Abstract
The role for the endothelial sphingosine-1-phosphate 1 receptor (S1P1R) in acute kidney injury (AKI) remains unclear as germline endothelial S1P1R deletion is embryonically lethal. Here, we generated conditional endothelial S1P1R deficiency by crossing mice with floxed S1P1R with mice expressing a tamoxifen-inducible form of Cre recombinase under the transcriptional control of the platelet-derived growth factor-β gene. Mice with tamoxifen-induced deletion of endothelial S1P1R had increased renal tubular necrosis, inflammation, impaired vascular permeability as well as exacerbated renal tubular apoptosis after ischemic AKI compared to tamoxifen-treated wild type mice. Moreover, endothelial S1P1R deletion resulted in increased hepatic injury after ischemic AKI. As a potential mechanism for exacerbated renal injury, conditional endothelial S1P1R null mice had markedly reduced endothelial HSP27 expression compared to wild type mice. Cultured glomerular endothelial cells treated with a specific S1P1R antagonist (W146) for 3 days also showed reduced HSP27 expression compared to vehicle treated cells. Finally, mice treated with W146 for 3 days also showed reduced endothelial HSP27 expression as well as exacerbated renal and hepatic injury after ischemic AKI. Thus, our studies demonstrate a protective role for endothelial S1P1R against ischemic AKI most likely by regulating endothelial barrier integrity and endothelial HSP27 expression.
Keywords: Apoptosis, Cre-recombinase, inflammation, necrosis, tamoxifen
Introduction
Renal ischemia and reperfusion (IR) injury is a major cause of perioperative acute kidney injury (AKI) for patients undergoing major vascular, cardiac or transplantation surgery and is directly associated with high mortality, morbidity and cost.1,2 Furthermore, patients who suffer from AKI also frequently develop extra-renal organ dysfunction including hepatic dysfunction, intestinal barrier disruption, respiratory failure and the systemic inflammatory response syndrome with the eventual development of sepsis and multi-organ failure.3,4 These extra-renal systemic complications secondary to AKI are the leading causes of mortality in the intensive care unit.5 Unfortunately, the severity and incidence of AKI has been increasing without effective therapy or improvements in patient survival for the past 60 years.6
Sphingolipids are integral components of the plasma membrane and modulate diverse pathways of cell death including necrosis, apoptosis, inflammation, and immunity.7,8 In particular, sphingosine-1-phosphate (S1P), produced by phosphorylation of sphingosine by sphingosine kinases, is the natural ligand for a family of five lysophospholipid targeted G-protein coupled receptors (S1P1–5Rs) and is a powerful modulator of cell death and stress.8 Encouraging preclinical studies suggest that modulation of S1PRs in renal proximal tubule cells reduces ischemic AKI. Specifically, we and others showed previously that activation of S1P1R or inhibition of S1P2R in renal proximal tubules provided powerful renal protection against ischemic AKI in mice.9,10
However, unlike the well characterized role for proximal tubule S1P1R modulation against ischemic AKI, the exact role of endothelial S1P1R in ischemic AKI remains unclear as germline global endothelial S1P1R deletion results in embryonic lethality. Furthermore, the role for S1P in modulating endothelial cell function and survival remain controversial. Some studies suggest that endothelial S1P serves to strengthen the vascular endothelial cell barrier whereas other studies suggest detrimental, pro-inflammatory roles for S1P in endothelial cells.11–13
In this study, we aimed to test whether endothelial S1P1Rs play a protective role against AKI due to renal IR injury. In order to do this, we generated mice with an inducible deletion of endothelial S1P1R by crossing mice with floxed S1P1R (S1P1Rf/f) with mice that express a tamoxifen-inducible form of Cre recombinase (iCreERT2) under the transcriptional control of the Platelet-derived growth factor-b (Pdgfb) gene. We subjected these mice as well as wild type (S1P1Rf/f) mice to mild (20 min) renal IR injury. We also explored the potential mechanisms for exacerbated renal injury in endothelial S1P1R null mice subjected to renal IR injury. Since S1P1R modulates heat shock protein 27 (HSP27) expression in some cell lines14,15 and since HSP27 is a well-known cytoprotective protein in endothelial cells16, we also tested the hypothesis that genetic deletion or chronic blockade of S1P1R decreases endothelial HSP27 expression.
Results
Generation of conditional endothelial S1P1R deficient mice
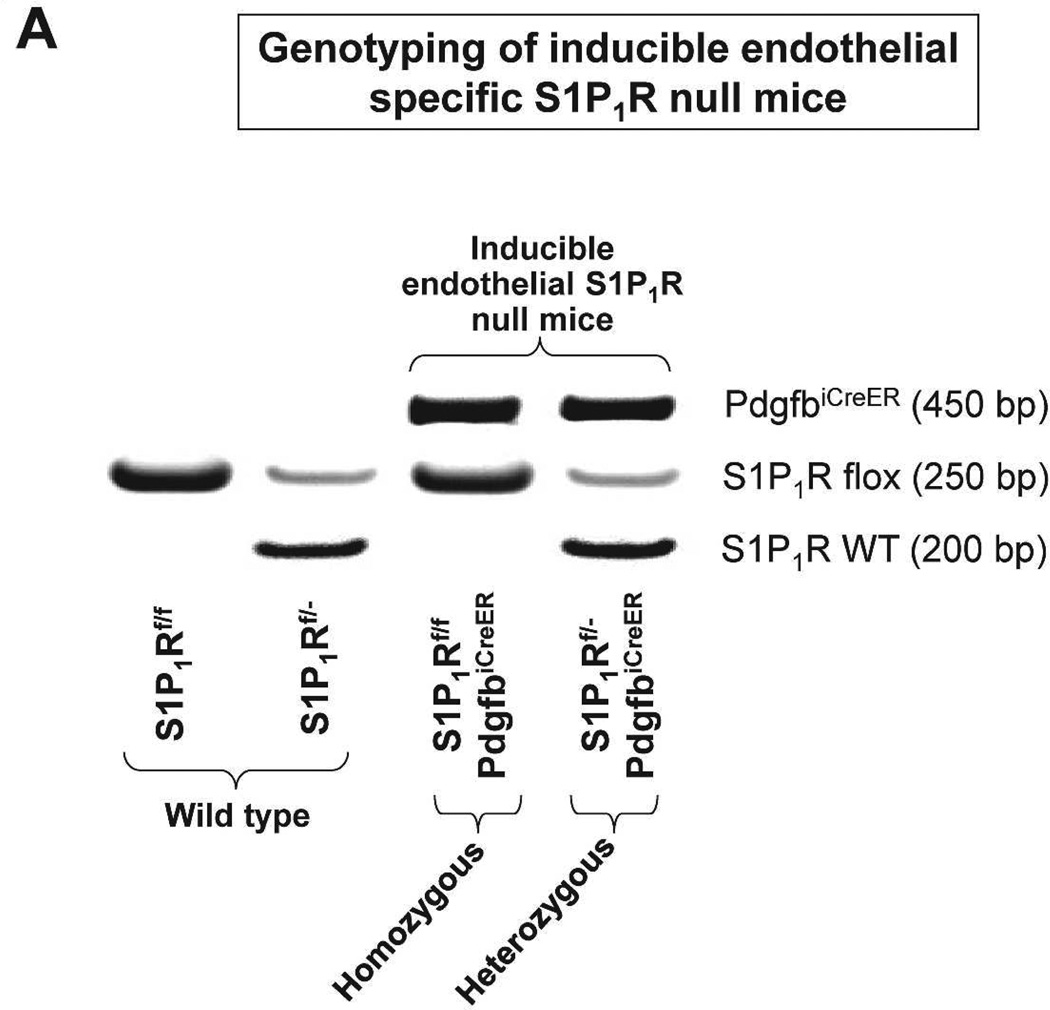
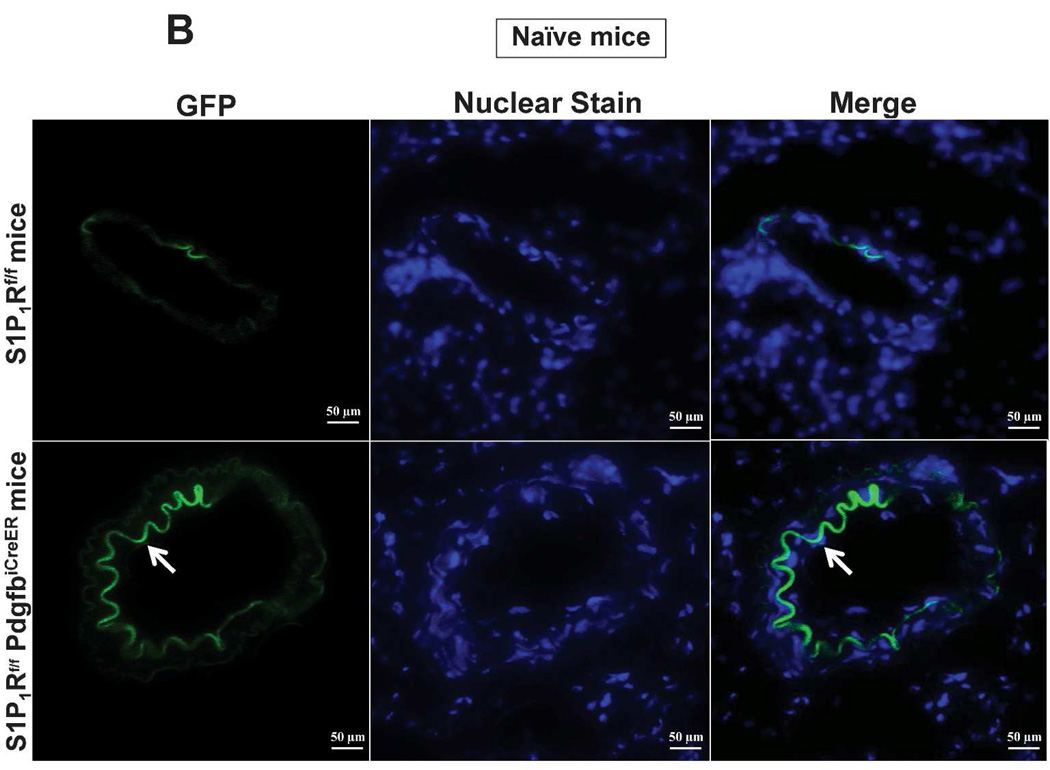
In order to conditionally delete S1P1R in endothelial cells of adult mice, we crossed floxed S1P1R (S1P1Rf/f) mice with mice that express a Platelet-derived growth factor-b (Pdgfb) promoter driven by a tamoxifen-inducible form of Cre-recombinase (iCreERT2) in vascular endothelial cells (PdgfbiCreER mice).17,18 Tail PCR provided genotypes of conditional endothelial S1P1R null (S1P1Rf/f PdgfbiCreER) mice and wild type (S1P1Rf/f) mice generated from our breeding (Figure 1A). Since the PdgfbiCreER mice also express an internal ribosomal entry site element and a sequence coding for enhanced green fluorescent protein (EGFP), we detected kidney endothelial cell EGFP expression in S1P1Rf/f PdgfbiCreER mice with fluorescent microscopy (Figure 1B).17 EGFP expression was strong in kidney endothelial cells from S1P1Rf/f PdgfbiCreER mice but was not visible (beyond faint background auto-fluorescence from external elastic membrane) in kidney sections from S1P1Rf/f mice.
Figure 1. Generation of mice with conditional endothelial specific S1P1R deletion.
A. Tail PCR confirming the genotypes of conditional endothelial specific S1P1R null (S1P1Rf/f PdgfbiCreER or S1P1Rf/- PdgfbiCreER) mice and the wild type (S1P1Rf/f or S1P1Rf/-) mice. B. As PdgfbiCreER mice express an internal ribosomal entry site element and a sequence coding for enhanced green fluorescent protein (EGFP), we detected the expression of EGFP in kidney endothelial cells from in S1P1Rf/f PdgfbiCreER mice with fluorescent microscopy. EGFP expression was strong in endothelial cells from kidneys of S1P1Rf/f PdgfbiCreER mice (arrow). Besides faint auto-fluorescence from external elastic membrane, EGFP expression was not detected in S1P1Rf/f mice kidney endothelial cells. We also performed DAPI nuclear staining in both S1P1Rf/f PdgfbiCreER mice and in S1P1Rf/f mice (blue).
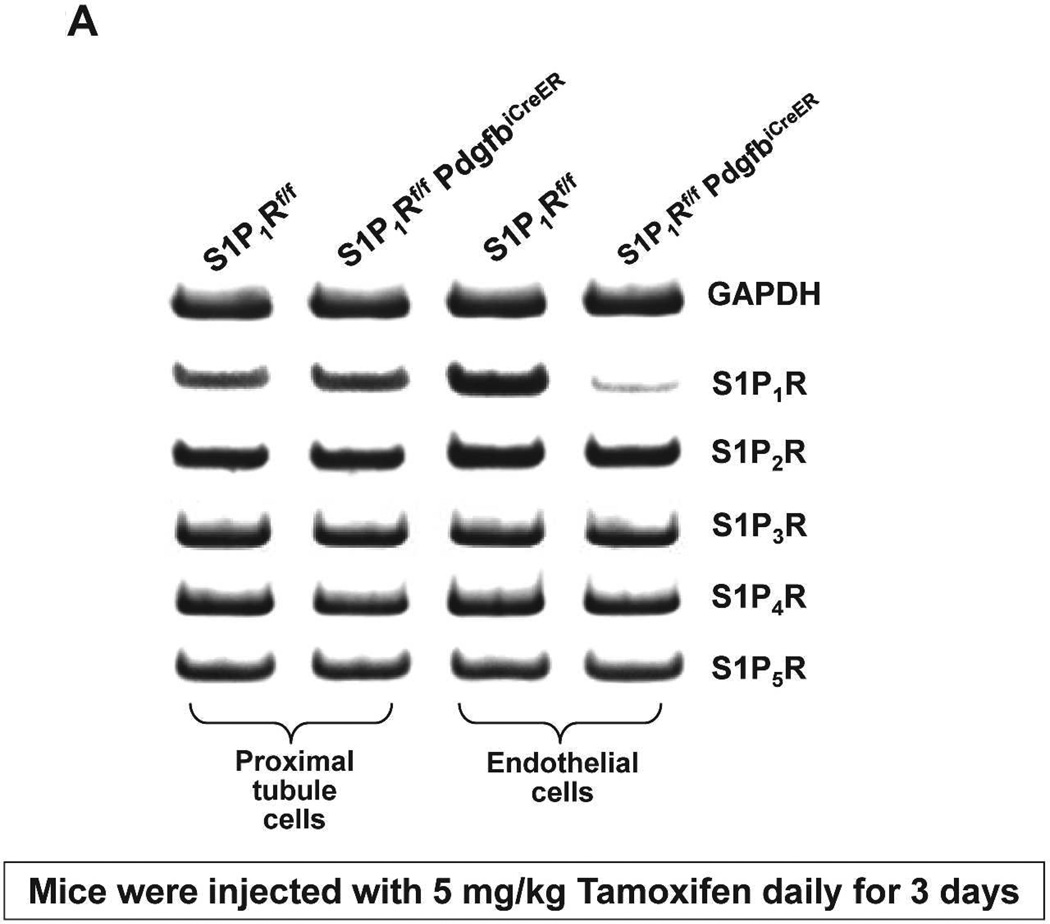
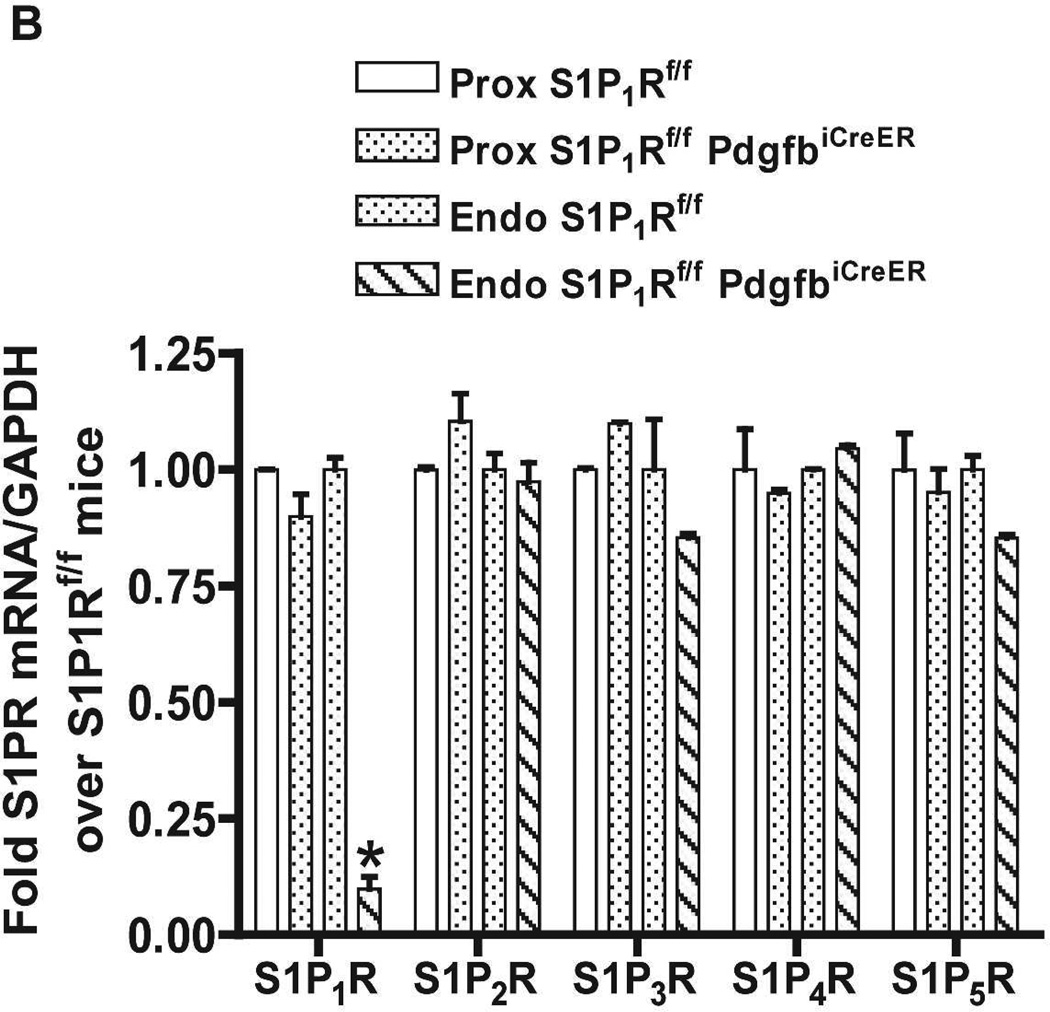
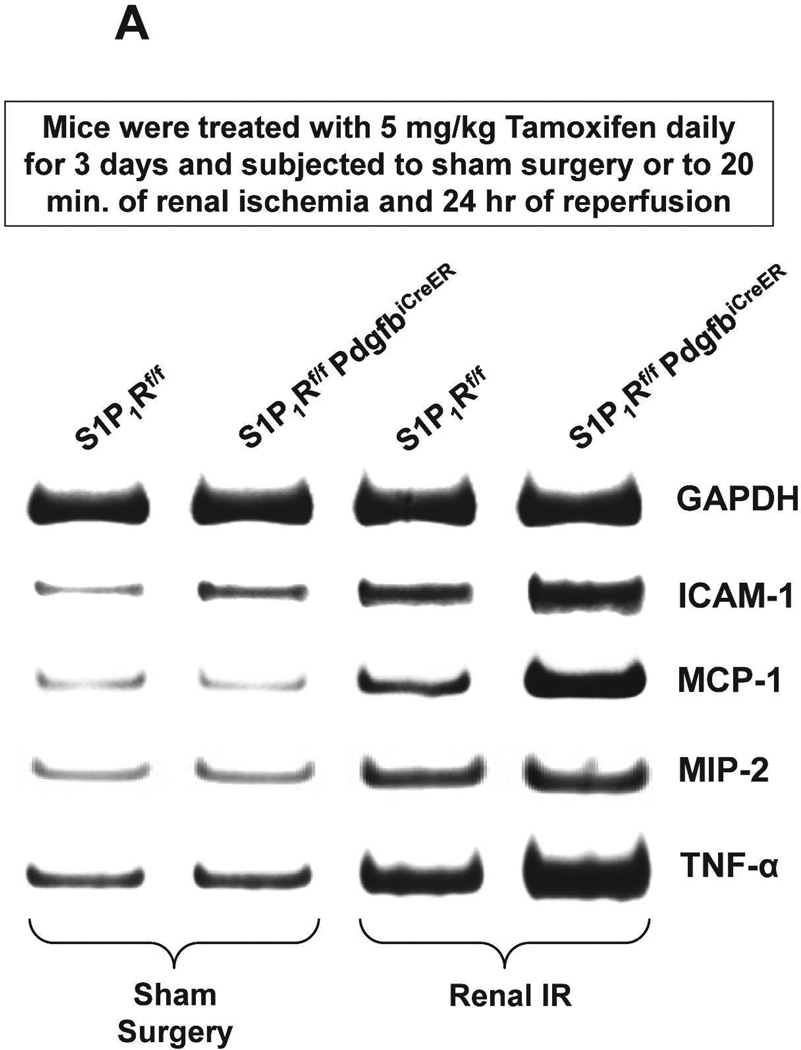
S1P1Rf/f PdgfbiCreER mice were treated daily with 5 mg/kg tamoxifen (i.p.) for 3 days for Cre-recombinase mediated deletion of endothelial S1P1R. S1Pf/f mice were also treated with tamoxifen for 3 days and used as controls. We confirmed deletion of endothelial S1P1R in S1P1Rf/f PdgfbiCreER mice with RTPCR. S1P1–5R mRNA levels in freshly isolated endothelial cells (prepared with magnetic bead coupled antibody capture) and proximal tubules (prepared with percoll gradient separation) were measured with conventional and real time RTPCR. A representative gel image of PCR products and the quantified mRNA levels (Figure 2) show a drastically decreased S1P1R mRNA (∼90%) in endothelial cells isolated from kidneys of S1P1Rf/f PdgfbiCreER mice compared to the wild type (S1P1Rf/f) mice endothelial cells. Other S1PR subtypes (S1P2–5R) did not change with conditional endothelial S1P1R deletion (Figure 2). Figure 2 also shows that renal proximal tubular S1P1–5R mRNA expression was similar between tamoxifen-treated S1P1Rf/f PdgfbiCreER mice and S1P1Rf/f mice.
Figure 2. Conditional deletion of renal endothelial S1P1R in S1P1Rf/f PdgfbiCreER mice.
S1P1–5R mRNA levels measured with RTPCR in freshly isolated renal endothelial cells (with Dynabeads coupled to CD144 antibody) and renal proximal tubules (with Percoll density gradient separation) from conditional S1P1R null mice (S1P1Rf/f PdgfbiCreER) and wild type mice (S1P1Rf/f). Representative gel image of PCR products (A) and densitometric quantification of relative band intensities normalized to GAPDH (B, N=4). Endothelial cells from conditional endothelial S1P1R null mice show significantly (∼90%) reduced S1P1R mRNA expression compared to wild type mice without any changes in other S1PR subtype (S1P2–5R) mRNA expression (N=4). Furthermore, proximal tubule S1P1R mRNA levels were similar between conditional endothelial S1P1R null mice and wild type mice. *P<0.05 vs. S1P1Rf/f mice.
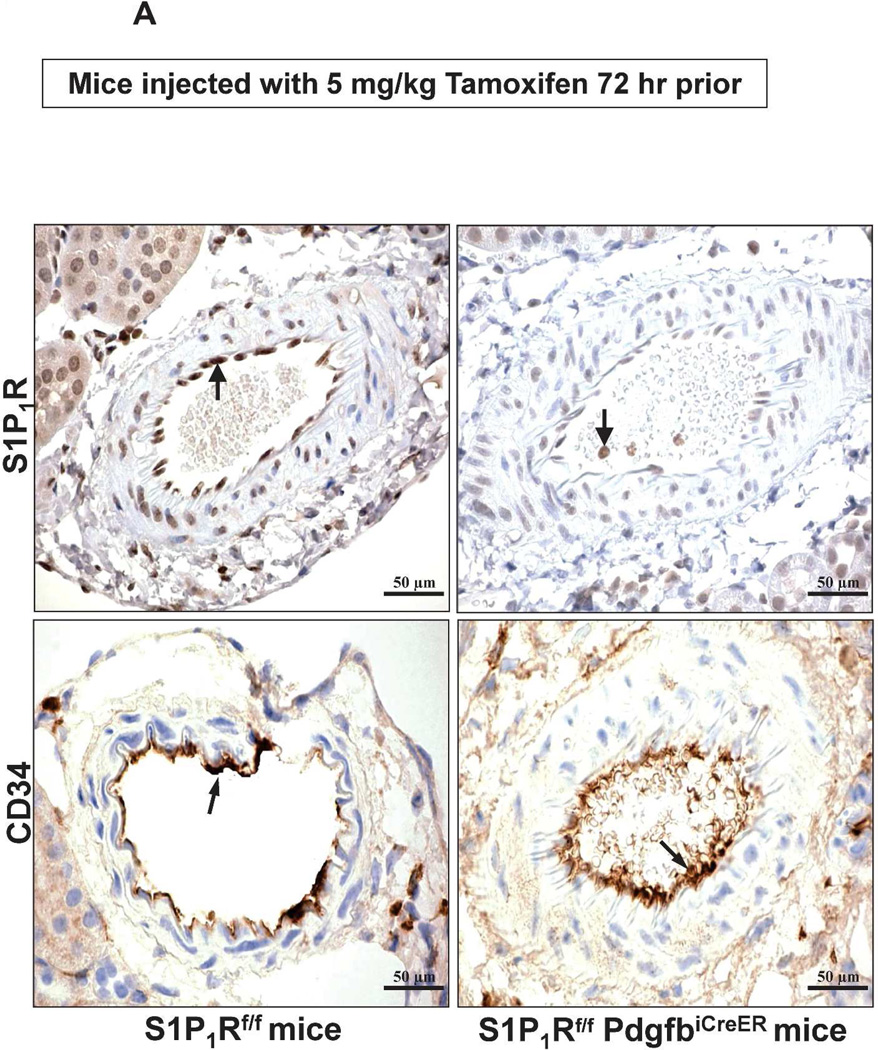
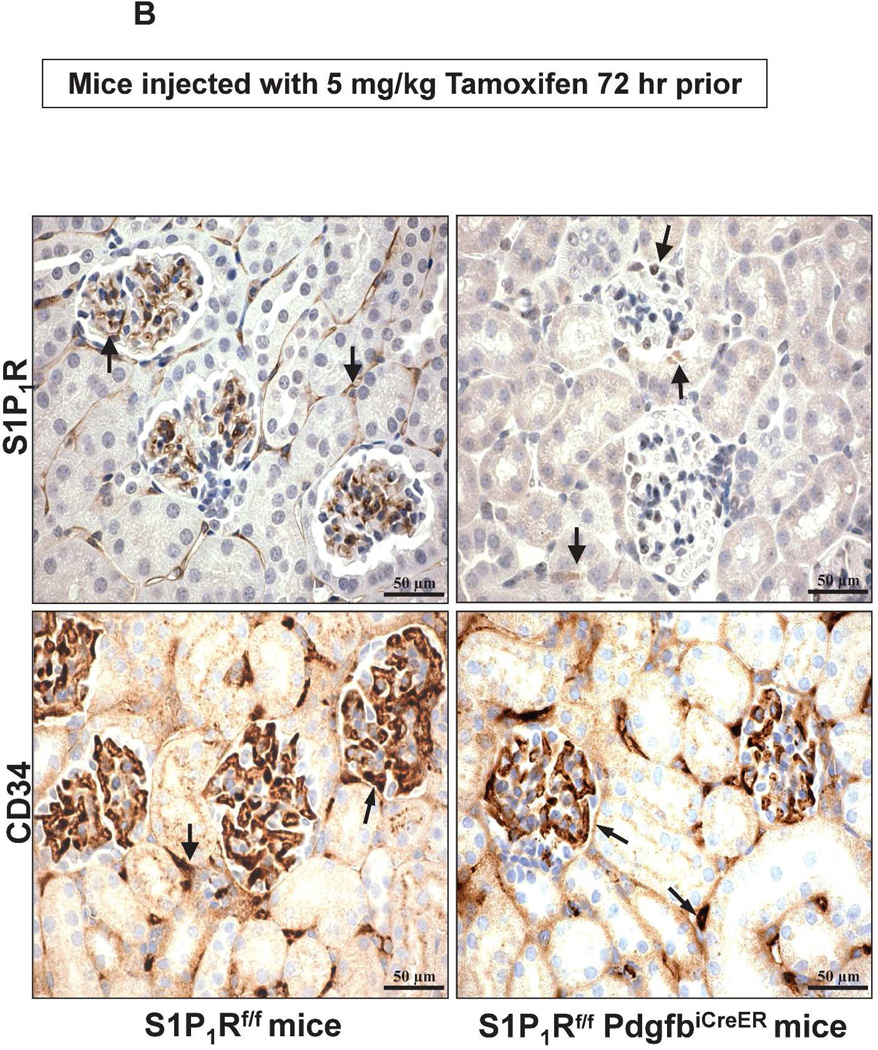
We also performed immunohistochemistry for kidney S1P1R and for CD34 (an endothelial cell marker) in tamoxifen-treated S1P1Rf/f PdgfbiCreER and S1P1Rf/f mice. Figure 3 show representative images for S1P1R and CD34 immunoreactivity in the kidney. Figure 3A shows images from kidney blood vessels and Figure 3B shows immunohistochemistry images for peritubular capillaries and glomeruli. As shown in Figure 3A, S1P1R immunoreactivity was markedly attenuated in kidney blood vessels from tamoxifen-treated S1P1Rf/f PdgfbiCreER mice when compared to S1P1Rf/f mice. CD34 immunoreactivity was similar between tamoxifen-treated S1P1Rf/f PdgfbiCreER mice and S1P1Rf/f mice in kidney blood vessels. Furthermore, Figure 3B shows that S1P1R is robustly expressed in peritubular capillaries and glomeruli from S1P1Rf/f mice but not from S1P1Rf/f PdgfbiCreER mice. Equivalent CD34 immunoreactivity was detected in peritubular capillaries and glomeruli from S1P1Rf/f mice and S1P1Rf/f PdgfbiCreER mice. These finds are consistent with previous studies that pericytes and smooth muscle cells do not produce Pdgfb in vivo.17 S1P1R immunoreactivity was positive in kidney leukocytes from conditional endothelial S1P1R null mice (Figure 3).
Figure 3. Reduced kidney endothelial S1P1R protein expression in conditional endothelial S1P1R null (S1P1Rf/f PdgfbiCreER) mice.
A. Representative images (magnification 600X) for S1P1R and CD34 immunoreactivity (brown stain, arrows) in kidney blood vessels from tamoxifen-treated S1P1Rf/f PdgfbiCreER and S1P1Rf/f mice. S1P1R immunoreactivity was drastically reduced (near absent) in tamoxifen-treated S1P1Rf/f PdgfbiCreER mice when compared to S1P1Rf/f mice. S1P1R immunoreactivity was still visible from intravascular leukocytes in conditional endothelial S1P1R null mice. CD34 immunoreactivity was similar between tamoxifen-treated S1P1Rf/f PdgfbiCreER mice and S1P1Rf/f mice. Representative of 4 experiments. B. Representative images (magnification 600X) for S1P1R and CD34 immunoreactivity (brown stain, arrows) in peritubular capillaries and glomeruli from tamoxifen-treated S1P1Rf/f PdgfbiCreER and S1P1Rf/f mice. S1P1R immunoreactivity was absent in peritubular capillaries and glomeruli from tamoxifen-treated S1P1Rf/f PdgfbiCreER mice when compared to S1P1Rf/f mice. Again, leukocyte S1P1R immunoreactivity was visible in kidneys from conditional endothelial S1P1R null mice. CD34 immunoreactivity was similar between tamoxifen-treated S1P1Rf/f PdgfbiCreER mice and S1P1Rf/f mice. Representative of 4 experiments.
Endothelial S1P1R deletion exacerbates renal and hepatic injury after ischemic AKI in mice
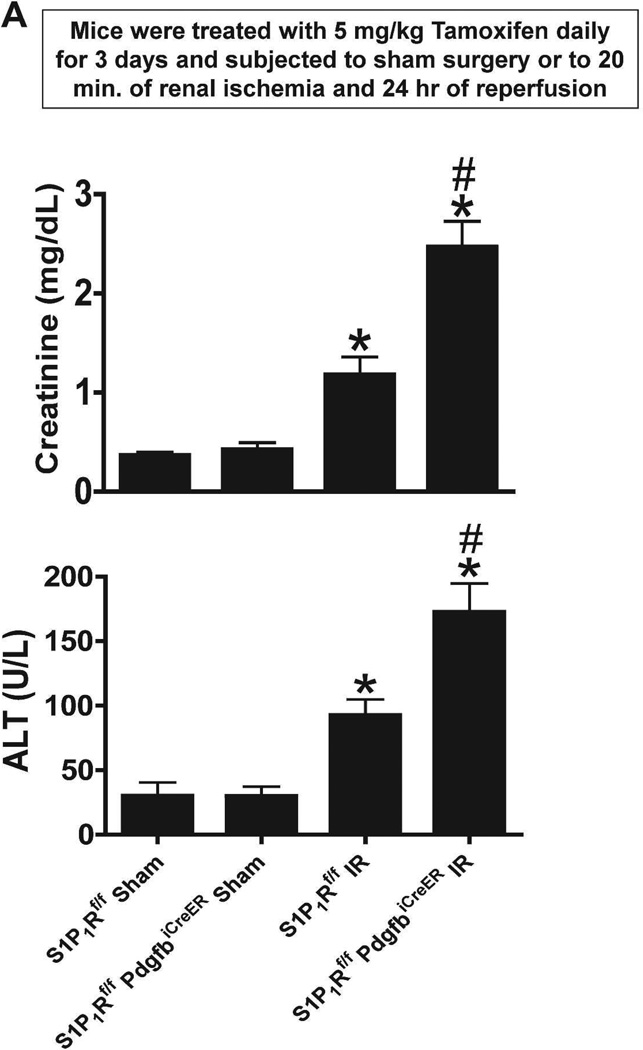
Next, we tested whether endothelial S1P1R protects against ischemic AKI and remote hepatic injury in mice. We previously showed that ischemic AKI also results in hepatic necrosis, vacuolization and elevated plasma transaminase levels in mice.19 Plasma creatinine and ALT values were similar between sham-operated (anesthesia, laparotomy, right nephrectomy and recovery) tamoxifen-treated S1P1Rf/f PdgfbiCreER mice and S1P1Rf/f mice (Figure 4A). As expected plasma creatinine and ALT levels increased mildly but significantly in tamoxifen-treated wild type (S1P1Rf/f) mice 24 hr after mild (20 min) renal IR. However, conditional endothelial S1P1R deficient (S1P1Rf/f PdgfbiCreER) mice subjected to renal IR had significantly increased renal and hepatic injury as evidenced by significantly higher plasma creatinine and ALT levels compared to S1P1Rf/f mice subjected to renal IR.
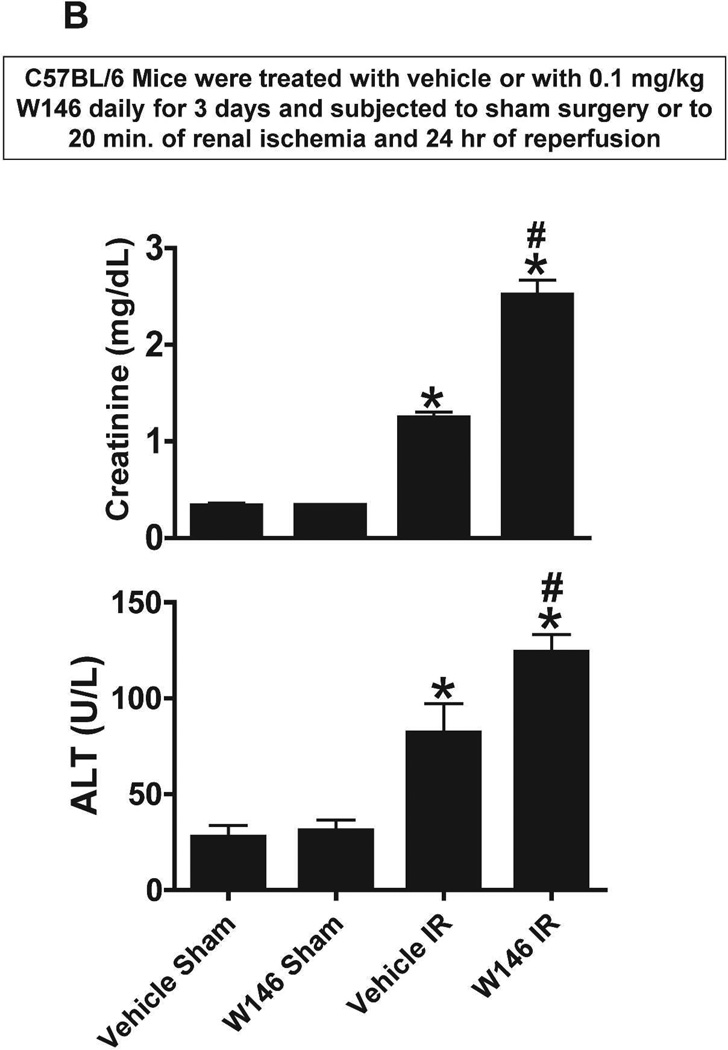
Figure 4. Endothelial S1P1R deficiency or chronic S1P1R antagonist treatment exacerbates renal and hepatic injury after renal IR.
A. Plasma markers for renal and hepatic injury from tamoxifen-treated endothelial S1P1R null (S1P1Rf/f PdgfbiCreER) mice or wild type (S1P1Rf/f) mice subjected to sham-operation or to 20 min of renal ischemia and 24 hr reperfusion (N=5–7). Endothelial S1P1R deletion significantly increased renal and hepatic injury in mice. B. Plasma markers for renal and hepatic injury from vehicle-or W146-treated (0.1 mg/kg daily for 3 days) C57BL/6 mice subjected to sham-operation or to 20 min of renal ischemia and 24 hr of reperfusion (N=6). Chronic S1P1R antagonist treatment increased renal and hepatic injury after renal IR injury. *P<0.05 vs. respective sham-operated mice. #P<0.05 vs. wild type (S1P1Rf/f) or vehicle-treated C57BL/6 mice subjected to renal IR injury.
We also assessed renal function by estimating glomerular filtration rate 24 hours after renal IR injury in mice with the FITC-inulin clearance technique as described by Qi et al.20 The glomerular filtration rate in tamoxifen-treated S1P1Rf/f PdgfbiCreER mice subjected to renal IR was lower (0.22±0.08 ml/min/100g body weight, N=3) than the glomerular filtration rate in tamoxifen-treated S1P1Rf/f mice subjected to renal IR (0.66±0.21 ml/min/100g body weight, N=3).
Chronic S1P1R blockade exacerbates renal and hepatic injury after ischemic AKI in mice
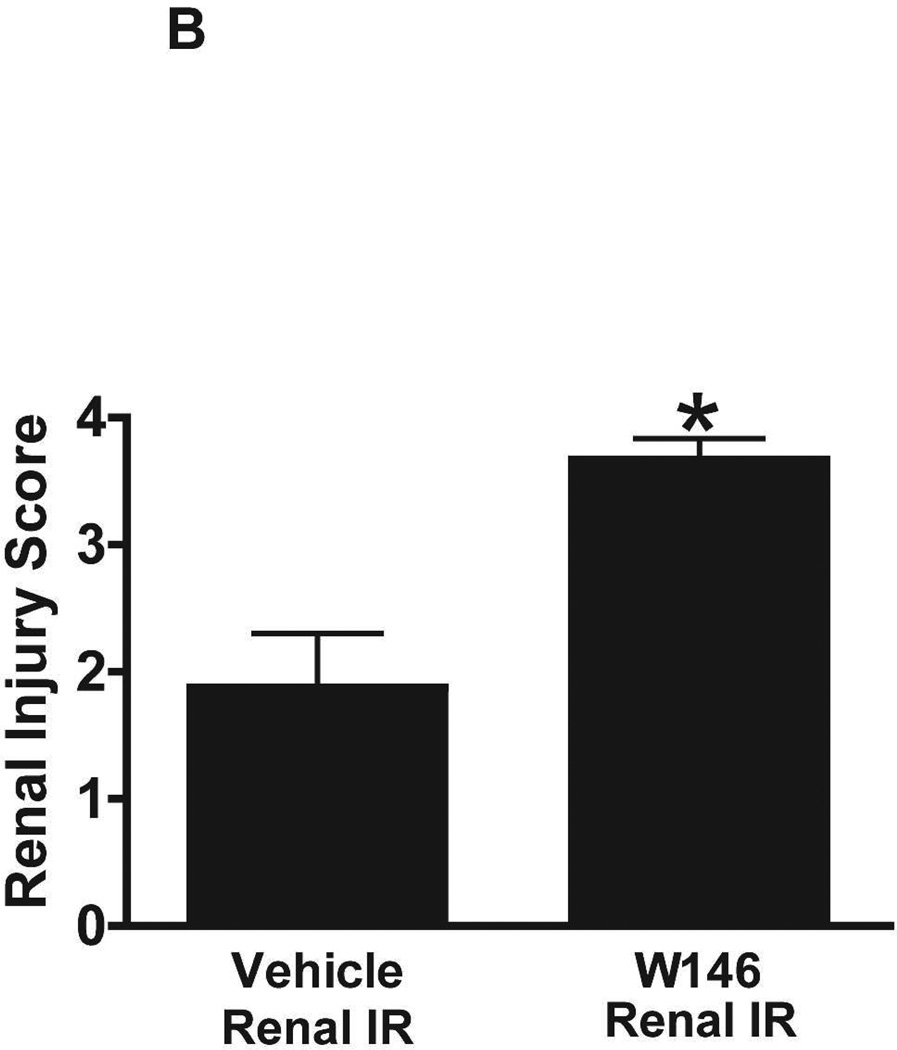
We then tested the effects of chronic S1P1R blockade (W146 treatment daily for 3 days) in C57BL/6 mice subjected to sham-operation or to 20 min renal IR injury. Again, sham-operated vehicle-treated and W146-treated mice had similar baseline renal and hepatic markers of injury (Figure 4B). Vehicle-treated mice showed increased renal and hepatic injury after 20 min renal IR. However, chronic W146-treated mice had significantly elevated plasma markers of renal and hepatic injury.
Endothelial S1P1R deficient mice have increased kidney necrosis, neutrophil infiltration, and apoptosis and liver necrosis and vacuolization after renal IR
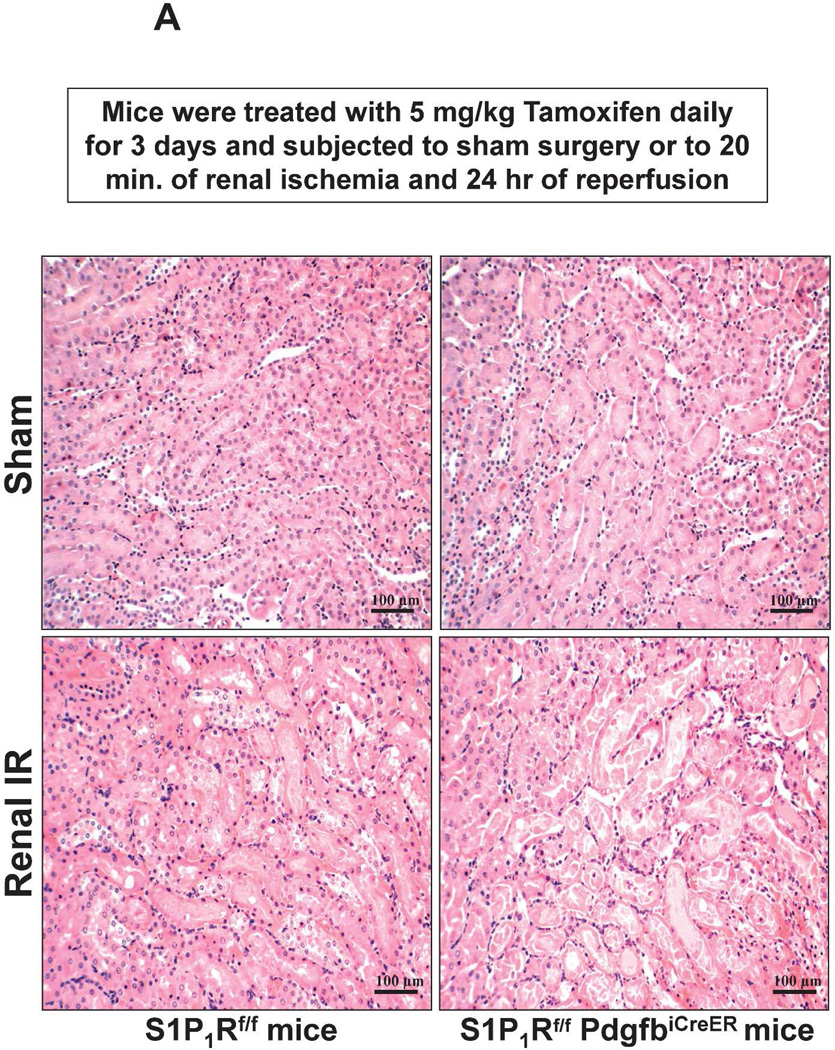
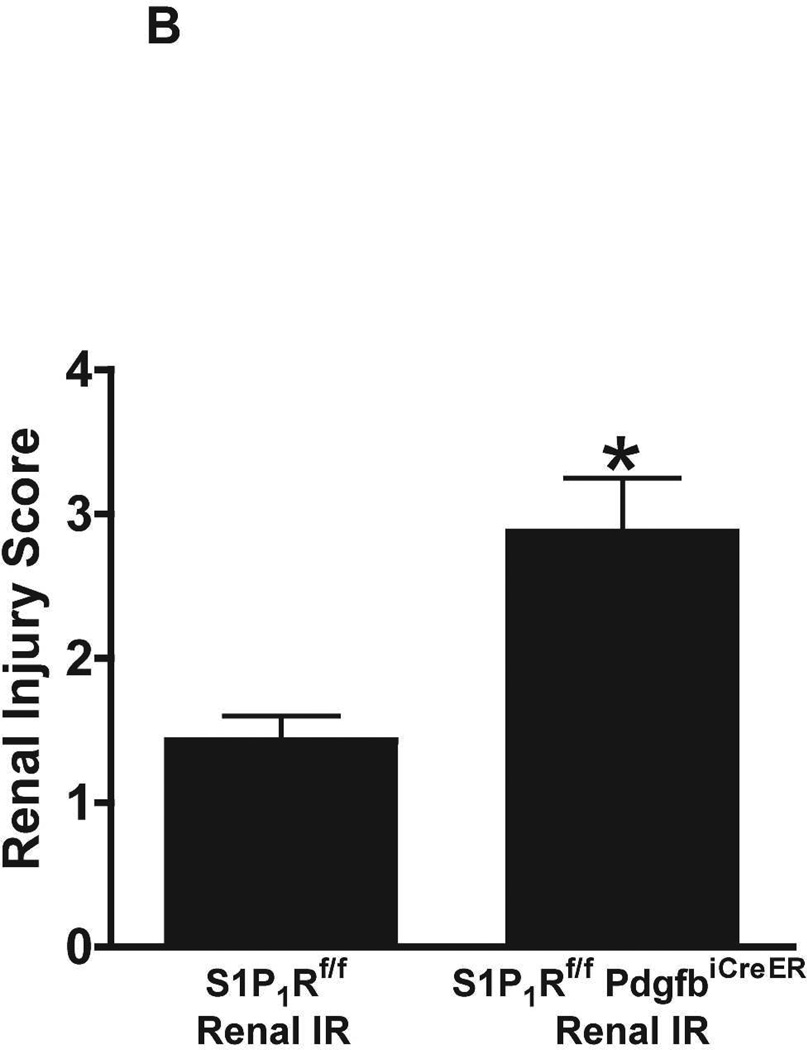
Figure 5A shows representative H&E images (from 6 experiments) of tamoxifen-treated mice subjected to sham-operation or to 20 min renal ischemia and 24 hr of reperfusion (magnification 200X). Sham-operated tamoxifen-treated wild type (S1P1Rf/f) mice and tamoxifen-treated endothelial S1P1R deficient mice had normal renal histology (Figure 5A). Compared to sham-operated S1P1Rf/f mice, the kidneys of tamoxifen-treated S1P1Rf/f mice subjected to renal IR showed moderate tubular vacuolization, proteinaceous casts with increased tubular dilatation and congestion (Figure 5A). Consistent with the plasma creatinine data, tamoxifen-treated endothelial S1P1R deficient (S1P1Rf/f PdgfbiCreER) mice had increased renal tubular necrosis, congestion and cast formation. The Jablonski scale21 renal injury score (scale: 0–4) for histology grading was used to grade renal tubular necrosis 24 hr after 20 min renal IR (Figure 5B). Twenty min of renal ischemia and 24 hr of reperfusion resulted in mild to moderate acute tubular necrosis in tamoxifen-treated wild type (S1P1Rf/f) mice. In contrast, tamoxifen-treated endothelial S1P1R deficient mice had significantly higher renal injury scores (Figure 5B).
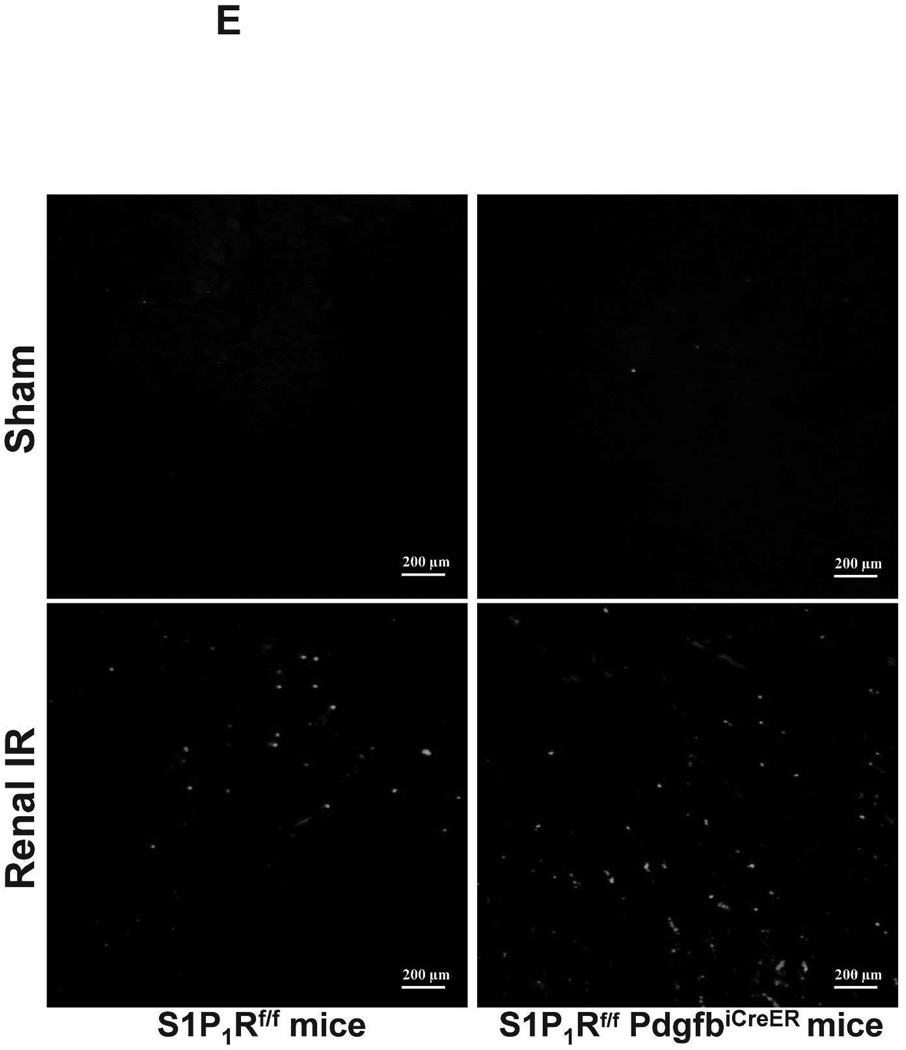
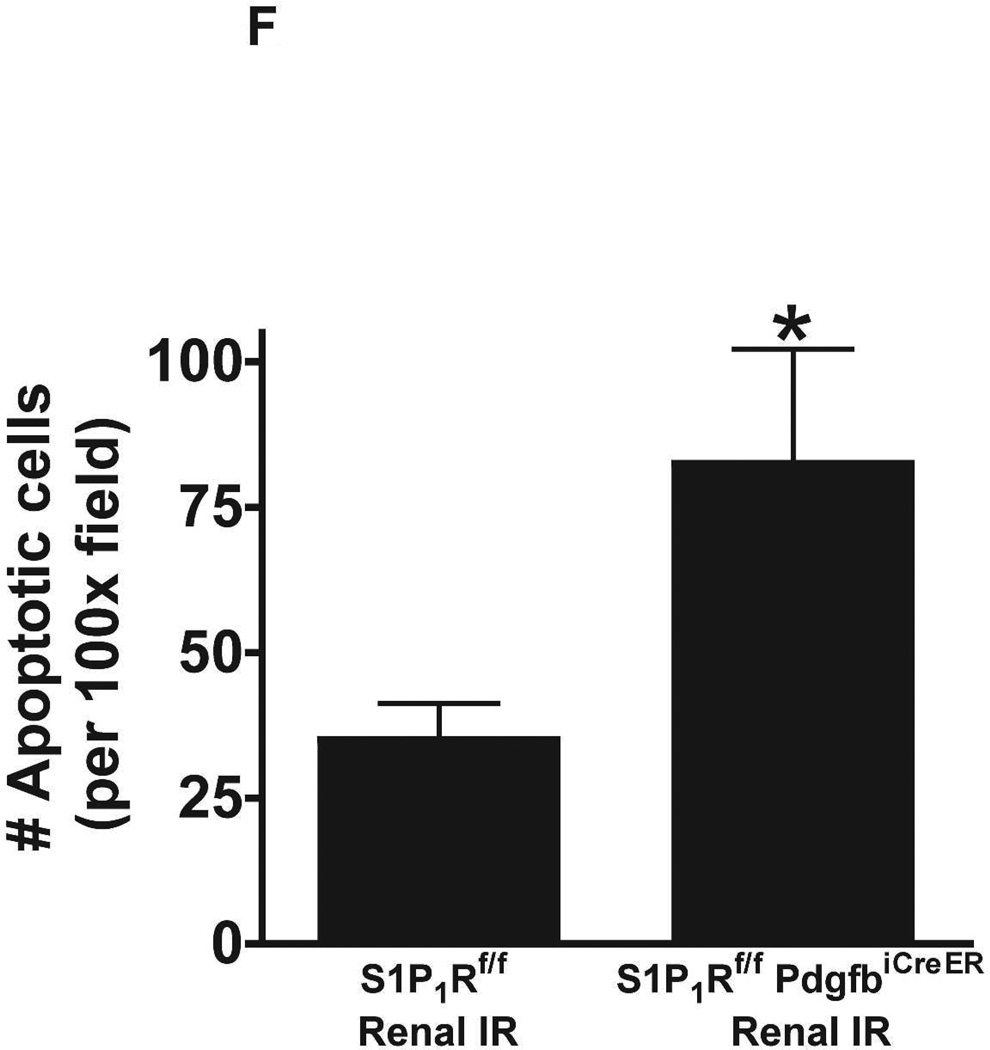
Figure 5. Endothelial S1P1R deletion increases renal necrosis, neutrophil infiltration and apoptosis as well as hepatic vacuolization after renal IR.
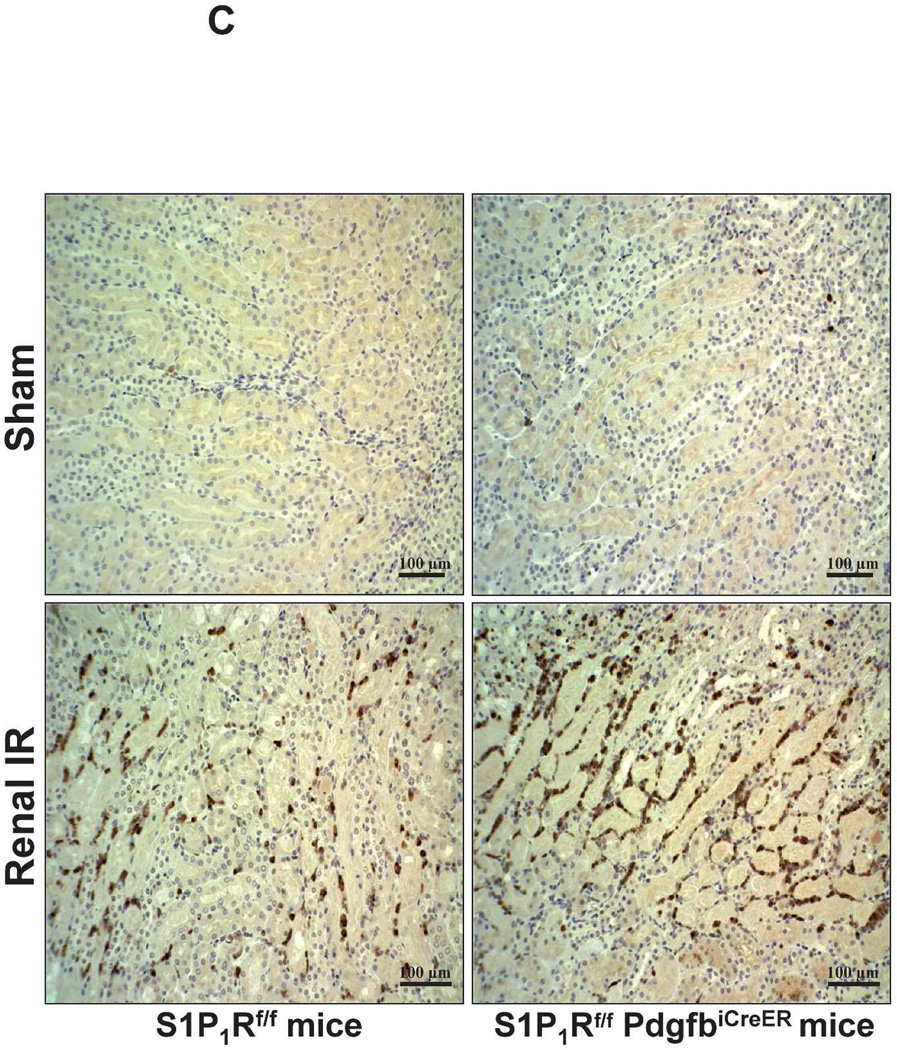
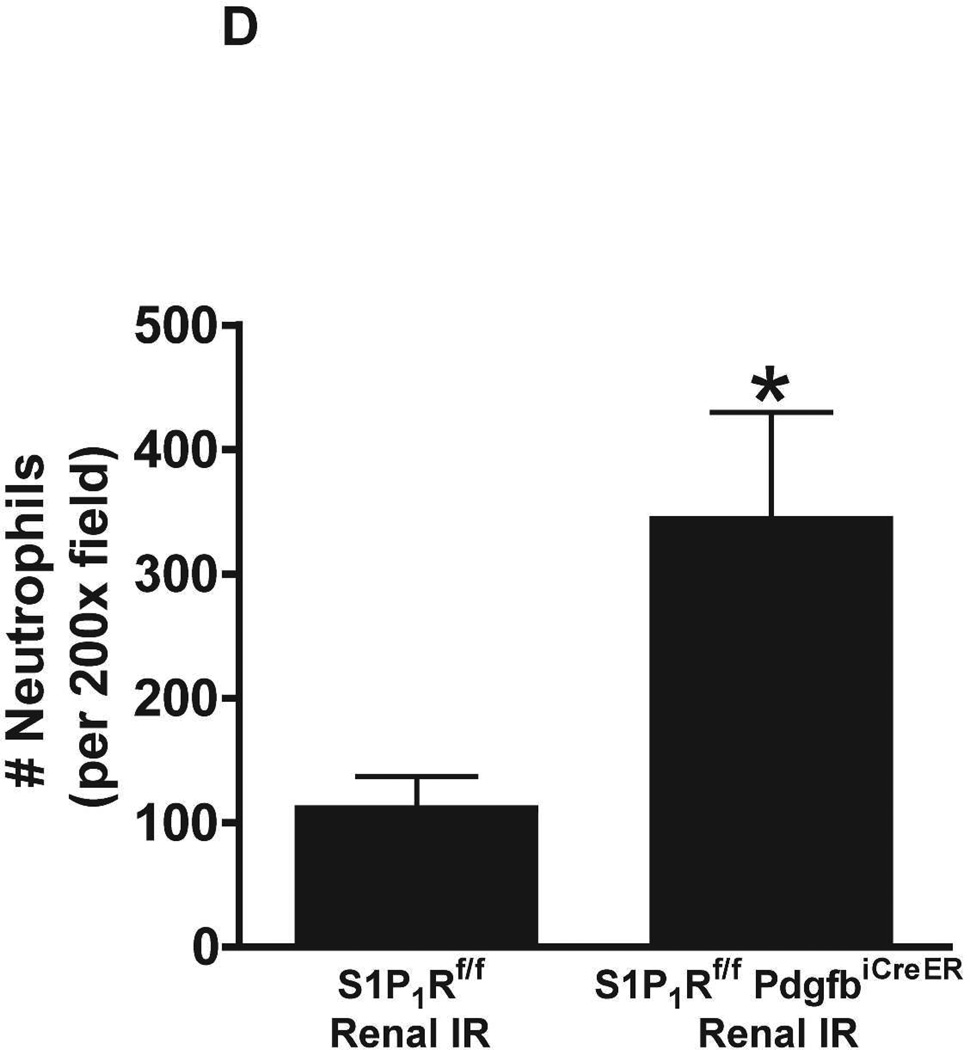
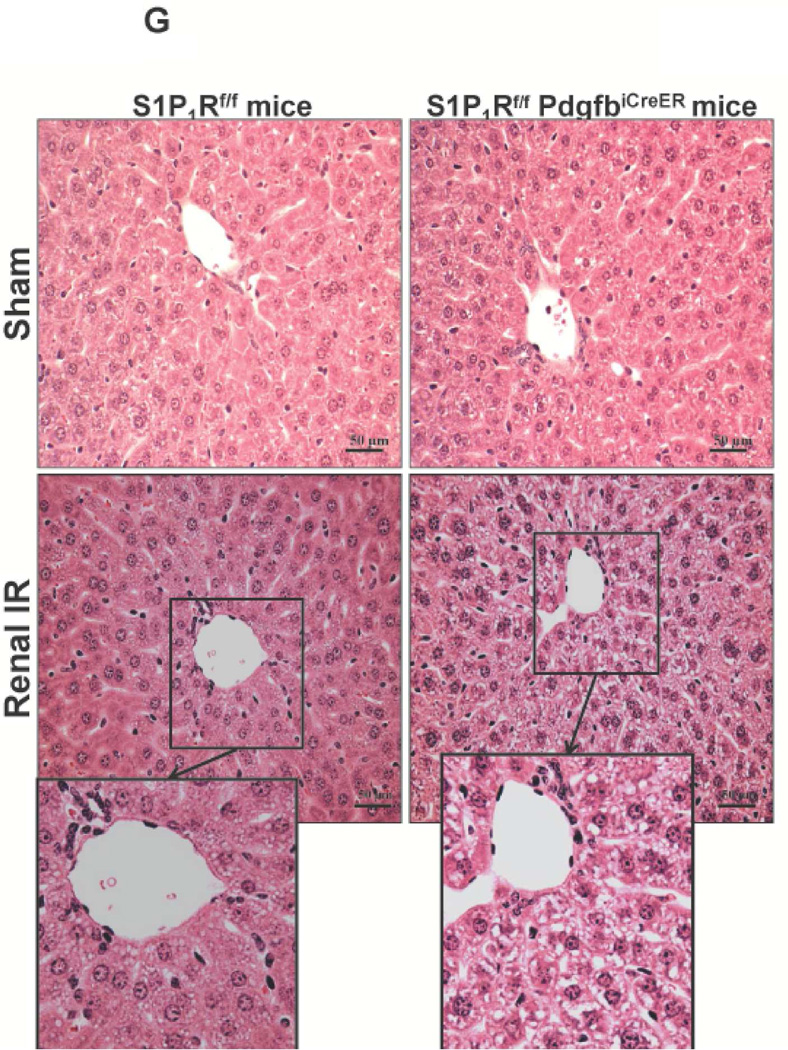
A. Representative hematoxylin and eosin staining (magnification 200X) of kidney sections of tamoxifen-treated (5 mg/kg i.p. daily for 3 days) endothelial S1P1R null (S1P1Rf/f PdgfbiCreER) mice or wild type (S1P1Rf/f) mice subjected to sham-operation or to 20 min of renal ischemia and 24 hr of reperfusion. Photographs are representative of 6 independent experiments. B. Summary of Jablonski scale renal injury scores (scale 0–4) for mice subjected to renal IR. Tamoxifen-treated wild type (S1P1Rf/f) mice showed increased renal tubular necrosis after renal IR. Endothelial S1P1R deficiency further exacerbated renal tubular necrosis in mice subjected to renal IR injury. C and E. Representative photomicrographs for immunohistochemistry (brown staining) for neutrophil infiltration (C, magnification 200X) and TUNEL staining (E, representing apoptotic nuclei, magnification 100X) from kidneys of tamoxifen-treated endothelial S1P1R null (S1P1Rf/f PdgfbiCreER) mice or wild type (S1P1Rf/f) mice subjected to sham-operation or to 20 min of renal ischemia followed by 24 hr of reperfusion (IR). Tamoxifen-treated wild type mice showed extensive neutrophil infiltration and TUNEL positive cells after 20 min of renal IR. Tamoxifen-treated endothelial S1P1R null (S1P1Rf/f PdgfbiCreER) mice had increased neutrophil infiltration after renal IR. Few neutrophils were also visible in tamoxifen-treated sham-operated endothelial S1P1R null mice. D and F. Quantifications of infiltrated neutrophils per 200X field (D, N=4–6) and apoptotic cells per 100X field (F, N=5) in the kidneys of tamoxifen-treated endothelial S1P1R null (S1P1Rf/f PdgfbiCreER) mice or wild type (S1P1Rf/f) mice subjected to 20 min of renal ischemia followed by 24 hr of reperfusion. *P<0.05 vs. S1P1Rf/f mice subjected to renal IR. Error bars represent 1 SEM. G. Liver histology was assessed by H&E staining (representative of 5–6 slides, 400X). Sham-operated wild type mice had normal liver histology. Twenty min of renal ischemia and 24 hr of reperfusion resulted in mild to moderate nuclear and cytoplasmic degenerative changes, cellular vacuolization, congestion as well as individual hepatocyte necrosis and focal apoptotic, pyknotic nuclei were observed. The severity of liver injury was increased for conditional endothelial S1P1R deficient mice manifested by increased of cellular degenerative changes including increased individual hepatocyte necrosis, apoptosis and vacuolization.
Figure 5C shows representative images (from 4–6 experiments) of immunohistochemistry of neutrophil infiltration (dark brown) in the kidneys (corticomedullary junction) of tamoxifen-treated mice subjected to sham-operation or to 20 min of renal ischemia and 24 hr of reperfusion (magnification 200X). In sham-operated tamoxifen-treated wild type (S1P1Rf/f) mice, we were unable to detect any neutrophils in the kidney. However, few neutrophils were detected in sham-operated tamoxifen-treated endothelial S1P1R deficient mice. Neutrophil infiltration increased in tamoxifen-treated wild type mice concentrated near the corticomedullary junction (Figure 5C). Again, tamoxifen-treated endothelial S1P1R deficient mice had increased neutrophil infiltration after 20 min of renal IR and 24 hr of reperfusion (Figure 5C and Figure 5D).
TUNEL staining detected apoptotic renal tubular cells in kidneys of mice (representative of 5 experiments). In tamoxifen-treated sham-operated mice, very few TUNEL positive cells were detected in the kidneys of both wild type and endothelial S1P1R deficient mice (Figure 5E, 100X). TUNEL staining showing renal tubule cell apoptosis increased in tamoxifen-treated wild type mice subjected to 20 min of renal IR (Figure 5E and Figure 5F). We show here that tamoxifen-treated endothelial S1P1R deficient mice had significantly increased number of apoptotic TUNEL-positive cells in the kidney 24 hr after IR.
Liver histology was also assessed by H&E staining of liver sections (representative of 5–6 slides). As shown in Figure 5G, sham-operated wild type mice had normal liver histology. Twenty min renal ischemia and 24 hr reperfusion resulted in mild-moderate nuclear and cytoplasmic degenerative changes, cellular vacuolization, congestion as well as individual hepatocyte necrosis and focal apoptotic, pyknotic nuclei were observed (Figure 5G). The severity of liver injury was increased for conditional endothelial S1P1R deficient mice consistent with higher plasma ALT and manifested by increased of cellular degenerative changes including increased individual hepatocyte necrosis, apoptosis and vacuolization. Some degree of centrilobular necrosis was observed in endothelial S1P1R deficient mice. Vascular congestion and leukocyte (mainly neutrophil) infiltration were also detected after renal IR in endothelial S1P1R deficient mice.
Chronic S1P1R blockade increases renal tubular necrosis, neutrophil infiltration, and apoptosis and liver necrosis and vacuolization after renal IR
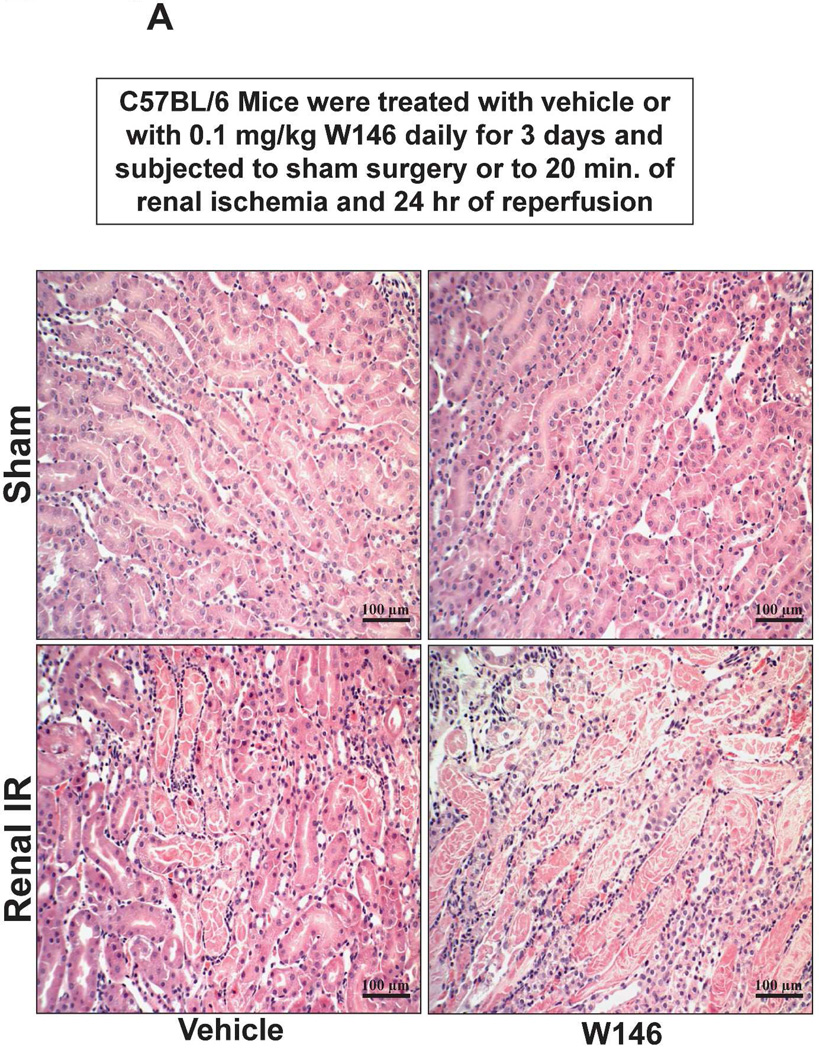
Figure 6A shows representative H&E images (from 5–6 experiments) of vehicle or W146-treated C57BL/6 mice subjected to sham-operation or to 20 min of renal ischemia and 24 hr of reperfusion (magnification 200X). Sham-operated vehicle-treated and W146-treated C57BL/6 mice had normal renal histology (Figure 6A). Compared to sham-operated mice, the kidneys of vehicle-treated C57BL/6 mice subjected to 20 min renal IR showed increased renal injury (increased tubular vacuolization, proteinaceous casts with increased tubular dilatation and congestion, Figure 6A). Furthermore, C57BL/6 mice treated with W146 for 3 days mice had increased renal tubular necrosis, congestion and cast formation after 20 min renal IR. The Jablonski scale renal injury scores again show that 20 min of renal ischemia and 24 hr of reperfusion resulted in mild to moderate acute tubular necrosis in vehicle-treated C57BL/6 mice. In contrast, W146-treated C57BL/6 mice had significantly higher renal injury scores (Figure 6B).
Figure 6. Chronic S1P1R antagonist treatment in C57BL/6 mice increases renal necrosis, neutrophil infiltration and apoptosis as well as hepatic vacuolization after renal IR.
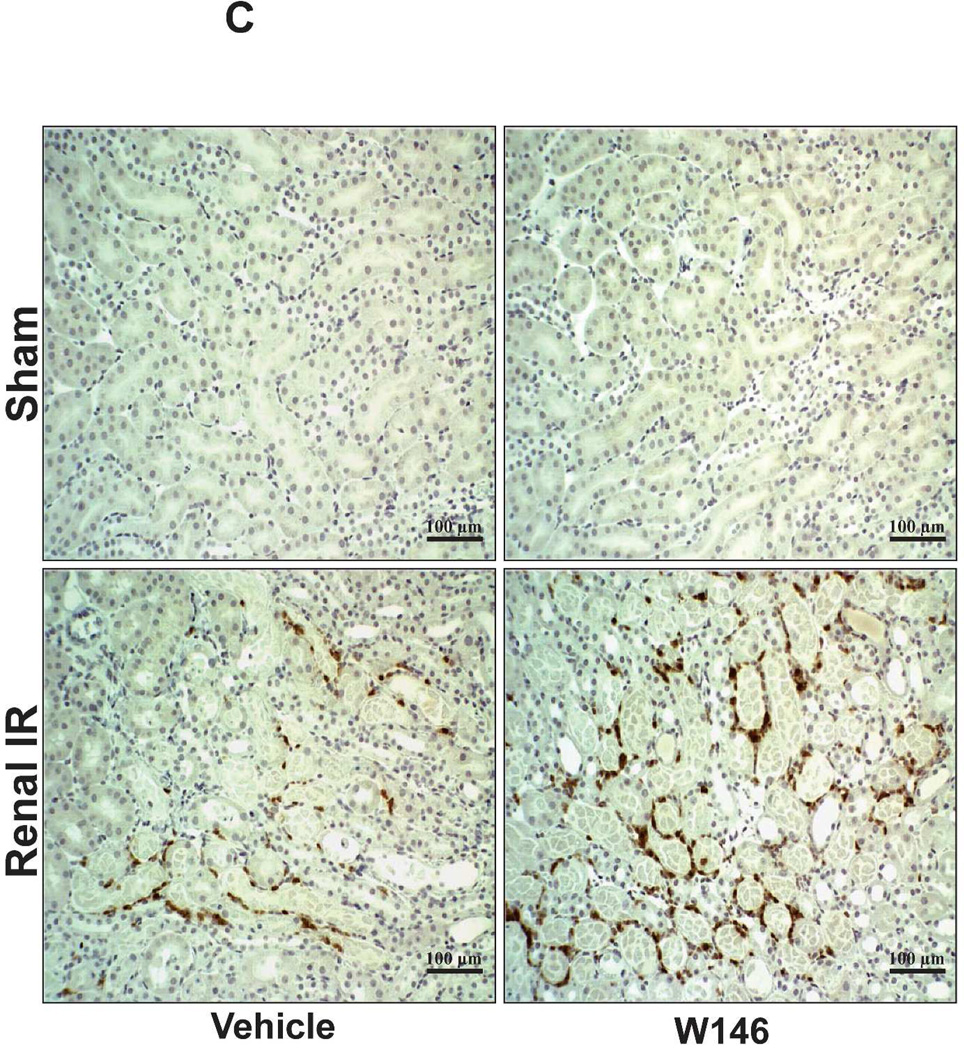
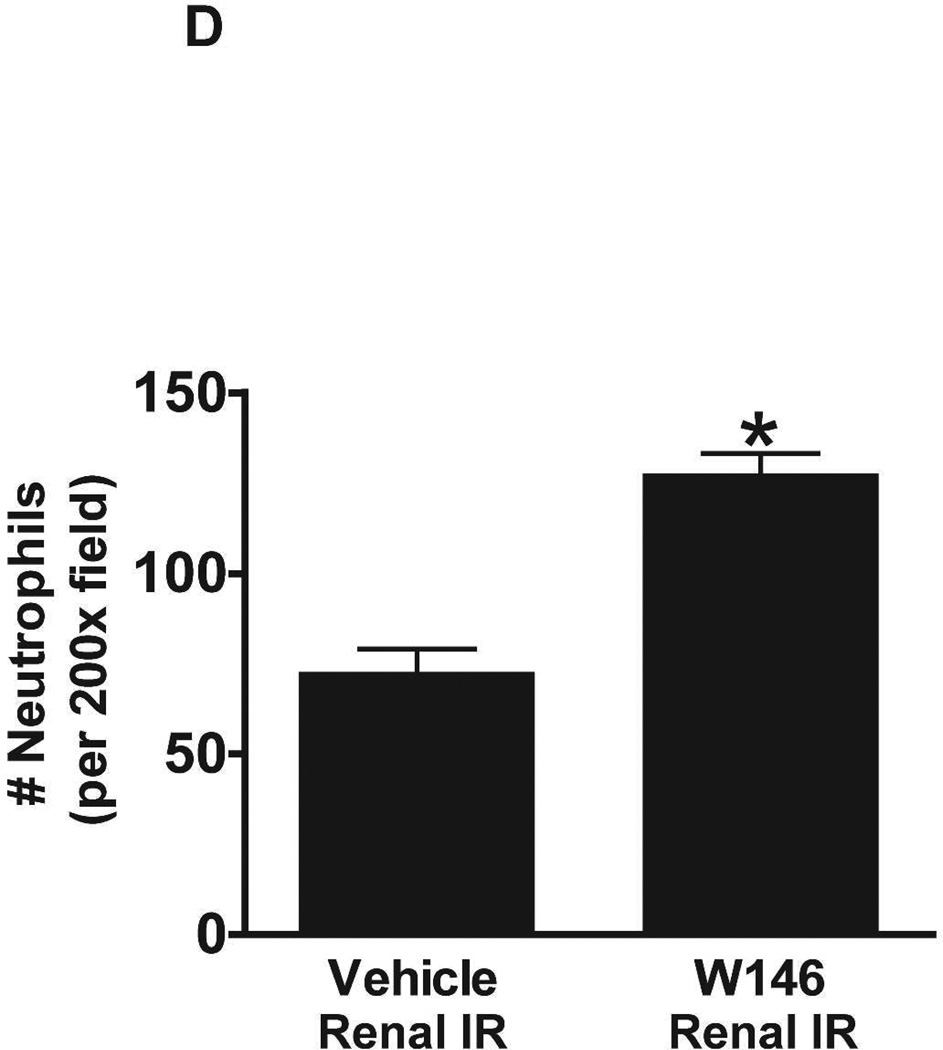
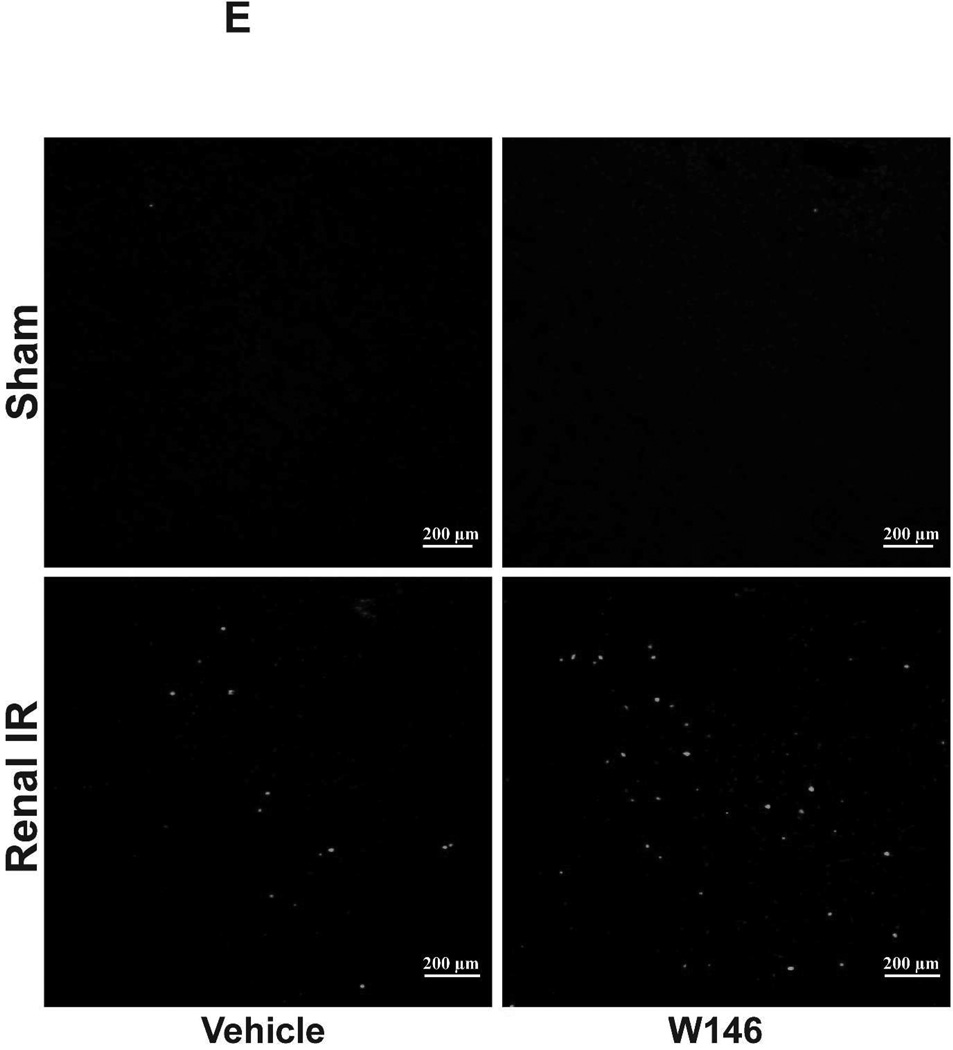
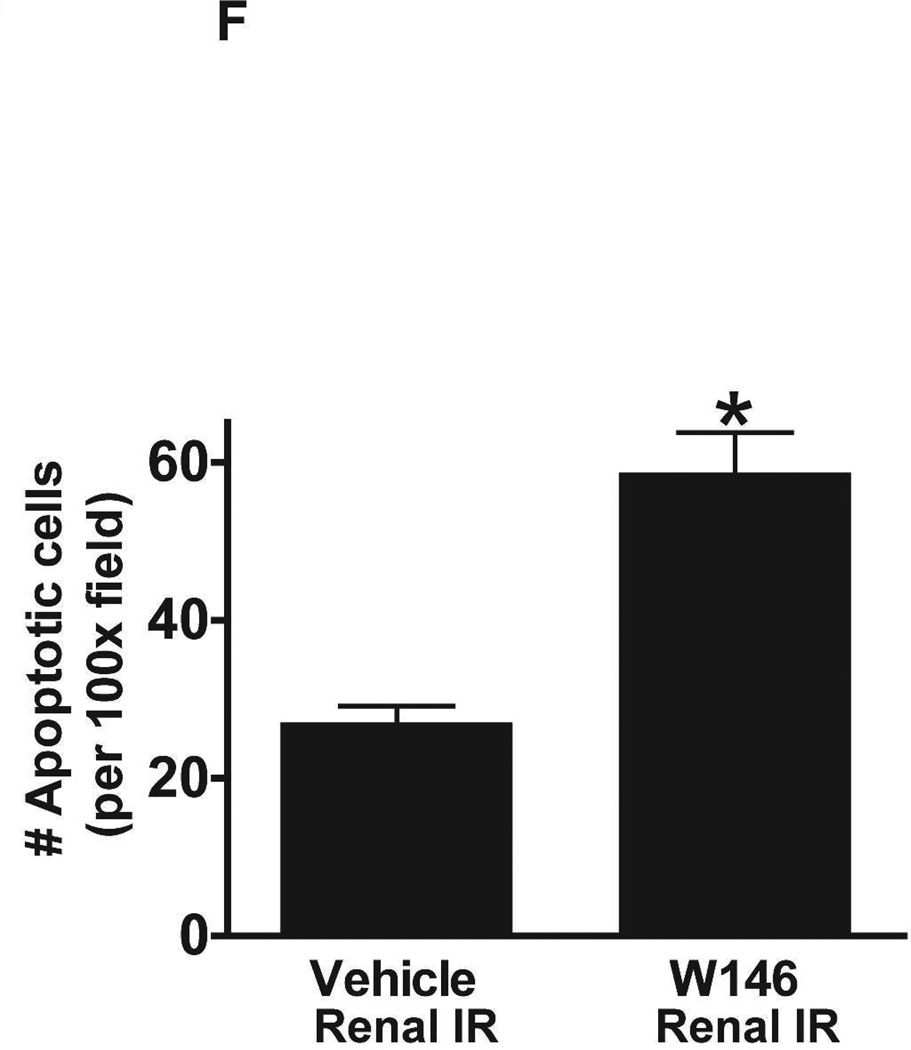
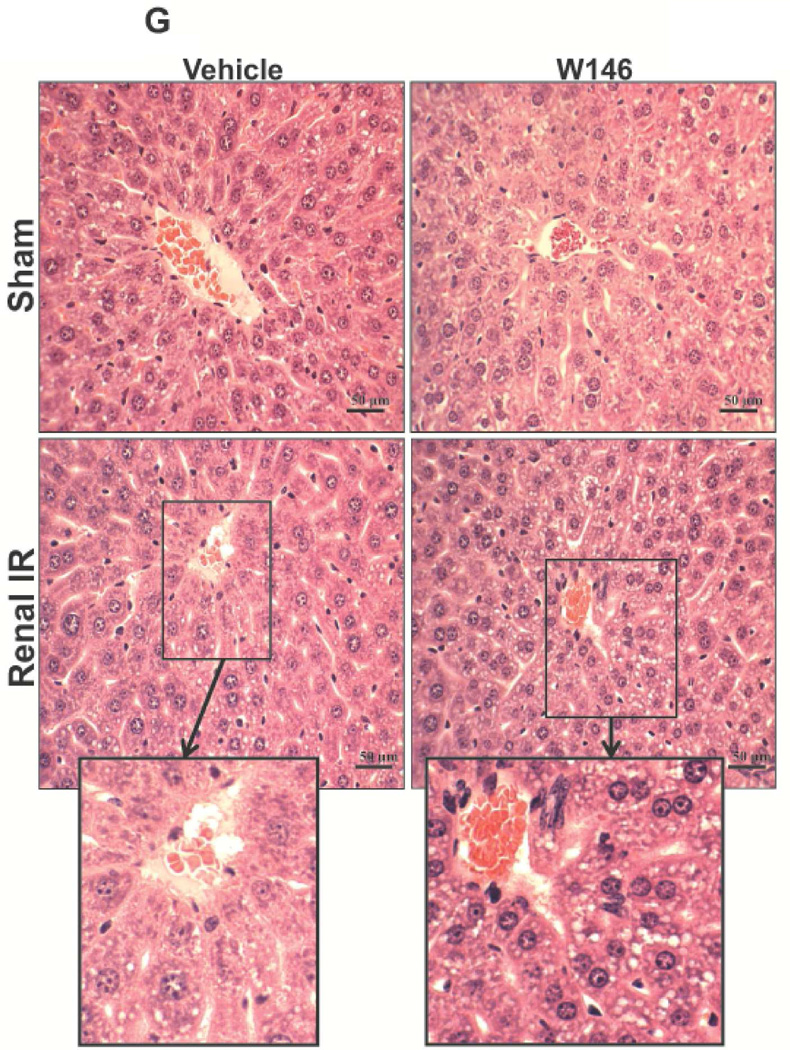
A. Representative hematoxylin and eosin staining (magnification 200X) of kidney sections of vehicle (1% DMSO)- or W146-treated (0.1 mg/kg i.p. daily for 3 days) C57BL/6 mice subjected to sham-operation or to 20 min of renal ischemia followed by 24 hr of reperfusion. Photographs are representative of 5–6 independent experiments. B. Summary of Jablonski scale renal injury scores (scale 0–4) for mice subjected to renal IR. W146-treated mice showed increased renal tubular necrosis after renal IR compared to vehicle-treated C57BL/6 mice subjected to renal IR injury. C and E. Representative photomicrographs for immunohistochemistry (brown staining) for neutrophil infiltration (C, magnification 200X) and TUNEL staining (E, representing apoptotic nuclei, magnification 100X) from kidneys of vehicle-treated or W146-treated C57BL/6 mice subjected to sham-operation or to 20 min of renal ischemia followed by 24 hr of reperfusion (IR). Vehicle-treated C57BL/6 mice had numerous neutrophil infiltration and TUNEL positive cells after 20 min renal IR. W146-treated C57BL/6 mice had even higher number of neutrophil infiltration and TUNEL-positive cells after renal IR. D and F. Quantifications of infiltrated neutrophils per 200X field (D, N=4–6) and apoptotic cells per 100X field (F, N=4–6) in the kidneys of vehicle- or W146-treated C57BL/6 mice subjected to 20 min of renal ischemia followed by 24 hr of reperfusion. *P<0.05 vs. vehicle-treated C57BL/6 mice subjected to renal IR. Error bars represent 1 SEM. G. Liver histology was assessed by H&E staining. Sham-operated C57BL/6 mice had normal liver histology (representative of 5–6 slides, 400X). Vehicle-treatment and 20 min renal ischemia and 24 hr reperfusion in C57BL/6 mice resulted in mild to moderate hepatic injury (nuclear and cytoplasmic degenerative changes, cellular vacuolization, congestion as well as individual hepatocyte necrosis and focal apoptotic, pyknotic nuclei). Similar to conditional endothelial S1P1R deficient mice, the severity of liver injury was increased for W146-treated C57BL/6 mice subjected to renal IR injury (increased hepatocyte degenerative changes including increased individual hepatocyte necrosis, apoptosis and vacuolization).
Figure 6C shows representative images (from 4–6 experiments) of immunohistochemistry of kidney neutrophil infiltration (dark brown) of vehicle- or W146-treated C57BL/6 mice subjected to sham-operation or to 20 min of renal ischemia and 24 hr of reperfusion (magnification 200X). In sham-operated mice, we were unable to detect any neutrophils in the kidney. Neutrophil infiltration increased in vehicletreated mice subjected to renal IR (Figure 6C). Chronic W146-treament further increased neutrophil infiltration after 20 min of renal ischemia and 24 hr of reperfusion (Figure 6C and Figure 6D).
Chronic S1P1R antagonist treatment also increased renal apoptosis after IR. In sham-operated C57BL/6 mice, very few TUNEL positive cells were detected in the kidneys from vehicle- and W146-treated mice (Figure 6E, 100X, representative of from 4–6 experiments). TUNEL staining increased in vehicle-treated mice subjected to 20 min renal IR (Figure 6E and Figure 6F, N=4–6). Furthermore, W146-treated mice had significantly increased numbers of apoptotic TUNEL-positive cells in the kidney 24 hr after IR.
Liver histology was also assessed by H&E staining of liver sections. As shown in Figure 5G (representative of 5–6 slides), sham-operated C57BL/6 mice had normal liver histology. Vehicle-treatment and 20 min renal ischemia and 24 hr reperfusion resulted in mild-moderate hepatic injury (nuclear and cytoplasmic degenerative changes, cellular vacuolization, congestion as well as individual hepatocyte necrosis and focal apoptotic, pyknotic nuclei, Figure 5G). Similar to conditional endothelial S1P1R deficient mice, the severity of liver injury was increased for W146-treated C57BL/6 mice subjected to renal IR injury (increased hepatocyte degenerative changes including increased individual hepatocyte necrosis, apoptosis and vacuolization). Some degree of centrilobular necrosis was again observed in W146-treated mice subjected to 20 min renal IR injury.
Endothelial S1P1R deficiency increases pro-inflammatory gene expression in the kidney after IR
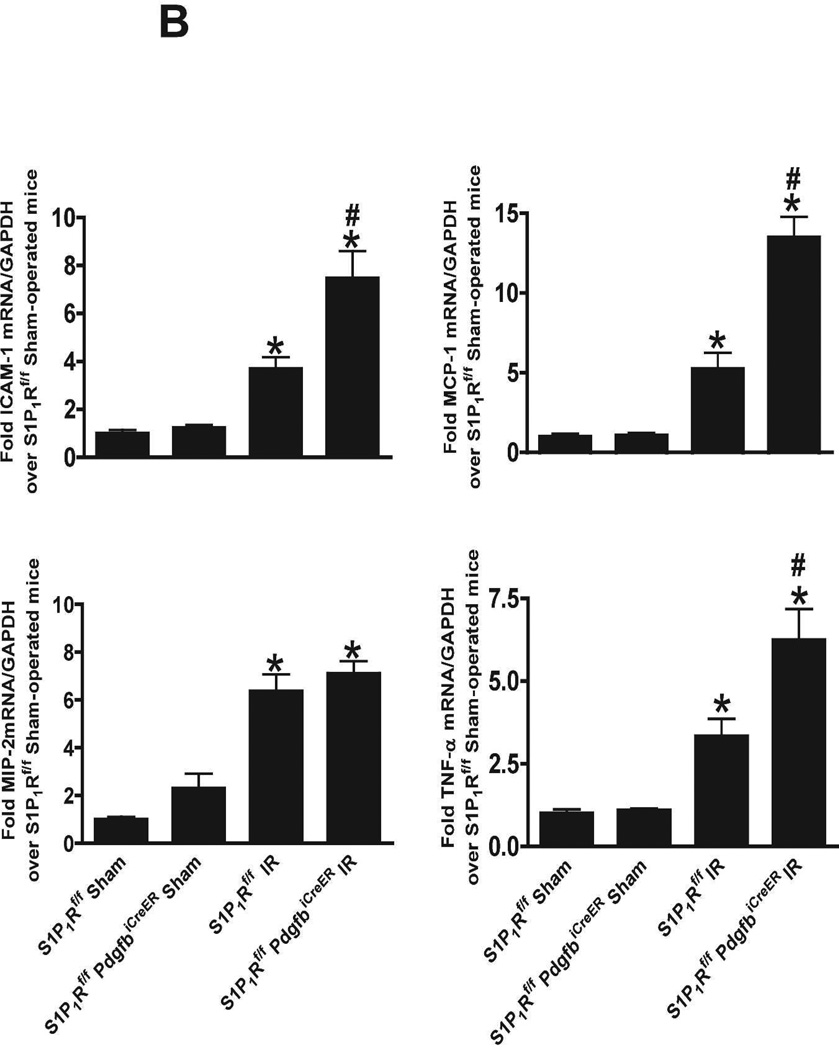
We measured the expression of pro-inflammatory cytokine mRNAs in the kidney (TNF-α, ICAM-1, MCP-1 and MIP-2) 24 hr after renal IR by RTPCR (primer sequences listed in Table 1). We show that tamoxifen-treated wild type mice subjected to renal IR had significantly increased expression of all pro-inflammatory mRNAs examined compared to the tamoxifen-treated sham-operated wild type mice (Figure 7). Moreover, tamoxifen-treated endothelial S1P1R deficient mice had even greater increases in TNF-α, MCP-1 and ICAM-1 expression without any changes in MIP-2 expression compared to tamoxifen-treated wild type mice subjected to renal IR.
Table 1.
Primers used to amplify cDNAs based on published GenBank sequences for mice. bp, base pairs; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; ICAM-1, intercellular adhesion molecule-1; iCreER, tamoxifen inducible Cre recombinase, MCP-1, monocyte chemoattractive protein-1; MIP-2, macrophage inflammatory protein-2; Pdgfb, platelet derived growth factor-b; S1PR, sphingosine 1-phosphare receptor; SK, sphingosine kinase; TNF-α, tumor necrosis factor-alpha; HSP, heat shock protein; HO-1, heme oxygenase-1.
| Primers | Species | Sequence (Sense/Antisense) | Product Size (bp) |
Cycle Number |
Annealing Temp (°C) |
|---|---|---|---|---|---|
| S1P1R | Mouse | 5 ′-CGGTGT AGACCC AGAGTCCT-3′ 5′-AGC AGC AGATGAGAATGAAC-3′ |
393 | 20 | 64 |
| S1P2R | Mouse | 5′- AAAACC AACC ACTGGCTGTC-3′ 5 ′-GAGTGGAACTTGCTGTT-3′ |
317 | 25 | 60 |
| S1P3R | Mouse | 5′-AAGCCTAGCGGGAGAGAAAC-3′ 5 ′-GGCAATC AAAACC ATCAGGT-3′ |
452 | 22 | 64 |
| S1P4R | Mouse | 5 ′-GC AGAAGTCTCC ACGTCCTC-3′ 5 ′-GCTGAGTGACCGAGAAGTCC-3′ |
403 | 23 | 62 |
| S1P5R | Mouse | 5′-ACACCAAATGCCCAGCTTAC- 3′ 5′- ACC AAGAGC AC AGCC AAGTTC-3′ |
335 | 32 | 62 |
| S1P1R FLOX | Mouse | 5′-GAGCGGAGGAAGTTAAAAGTG- 3′ 5′-CCTCCTAAGAGATTGCAGCAA- 3′ |
Flox 250, Cont 200 |
45 | 56 |
| PdgfbiCreER | Mouse | 5′-CCAGCCGCCGTCGCAACT- 3′ 5′-GCCGCCGGGATCACTCTCG- 3′ |
450 | 35 | 60 |
| TNF-α | Mouse | 5 ′-TACTGAACTTCGGGGTGATTGGTCC-3′ 5 ′-C AGCCTTGTCCCTTGAAGAGAACC-3′ |
290 | 24 | 65 |
| ICAM-1 | Mouse | 5 ′-TGTTTCCTGCCTCTGAAGC-3′ 5 ′-CTTCGTTTGTGATCCTCCG-3′ |
409 | 21 | 60 |
| MCP-1 | Mouse | 5′- ACCTGCTGCTACTC ATTC AC-3′ 5 ′-TTGAGGTGGTTGTGGAAAAG-3′ |
312 | 22 | 60 |
| MIP-2 | Mouse | 5 ′-CC AAGGGTTGACTTC AAGAAC-3′ 5′-AGCGAGGC AC ATC AGGTACG-3′ |
282 | 28 | 60 |
| HSP27 | Mouse | 5 ′-CCTAAGGTCTGGC ATGGT A-3′ 5′-AGGAAGCTCGTTGTTGAAGC-3′ |
373 | 25 | 66 |
| HSP27 | Human | 5 ′-GTCCCTGGATGTC AACC AC-3′ 5 ′-GACTGGGATGGTGATCTCG-3′ |
286 | 15 | 65 |
| HSP32 (HO-1) |
Mouse and human | 5 ′-TGAAGGAGGCC ACC AAGGAG-3′ 5 ′-GTGGGCC ACC AGC AGCTC-3′ |
320 | 15 | 65 |
| HSP70 | Mouse and human | 5 ′-GATC ACC ATC ACC AACGAC AA-3′ 5 ′-TTGTCC AGC ACCTTCTTCTTGTC-3′ |
218 | 15/18 | 65(H)/ 60(M) |
| GAPDH | Mouse and human | 5’-ACCACAGTCCATGCCATCAC-3’ 5’-CACCACCCTGTTGCTGTAGCC-3’ |
450 | 15 | 65 |
Respective anticipated PCR product size (bp, base pairs), PCR cycle number for linear amplification, and annealing temperatures used for each primer are provided.
Figure 7. Endothelial S1P1R deficiency increases pro-inflammatory gene expression in the kidney after renal IR.
Representative gel images (A) of RTPCR and densitometric quantification of relative band intensities normalized to GAPDH (B) of pro-inflammatory markers TNF-α, ICAM-1, MCP-1 and MIP-2 in kidneys from tamoxifen-treated (5 mg/kg i.p. daily for 3 days) endothelial S1P1R null (S1P1Rf/f PdgfbiCreER) or wild type (S1P1Rf/f) mice. Mice were subjected to sham-operation or to 20 min of renal ischemia followed by 24 hr of reperfusion (N=4–5 per group). Conditional deletion of endothelial S1P1R significantly increased the expression of TNF-α, MCP-1 and ICAM-1 mRNAs examined compared to the wild type mice subjected to renal IR. *P<0.05 vs. Sham-operated mice. #P<0.05 vs. wild type mice subjected to renal IR. Error bars represent 1 SEM.
Endothelial S1P1R deficiency increases kidney vascular permeability after renal IR
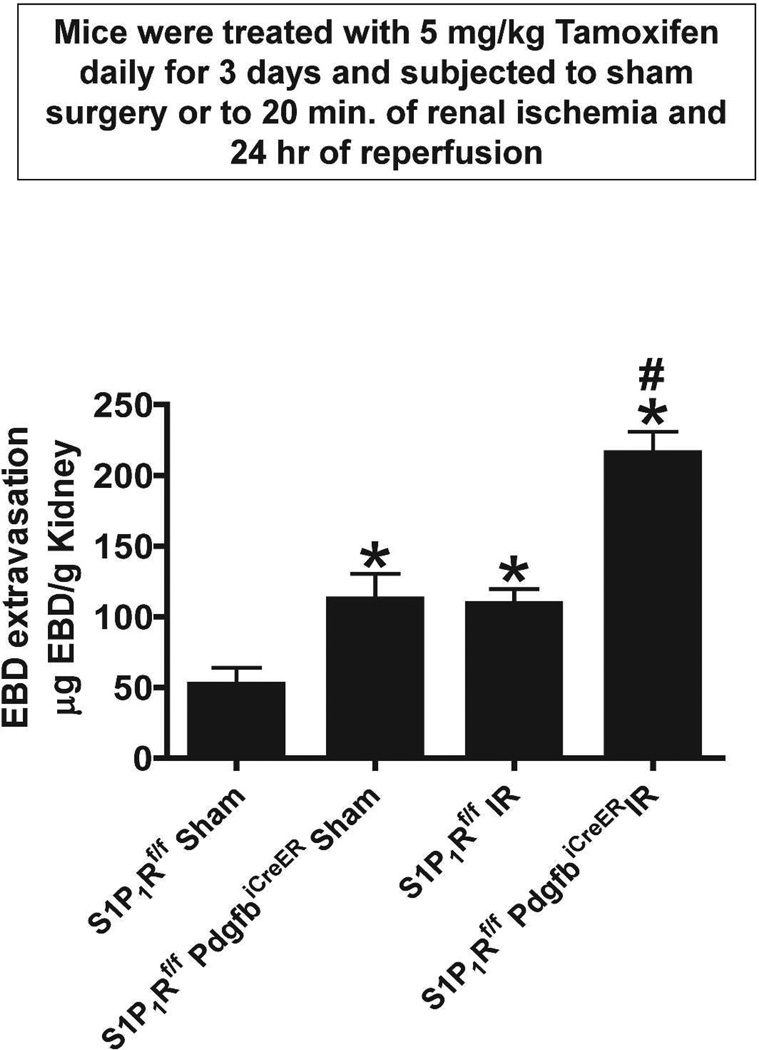
We measured kidney vascular permeability after 20 min renal IR with EBD injection. EBD binds to plasma proteins and its appearance in extravascular tissues reflects an increase in vascular permeability. Analysis of EBD extravasations in kidneys from tamoxifen-treated wild type (S1P1Rf/f) or endothelial S1P1R deficient mice subjected to either sham-operation or to renal IR is shown in Figure 8. We show that renal IR caused significant increases in kidney vascular permeability. However, tamoxifen-treated endothelial S1P1R deficient mice had significantly increased EBD extravasation after sham-operation or after renal IR.
Figure 8. Endothelial S1P1R deficiency increases kidney vascular permeability after IR.
Quantification of EBD extravasations as indices of kidney vascular permeability from tamoxifen-treated wild type (S1P1Rf/f) or endothelial S1P1R null mice subjected to either sham-operation or to renal IR 5 hr prior (N=5). Wild type mice subjected to renal IR had increased kidney vascular permeability. Moreover, tamoxifen-treated endothelial S1P1R null mice had significantly increased kidney EBD extravasation after sham-operation or after renal IR. Data are presented as means ± SEM. *P<0.05 vs. sham-operated wild type (S1P1Rf/f) mice. #P<0.05 vs. wild type (S1P1Rf/f) mice subjected to renal IR.
Conditional endothelial S1P1R deficiency or chronic S1P1R blockade decreases endothelial HSP27 mRNA expression in mice
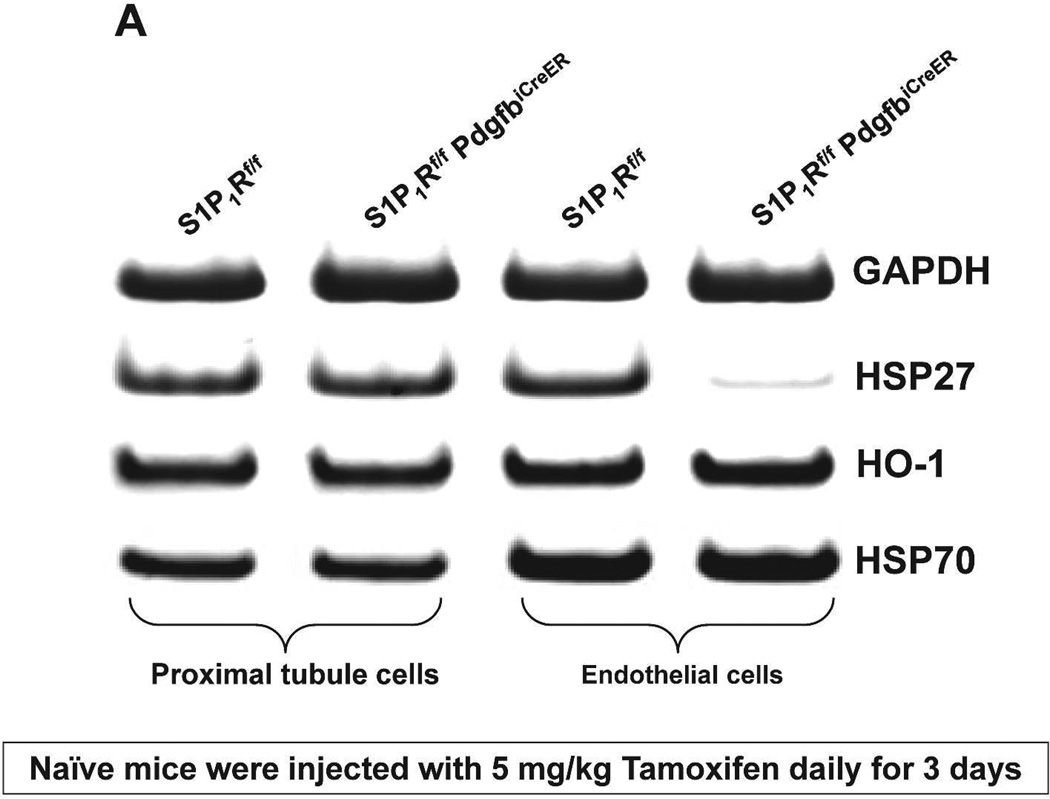
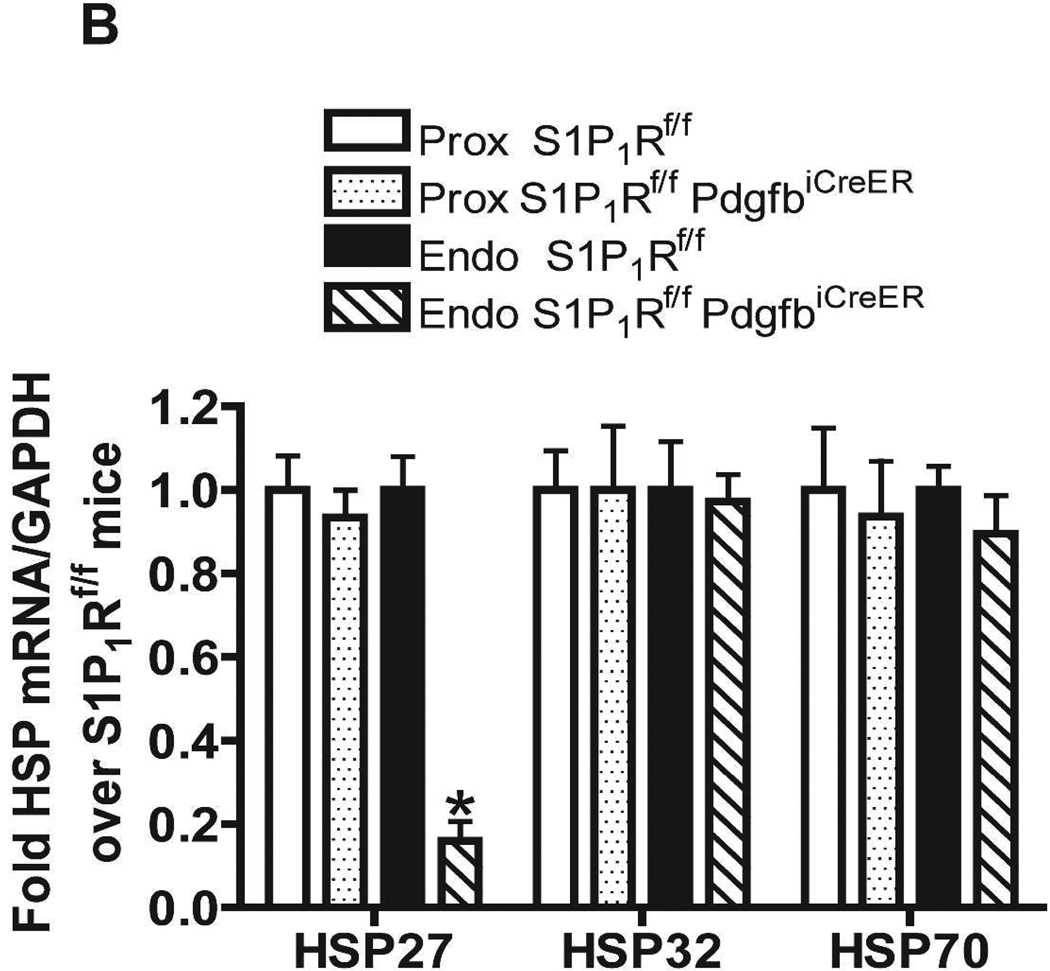
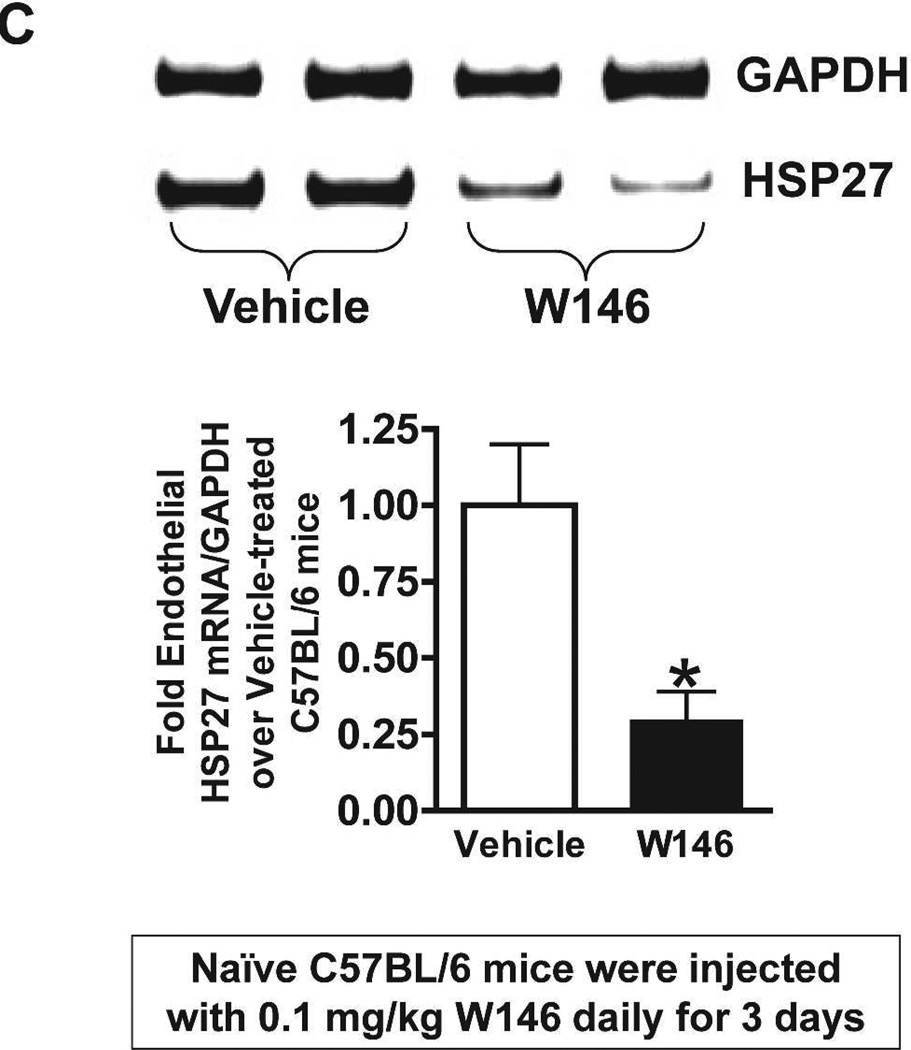
We probed for the potential mechanisms for increased renal injury and increased impairment of vascular permeability with endothelial S1P1R deficiency or antagonism. Since S1P1R induces HSP27 expression in certain cell types14,15 and since HSP27 is a well-known cytoprotective protein in endothelial cells16, we hypothesized that deletion or chronic blockade of S1P1R decreases endothelial HSP27 expression. Figure 9A and Figure 9B show that freshly isolated renal endothelial cells from naïve conditional endothelial S1P1R deficient mice express significantly reduced HSP27 mRNA compared with tamoxifen-treated wild type (S1P1Rf/f) mice without any changes in HSP32 or HSP70 expression. Furthermore, we show that HSP27, HSP32 and HSP70 mRNA levels in freshly isolated proximal tubule cells were similar between conditional S1P1R deficient mice and wild type mice. Finally, we determined that endothelial cells isolated from C57BL/6 mice chronically treated with W146 (0.1 mg/kg for 3 days) also express significantly reduced HSP27 mRNA compared to vehicle-treated C57BL/6 mice (Figure 9C). However, HSP27 mRNA expression did not change in endothelial cells isolated from kidney of C57BL/6 mice treated with W146 (0.1 mg/kg) for 1 hr compared to vehicle-treated mice (Figure 9D). Consistent with this, W146 treatment for 1 hour did not exacerbate ischemic AKI in C57BL/6 mice (Cr=1.44±0.14 mg/dL, N=4) compared to vehicle-treated mice (Cr=1.30±0.09 mg/dL, N=4).
Figure 9. Endothelial S1P1R deficiency or chronic S1P1R blockade decreases endothelial HSP27 mRNA expression in mice.
Representative gel images of RTPCR (A) and densitometric quantification of relative band intensities normalized to GAPDH (B) of HSP27, HO-1 (HSP32) and HSP70 mRNA expression in freshly isolated endothelial and proximal tubule cells from conditional endothelial S1P1R null mice or from wild type (S1P1Rf/f) mice. Renal endothelial cells from endothelial S1P1R null mice express significantly reduced HSP27 mRNA compared wild type mice without any changes in HO-1 or HSP70 expression. Furthermore, we show that HSP27, HSP32 and HSP70 mRNA levels in freshly isolated proximal tubule cells were similar between endothelial S1P1R null mice and wild type mice. C and D. Representative gel images of RTPCR (top) and densitometric quantification of relative band intensities normalized to GAPDH (bottom) of HSP27 mRNA expression in freshly isolated endothelial cells from C57BL/6 mice treated for 3 days (C) or for 1 hr (D) with vehicle or with W146 (a selective S1P1R antagonist, 0.1 mg/kg). S1P1R antagonist treatment significant reduced endothelial HSP27 expression only after treatment for 3 days with W146 (C). W146 treatment for 1 hr had no effect on endothelial HSP27 expression (D). *P<0.05 vs. wild type (S1P1Rf/f) mice or vehicle-treated C57BL/6 mice.
S1P1R-mediated modulation of cultured endothelial cell HSP27 mRNA expression
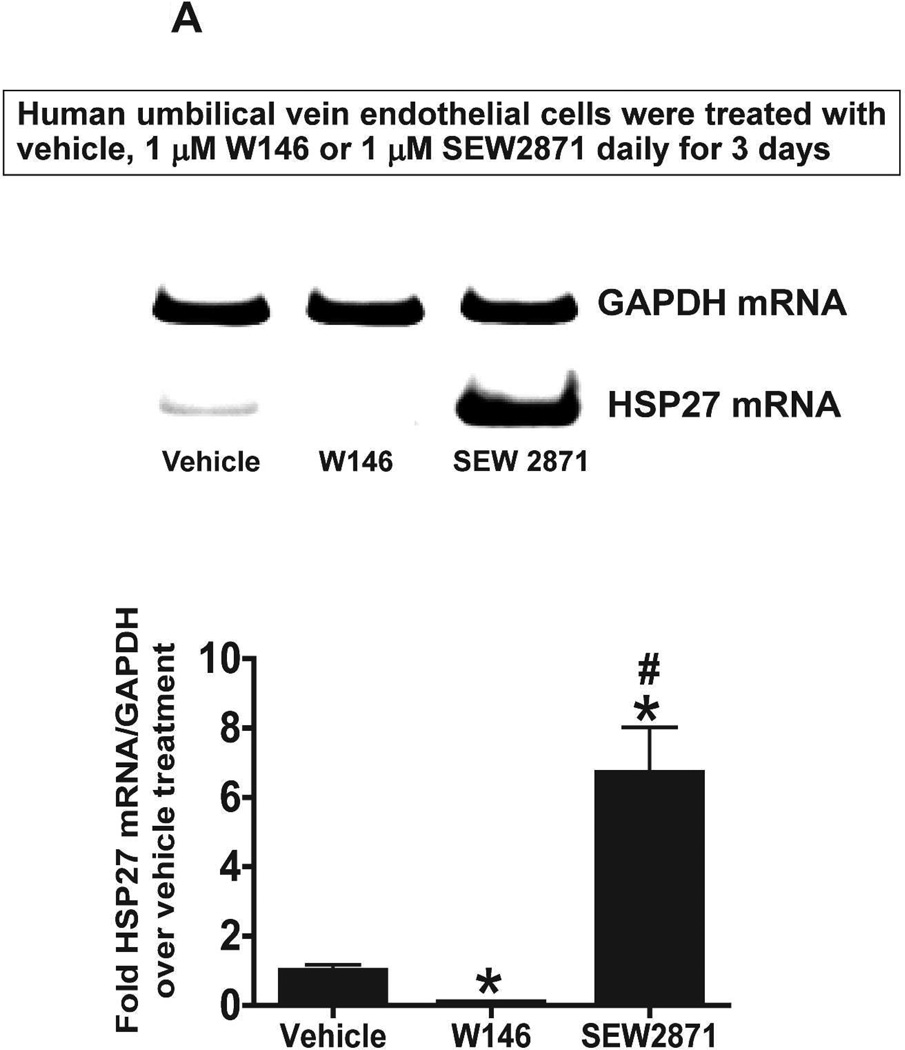
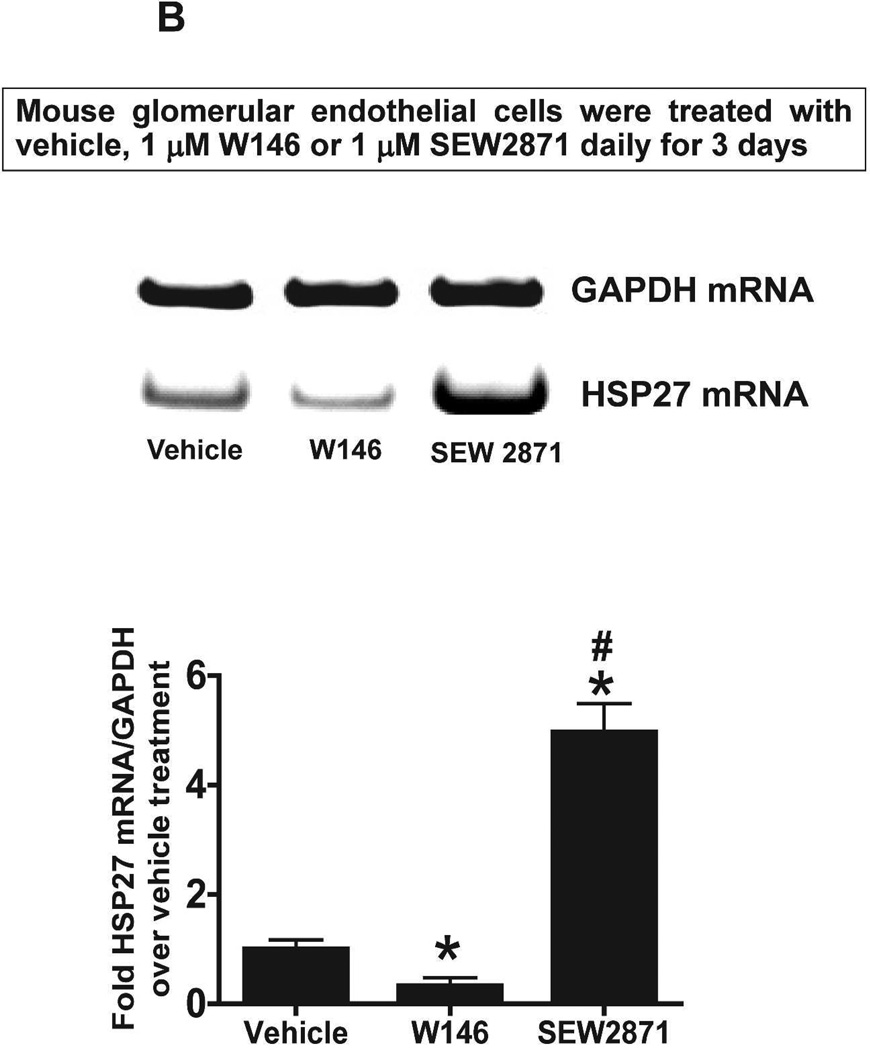
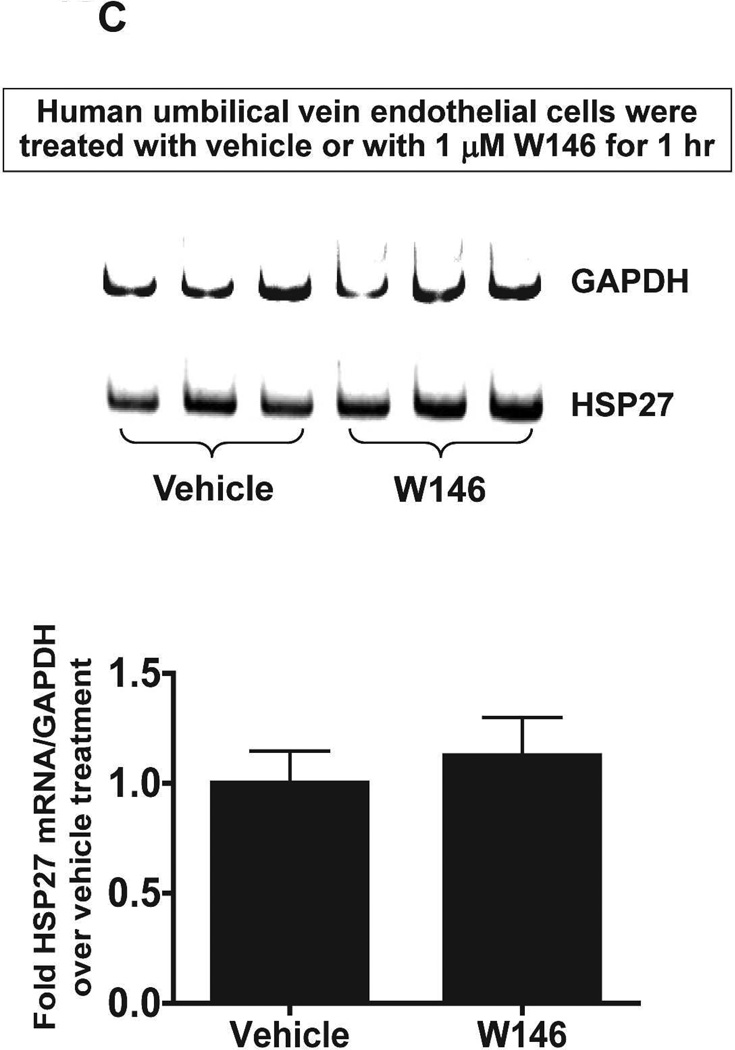
We next tested whether S1P1R antagonism or activation modulates HSP27 mRNA expression in cultured endothelial cells. Human umbilical vein endothelial (EA.hy926) cells (Figure 10A) or mouse glomerular endothelial cells (Figure 10B) were treated with a selective S1P1R antagonist (1 µM W146) or agonist (1 µM SEW2871) for 3 days and probed for HSP27 mRNA expression with RTPCR. Therefore, Figure 10 and Figure 10B show that human and mouse endothelial cells treated with W146 for 3 days had significantly reduced HSP27 mRNA expression. Conversely, chronic S1P1R activation with SEW2871 significantly increased HSP27 mRNA expression in human and mouse endothelial cells. In contrast, W146 treatment for 1 hr did not change HSP27 mRNA expression in human endothelial cells (Figure 10C).
Figure 10. S1P1R modulates HSP27 mRNA expression in cultured endothelial cells.
A and B. Representative gel images of RTPCR (top) and densitometric quantification of relative band intensities normalized to GAPDH (bottom) of HSP27 mRNA expression in human umbilical vein endothelial cells (A, N=6) or mouse glomerular endothelial cells (B, N=5) treated with vehicle (1% DMSO), with a selective S1P1R agonist (1 µM SEW2871) or with a selective antagonist (1 µM W146) for 3 days. Endothelial cells treated with W146 for 3 days had significantly reduced HSP27 mRNA expression. In contrast, chronic S1P1R activation with SEW2871 significantly increased HSP27 mRNA expression in human and mouse endothelial cells. C. Representative gel images of RTPCR (top) and densitometric quantification of relative band intensities normalized to GAPDH (bottom) of HSP27 mRNA expression in human umbilical vein endothelial cells (N=4) treated with vehicle (1% DMSO) or with a selective antagonist (1 µM W146) for 1 hr. W146 treatment for 1 hr did not change endothelial HSP27 expression. *P<0.05 vs. vehicle-treated cells.
Chronic S1P1R antagonism or HSP27 knockdown decreases endothelial cell viability
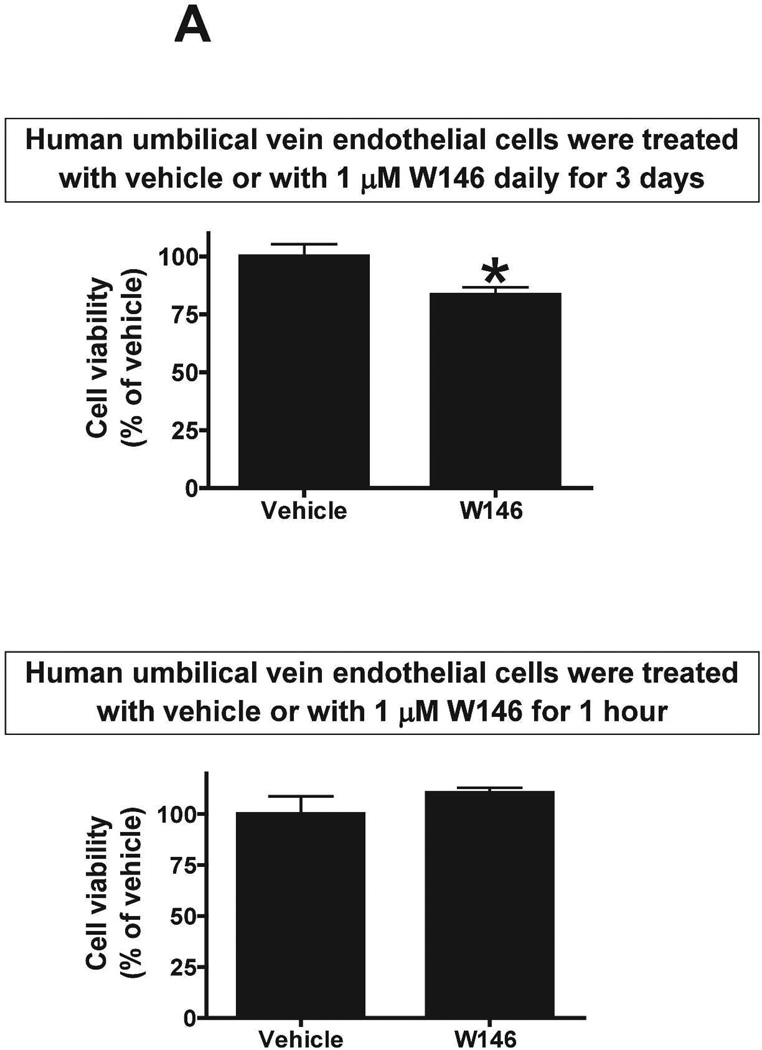
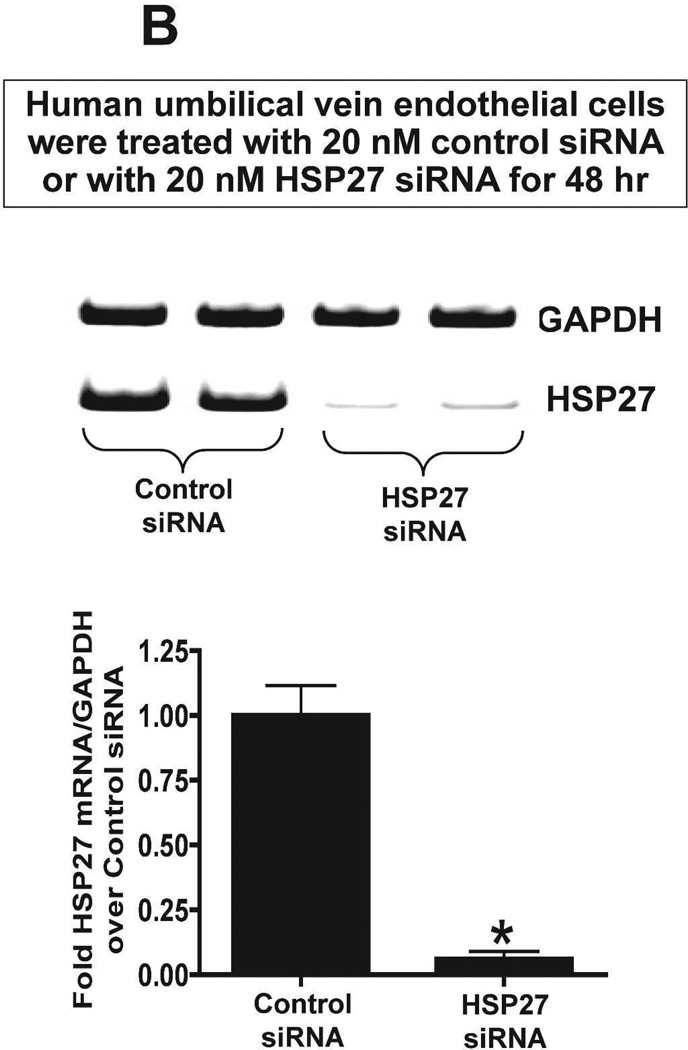
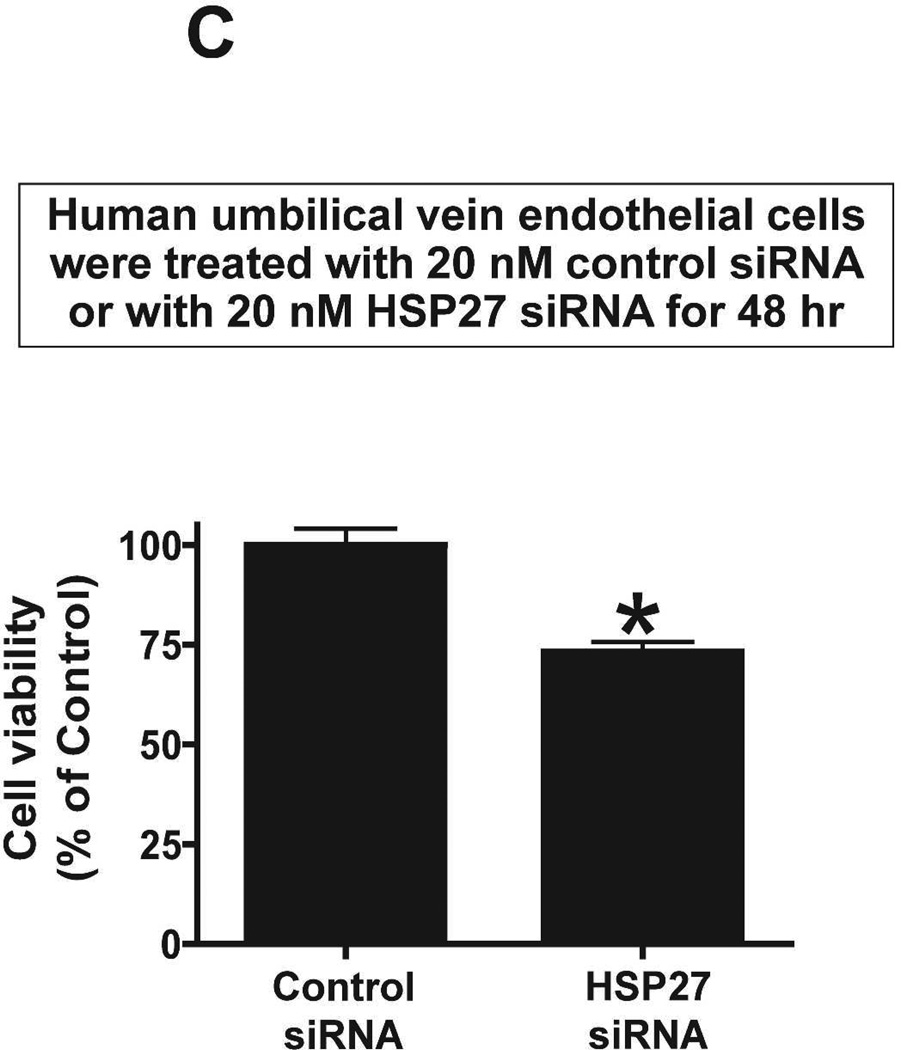
We next tested whether chronic (3 days) S1P1R antagonism or HSP27 knockdown modulates endothelial cell viability in culture. MTT assay was performed to measure endothelial cell viability. Human umbilical vein endothelial cells treated with W146 for 3 days had significantly reduced endothelial cell viability (∼83% of vehicle-treated group) whereas 1 hr treatment had no effect (Figure 11A). We then tested whether knockdown of HSP27 expression also modulates endothelial cell viability in culture. Figure 11B shows that HSP27 siRNA treatment for 48 hr significantly attenuated HSP27 mRNA expression in endothelial cells compared to control siRNA-treated cells. Furthermore, HSP27 siRNA induced HSP27 knockdown significantly reduced endothelial cell viability in culture (∼73% of control siRNA group). Therefore, from these studies, we conclude that downregulation of HSP27 expression decrease endothelial cell survival.
Figure 11. Chronic S1P1R antagonism or HSP27 knockdown decreases endothelial cell viability.
MTT assay measured endothelial cell viability. A. Human umbilical vein endothelial cells were treated with vehicle (1% DMSO) with a selective S1P1R antagonist (1 µM W146) for 3 days (N=4, top) or for 1 hr (N=4, bottom). Treatment with W146 for 3 days significantly reduced endothelial cell viability whereas 1 hr treatment had no effect. B. Representative gel images of RTPCR (top) and densitometric quantification of relative band intensities normalized to GAPDH (bottom) of HSP27 mRNA expression in human umbilical vein endothelial cells (N=4) treated with 20 nM control siRNA or with 20nM HSP27 siRNA for 48 hr (N=4). HSP27 siRNA treatment significantly attenuated HSP27 mRNA expression in endothelial cells. C. siRNA induced HSP27 knockdown significantly reduced endothelial cell viability in culture (N=4). *P<0.05 vs. vehicletreated cells.
Discussion
Acute kidney injury (AKI) remains a significant clinical complication with high mortality and morbidity despite more than 6 decades of research.1,4 Due to extreme susceptibility to ischemia induced necrosis and apoptosis, renal proximal tubular injury has been considered the hallmark of AKI pathology.22 However, it is becoming increasingly clear that complex and close orchestration of signaling events between proximal tubule cells, endothelial cells, pericytes and resident dendritic cells play a major role in ischemic AKI.23,24 In particular, endothelial cell injury after ischemic AKI maintains and even extends renal dysfunction and may play a critical role in the development of chronic kidney disease.25 Indeed, renal vascular endothelial dysfunction results in reduction in post-ischemic peritubular capillary blood flow as well as in increased coagulation, leukocyte infiltration and tubular inflammation. Therefore, therapeutic strategies to protect against endothelial dysfunction would reduce the extent and maintenance phase of AKI after renal IR injury.
Sphingosine-1-Phosphate (S1P) is an endogenous lysophospholipid ligand that regulates diverse cellular function in many organs by binding to cell surface G-protein coupled high affinity S1P receptors.26,27 There are 5 identified S1PRs that produce distinct as well as overlapping intracellular signaling events. S1P1R, S1P2R and S1P3R in particular are widely expressed in many cell types including the brain, liver, heart, kidney and the immune system.26 Of 5 S1P receptor subtypes, the S1P1R has been the most extensively studied. Activation of the S1P1R protects against cardiac and hepatic IR injury and reduces systemic and tissue inflammation by directly affecting lymphocyte egress.28–30 In the kidney, activation of the S1P1R with selective agonists (SEW2871 or FTY720) protects against renal IR injury by reducing inflammation, attenuating pro-inflammatory T-lymphocyte function as well as by eliciting direct cytoprotective effects on renal proximal tubule cells.28,31,32
Although the renal tubular protective role for S1P1R activation is well established, the role for endothelial S1P1R in modulating ischemic AKI is not entirely clear.10,28,32 This is mainly because germline global or conditional endothelial S1P1R deletion results in embryonic lethality due to immature formation of blood vessels and excessive hemorrhage.18,33 In addition, conflicting data exists in the literature regarding the role of S1P in endothelial cell function and survival. While many studies implicate a cytoprotective and barrier-strengthening role for endothelial S1P and S1P1R activation, other studies suggest that S1P promotes inflammation and leukocyte adhesion in endothelial cells.11–13 For example, S1P promotes adhesion molecule VCAM-1 and E-selectin induction in endothelial cells via NF-kB activation.34,35 Furthermore, chronic overexpression of sphingosine kinase-1 and increased S1P synthesis in cultured endothelial cells increases VCAM-1 expression and neutrophil adhesion in response to TNF-α stimulation.36
In this study, we crossed floxed S1P1R mice with mice that express a tamoxifen-inducible form of Cre recombinase under the transcriptional control of Pdgfb to selectively delete endothelial S1P1R in adult mice.17,18 PdgfbiCreER mice express a tamoxifen inducible form of Cre recombinase (iCreERT2) under the transcriptional control of the Pdgfb gene. As Pdgfb is predominantly expressed in endothelial cells, use of PdgfbiCreER mice would result in more selective expression of Cre recombinase in endothelial cells when compared to Tie-2Cre mice.17 Although Tie-2Cre mice are widely used to delete the gene of interest in endothelial cells as Tie-2 expression was thought to be restricted to endothelial cells, recent studies suggest that certain myeloid cells also express Tie-2.37 Indeed, Cre driven by Tie-2 will recombine genomic DNA in both endothelial and myeloid leukocytes as some leukocytes, in particular monocytes and eosinophils, also express Tie-2.38,39 We performed qRTPCR for S1P1–5R subtypes in splenocytes and peritoneal macrophages and determined that conditional endothelial S1P1R deletion did not change expression of S1P1–5R subtypes in leukocytes (data not shown). Furthermore, PdgfbiCreER mice has the added advantage as they express Cre recombinase fused to a mutant form of the human estrogen receptor that is insensitive to endogenous estrogen but is responsive to tamoxifen.17
With immunohistochemistry as well as RTPCR, we showed that S1P1Rf/f PdgfbiCreER mice have selectively reduced S1P1R expression in endothelial cells after 3 days of tamoxifen treatment. We also complemented our inducible endothelial S1P1R studies by treating C57BL/6 mice with a selective S1P1R antagonist (W146). We demonstrate in this study that either inducible deletion of endothelial S1P1R or chronic pharmacological blockade of S1P1R exacerbated renal IR injury and remote hepatic dysfunction in mice. Furthermore, inducible genetic deletion of endothelium or chronic S1P1R antagonist treatment increased renal tubular necrosis, neutrophil infiltration, and apoptosis. These data prove that endothelial S1P1R activation serves a protective role against ischemic AKI.
In addition to increased kidney neutrophil infiltration, we determined that inducible endothelial S1P1R null mice had increased expression of several key pro-inflammatory mRNAs including ICAM-1, MCP-1 and TNF-α, in the kidney after renal IR. ICAM-1 is an important mediator of neutrophil trans-endothelial migration and MCP-1 acts as a chemoattractant for monocytes and lymphocytes to areas of injury. 26,27 These findings suggest that endothelial S1P1R serves to protect against pro-inflammatory leukocyte attachment and transmigration after renal IR injury.
Since heat shock proteins are well recognized cytoprotective proteins and regulate endothelial cell survival, cytoskeleton structure and differentiation, we tested whether endothelial S1P1R deficiency leads to depletion of one of these cytoprotective chaperone heat shock proteins.16,40 We showed in this study that selective endothelial S1P1R deletion or chronic S1P1R blockade with W146 decreased endothelial HSP27 expression in vivo. Indeed, S1P1R activation has been shown to modulate HSP27 expression in other cell types. S1P has been shown to induce HSP27 via the p38 MAP-kinase and the PI3-kinase/Akt pathways in osteoblasts.14,15 We propose that marked reduction in endothelial HSP27 may be responsible for increased renal and hepatic injury after ischemic AKI in endothelial S1P1R null mice. Further study will be necessary to determine the precise relationship between S1P1R and HSP27 in mediating endothelial protection after ischemic AKI.
In contrast to increased renal and hepatic injury with downregulation of endothelial HSP27 in endothelial S1P1R null mice, we showed previously that overexpression of sphingosine kinase-1 and enhanced activation of S1P1R induced HSP27 in renal proximal tubules in mice and in HK-2 cells. Furthermore, we recently demonstrated that HSP27 overexpression protected mice against hepatic IR induced renal endothelial cell apoptosis by promoting F-actin cytoskeletal preservation.41,42 HSP27 is a member of a family of chaperone proteins that are up-regulated in response to a wide range of cellular stresses including hypoxia, ischemia and exposure to toxic drugs.43,44 Increased expression of HSP27 serves to defend a cell against injury or death by acting as chaperones facilitating proper polypeptide folding and aberrant protein removal.45–47 In addition, HSP27 is a potent anti-apoptotic protein and is a key stabilizer of the actin cytoskeleton; both of these cellular effects lead to increased resistance against cell death.40,48 Furthermore, HSP27 is directly responsible for increased tolerance for endothelial cell death against apoptosis and heat stress.16,40 We also provide evidence for a direct link between HSP27 expression and endothelial viability as knockdown of HSP27 expression with HSP27 siRNA or with chronic W146 treatment reduced viability of human endothelial cells in culture whereas short term (1 hr) W146 treatment did not reduce HSP27 expression and failed to reduce endothelial cell viability. Collectively, these findings suggest an important role for HSP27 in S1P1R-mediated endothelial cell protection.
In addition to S1P1R-mediated endothelial cell protection, additional mechanisms may regulate endothelial function during and after ischemic AKI. Grenz et al.49 eloquently demonstrated that increased adenosine generation and transport via transcriptional regulation of equilibrative nucleoside transporters regulate post-ischemic no-reflow phenomenon by modulating renal endothelial A2b adenosine receptors. Interestingly, we also recently showed that increased adenosine generation after ischemic AKI enhances sphingosine kinase-1 activity leading to increased S1P generation and S1P1R activation in the kidney.50 Future studies are required to determine whether increased vascular adenosine by transcriptional regulation of equilibrative nucleoside transporters modulate endothelial S1P1R activity to regulate endothelial function after ischemic AKI.
Ischemia and reperfusion injury is characterized by intensive tissue hypoxia followed by profound inflammation.51 Previous studies have shown that hypoxia inducible factor (HIF)-1α dependent transcriptional regulation plays a critical role in modulating tissue hypoxia and inflammation as well as promoting cellular repair.51 Interestingly, S1P signaling can directly modulate HIF-1α activity.52 It remains to determined in future studies whether endothelial S1P1R signaling can directly modulate HIF-1α signaling to protect against ischemic AKI induced tissue hypoxia and inflammation.
Plasma creatinine measurement as an index of renal function after AKI has several limitations especially in non-steady state conditions due to renal tubular secretion of creatinine and plasma non-creatinine chromagen interference.53,54 In fact, Eisner et al. demonstrated that ∼35–50% of creatinine clearance is mediated by renal tubular secretion of creatinine rather than glomerular filtration in mice.54 Their findings indicate that utilizing creatinine would lead to overestimation of renal glomerular filtration rate. Their findings also suggest that renal tubular damage due to IR injury would decrease renal tubular creatinine secretion in mice leading to even greater increase in plasma creatinine levels. Therefore, we also estimated glomerular filtration rate with FITC-inulin technique.20 These 2 indices of renal function together with assessment of renal tubular necrosis, inflammation and apoptosis allow us to support our conclusion that mice with conditional deletion of endothelial S1P1R have increased renal injury after ischemic AKI.
One of the most exciting aspects of sphingolipid research is small molecule therapy targeting S1P1R to protect renal proximal tubules and endothelial cells against ischemic AKI. However, multiple S1PR subtypes are expressed in a single cell type including renal tubules and endothelial cells that may produce divergent and undesirable side effects. Therefore, specific targeting of S1P1R will be critical to prevent undesirable S1PR receptor signaling that may limit therapeutic potential of S1P1R activation. Several compounds specifically targeting S1P1R have been already developed and are being tested in preclinical and clinical trials.55
In summary, we demonstrate in this study that inducible and selective endothelial S1P1R deletion in adult mice exacerbates renal and hepatic injury after ischemic AKI by exacerbating kidney vascular permeability impairment, renal tubular necrosis, apoptosis and inflammation. We also propose that a selective downregulation of endothelial HSP27, a key cytoprotective protein, may explain increased organ injury in endothelial S1P1R null mice.
Methods
Generation of mice with conditional tamoxifen inducible deletion of endothelial S1P1R
Mice with global or endothelial specific deletion of S1P1R are embryonically lethal due to immature formation of blood vessels and excessive hemorrhage.33 Therefore, we generated conditional endothelial S1P1R deficient mice by crossing mice with floxed S1P1R (S1P1Rf/f, provided by Dr Rick L. Proia (NIH)) with mice that express a tamoxifen-inducible form of Cre recombinase (iCreERT2) under the transcriptional control of the Pdgfb gene.17,18 Resulting S1P1Rf/f PdgfbiCreER (endothelial S1P1R deficient) or S1P1Rf/f (wild type) mice on a C57BL6/129 background were genotyped by tail biopsy PCR using Pdgfb-Cre transgene and S1P1R loxP-specific primers (Table 1). Pdgfb-Cre primers generated a 450-bp fragment. S1P1R loxP primers amplified a 250-bp fragment for the S1P1R floxed allele and a 200-bp fragment for the S1P1R wild-type allele. Adult (∼20g) mice were injected with 5 mg Tamoxifen in filter-sterilized olive oil (i.p.) daily for 3 days to conditionally delete endothelial S1P1R.
EGFP detection in S1P1R PdgfbiCreER mice
PdgfbiCreER mice also express an internal ribosomal entry site element and a sequence coding for enhanced green fluorescent protein (EGFP).17 To confirm that Pdgfb-iCreER transgene expression is specific for endothelial cells in the kidney, we detected EGFP expression in kidney cryosections from S1P1Rf/f PdgfbiCreER or S1P1Rf/f mice. Kidneys were fixed in 30% sucrose solution for 4 hr and embedded into optimal cutting temperature compound (Tissue-Tek, Sakura Finetek USA, Trannce, CA). The 8 µm cryosections were mounted in ProLong Gold antifade reagent-containing DAPI from Molecular Probes (Invitrogen, Carlsbad, CA) and imaged under a fluorescence microscope.
Murine model of renal IR injury
After Institutional Animal Care and Use Committee approval, we subjected adult (∼20g) male mice to 20 min. of renal IR as previously described.56,57 Sham-operated animals underwent the same surgical procedures (anesthesia, laparotomy, bowel manipulations and wound closure) without renal ischemia. We collected kidney, liver and plasma 24 hr after renal IR or sham-operation to examine the severity of renal and hepatic dysfunction. We also collected kidney 5 hr after renal IR or sham-operation in a separate cohort of mice to measure impairments in kidney vascular permeability.
Analyses for plasma markers for renal and hepatic injury
Plasma creatinine was measured by an enzymatic creatinine reagent kit according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA). This method of creatinine measurement largely eliminates the interferences from mouse plasma chromagens well known to the Jaffe method.58 Renal function was also assessed by estimating glomerular filtration rate 24 hours after renal IR injury. The glomerular filtration rate was estimated with fluorescein isothiocyanate (FITC-inulin) clearance technique as described by Qi et al.20 The plasma alanine aminotransferase (ALT) activities were measured by using the Infinity ALT assay kit according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA).
Histological detection of kidney and liver injury
Kidney and liver morphological assessment was performed by an experienced pathologist (V.DA.) who was unaware of the treatment that each animal had received. An established grading scale of necrotic injury (0–4, Renal Injury Score) to the proximal tubules was used for the histopathological assessment of IR-induced damage as outlined by Jablonski et al.21 and as described previously in our studies.59,60 Liver H&E sections were graded for hepatocyte hypereosinophilia, cytoplasmic vacuolization, coagulation necrosis, sinusoidal congestion and sinusoidal inflammation as described by us previously.19
Histological detection of kidney apoptosis and neutrophil infiltration
We detected apoptosis after renal IR with TUNEL staining as described using a commercially available in situ cell death detection kit (Roche, Indianapolis, IN) according to the instructions provided by the manufacturer.29,61 Apoptotic TUNEL positive cells were quantified in 5–7 randomly chosen 100X microscope images fields in the corticomedullary junction and results were expressed as apoptotic cells counted per 100X field. Renal inflammation after IR injury was determined by detecting neutrophil infiltration with immunohistochemistry 24 hr after renal IR as described previously.29,61 Neutrophils infiltrating the kidney were quantified in 5–7 randomly chosen 200X microscope image fields in the corticomedullary junction and results were expressed as neutrophils counted per 200X field.
Kidney vascular permeability after ischemic AKI
Changes in the kidney vascular permeability 5 hr after renal IR were assessed by quantifying extravasations of Evans blue dye (EBD) into the tissue as described by Awad et al. with some modifications as described by us. 10,61
Immunohistochemistry for S1P1R and CD34
S1P1R and CD34 immunohistochemistry were performed in paraffin-embedded kidney sections from tamoxifen-treated conditional endothelial S1P1R deficient mice and wild type (S1P1Rf/f) mice with rabbit anti-mouse S1P1R (Cayman, Ann Arbor, MI, USA, 1:100) or with rat anti-mouse CD34 (Cedarlane labs, Burlington, NC, USA, 1:100). A primary antibody that recognized IgG2a (Serotec, Raleigh, NC, USA) was used as a negative isotype control in all experiments.
Isolation of mouse kidney proximal tubule cells and endothelial cells
Adult S1P1Rf/f PdgfbiCreER or S1P1Rf/f mice were pretreated with tamoxifen for 3 days. Mouse kidney proximal tubules were isolated using Percoll density gradient separation as described previously.9,62 Renal endothelial cells were isolated with CD144 antibody (eBioscience inc, San Diego, CA) capture and magnetic bead separation (Dynabeads®, Invitrogen) according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA) and as described by us previously.9
Cell culture
Immortalized mouse glomerular endothelial cells were obtained from Dr. M. Madaio (Georgia Regents University) and grown in low glucose DMEM/Ham’s F12 medium plus 10% FBS, 2 mM l-glutamine, and 10 mM HEPES.63 Immortalized human umbilical vein endothelial cells (EA.hy926, American Type Culture Collection, Manassas, VA) cells were grown in high-glucose DMEM plus 10% FBS. Endothelial cells were pretreated with vehicle (1% DMSO), 1 µM W146 (3-amino-4-(3-hexylphenylamino)-4-oxobutyl phosphonic acid, a selective S1P1R antagonist) or SEW2871 (5-[4-phenyl-5-(trifluoromethyl)-2-thienyl]-3-[3-(trifluoromethyl)phenyl]-1,2,4-oxadiazole, a selective S1P1R agonist) for 3 days to chronically antagonize or activate S1P1R in vitro. Some EA.hy926 cells were also pretreated for 1 hr with W146 before harvest.
HSP27 siRNA Transfection
Immortalized human umbilical vein endothelial cells were transfected with 20–100 nM HSP27 siRNA (sc-29350) or with control siRNA (sc-36869) from Santa Cruz Biotechnology (Santa Cruz, CA) with siRNA transfection reagent (sc-29528) in siRNA transfection medium (sc-36868) for 48 hr according to the manufacturer’s instructions.
Reverse transcription PCR analyses (RTPCR)
Kidney inflammation after renal ischemia was also determined by measuring mRNA encoding markers of inflammation, including keratinocyte derived cytokine (KC), intercellular adhesion molecule-1 (ICAM-1), monocyte chemoattractive protein-1 (MCP-1), macrophage inflammatory protein-2 (MIP-2), and tumor necrosis factor-alpha (TNF-α) 24 hr after renal IR. We also performed RTPCR for heat shock proteins (HSP27, heme oxygenase-1 [HO-1 or HSP32] and HSP70) and S1P1–5R in kidneys isolated from naive mice. Finally, we measured HSP27 mRNA expression in cultured endothelial cells with RTPCR. RTPCR was performed as described previously with the primers listed in Table 1.29,61
Measurement of endothelial cell viability
Endothelial cell viability was tested with a 3-[4,5-dimethyl(thiazol-2-yl)-3,5-diphery]tetradium bromide (MTT) cytotoxicity assay as described.64 The MTT cytotoxicity assay measures cell viability by quantifying the mitochondrial formation of formazan product (dark blue) via dehydrogenase-mediated reduction of the tetrazolium ring of MTT.
Statistical analysis
The data were analyzed with Student's t-test when comparing means between two groups or one-way ANOVA plus Tukey’s post hoc multiple comparison test when comparing multiple groups. The ordinal values of the renal and hepatic injury scores were analyzed by the Mann–Whitney nonparametric test. In all cases, a probability statistic P<0.05 was taken to indicate significance. All data are expressed throughout the text as means ± SEM.
Acknowledgments
Funding: This work was supported in part by Department of Anesthesiology, Columbia University and by R01 DK-058547 and R01 GM-067081 (to H.T.L.).
We thank Dr. Rick L. Proia for providing us with the floxed S1P1R mice.
Footnotes
Disclosures: No conflict of interest exists for each author.
References
- 1.Jones DR, Lee HT. Perioperative renal protection. Best Pract Res Clin Anaesthesiol. 2008;22:193–208. doi: 10.1016/j.bpa.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Aronson S, Blumenthal R. Perioperative renal dysfunction and cardiovascular anesthesia: concerns and controversies. J Cardiothorac Vasc Anesth. 1998;17:117–130. doi: 10.1016/s1053-0770(98)90106-9. [DOI] [PubMed] [Google Scholar]
- 3.Yap SC, Lee HT. Acute kidney injury and extrarenal organ dysfunction: new concepts and experimental evidence. Anesthesiology. 2012;116:1139–1148. doi: 10.1097/ALN.0b013e31824f951b. [DOI] [PubMed] [Google Scholar]
- 4.Paladino JD, Hotchkiss JR, Rabb H. Acute kidney injury and lung dysfunction: a paradigm for remote organ effects of kidney disease? Microvasc Res. 2009;77:8–12. doi: 10.1016/j.mvr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faubel S. Pulmonary complications after acute kidney injury. Adv Chronic Kidney Dis. 2008;15:284–296. doi: 10.1053/j.ackd.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven't worked and what is on the horizon. Clin J Am Soc Nephrol. 2007;2:356–365. doi: 10.2215/CJN.03280906. [DOI] [PubMed] [Google Scholar]
- 7.Hait NC, Oskeritzian CA, Paugh SW, et al. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Jo SK, Bajwa A, Awad AS, et al. Sphingosine-1-phosphate receptors: biology and therapeutic potential in kidney disease. Kidney Int. 2008;73:1220–1230. doi: 10.1038/ki.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SW, Kim M, Brown KM, et al. Inhibition of sphingosine 1-phosphate receptor 2 protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2012;23:266–280. doi: 10.1681/ASN.2011050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Awad AS, Ye H, Huang L, et al. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2006;290:F1516–F1524. doi: 10.1152/ajprenal.00311.2005. [DOI] [PubMed] [Google Scholar]
- 11.Singleton PA, Chatchavalvanich S, Fu P, et al. Akt-mediated transactivation of the S1P1 receptor in caveolin-enriched microdomains regulates endothelial barrier enhancement by oxidized phospholipids. Circ Res. 2009;104:978–986. doi: 10.1161/CIRCRESAHA.108.193367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucke S, Levkau B. Endothelial functions of sphingosine-1-phosphate. Cell Physiol Biochem. 2010;26:87–96. doi: 10.1159/000315109. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Pisonero I, Duenas AI, Barreiro O, et al. Lipopolysaccharide and sphingosine-1-phosphate cooperate to induce inflammatory molecules and leukocyte adhesion in endothelial cells. J Immunol. 2012;189:5402–5410. doi: 10.4049/jimmunol.1201309. [DOI] [PubMed] [Google Scholar]
- 14.Takai S, Tokuda H, Matsushima-Nishiwaki R, et al. Phosphatidylinositol 3-kinase/Akt plays a role in sphingosine 1-phosphate-stimulated HSP27 induction in osteoblasts. J Cell Biochem. 2006;98:1249–1256. doi: 10.1002/jcb.20846. [DOI] [PubMed] [Google Scholar]
- 15.Kozawa O, Tanabe K, Ito H, et al. Sphingosine 1-phosphate regulates heat shock protein 27 induction by a p38 MAP kinase-dependent mechanism in aortic smooth muscle cells. Exp Cell Res. 1999;250:376–380. doi: 10.1006/excr.1999.4536. [DOI] [PubMed] [Google Scholar]
- 16.Gill RR, Gbur CJ, Jr, Fisher BJ, et al. Heat shock provides delayed protection against oxidative injury in cultured human umbilical vein endothelial cells. J Mol Cell Cardiol. 1998;30:2739–2749. doi: 10.1006/jmcc.1998.0837. [DOI] [PubMed] [Google Scholar]
- 17.Claxton S, Kostourou V, Jadeja S, et al. Efficient, inducible Cre-recombinase activation in vascular endothelium. Genesis. 2008;46:74–80. doi: 10.1002/dvg.20367. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Wada R, Yamashita T, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SW, Chen SW, Kim M, et al. Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy. Lab Invest. 2011;91:63–84. doi: 10.1038/labinvest.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qi Z, Whitt I, Mehta A, et al. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol. 2004;286:F590–F596. doi: 10.1152/ajprenal.00324.2003. [DOI] [PubMed] [Google Scholar]
- 21.Jablonski P, Howden BO, Rae DA, et al. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983;35:198–204. doi: 10.1097/00007890-198303000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Sheridan AM, Bonventre JV. Cell biology and molecular mechanisms of injury in ischemic acute renal failure. Curr Opin Nephrol Hypertens. 2000;9:427–434. doi: 10.1097/00041552-200007000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Bajwa A, Kinsey GR, Okusa MD. Immune mechanisms and novel pharmacological therapies of acute kidney injury. Curr Drug Targets. 2009;10:1196–1204. doi: 10.2174/138945009789753174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol. 2008;109:e102–e107. doi: 10.1159/000142934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 2007;72:151–156. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 26.Chae SS, Proia RL, Hla T. Constitutive expression of the S1P1 receptor in adult tissues. Prostaglandins Other Lipid Mediat. 2004;73:141–150. doi: 10.1016/j.prostaglandins.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279:15396–15401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 28.Lien YH, Yong KC, Cho C, et al. S1P(1)-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int. 2006;69:1601–1608. doi: 10.1038/sj.ki.5000360. [DOI] [PubMed] [Google Scholar]
- 29.Park SW, Kim M, Chen SW, et al. Sphinganine-1-phosphate protects kidney and liver after hepatic ischemia and reperfusion in mice through S1P(1) receptor activation. Lab Invest. 2010;90:1209–1224. doi: 10.1038/labinvest.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SW, Kim M, Kim M, et al. Sphingosine kinase 1 protects against renal ischemia-reperfusion injury in mice by sphingosine-1-phosphate1 receptor activation. Kidney Int. 2011;80:1315–1327. doi: 10.1038/ki.2011.281. [DOI] [PubMed] [Google Scholar]
- 31.Lai LW, Yong KC, Igarashi S, Lien YH. A sphingosine-1-phosphate type 1 receptor agonist inhibits the early T-cell transient following renal ischemia-reperfusion injury. Kidney Int. 2007 doi: 10.1038/sj.ki.5002203. [DOI] [PubMed] [Google Scholar]
- 32.Bajwa A, Jo SK, Ye H, et al. Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J Am Soc Nephrol. 2010;21:955–965. doi: 10.1681/ASN.2009060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allende ML, Yamashita T, Proia RL. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood. 2003;102:3665–3667. doi: 10.1182/blood-2003-02-0460. [DOI] [PubMed] [Google Scholar]
- 34.Xia P, Gamble JR, Rye KA, et al. Tumor necrosis factor-alpha induces adhesion molecule expression through the sphingosine kinase pathway. Proc Natl Acad Sci U S A. 1998;95:14196–14201. doi: 10.1073/pnas.95.24.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimamura K, Takashiro Y, Akiyama N, et al. Expression of adhesion molecules by sphingosine 1-phosphate and histamine in endothelial cells. Eur J Pharmacol. 2004;486:141–150. doi: 10.1016/j.ejphar.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 36.Limaye V, Xia P, Hahn C, et al. Chronic increases in sphingosine kinase-1 activity induce a pro-inflammatory, pro-angiogenic phenotype in endothelial cells. Cell Mol Biol Lett. 2009;14:424–441. doi: 10.2478/s11658-009-0009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De PM, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;9:789–795. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]
- 38.Lemieux C, Maliba R, Favier J, et al. Angiopoietins can directly activate endothelial cells and neutrophils to promote proinflammatory responses. Blood. 2005;105:1523–1530. doi: 10.1182/blood-2004-09-3531. [DOI] [PubMed] [Google Scholar]
- 39.Feistritzer C, Mosheimer BA, Sturn DH, et al. Expression and function of the angiopoietin receptor Tie-2 in human eosinophils. J Allergy Clin Immunol. 2004;114:1077–1084. doi: 10.1016/j.jaci.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 40.Huot J, Houle F, Marceau F, Landry J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ Res. 1997;80:383–392. doi: 10.1161/01.res.80.3.383. [DOI] [PubMed] [Google Scholar]
- 41.Chen SW, Park SW, Kim M, et al. Human heat shock protein 27 overexpressing mice are protected against hepatic ischemia and reperfusion injury. Transplantation. 2009;87:1478–1487. doi: 10.1097/TP.0b013e3181a3c691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park SW, Chen SW, Kim M, et al. Human heat shock protein 27 overexpressing mice are protected against acute kidney injury after hepatic ischemia and reperfusion. Am J Physiol Renal Physiol. 2009;297:F885–F894. doi: 10.1152/ajprenal.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arrigo AP. Hsp27: novel regulator of intracellular redox state. IUBMB Life. 2001;52:303–307. doi: 10.1080/152165401317291156. [DOI] [PubMed] [Google Scholar]
- 44.Bruey JM, Ducasse C, Bonniaud P, et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- 45.Wyttenbach A, Sauvageot O, Carmichael J, et al. Heat shock protein 27 prevents cellular polyglutamine toxicity and suppresses the increase of reactive oxygen species caused by huntingtin. Hum Mol Genet. 2002;11:1137–1151. doi: 10.1093/hmg/11.9.1137. [DOI] [PubMed] [Google Scholar]
- 46.Samali A, Robertson JD, Peterson E, et al. Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones. 2001;6:49–58. doi: 10.1379/1466-1268(2001)006<0049:hpmotc>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paul C, Manero F, Gonin S, et al. Hsp27 as a negative regulator of cytochrome C release. Mol Cell Biol. 2002;22:816–834. doi: 10.1128/MCB.22.3.816-834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guay J, Lambert H, Gingras-Breton G, et al. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci. 1997;110(Pt 3):357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- 49.Grenz A, Bauerle JD, Dalton JH, et al. Equilibrative nucleoside transporter 1 (ENT1) regulates postischemic blood flow during acute kidney injury in mice. J Clin Invest. 2012;122:693–710. doi: 10.1172/JCI60214. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Park SW, Kim M, Brown KM, et al. Proximal tubule sphingosine kinase-1 has a critical role in A1 adenosine receptor-mediated renal protection from ischemia. Kidney Int. 2012 doi: 10.1038/ki.2012.224. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cuvillier O, Ader I, Bouquerel P, et al. Hypoxia, therapeutic resistance, and sphingosine 1-phosphate. Adv Cancer Res. 2013;117:117–141. doi: 10.1016/B978-0-12-394274-6.00005-4. [DOI] [PubMed] [Google Scholar]
- 53.Breyer MD, Qi Z. Better nephrology for mice--and man. Kidney Int. 2010;77:487–489. doi: 10.1038/ki.2009.544. [DOI] [PubMed] [Google Scholar]
- 54.Eisner C, Faulhaber-Walter R, Wang Y, et al. Major contribution of tubular secretion to creatinine clearance in mice. Kidney Int. 2010;77:519–526. doi: 10.1038/ki.2009.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang ZY, Zhang Z, Zug C, et al. AUY954, a selective S1P(1) modulator, prevents experimental autoimmune neuritis. J Neuroimmunol. 2009;216:59–65. doi: 10.1016/j.jneuroim.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 56.Gallos G, Ruyle TD, Emala CW, Lee HT. A1 adenosine receptor knockout mice exhibit increased mortality, renal dysfunction, and hepatic injury in murine septic peritonitis. Am J Physiol Renal Physiol. 2005;289:F369–F376. doi: 10.1152/ajprenal.00470.2004. [DOI] [PubMed] [Google Scholar]
- 57.Kim M, Kim M, Kim N, et al. Isoflurane mediates protection from renal ischemia-reperfusion injury via sphingosine kinase and sphingosine-1-phosphate-dependent pathways. Am J Physiol Renal Physiol. 2007;293:F1827–F1835. doi: 10.1152/ajprenal.00290.2007. [DOI] [PubMed] [Google Scholar]
- 58.SLOT C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest. 1965;17:381–387. doi: 10.3109/00365516509077065. [DOI] [PubMed] [Google Scholar]
- 59.Kim M, Park SW, Kim M, et al. Selective Renal Over-Expression of Human Heat Shock Protein 27 Reduces Renal Ischemia-Reperfusion Injury in Mice. Am J Physiol Renal Physiol. 2010 doi: 10.1152/ajprenal.00194.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim M, Kim M, Park SW, et al. Isoflurane protects human kidney proximal tubule cells against necrosis via sphingosine kinase and sphingosine-1-phosphate generation. Am J Nephrol. 2010;31:353–362. doi: 10.1159/000298339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park SW, Kim M, Chen SW, et al. Sphinganine-1-phosphate attenuates both hepatic and renal injury induced by hepatic ischemia and reperfusion in mice. Shock. 2010;33:31–42. doi: 10.1097/SHK.0b013e3181c02c1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vinay P, Gougoux A, Lemieux G. Isolation of a pure suspension of rat proximal tubules. Am J Physiol. 1981;241:F403–F411. doi: 10.1152/ajprenal.1981.241.4.F403. [DOI] [PubMed] [Google Scholar]
- 63.Akis N, Madaio MP. Isolation, culture, and characterization of endothelial cells from mouse glomeruli. Kidney Int. 2004;65:2223–2227. doi: 10.1111/j.1523-1755.2004.00634.x. [DOI] [PubMed] [Google Scholar]
- 64.Lee HT, Jan M, Bae SC, et al. A1 adenosine receptor knockout mice are protected against acute radiocontrast nephropathy in vivo. Am J Physiol Renal Physiol. 2006;290:F1367–F1375. doi: 10.1152/ajprenal.00347.2005. [DOI] [PubMed] [Google Scholar]