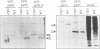Abstract
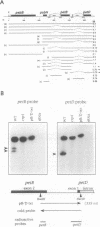
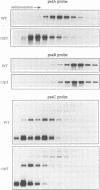
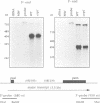
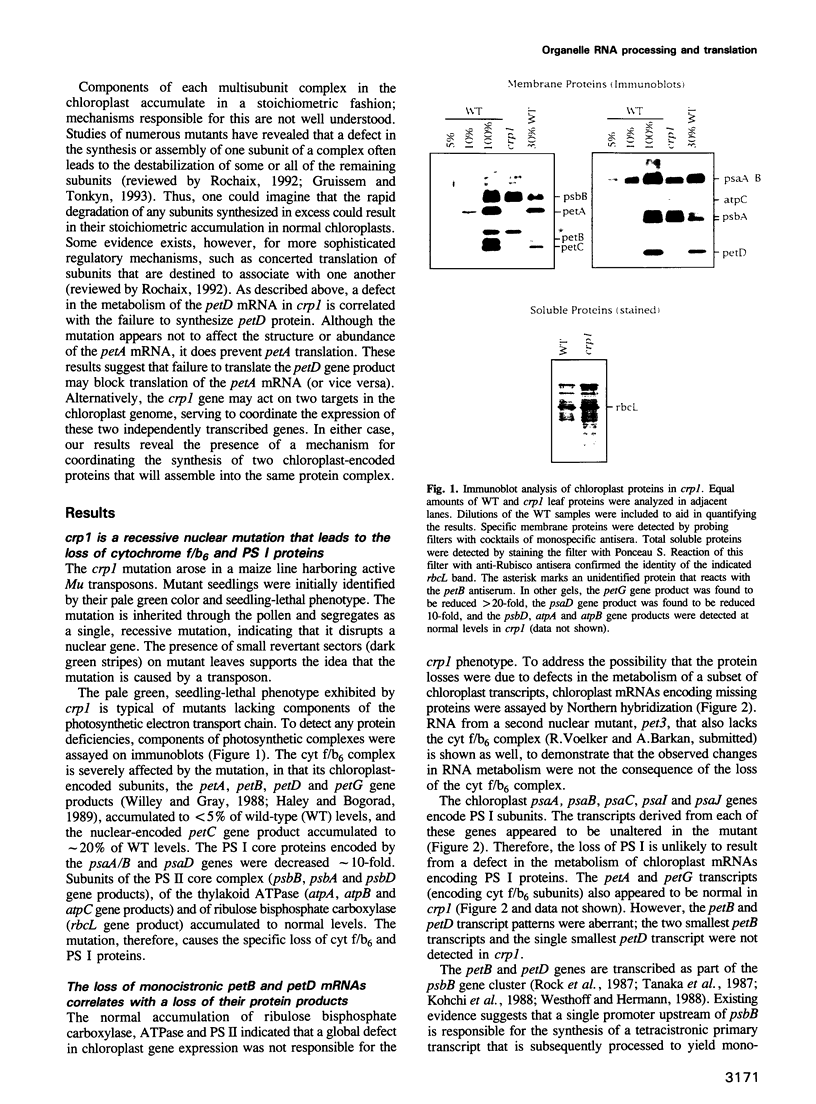
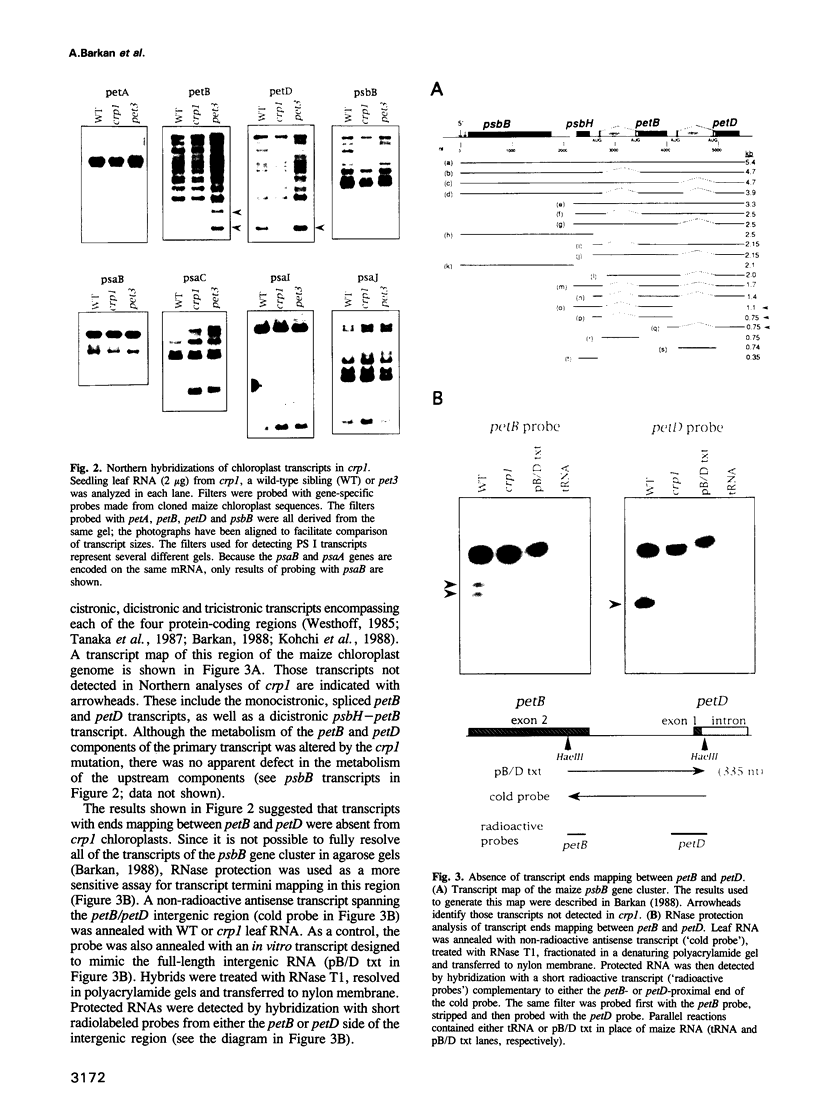
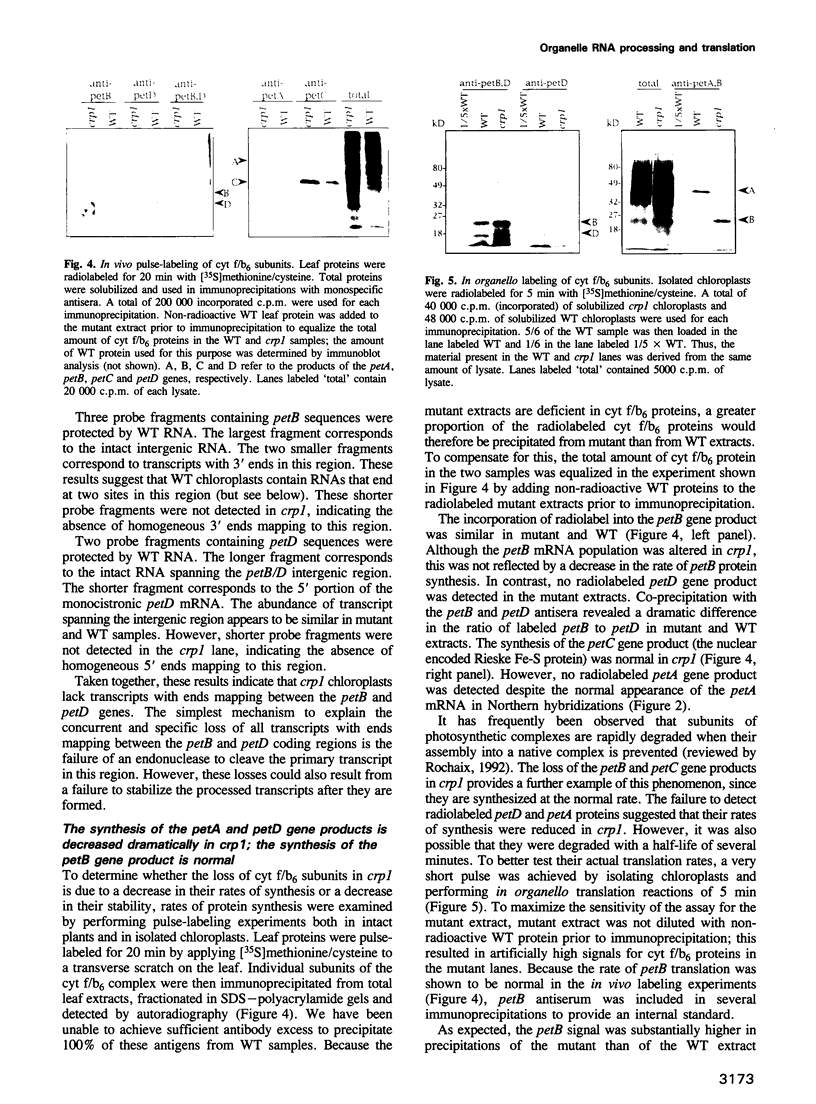
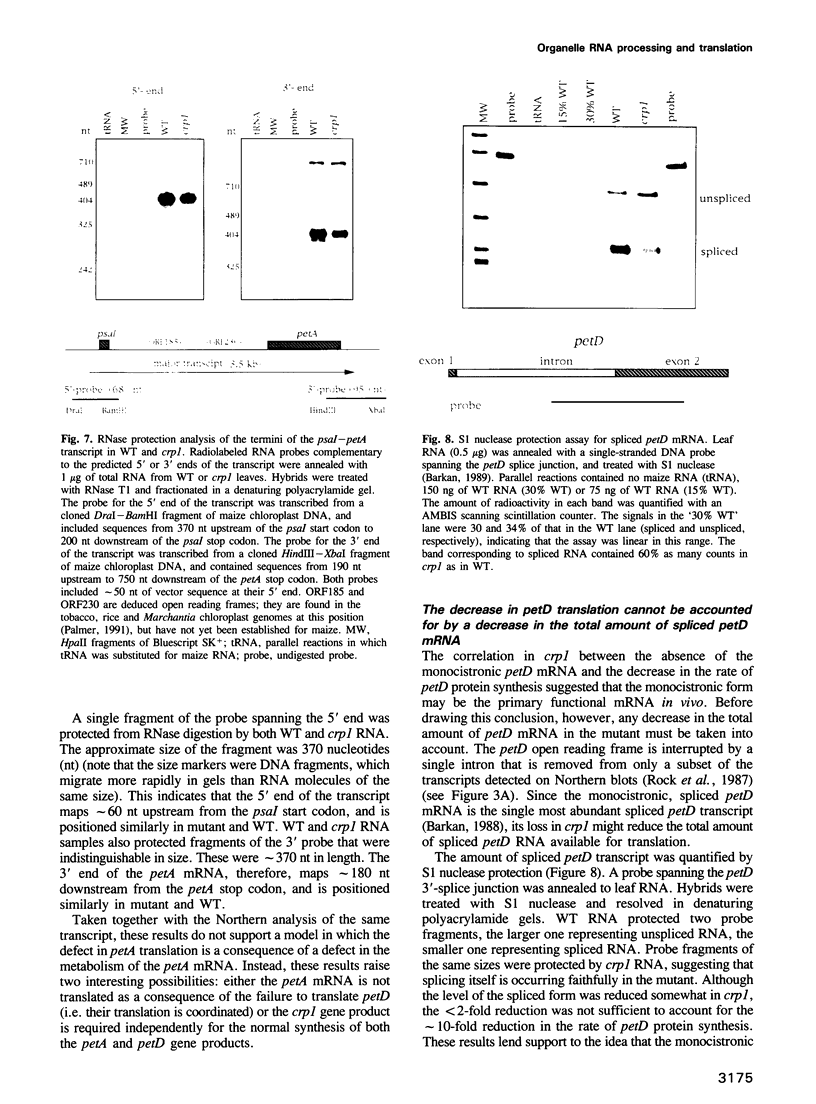
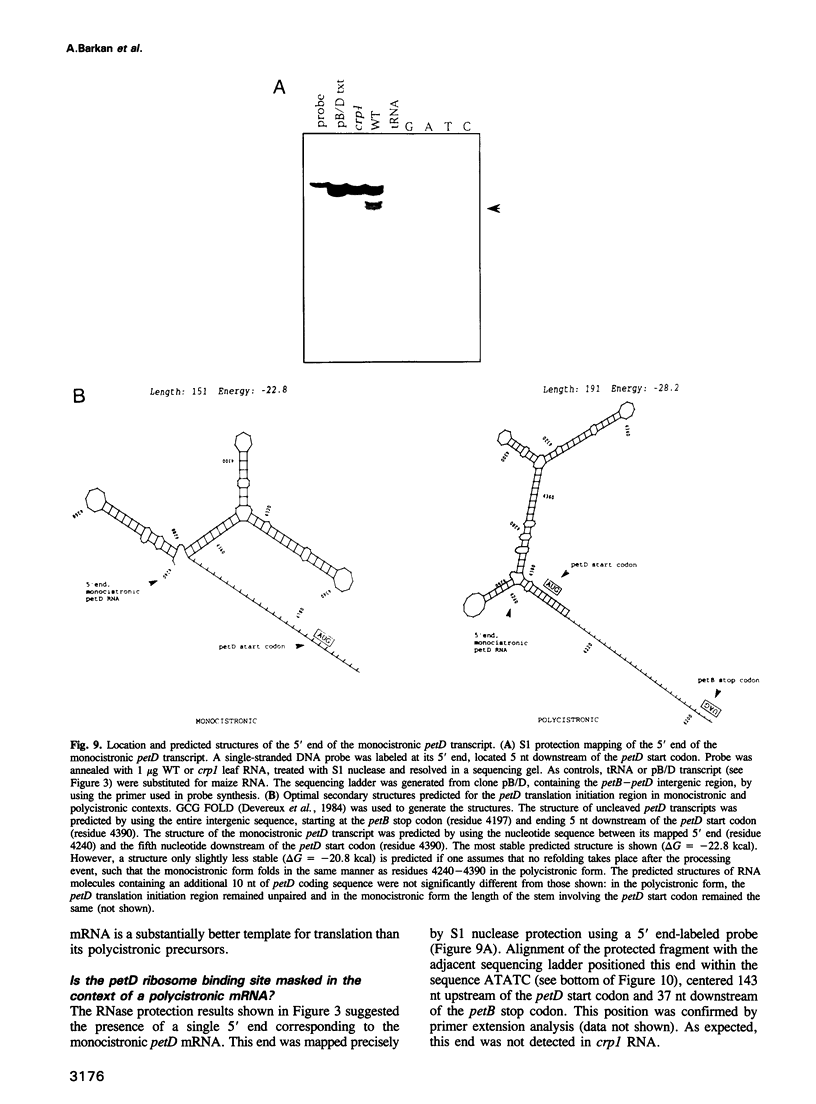
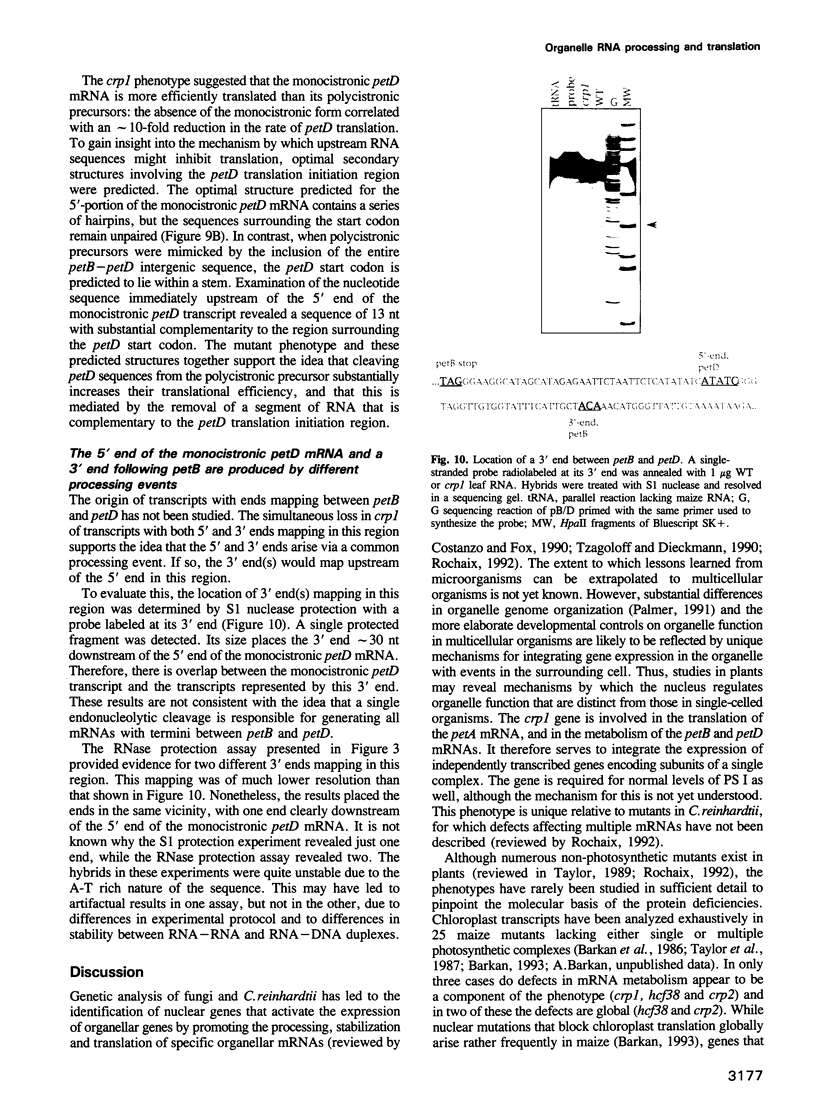
A mutant designated crp1 (chloroplast RNA processing 1) was identified in a screen for transposon-induced maize mutants with defects in chloroplast gene expression. crp1 is a recessive, nuclear mutation that causes the loss of the cytochrome f/b6 complex and a reduction in photosystem I. The molecular basis for these protein losses is unique relative to previously described mutants with defects in organelle gene expression; it involves defects in the metabolism of two organellar mRNAs and in the translation of two organellar proteins. Mutants lack the monocistronic forms of the petB and petD mRNAs (encoding cytochrome f/b6 subunits), but contain normal levels of their polycistronic precursors. Pulse-labeling experiments revealed normal synthesis of the petB gene product, but a large decrease in synthesis of the petD gene product. These results suggest that petD sequences are more efficiently translated in a monocistronic than in a polycistronic context, thereby providing evidence that the elaborate RNA processing typical of chloroplast transcripts can play a role in controlling gene expression. Structural predictions suggest that the petD start codon lies in a stable hairpin in the polycistronic RNA, but remains unpaired in the monocistronic transcript. Thus, processing to a monocistronic form may increase translational efficiency by releasing the translation initiation region from inhibitory interactions with upstream RNA sequences. Synthesis of a third cytochrome f/b6 subunit, encoded by the petA gene, was undetectable in crp1, although its mRNA appeared unaltered. Two mechanisms are consistent with the simultaneous loss of both petA and petD protein synthesis: the translation of the petA and petD mRNAs might be coupled via a mechanism independent of crp1, or the crp1 gene may function to coordinate the expression of the two genes, which encode subunits of the same complex.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barkan A., Miles D., Taylor W. C. Chloroplast gene expression in nuclear, photosynthetic mutants of maize. EMBO J. 1986 Jul;5(7):1421–1427. doi: 10.1002/j.1460-2075.1986.tb04378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A. Nuclear Mutants of Maize with Defects in Chloroplast Polysome Assembly Have Altered Chloroplast RNA Metabolism. Plant Cell. 1993 Apr;5(4):389–402. doi: 10.1105/tpc.5.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A. Proteins encoded by a complex chloroplast transcription unit are each translated from both monocistronic and polycistronic mRNAs. EMBO J. 1988 Sep;7(9):2637–2644. doi: 10.1002/j.1460-2075.1988.tb03116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A. Tissue-dependent plastid RNA splicing in maize: transcripts from four plastid genes are predominantly unspliced in leaf meristems and roots. Plant Cell. 1989 Apr;1(4):437–445. doi: 10.1105/tpc.1.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner B. J., Rapp J. C., Mullet J. E. Plastid Genes Encoding the Transcription/Translation Apparatus Are Differentially Transcribed Early in Barley (Hordeum vulgare) Chloroplast Development (Evidence for Selective Stabilization of psbA mRNA). Plant Physiol. 1993 Mar;101(3):781–791. doi: 10.1104/pp.101.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner B. J., Rapp J. C., Mullet J. E. Plastid transcription activity and DNA copy number increase early in barley chloroplast development. Plant Physiol. 1989 Mar;89(3):1011–1018. doi: 10.1104/pp.89.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J. O., Breiding D. E., Klessig D. F. Light-mediated control of translational initiation of ribulose-1, 5-bisphosphate carboxylase in amaranth cotyledons. Plant Cell. 1990 Aug;2(8):795–803. doi: 10.1105/tpc.2.8.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock R., Hagemann R., Kössel H., Kudla J. Tissue- and stage-specific modulation of RNA editing of the psbF and psbL transcript from spinach plastids--a new regulatory mechanism? Mol Gen Genet. 1993 Aug;240(2):238–244. doi: 10.1007/BF00277062. [DOI] [PubMed] [Google Scholar]
- Christopher D. A., Kim M., Mullet J. E. A novel light-regulated promoter is conserved in cereal and dicot chloroplasts. Plant Cell. 1992 Jul;4(7):785–798. doi: 10.1105/tpc.4.7.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M. C., Fox T. D. Control of mitochondrial gene expression in Saccharomyces cerevisiae. Annu Rev Genet. 1990;24:91–113. doi: 10.1146/annurev.ge.24.120190.000515. [DOI] [PubMed] [Google Scholar]
- Deng X. W., Gruissem W. Constitutive transcription and regulation of gene expression in non-photosynthetic plastids of higher plants. EMBO J. 1988 Nov;7(11):3301–3308. doi: 10.1002/j.1460-2075.1988.tb03200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyer R., Hoch B., Neckermann K., Maier R. M., Kössel H. RNA editing in maize chloroplasts is a processing step independent of splicing and cleavage to monocistronic mRNAs. Plant J. 1993 Oct;4(4):621–629. doi: 10.1046/j.1365-313x.1993.04040621.x. [DOI] [PubMed] [Google Scholar]
- Gamble P. E., Mullet J. E. Translation and stability of proteins encoded by the plastid psbA and psbB genes are regulated by a nuclear gene during light-induced chloroplast development in barley. J Biol Chem. 1989 May 5;264(13):7236–7243. [PubMed] [Google Scholar]
- Haley J., Bogorad L. A 4-kDa maize chloroplast polypeptide associated with the cytochrome b6-f complex: subunit 5, encoded by the chloroplast petE gene. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1534–1538. doi: 10.1073/pnas.86.5.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno A., Hirai A. A transcription map of the chloroplast genome from rice (Oryza sativa). Curr Genet. 1993 Feb;23(2):166–174. doi: 10.1007/BF00352017. [DOI] [PubMed] [Google Scholar]
- Kim M., Christopher D. A., Mullet J. E. Direct evidence for selective modulation of psbA, rpoA, rbcL and 16S RNA stability during barley chloroplast development. Plant Mol Biol. 1993 Jun;22(3):447–463. doi: 10.1007/BF00015975. [DOI] [PubMed] [Google Scholar]
- Klaff P., Gruissem W. Changes in Chloroplast mRNA Stability during Leaf Development. Plant Cell. 1991 May;3(5):517–529. doi: 10.1105/tpc.3.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohchi T., Yoshida T., Komano T., Ohyama K. Divergent mRNA transcription in the chloroplast psbB operon. EMBO J. 1988 Apr;7(4):885–891. doi: 10.1002/j.1460-2075.1988.tb02892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuras R., Wollman F. A. The assembly of cytochrome b6/f complexes: an approach using genetic transformation of the green alga Chlamydomonas reinhardtii. EMBO J. 1994 Mar 1;13(5):1019–1027. doi: 10.1002/j.1460-2075.1994.tb06350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mullet J. E. Dynamic regulation of chloroplast transcription. Plant Physiol. 1993 Oct;103(2):309–313. doi: 10.1104/pp.103.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochaix J. D. Post-transcriptional steps in the expression of chloroplast genes. Annu Rev Cell Biol. 1992;8:1–28. doi: 10.1146/annurev.cb.08.110192.000245. [DOI] [PubMed] [Google Scholar]
- Rock C. D., Barkan A., Taylor W. C. The maize plastid psbB-psbF-petB-petD gene cluster: spliced and unspliced petB and petD RNAs encode alternative products. Curr Genet. 1987;12(1):69–77. doi: 10.1007/BF00420729. [DOI] [PubMed] [Google Scholar]
- Saito H., Richardson C. C. Processing of mRNA by ribonuclease III regulates expression of gene 1.2 of bacteriophage T7. Cell. 1981 Dec;27(3 Pt 2):533–542. doi: 10.1016/0092-8674(81)90395-0. [DOI] [PubMed] [Google Scholar]
- Sexton T. B., Christopher D. A., Mullet J. E. Light-induced switch in barley psbD-psbC promoter utilization: a novel mechanism regulating chloroplast gene expression. EMBO J. 1990 Dec;9(13):4485–4494. doi: 10.1002/j.1460-2075.1990.tb07899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Obokata J, Chunwongse J, Shinozaki K, Sugiura M. Rapid splicing and stepwise processing of a transcript from the psbB operon in tobacco chloroplasts: determination of the intron sites in petB and petD. Mol Gen Genet. 1987 Oct;209(3):427–431. doi: 10.1007/BF00331145. [DOI] [PubMed] [Google Scholar]
- Taylor W. C., Barkan A., Martienssen R. A. Use of nuclear mutants in the analysis of chloroplast development. Dev Genet. 1987;8(5-6):305–320. doi: 10.1002/dvg.1020080503. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Dieckmann C. L. PET genes of Saccharomyces cerevisiae. Microbiol Rev. 1990 Sep;54(3):211–225. doi: 10.1128/mr.54.3.211-225.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencik M. L., McEwen J. E. Genetic evidence that different functional domains of the PET54 gene product facilitate expression of the mitochondrial genes COX1 and COX3 in Saccharomyces cerevisiae. Mol Cell Biol. 1991 May;11(5):2399–2405. doi: 10.1128/mcb.11.5.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff P., Herrmann R. G. Complex RNA maturation in chloroplasts. The psbB operon from spinach. Eur J Biochem. 1988 Feb 1;171(3):551–564. doi: 10.1111/j.1432-1033.1988.tb13824.x. [DOI] [PubMed] [Google Scholar]