error resulting to inadequate power. It is not known if our observations would generalize to patients who were not treatment resistant. Third, neurocognitive assessments were repeated only once, immediately following ketamine administration and were limited to only a few tests. While our intention was to measure potential adverse neurocognitive effects of ketamine – hence the selected time-point corresponding to the peak acute neuropsychiatric effects – a critical question not addressed by the current study is whether there are longer-term neurocognitive effects of ketamine administered at low doses in patients with TRD. Future studies deploying rigorous neurocognitive measurements in the context of longer (e.g. weeks) treatment studies in TRD will be required before a thorough risk/benefit assessment regarding the safety of ketamine for depression can be undertaken. An additional limitation is that our observed association between baseline neurocognition and antidepressant outcome may be driven by other, unmeasured variables. Prior studies have found an association between a family history of alcohol abuse and antidepressant response to ketamine (Phelps et al. 2009; Luckenbaugh et al 2012), although this data was not available for our sample.
In conclusion, our study demonstrated specific associations between baseline neurocognition and antidepressant outcomes following ketamine and characterized the acute neurocognitive impact of ketamine in TRD. Future studies will be required to determine more durable neurocognitive effects if ketamine is to be considered as a future treatment for severe and refractory forms of depression.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant number UL1TR000067 (Mount Sinai Clinical and Translational Science Award) and by a NARSAD award from the Brain and Behavior Research Foundation (Mathew SJ). Dr. Murrough is supported by a Career Development Award from NIH/NIMH (K23MH094707).
Footnotes
Conflict of Interest
In the past two years, Dr. Murrough has received research support from Evotec, Janssen Pharmaceuticals and Avanir. Dr. Iosifescu has consulted for CNS Response, Inc and as received grant/research support through Mount Sinai School of Medicine from Brainsway, Euthymics Bioscience Inc, Neosync and Shire. In the next two years it is likely he will receive grants from Hoffmann-La Roche Inc and Astrazeneca LP. Dr. Charney has been named as an inventor on a pending use-patent of ketamine for the treatment of depression. If ketamine were shown to be effective in the treatment of depression and received approval from the Food and Drug Administration for this indication, Dr. Charney and Mount Sinai School of Medicine could benefit financially. Dr. Mathew has received consulting fees or research support from Allergan, AstraZeneca, Bristol-Myers Squibb, Cephalon, Inc, Corcept, Johnson & Johnson, Noven, Roche, Takeda. All other authors declare no conflict of interest.
References
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, Krystal JH. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57:270–276. doi: 10.1001/archpsyc.57.3.270. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Narasimhan M, Sanacora G, Miano AP, Hoffman RE, Hu XS, Charney DS, Boutros NN. A randomized clinical trial of repetitive transcranial magnetic stimulation in the treatment of major depression. Biol Psychiatry. 2000;47:332–337. doi: 10.1016/s0006-3223(99)00243-7. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Trauma Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkin JJ, Leuchter AF, Cook IA, Kasl-Godley JE, Abrams M, Rosenberg-Thompson S. Executive dysfunction predicts nonresponse to fluoxetine in major depression. J Affect Disord. 2000;60:13–23. doi: 10.1016/s0165-0327(99)00157-3. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis Disorders (SCID) New York State Psychiatric Institute, Biometrics Research; New York: 1995. [Google Scholar]
- Gorlyn M, Keilp JG, Grunebaum MF, Taylor BP, Oquendo MA, Bruder GE, Stewart JW, Zalsman G, Mann JJ. Neuropsychological characteristics as predictors of SSRI treatment response in depressed subjects. J Neural Transm. 2008;115:1213–1219. doi: 10.1007/s00702-008-0084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SM, Li J. Ketamine in adults: what emergency physicians need to know about patient selection and emergence reactions. Acad Emerg Med. 2000;7:278–281. doi: 10.1111/j.1553-2712.2000.tb01076.x. [DOI] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA., Jr Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37:1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Perry EB, Jr, Gueorguieva R, Belger A, Madonick SH, Abi-Dargham A, Cooper TB, Macdougall L, Abi-Saab W, D’Souza DC. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch Gen Psychiatry. 2005;62:985–994. doi: 10.1001/archpsyc.62.9.985. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D’Souza DC, Karper LP, Bennett A, Abi-Dargham A, Abi-Saab D, Cassello K, Bowers MB, Jr, Vegso S, Heninger GR, Charney DS. Interactive effects of subanesthetic ketamine and haloperidol in healthy humans. Psychopharmacology (Berl) 1999;145:193–204. doi: 10.1007/s002130051049. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, Kelmendi B, Simen A, McMahon FJ, Sanacora G, Zarate C., Jr Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry. 2012;72:e27–28. doi: 10.1016/j.biopsych.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckenbaugh DA, Ibrahim L, Brutsche N, Franco-Chaves J, Mathews D, Marquardt CA, Cassarly C, Zarate CA., Jr Family history of alcohol dependence and antidepressant response to an N-methyl-D-aspartate antagonist in bipolar depression. Bipolar Disord. 2012;14:880–887. doi: 10.1111/bdi.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ, Shah A, Lapidus K, Clark C, Jarun N, Ostermeyer B, Murrough JW. Ketamine for treatment-resistant unipolar depression: current evidence. CNS Drugs. 2012;26:189–204. doi: 10.2165/11599770-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010;13:71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan SN, Mathias JL. The depression-executive dysfunction (DED) syndrome and response to antidepressants: a meta-analytic review. Int J Geriatr Psychiatry. 2010;25:933–944. doi: 10.1002/gps.2431. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Mony L, Kew JN, Gunthorpe MJ, Paoletti P. Allosteric modulators of NR2B-containing NMDA receptors: molecular mechanisms and therapeutic potential. Br J Pharmacol. 2009;157:1301–1317. doi: 10.1111/j.1476-5381.2009.00304.x;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJ, Muetzelfeldt L, Curran HV. Ketamine use, cognition and psychological wellbeing: a comparison of frequent, infrequent and ex-users with polydrug and non-using controls. Addiction. 2009;104:77–87. doi: 10.1111/j.1360-0443.2008.02394.x. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Mofeez A, Brandner B, Bromley L, Curran HV. Acute effects of ketamine on memory systems and psychotic symptoms in healthy volunteers. Neuropsychopharmacology. 2004;29:208–218. doi: 10.1038/sj.npp.1300342. [DOI] [PubMed] [Google Scholar]
- Murrough JW. Ketamine as a novel antidepressant: from synapse to behavior. Clin Pharmacol Ther. 2012;91:303–309. doi: 10.1038/clpt.2011.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, Aan Het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV. Rapid and Longer-Term Antidepressant Effects of Repeated Ketamine Infusions in Treatment-Resistant Major Depression. Biol Psychiatry. 2013;74:250–256. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorham DR, Shawver JR. Basic dimensions of change in the symptomatology of chronic schizophrenics. J Abnorm Soc Psychol. 1961;63:597–602. doi: 10.1037/h0039893. [DOI] [PubMed] [Google Scholar]
- Parwani A, Weiler MA, Blaxton TA, Warfel D, Hardin M, Frey K, Lahti AC. The effects of a subanesthetic dose of ketamine on verbal memory in normal volunteers. Psychopharmacology (Berl) 2005;183:265–274. doi: 10.1007/s00213-005-0177-2. [DOI] [PubMed] [Google Scholar]
- Perry EB, Jr, Cramer JA, Cho HS, Petrakis IL, Karper LP, Genovese A, O’Donnell E, Krystal JH, D’Souza DC Yale Ketamine Study Group. Psychiatric safety of ketamine in psychopharmacology research. Psychopharmacology (Berl) 2007;192:253–260. doi: 10.1007/s00213-007-0706-2. [DOI] [PubMed] [Google Scholar]
- Phelps LE, Brutsche N, Moral JR, Luckenbaugh DA, Manji HK, Zarate CA., Jr Family history of alcohol dependence and initial antidepressant response to an N-methyl-D-aspartate antagonist. Biol Psychiatry. 2009;65:181–184. doi: 10.1016/j.biopsych.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich DL, Silvay G. Ketamine: an update on the first twenty-five years of clinical experience. Can J Anaesth. 1989;36:186–197. doi: 10.1007/BF03011442. [DOI] [PubMed] [Google Scholar]
- Rowland LM, Astur RS, Jung RE, Bustillo JR, Lauriello J, Yeo RA. Selective cognitive impairments associated with NMDA receptor blockade in humans. Neuropsychopharmacology. 2005;30:633–639. doi: 10.1038/sj.npp.1300642. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):10–17. [PubMed] [Google Scholar]
- Smith GS, Schloesser R, Brodie JD, Dewey SL, Logan J, Vitkun SA, Simkowitz P, Hurley A, Cooper T, Volkow ND, Cancro R. Glutamate modulation of dopamine measured in vivo with positron emission tomography (PET) and 11C-raclopride in normal human subjects. Neuropsychopharmacology. 1998;18:18–25. doi: 10.1016/S0893-133X(97)00092-4. [DOI] [PubMed] [Google Scholar]
- Swardfager W, Herrmann N, Marzolini S, Saleem M, Shammi P, Oh PI, Albert PR, Daigle M, Kiss A, Lanctot KL. Brain derived neurotrophic factor, cardiopulmonary fitness and cognition in patients with coronary artery disease. Brain Behav Immun. 2011;25:1264–1271. doi: 10.1016/j.bbi.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BP, Bruder GE, Stewart JW, McGrath PJ, Halperin J, Ehrlichman H, Quitkin FM. Psychomotor slowing as a predictor of fluoxetine nonresponse in depressed outpatients. Am J Psychiatry. 2006;163:73–78. doi: 10.1176/appi.ajp.163.1.73. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vontobel P, Oye I, Hell D, Leenders KL. Effects of (S)-ketamine on striatal dopamine: a [11C]raclopride PET study of a model psychosis in humans. J Psychiatr Res. 2000;34:35–43. doi: 10.1016/s0022-3956(99)00031-x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) Harcourt Assessment; San Antonio: 1999. [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]