Abstract
Background
The relation of food insecurity (inability to acquire nutritionally adequate and safe foods) and chronic kidney disease (CKD) is unknown. We examined whether food insecurity is associated with prevalent CKD among lower income individuals in both the general U.S. adult population and an urban population.
Methods
We conducted cross-sectional analyses of lower income participants of the National Health and Nutrition Examination Survey (NHANES) 2003–2008 (n=9,126); and the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study (n=1,239). Food insecurity was defined based on questionnaires and CKD was defined by reduced estimated glomerular filtration rate or albuminuria; adjustment was performed with multivariable logistic regression.
Results
In NHANES, the age-adjusted prevalence of CKD was 20.3%, 17.6% and 15.7% for the high, marginal and no food insecurity groups, respectively. Analyses adjusting for sociodemographics and smoking status revealed high food insecurity to be associated with greater odds of CKD only among participants with either diabetes [odds ratio (OR) 1.67, 95% confidence interval (CI) 1.14–2.45 comparing high to no food insecurity group] or hypertension (OR 1.37, 95% CI 1.03–1.82). In HANDLS, the age-adjusted CKD prevalence was 5.9% and 4.6% for those with and without food insecurity, respectively (P=0.33). Food insecurity was associated with a trend towards greater odds of CKD (OR 1.46, 95% CI 0.98–2.18) with no evidence of effect modification across diabetes, hypertension or obesity subgroups.
Conclusion
Food insecurity may contribute to disparities in kidney disease, especially among persons with diabetes or hypertension, and is worthy of further study.
Keywords: renal, socioeconomic status, disparity, nutrition
INTRODUCTION
Low socioeconomic status (SES) is associated with prevalent chronic kidney disease (CKD)(1–7) and increased risk of end-stage renal disease (ESRD)(8, 9). However, few studies have addressed the correlates of low SES which might lead to CKD, thus little progress has been made towards the elimination of socioeconomic disparities in CKD.
Food insecurity may accompany low SES, and has been defined as “limited or uncertain ability to acquire nutritionally adequate and safe foods in socially acceptable ways”(10). In 2010, 17 million U.S. households were food insecure.(11) Food insecurity is associated with greater prevalence of several diet-related chronic conditions, including diabetes, hypertension, and obesity.(12, 13) However, little is known about the relation of food insecurity and CKD.
Food insecurity might lead to CKD through its influence on the development and management of risk factors for CKD (e.g. diabetes, hypertension) or through direct effects of diet on the kidney.(14) For example, acid-inducing diets (i.e. limited fruits and vegetables) may lead to endothelin-mediated glomerulosclerosis and fibrosis.(15, 16) In contrast, diets rich in fruits and vegetables have been associated with attenuated kidney injury(17, 18) and lower risk of CKD progression(19). Food insecurity is associated with limited fruit and vegetable intake.(20) Elucidating the relation of food insecurity and CKD could advance understanding of the mechanisms through which low SES may lead to CKD. Therefore, we sought to describe this association among lower income individuals in the general US adult population and in an urban population selected on the basis of SES. We hypothesized that, because the types and cost of foods available to urban versus other population groups may differ (e.g. greater availability of ‘fast foods’ in urban areas), food insecurity may be more strongly associated with prevalent CKD in an urban population.
METHODS
The National Health and Nutrition Examination Survey (NHANES): Design and Population
We examined combined data from the 2003–2004, 2005–2006, and 2007–2008 survey periods of the NHANES, which were conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention to examine disease trends in representative samples of non-institutionalized U.S. civilian residents.(21) The survey consists of a standardized in-home interview and a physical examination and blood and urine collection at a mobile examination center (MEC). Participants gave informed consent. The protocol was approved by the NCHS Research Ethics Review Board.
Our population of interest was U.S. adults aged 20 years or older who had a household income less than 400% of the federal poverty level [in order to exclude higher income persons unlikely to experience food insecurity (as in previous studies(13, 22))]. The MEC sample included 15,222 participants who were at least 20 years of age. Of these, 14,358 had food insecurity data available and were not pregnant. A total of 10,354 participants from this sample met our household income criteria. We excluded those with missing serum creatinine, urine albumin or urine creatinine (n=841) and those with eGFR≥15 ml/min/1.73m2 (n=387), resulting in a final analytic sample of 9,126 participants.
NHANES: Measurements and Definitions
Food insecurity was assessed using a 10-item questionnaire, and scored responses were released by the NHANES as 4 categories, which we regrouped into 3 categories and labeled as: no (0 affirmative responses), marginal (1–2), and high (3–10) food insecurity. We used only those items pertaining to the adults in the household. Questionnaire items included, “In the last 12 months…I couldn’t afford to eat balanced meals” and “Did you ever cut the size of your meals or skip meals because there wasn’t enough money for food”. Self-reported demographics (age, sex, race/ethnicity and marital status), socioeconomic status (education, insurance, and income), health behaviors (smoking) and health conditions (hypertension and diabetes mellitus) were obtained during the interview portions of the surveys. Income was assessed using the poverty income ratio, a ratio of household income to household poverty level(23). Height and weight, used to calculate body mass index (BMI), were measured on the MEC, and those with BMI>30 kg/m2 were classified as obese. Participants with self-reported diabetes or measured non-fasting plasma glucose ≥200 mg/dl were classified as having diabetes. Participants with a self-report of hypertension or measured average systolic blood pressure (SBP) ≥140 or diastolic blood pressure (DBP) ≥90 mmHg were classified as having hypertension(24). Serum creatinine was measured by the modified kinetic method of Jaffe using different analyzers in different survey years. Random spot urine samples were obtained, and urine albumin and creatinine were measured using frozen specimens. Urine albumin was measured using a solid-phase fluorescence immunoassay; urine creatinine was measured using the modified Jaffe kinetic method in the same laboratory.
CKD was defined using estimated glomerular filtration rate (eGFR) and the presence of albuminuria, based on Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines(25). Estimated GFR (eGFR) was calculated using the modified Modification of Diet in Renal Disease Study equation for calibrated creatinine.(26, 27) Calibration equations adhering to the Cleveland Clinic protocol were applied to serum creatinine values obtained in 2005–2006 and to urine creatinine values obtained in 2003–2004 and 2005–2006.(28) Albuminuria was considered present at urinary albumin-to-creatinine ratios ≥ 30mg/g. CKD was defined as eGFR <60 ml/min per 1.73m2 or albuminuria.
Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS): Design and Population
We examined cross-sectional data from the National Institute on Aging (NIA), HANDLS Study. HANDLS is a population-based cohort study of the influences and interaction of race and SES on the development of health disparities among minority and lower SES subgroups.(29) Participants are community-dwelling African Americans and whites age 30–64 years at enrollment, drawn from 13 neighborhoods, each composed of contiguous U.S. census tracts in Baltimore City, Maryland to reflect socioeconomic and racial diversity. HANDLS participants were sampled representatively using a factorial cross of 4 factors (age, sex, race and SES) with approximately equal numbers of participants in each “cell”. Enrollment was from 2004 to 2008. Each participant provided informed consent. The MedStar Research Institute Institutional Review Board approved the study protocol.
Although the total HANDLS population is 3,720, in the present study, we restricted our analysis to participants who (1) completed fasting laboratory testing (n=2,708), (2) had available data on food security (n=2,191), (3) had an annual household income less than $75,000 (400% of the poverty level for a family of 4)(30) (n=1923), (4) had available data on urinary albumin-tocreatinine (n=1,240) and (5) had an eGFR ≥15 ml/min per 1.73 m2 (final n=1,239).
HANDLS: Measurements and Definitions
Food insecurity was assessed using the NHANES 1999–2000 food insecurity questionnaire.(31) Because this early version of the questionnaire did not include a comparable scoring system to that of the more recent NHANES questionnaire, we defined food insecurity based upon an affirmative response to, “In the last 12 months, did you or your household ever cut the size of your meals or skip meals because there wasn’t enough money for food?” This question was included in both the NHANES and HANDLS questionnaires (Appendix A), and in 2011, 97% of US adults who had food insecurity with hunger answered this question affirmatively.(32) Demographic data including, age, sex, race, employment and educational status were assessed during an initial household survey. Poverty status was based on reported annual household income below or above 125% of the 2004 Department of Health and Human Services poverty guideline(30), and was determined at baseline in the HANDLS study to allow for selection of a representative sample(29). A mobile research vehicle (MRV) was the site of health care provider ascertained medical and social history (including smoking history) and physical examination. Fasting venous blood specimen and spot urine samples were collected on the MRV and analyzed at the NIA Clinical Research Branch Core Laboratory (Baltimore, MD) and Quest Diagnostics, Inc. (Baltimore, MD and Chantilly, VA). Hypertension was defined as an average of seated and standing systolic blood pressure ≥ 140 mmHg, an average of seated and standing diastolic blood pressure ≥ 90 mmHg,(24) a history of blood pressure medication use, and/or a self-report of hypertension. Diabetes mellitus was defined as a fasting plasma glucose concentration of ≥ 126 mg/dl (7.0 mmol/l)(33), or self-report of diabetes. Height and weight were measured and used to calculate BMI to determine the presence of obesity (BMI ≥30 kg/m2).
Serum creatinine was measured at Quest Diagnostics, Inc. by isotope dilution mass spectrometry (IDMS) (Olympus America Inc., Melville, NY) and standardized to the reference laboratory at the Cleveland Clinic. Urine microalbumin concentration was measured at Quest Diagnostics, Inc. using an immunoturbimetric assay (Kamiya Biomedical Co., Seattle, WA). CKD was defined as in NHANES.
Statistical Methods
To account for the complex design in NHANES, including weights, clusters and strata, all analyses for NHANES were performed using survey procedures. Six-year weights were calculated as the two-year MEC weight multiplied by 1/3. For both studies, participant characteristics stratified by food insecurity status were compared using descriptive statistics. In NHANES, for categorical variables, survey weighted percentages and standard errors were calculated and tested for study group differences using Rao-Scott chi-square tests. For continuous variables, weighted means and standard errors were calculated, and tested for study group differences using analysis of variance. In HANDLS, participant characteristics were compared using Fisher exact tests for categorical variables and t tests for continuous variables.
Multivariable logistic regression was used to calculate age-adjusted prevalence of CKD, stratified by food insecurity status, and to determine the magnitude and direction of the relation between food insecurity and CKD. In NHANES, we compared the high and marginal food insecurity groups to the no food insecurity group, and sequentially adjusted for age, sex, race, education, marital status, health insurance, poverty income ratio, smoking status, diabetes, hypertension, and obesity. In HANDLS, we compared the food insecurity to the no food insecurity group and adjusted for similar variables to NHANES (poverty status was used, as opposed to poverty income ratio). Selected confounders were related to either food insecurity or CKD in previous studies, including reports from NHANES(26) and HANDLS(34). Model-wise deletion was used to handle missing data in the HANDLS models (variables were missing at 3.6% or less).
Subgroup analyses by diabetes, hypertension and obesity status were performed to determine if food insecurity was differentially related to CKD across these groups. An interaction between food insecurity and each subgroup was considered in the aforementioned models.
In a sensitivity analysis, we used the CKD Epidemiology Collaboration (CKD-EPI) equation(35) to estimate GFR in our primary definition of CKD. Stata, version 11 (StataCorp, College Station, TX) was used for all analyses. In HANDLS, the possibility of confounding by neighborhood was controlled with fixed-effects modeling(36). A two-sided p <0.05 was used as the level of significance for all tests.
RESULTS
NHANES
Among U.S. adults aged 20 years and older and with a household income <400% of the poverty level in 2003–2008, 74% reported no food insecurity, 11% marginal food insecurity, and 15% high food insecurity. Those with high or marginal food insecurity were younger, less likely to be of Non-Hispanic White race/ethnicity, less likely to be insured and/or have completed at least a 12th grade education than were those with no food insecurity. Conversely, individuals with high or marginal food insecurity were more likely to be smokers, and have obesity and/or hypertension. Income level declined across food insecurity categories, with the high food insecurity group having the lowest mean poverty income ratio (Table 1).
Table 1.
Population Characteristics by Food Insecurity Status, NHANES 2003–2008
(U.S. adults age >=20 years with annual household income <400% of the federal poverty guideline)
| Characteristic | All | No Food Insecurity | Marginal Food Insecurity | High Food Insecurity | p-value |
|---|---|---|---|---|---|
| Total N (%) | 9126 | (74%) | (11%) | (15%) | -- |
| Age in years: mean (SE) | 46.6 (0.4) | 48.4 (0.5) | 41.6 (0.7) | 41.6 (0.5) | <0.001 |
| Male, % | 47 | 47 | 46 | 47 | 0.59 |
| Race/ethnicity, % | <0.001 | ||||
| Mexican American | 11 | 8 | 21 | 17 | |
| Non-Hispanic White | 65 | 71 | 46 | 49 | |
| Non-Hispanic Black | 13 | 11 | 19 | 18 | |
| * Poverty income ratio: mean (SE) | 2.0 (0.03) | 2.2 (0.03) | 1.6 (0.05) | 1.3 (0.04) | <0.001 |
| Insured, % | 74 | 79 | 61 | 59 | <0.001 |
| Education>= 12th grade, % | 74 | 78 | 65 | 60 | <0.001 |
| Current Tobacco use, % | 28 | 24 | 36 | 41 | <0.001 |
| Comorbid Conditions, % | |||||
| Hypertension | 39 | 41 | 34 | 36 | 0.006 |
| Diabetes | 9 | 9 | 9 | 10 | 0.292 |
| Obesity | 35 | 33 | 38 | 38 | 0.009 |
Abbreviations: SE, standard error.
Poverty income ratio is a ratio of household income to household poverty level (higher values indicate greater income).
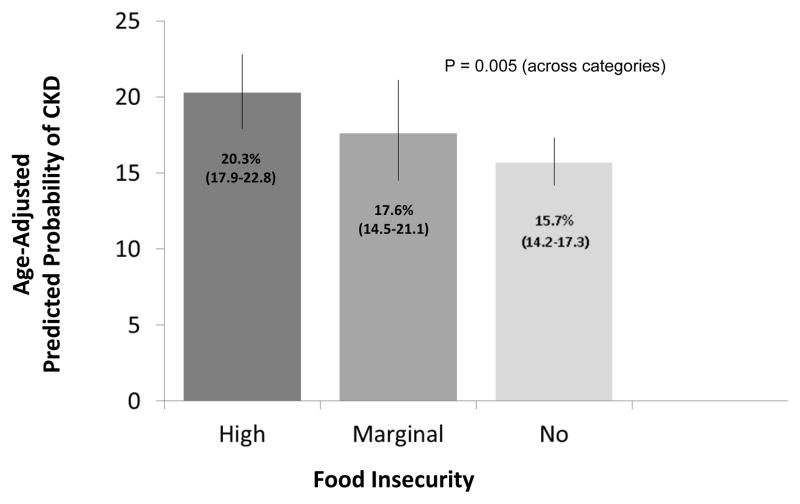
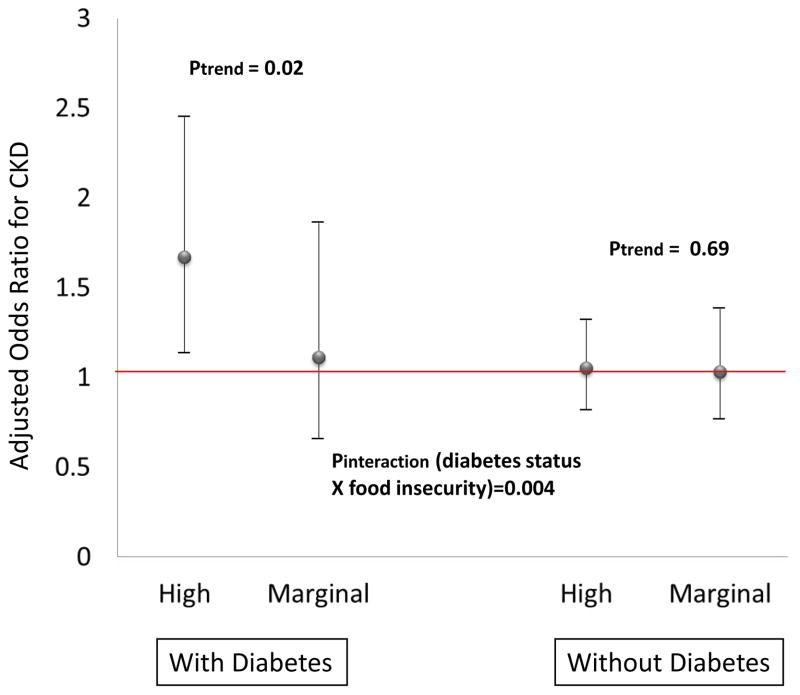
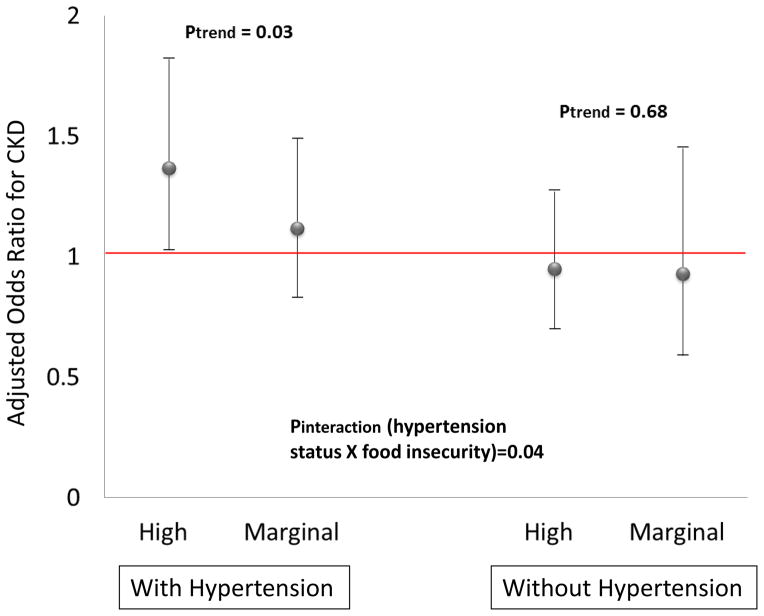
Age-adjusted prevalence of CKD was 20.3%, 17.6%, and 15.7% for the high, marginal and no food insecurity individuals, respectively (Figure 1). Logistic regression models including adjustment for age, sex and race revealed greater food insecurity to be associated with statistically significantly greater odds of CKD (Table 2). Upon further adjustment for sociodemographic factors and comorbid conditions, there was no significant association between food insecurity and CKD. Logistic regression models inclusive of adjustment for sociodemographic factors (age, race/ethnicity, sex, education, marital status, insurance, poverty income ratio, and smoking status) and stratified by diabetes, hypertension and obesity status revealed high food insecurity to be associated with greater odds of CKD only among the subgroups with either diabetes [odds ratio (OR) 1.67, 95% confidence interval (CI) 1.14–2.45] or hypertension (OR 1.37, 95% CI 1.03–1.82) (Figure 2).
Figure 1.
Age-Adjusted Prevalence of CKD by Food Insecurity Status, NHANES 2003–2008
(US adults age >=20 years with income <400% of the federal poverty guideline)
Table 2.
Relation of Food Insecurity Status to CKD, NHANES 2003–2008 from Logistic Regression Analyses
| Model | Adjusted for | Food Insecurity | Odds Ratio (95% Confidence Interval) for CKD | p-value for trend |
|---|---|---|---|---|
| 1 | age, sex, race | High | 1.35 (1.09–1.67) | 0.007 |
| Marginal | 1.14 (0.88–1.48) | |||
| No | 1.0 (reference) | |||
| 2 | + education, marital status, insurance, * poverty income ratio, smoking status | High | 1.24 (1.00–1.53) | 0.05 |
| Marginal | 1.09 (0.84–1.41) | |||
| No | 1.0 (reference) | |||
| 3 | +diabetes, hypertension, obesity | High | 1.06 (0.86–1.31) | 0.59 |
| Marginal | 1.00 (0.74–1.35) | |||
| No | 1.0 (reference) |
Poverty income ratio is a ratio of household income to household poverty level (higher values indicate greater income).
Figure 2.
(Panel a) Adjusted Odds Ratios for CKD Comparing High or Marginal Food Insecurity Groups to the No Food Insecurity Group (Reference), Among Lower Income Persons With or Without Diabetes, NHANES 2003–2008 [Adjusted for sociodemographic factors (age, race/ethnicity, sex, education, marital status, insurance, poverty income ratio, and smoking status)].
(Panel b) Adjusted Odds Ratios for CKD Comparing High or Marginal Food Insecurity Groups to the No Food Insecurity Group (Reference), Among Lower Income Persons With or Without Hypertension, NHANES 2003–2008 [Adjusted for sociodemographic factors (age, race/ethnicity, sex, education, marital status, insurance, poverty income ratio, and smoking status)].
HANDLS
There were 1,239 HANDLS participants included in our analysis. Among them, 27% reported food insecurity. The age range of participants was 30 to 64 years. Participants with food insecurity were younger, less likely to be male, employed or insured; and less likely to have completed at least a 12th grade education than were participants with no food insecurity. Conversely, participants with food insecurity were more likely to live in poverty or use tobacco. There were no differences in race or comorbid conditions across food insecurity groups in HANDLS (Table 3).
Table 3.
Participant Characteristics by Food Insecurity Status, HANDLS
| Characteristic | N for analysis | All | No Food Insecurity | Food Insecurity | p-value |
|---|---|---|---|---|---|
| Total N (%) | 1239 | 1240 | 902 (73) | 338 (27) | -- |
| Age in years: mean (SD) | 1239 | 47.8 (9.4) | 48.6 (9.4) | 45.9 (9.0) | <0.001 |
| Male | 1239 | 544 (44) | 417 (46) | 127 (38) | 0.007 |
| Black Race | 1239 | 633 (51) | 462 (51) | 171 (51) | 0.85 |
| * Poverty Status | 1239 | (412) 33 | 260 (29) | 152 (45) | <0.001 |
| Ever Married | 1192 | 723 (61) | 533 (62) | 190 (58) | 0.19 |
| Currently Employed | 1193 | 735 (62) | 574 (67) | 161 (49) | <0.001 |
| Education>= 12th grade | 1193 | 833 (70) | 628 (73) | 205 (62) | <0.001 |
| Insured | 1193 | 804 (67) | 618 (72) | 186 (56) | <0.001 |
| Current Tobacco Use | 1199 | 581 (48) | 394 (45) | 187 (57) | <0.001 |
| Comorbid Conditions | |||||
| Hypertension | 1237 | 550 (44) | 393 (440) | 157 (46) | 0.40 |
| Diabetes | 1236 | 272 (22) | 199 (22) | 73 (22) | 0.88 |
| Obesity | 1237 | 550 (44) | 393 (44) | 157 (46) | 0.40 |
Poverty status defined as annual household income below 125% of the 2004 Department of Health and Human Services poverty guideline (30).
The age-adjusted prevalence of CKD was 5.9% for the food insecurity and 4.6% for the no food insecurity participants (P= 0.33). A logistic regression model (model 1) including adjustment for age, sex and race revealed food insecurity to be associated with greater odds of CKD, that was not statistically significant [odds ratio (OR) 1.27, 95% confidence interval (CI) 0.84–1.92]. Upon further adjustment (model 2) for other sociodemographic factors (education, poverty status, insurance and marital status), there was a significant association between food insecurity and CKD (OR 1.54, 95% CI 1.10–2.16), largely influenced by the addition of insurance status to model 1. Insured participants were more likely to have CKD than uninsured individuals. Upon further adjustment for tobacco use and comorbid conditions (diabetes, hypertension and obesity), the association of food insecurity and CKD was attenuated to statistical non-significance (OR 1.46, 95% CI 0.98–2.18). Logistic regression models stratified by diabetes, hypertension and obesity status revealed no statistically significant effect modification; although there was a trend towards a greater association of food insecurity with CKD among participants with either hypertension or obesity (Appendix B).
Sensitivity Analysis
In NHANES, the age-adjusted prevalence of CKD by the CKD-EPI equation was 18.9%, 17.1%, and 14.2% for the high, marginal and no food insecurity participants, respectively (P<0.001), and logistic regression models yielded similar results to our primary analysis, as they did in HANDLS (data not shown).
DISCUSSION
Among both the general and an urban population of lower income U.S. adults, we found that approximately one-quarter of individuals were food insecure, and food insecurity was associated with CKD. The relation was nuanced, with food insecurity having an independent relation with greater prevalence of CKD only among individuals with diabetes or hypertension in the general population, but with no statistically significant, independent relation with CKD in an urban population. Our results persisted with adjustment for correlates of low SES, and were similar using both the MDRD study and CKD-EPI equations for GFR estimation.
To our knowledge, this is the first report describing the relationship between food insecurity and CKD. In developing countries, food insecurity often leads to undernutrition and frank starvation.(33, 36) However, in the U.S., it is correlated with overnutrition (severe overconsumption of energy).(37) Thus, individuals with food insecurity are more likely to be overweight or obese due to increased intake of energy-dense foods (ie. rich in fat and sugar) and limited intake of fruits, vegetables and fiber.(20, 38) Downstream from obesity, food insecure persons are at risk of type 2 diabetes(22) and have worse glycemic control than those with no food insecurity(39). This could potentially explain the differential relation between food insecurity and CKD we observed between diabetic and non-diabetic individuals in the general population. It is possible that food insecurity only plays an additive detrimental role in the development of CKD (beyond that conferred by low income status alone) in the setting conditions such as diabetes and hypertension.
Our observation of general consistency across clinical subgroups in the HANDLS study, in comparison to the effect modification noted above in NHANES, might reflect design differences across the two studies (i.e. differences in sample size; and in the age distribution of the 2 studies, which likely explains the lower prevalence of CKD among HANDLS participants); but could underscore variations in food availability and dietary practices in the general and urban populations. Food deserts (“areas in the US with limited access to affordable and nutritious food”) are common in Baltimore, MD(40, 41), where the HANDLS study is conducted. Moreover, ‘food swamps’ (geographic areas where the overabundance of high-energy foods inundate healthy food options(42)) are also common in metropolitan areas(40). Food insecure individuals in these areas may preferentially purchase such energy-dense foods because they are (1) often less expensive than healthier choices(43), (2) may be more palatable than healthy foods(44), and (3) may be easier to prepare in kitchens with inadequate appliances(12). These foods often contain sodium-based food additives, which in the US come primarily from processed breads, cereals, grains, meats, sauces and canned items(12) and account for 75% of total sodium intake(45). Excessive sodium intake is a risk factor for adverse CKD outcomes(46).
There were limitations of our study. First, as cross-sectional analyses, causality cannot be inferred, and reverse causality (CKD leading to food insecurity, e.g. from loss of employment due to disability) is possible. Second, we used two different approaches to measuring the presence of food insecurity given differences in data collection in our two study populations. Third, due to sample size considerations (especially in HANDLS) we lacked granularity in some covariates examined (e.g. health insurance status). Fourth, we did not account for differences in dietary patterns. Finally, we did not include a measure of food insecurity among children in the homes of participants (not assessed in HANDLS), which might influence adults’ approaches to managing food insecurity.(47)
Despite its limitations, our study findings could have important implications. The global prevalence of risk factors for CKD, including hypertension and diabetes, has been rising and poverty has been implicated as a contributor.(48) An estimated 1.2 billion persons worldwide live in extreme poverty (earning less than $1/day)(49), and over 900 million persons in the world are undernourished(50). While understudied, it is therefore conceivable that food insecurity could be a contributor to the worldwide prevalence of CKD, which is estimated to affect 10–16% of adults.(25, 51–55)
In the clinical setting, as providers aim to inform patients of their CKD risk profile and individually tailor their management plan, it may be important to assess potential barriers or competing priorities to following lifestyle recommendations. As such, a simple screening question regarding food insecurity (e.g. “have you had to skip meals because there wasn’t enough money?”) could allow identification of patients at increased risk for CKD and guide dietary recommendations. Longitudinal studies in this area are needed to more closely examine food insecurity, consequent dietary patterns and CKD. Further, tailored dietary interventions among food insecure individuals could serve to mitigate disparities in CKD.
In conclusion, food insecurity is associated with CKD, particularly among lower income individuals with either diabetes or hypertension. Thus, food insecurity may play a role in disparities in CKD, and its further study could present opportunities for intervention.
Acknowledgments
The Centers for Disease Control and Prevention (CDC) CKD Surveillance Team consists of members groups led by the University of California, San Francisco (Neil Powe [PI], Laura Plantinga, Chi-yuan Hsu, Kirsten Bibbins-Domingo, Charles McCulloch, Deidra Crews, Vanessa Grubbs, Delphine Tuot, Tanushree Banerjee, and Annie Rein-Weston), University of Michigan (Rajiv Saran [PI], Elizabeth Hedgeman, Brenda Gillespie, William Herman, Friedrich Port, Bruce Robinson, Vahakn Shahinian, Jerry Yee, Eric Young, William McClellan, Ann O’Hare, and Anca Tilea), and CDC (Desmond Williams [Technical Advisor], Nilka Ríos Burrows, Mark Eberhardt, Paul Eggers, Nicole Flowers, Linda Geiss, Susan Hailpern, Regina Jordan, Juanita Mondeshire, Bernice Moore, Gary Myers, Meda Pavkov, Deborah Rolka, Sharon Saydah, Anton Schoolwerth, Rodolfo Valdez, and Larry Waller).
We thank the participants and staff of the NHANES and HANDLS.
We acknowledge Laney Light, MS for her contributions to the statistical analyses.
Support:
This project was supported under a cooperative agreement from the CDC, grant 1U58DP003839-01. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
This work was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Dr. Crews and Dr. Grubbs were supported by the Harold Amos Medical Faculty Development Program of the Robert Wood Johnson Foundation. Dr. Grubbs was also supported by grant K23 DK093710 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Dr. Powe was partially supported by grant R01 DK78124 from the NIDDK.
Appendix A. Food Insecurity Questionnaire Items
NHANES
| Item Label (HH=Household) | Full Question |
|---|---|
| HH Worried run out of food | “…please tell me whether the statement was often true, sometimes true, or never true for {you/your household} in the last 12 months-- {I/we} worried whether {my/our} food would run out before {I/we} got money to buy more.” |
| HH Food didn’t last | “…please tell me whether the statement was often true, sometimes true, or never true for {you/your household} in the last 12 months-- The food that {I/we} bought just didn’t last, and {I/we} didn’t have money to get more.” |
| HH Couldn’t afford balanced meals | “…please tell me whether the statement was often true, sometimes true, or never true for {you/your household} in the last 12 months-- {I/we} couldn’t afford to eat balanced meals.” |
| HH Adults cut size or skip meals | “In the last 12 months, since last, did {you/you or other adults in your household} ever cut the size of your meals or skip meals because there wasn’t enough money for food?” |
| HH How often adults cut size/skip meals | “How often did this happen?” |
| HH Eat less than should | “In the last 12 months, did you ever eat less than you felt you should because there wasn’t enough money to buy food?” |
| HH Hungry, but didn’t eat | “[In the last 12 months], were you ever hungry but didn’t eat because you couldn’t afford enough food?” |
| HH Lost weight, no money for food | “[In the last 12 months], did you lose weight because you didn’t have enough money for food?” |
| HH Adults not eat whole day | “In the last 12 months], did {you/you or other adults in your household} ever not eat for a whole day because there wasn’t enough money for food?” |
| HH How often adults not eat for day | “How often did this happen? Would you say . . .” |
HANDLS
Which of these statements best describe the food eaten by you and your family in the last 12 months?
-
0
Alway have enough
-
1
Enough, not what I want
-
2
Sometimes not enough
-
3
Often not enough
-
7
DK
-
9
NA
I have transportation problems that prevent me from always having enough to eat.
-
0
No
-
1
Yes
-
7
DK
-
9
NA
I have transportation problems that prevent me from always having the kinds of food I want or need.
-
0
No
-
1
Yes
-
7
DK
-
9
NA
I do not have enough money, food stamps, or WIC vouchers to buy enough food to eat.
-
0
No
-
1
Yes
-
7
DK
-
9
NA
I do not have enough money, food stamps, or WIC vouchers to buy the kinds of food I want or need.
-
0
No
-
1
Yes
-
7
DK
-
9
NA
I do not have enough food to eat because I am on a diet.
-
0
No
-
1
Yes
-
7
DK
-
9
NA
I do not have the kinds of food I want or need because I am on a diet
-
0
No
-
1
Yes
-
7
DK
-
9
NA
I do not have enough food to eat because I do not have a working stove or refrigerator.
-
0
No
-
1
Yes
-
7
DK
-
9
NA
I do not have the kinds of food I want or need because I do not have a working stove or refrigerator.
-
0
No
-
1
Yes
-
7
DK
-
9
NA
I do not have enough food to eat because I have health problems that prevent me from cooking or eating.
-
0
No
-
1
Yes
-
7
DK
-
9
NA
I do not always have the kinds of food I want or need because I have health problems that prevent me from cooking or eating.
-
0
No
-
1
Yes
-
7
DK
-
9
NA
I do not have enough food to eat because the kinds of food I want are not available.
-
0
No
-
1
Yes
-
7
DK
-
9
NA
I do not always have the kinds of food I want or need because the kinds of food I want are not available.
-
0
No
-
1
Yes
-
7
DK
-
9
NA
I do not have enough food to eat because good quality food is not available.
-
0
No
-
1
Yes
-
7
DK
-
9
NA
I do not always have the kinds of food I want or need because good quality food is not available.
-
0
No
-
1
Yes
-
7
DK
-
9
NA
In the last 12 months, did you or your household ever cut the size of your meals or skip meals because there wasn’t enough money for food?
-
0
No
-
1
Yes
-
7
DK
-
9
NA
In the last 12 months, how often did you or your household cut the size of your meals or skip meals because there wasn’t enough money for food?
-
0
Almost every month
-
1
Some months, not every month
-
2
Only 1 or 2 months
-
7
DK
-
9
NA
Appendix B
Table B1.
Relation of Food Insecurity Status to CKD, HANDLS from Logistic Regression Analyses
| Model | Variables Included | Odds Ratio (95% Confidence Interval) for CKD comparing Food Insecurity to No Food Insecurity Groups |
|---|---|---|
| 1 | age, sex and race | 1.27 (0.84–1.92) |
| 2 | +education, * poverty status, insurance, marital status | 1.54 (1.10–2.16) |
| 3 | +tobacco use, diabetes, hypertension, obesity | 1.46 (0.98–2.18) |
Poverty status defined as annual household income below 125% of the 2004 Department of Health and Human Services poverty guideline
Table B2.
Adjusted Odd Ratios for CKD Comparing Food Insecurity and No Food Insecurity Groups among Chronic Condition Subgroups, HANDLS
[Adjusted for sociodemographic characteristics (age, sex, race, education, poverty status, insurance, marital status) and tobacco use]
| Subgroup | Food Insecurity | Odds Ratio for CKD (95% Confidence Interval) | Subgroup | Odds Ratio for CKD (95% Confidence Interval) | P interaction (chronic condition X food insecurity) |
|---|---|---|---|---|---|
| With Diabetes | Yes | 1.13 (0.50–2.55) | Without Diabetes | 1.88 (1.15–3.06) | 0.58 |
| No | reference | reference | |||
| With Hypertension | Yes | 1.69 (0.99–2.89) | Without Hypertension | 0.73 (0.30–1.76) | 0.28 |
| No | reference | reference | |||
| With Obesity | Yes | 2.54 (1.04–6.22) | Without Obesity | 0.89 (0.44–1.82) | 0.30 |
| No | reference | reference |
References
- 1.Crews DC, Charles RF, Evans MK, Zonderman AB, Powe NR. Poverty, race, and CKD in a racially and socioeconomically diverse urban population. Am J Kidney Dis. 2010;55:992–1000. doi: 10.1053/j.ajkd.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruce MA, Beech BM, Crook ED, Sims M, Wyatt SB, Flessner MF, Taylor HA, Williams DR, Akylbekova EL, Ikizler TA. Association of socioeconomic status and CKD among African Americans: the Jackson Heart Study. Am J Kidney Dis. 55:1001–1008. doi: 10.1053/j.ajkd.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martins D, Tareen N, Zadshir A, Pan D, Vargas R, Nissenson A, Norris K. The association of poverty with the prevalence of albuminuria: data from the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2006;47:965–971. doi: 10.1053/j.ajkd.2006.02.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McClellan WM, Newsome BB, McClure LA, Howard G, Volkova N, Audhya P, Warnock DG. Poverty and racial disparities in kidney disease: the REGARDS study. Am J Nephrol. 2010;32:38–46. doi: 10.1159/000313883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabanayagam C, Shankar A, Saw SM, Lim SC, Tai ES, Wong TY. Socioeconomic status and microalbuminuria in an Asian population. Nephrol Dial Transplant. 2009;24:123–129. doi: 10.1093/ndt/gfn447. [DOI] [PubMed] [Google Scholar]
- 6.Crews DC, McClellan WM, Shoham DA, Gao L, Warnock DG, Judd S, Muntner P, Miller ER, Powe NR. Low income and albuminuria among REGARDS (Reasons for Geographic and Racial Differences in Stroke) study participants. Am J Kidney Dis. 2012;60:779–786. doi: 10.1053/j.ajkd.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merkin SS, Diez Roux AV, Coresh J, Fried LF, Jackson SA, Powe NR. Individual and neighborhood socioeconomic status and progressive chronic kidney disease in an elderly population: The Cardiovascular Health Study. Soc Sci Med. 2007;65:809–821. doi: 10.1016/j.socscimed.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Volkova N, McClellan W, Klein M, Flanders D, Kleinbaum D, Soucie JM, Presley R. Neighborhood poverty and racial differences in ESRD incidence. J Am Soc Nephrol. 2008;19:356–364. doi: 10.1681/ASN.2006080934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarver-Carr ME, Powe NR, Eberhardt MS, LaVeist TA, Kington RS, Coresh J, Brancati FL. Excess risk of chronic kidney disease among African-American versus white subjects in the United States: a population-based study of potential explanatory factors. J Am Soc Nephrol. 2002;13:2363–2370. doi: 10.1097/01.asn.0000026493.18542.6a. [DOI] [PubMed] [Google Scholar]
- 10.Anderson SA. Core Indicators of Nutritional State for Difficult-to-Sample Populations. Journal of Nutrition. 1990;120:1559–1599. doi: 10.1093/jn/120.suppl_11.1555. [DOI] [PubMed] [Google Scholar]
- 11.Coleman-Jensen A, Nord M, Andrews A, Carlson S. Household Food Security in the United States in 2010. U.S. Department of Agriculture, Econimic Research Service; 2011. [Accessed 7/29/13]. Report-125. ( http://www.ers.usda.gov/Publications/err125) [Google Scholar]
- 12.Castillo DC, Ramsey NL, Yu SS, Ricks M, Courville AB, Sumner AE. Inconsistent Access to Food and Cardiometabolic Disease: The Effect of Food Insecurity. Current cardiovascular risk reports. 2012;6:245–250. doi: 10.1007/s12170-012-0236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. The Journal of nutrition. 2010;140:304–310. doi: 10.3945/jn.109.112573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crews DC, Pfaff T, Powe NR. Socioeconomic factors and racial disparities in kidney disease outcomes. Seminars in nephrology. 2013;33:468–475. doi: 10.1016/j.semnephrol.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Wesson DE. Endothelins and kidney acidification. Contributions to nephrology. 2011;172:84–93. doi: 10.1159/000328764. [DOI] [PubMed] [Google Scholar]
- 16.Scialla JJ, Anderson CA. Dietary Acid load: a novel nutritional target in chronic kidney disease? Adv Chronic Kidney Dis. 20:141–149. doi: 10.1053/j.ackd.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goraya N, Simoni J, Jo C, Wesson DE. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney international. 2012;81:86–93. doi: 10.1038/ki.2011.313. [DOI] [PubMed] [Google Scholar]
- 18.Goraya N, Simoni J, Jo CH, Wesson DE. A Comparison of Treating Metabolic Acidosis in CKD Stage 4 Hypertensive Kidney Disease with Fruits and Vegetables or Sodium Bicarbonate. Clinical journal of the American Society of Nephrology : CJASN. 2013;8:371–381. doi: 10.2215/CJN.02430312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin J, Fung TT, Hu FB, Curhan GC. Association of dietary patterns with albuminuria and kidney function decline in older white women: a subgroup analysis from the Nurses’ Health Study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;57:245–254. doi: 10.1053/j.ajkd.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popkin BM. Contemporary nutritional transition: determinants of diet and its impact on body composition. Proc Nutr Soc. 70:82–91. doi: 10.1017/S0029665110003903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Center for Health Statistics. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–1994. Series 1: Program and collection procedures. Vital Health Stat. 1994;32:1–407. [PubMed] [Google Scholar]
- 22.Seligman HK, Bindman AB, Vittinghoff E, Kanaya AM, Kushel MB. Food insecurity is associated with diabetes mellitus: results from the National Health Examination and Nutrition Examination Survey (NHANES) 1999–2002. Journal of general internal medicine. 2007;22:1018–1023. doi: 10.1007/s11606-007-0192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Center for Health Statistics, Centers for Disease Control and Prevention. Documentation, Codebook, Frequencies, and Related Documentation Frequencies. Hyattsville, MD: National Center for Health Statistics; 2009. [Accessed 11/1/2012]. National Health and Nutrition Examination Survey 1999–2000. ( http://www.cdc.gov/nchs/data/nhanes/frequency/demo.pdf.) [Google Scholar]
- 24.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 25.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 26.Levey A, Coresh J, Greene T, Marsh J, Stevens LA, Kusek J, et al. Expressing the MDRD study equation for estimating GFR with IDMS traceable (gold standard) serum creatinine values. J Am Soc Nephrol. 2005;16:69A. [Google Scholar]
- 27.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 28.Selvin E, Manzi J, Stevens LA, Van Lente F, Lacher DA, Levey AS, Coresh J. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50:918–926. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 29.Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, Zonderman AB. Healthy aging in neighborhoods of diversity across the life span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethnicity & disease. 2010;20:267–275. [PMC free article] [PubMed] [Google Scholar]
- 30.2004 Health and Human Services Poverty Guidelines. U.S. Department of Health and Human Services; 2012. [Accessed March 3, 2009]. Health and Human Services Poverty Guidelines. ( http://aspe.hhs.gov/poverty/04poverty.shtml.) [Google Scholar]
- 31.National Health and Nutrition Examination Survey 1999–2000 Data Documentation C, and Frequencies National Center for Health Statistics, Centers for Disease Control and Prevention. Data Documentation C, and Frequencies. Hyattsville, MD: National Center for Health Statistics; 2009. [Accessed 11/2/2013]. National Health and Nutrition Examination Survey 1999–2000. ( http://www.cdc.gov/nchs/data/nhanes/fq-fs.pdf) [Google Scholar]
- 32.Definition of food security. U.S. Department of Agriculture, Economic Research Service; 2012. [Accessed 3/15/2013]. ( http://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-theus/definitions-of-food-security.aspx.) [Google Scholar]
- 33.Pasricha SR, Biggs BA. Undernutrition among children in South and South-East Asia. J Paediatr Child Health. 46:497–503. doi: 10.1111/j.1440-1754.2010.01839.x. [DOI] [PubMed] [Google Scholar]
- 34.Crews DC, Charles RF, Evans MK, Zonderman AB, Powe NR. Poverty, Race, and CKD in a Racially and Socioeconomically Diverse Urban Population. American Journal of Kidney Diseases. 2010;55:992–1000. doi: 10.1053/j.ajkd.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanumihardjo SA, Anderson C, Kaufer-Horwitz M, Bode L, Emenaker NJ, Haqq AM, Satia JA, Silver HJ, Stadler DD. Poverty, obesity, and malnutrition: an international perspective recognizing the paradox. J Am Diet Assoc. 2007;107:1966–1972. doi: 10.1016/j.jada.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Sarlio-Lahteenkorva S, Lahelma E. Food insecurity is associated with past and present economic disadvantage and body mass index. J Nutr. 2001;131:2880–2884. doi: 10.1093/jn/131.11.2880. [DOI] [PubMed] [Google Scholar]
- 38.Shariff ZM, Khor GL. Obesity and household food insecurity: evidence from a sample of rural households in Malaysia. Eur J Clin Nutr. 2005;59:1049–1058. doi: 10.1038/sj.ejcn.1602210. [DOI] [PubMed] [Google Scholar]
- 39.Seligman HK, Jacobs EA, Lopez A, Tschann J, Fernandez A. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care. 35:233–238. doi: 10.2337/dc11-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casagrande SS, Franco M, Gittelsohn J, Zonderman AB, Evans MK, Fanelli Kuczmarski M, Gary-Webb TL. Healthy food availability and the association with BMI in Baltimore, Maryland. Public health nutrition. 2011;14:1001–1007. doi: 10.1017/S1368980010003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franco M, Diez Roux AV, Glass TA, Caballero B, Brancati FL. Neighborhood characteristics and availability of healthy foods in Baltimore. American journal of preventive medicine. 2008;35:561–567. doi: 10.1016/j.amepre.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richardson AS, Boone-Heinonen J, Popkin BM, Gordon-Larsen P. Are neighbourhood food resources distributed inequitably by income and race in the USA? Epidemiological findings across the urban spectrum. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2011-000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holben D. Position of the American Dietetic Association: Food Insecurity in the United States. Journal of the American Dietetic Association. 2010;110:1368–1377. doi: 10.1016/j.jada.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 44.Offer A, Pechey R, Ulijaszek S. Obesity under affluence varies by welfare regimes: the effect of fast food, insecurity, and inequality. Economics and human biology. 2010;8:297–308. doi: 10.1016/j.ehb.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 45.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Report of the Dietary Guidelines Advisory Committee on the dietary guidelines for Americans. 2010. [Google Scholar]
- 46.Gutierrez OM. Sodium- and phosphorus-based food additives: persistent but surmountable hurdles in the management of nutrition in chronic kidney disease. Advances in chronic kidney disease. 2013;20:150–156. doi: 10.1053/j.ackd.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castillo DC, Ramsey NL, Yu SS, Ricks M, Courville AB, Sumner AE. Inconsistent Access to Food and Cardiometabolic Disease: The Effect of Food Insecurity. Curr Cardiovasc Risk Rep. 6:245–250. doi: 10.1007/s12170-012-0236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hossain MP, Goyder EC, Rigby JE, El Nahas M. CKD and poverty: a growing global challenge. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009;53:166–174. doi: 10.1053/j.ajkd.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 49.The World Bank. Atlas of Global Development. Washington DC: Harper-Collins; 2007. [Google Scholar]
- 50.Food and Agriculture Organization of the United Nations. The state of food insecurity in the world 2010: Adressing food insecurity in protracted crises. 2010 http://www.fao.org/docrep/013/i1683e/i1683e.pdf.
- 51.Chadban SJ, Briganti EM, Kerr PG, Dunstan DW, Welborn TA, Zimmet PZ, Atkins RC. Prevalence of kidney damage in Australian adults: The AusDiab kidney study. Journal of the American Society of Nephrology : JASN. 2003;14:S131–138. doi: 10.1097/01.asn.0000070152.11927.4a. [DOI] [PubMed] [Google Scholar]
- 52.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 53.Hallan SI, Coresh J, Astor BC, Asberg A, Powe NR, Romundstad S, Hallan HA, Lydersen S, Holmen J. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. Journal of the American Society of Nephrology : JASN. 2006;17:2275–2284. doi: 10.1681/ASN.2005121273. [DOI] [PubMed] [Google Scholar]
- 54.Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, Chiang PH, Hsu CC, Sung PK, Hsu YH, Wen SF. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371:2173–2182. doi: 10.1016/S0140-6736(08)60952-6. [DOI] [PubMed] [Google Scholar]
- 55.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney international. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]