ABSTRACT
The 2009 pandemic H1N1 virus (pH1N1) was derived through reassortment of North American triple reassortant and Eurasian avian-like swine influenza viruses (SIVs). To date, when, how and where the pH1N1 arose is not understood. To investigate viral reassortment, we coinfected cell cultures and a group of pigs with or without preexisting immunity with a Eurasian H1N1 virus, A/Swine/Spain/53207/2004 (SP04), and a North American triple reassortant H1N1 virus, A/Swine/Kansas/77778/2007 (KS07). The infected pigs were cohoused with one or two groups of contact animals to investigate viral transmission. In coinfected MDCK or PK15 continuous cell lines with KS07 and SP04 viruses, more than 20 different reassortant viruses were found. In pigs without or with preexisting immunity (immunized with commercial inactivated swine influenza vaccines) and coinfected with both viruses, six or seven reassortant viruses, as well as the parental viruses, were identified in bronchoalveolar lavage fluid samples from the lungs. Interestingly, only one or two viruses transmitted to and were detected in contact animals. No reassortant containing a gene constellation similar to that of pH1N1 virus was found in either coinfected cells or pigs, indicating that the reassortment event that resulted in the generation of this virus is a rare event that likely involved specific viral strains and/or a favorable, not-yet-understood environment.
IMPORTANCE
The 2009 pandemic-like H1N1 virus could not be reproduced either in cell cultures or in pigs coinfected with North American triple reassortant H1N1 and Eurasian H1N1 swine influenza viruses. This finding suggests that the generation of the 2009 pandemic H1N1 virus by reassortment was a rare event that likely involved specific viral strains and unknown factors. Different reassortant viruses were detected in coinfected pigs with and without preexisting immunity, indicating that host immunity plays a relevant role in driving viral reassortment of influenza A virus.
INTRODUCTION
Influenza A virus (IAV) is an important zoonotic pathogen that poses a severe threat to animal and human health. According to a report from the World Health Organization, annual IAV epidemics result in 250 to 500 million human infections, which cause 250,000 to 500,000 fatalities worldwide (1). IAVs belong to the family of Orthomyxoviridae, whose members have negative-, single-stranded RNA genomes and evolve very rapidly via antigenic drift and antigenic shift. According to the antigenic differences in two surface proteins, hemagglutinin (HA) and neuraminidase (NA), IAVs are divided into different subtypes. To date, 18 HA and 11 NA subtypes have been identified; both H17N10 and H18N11 subtypes were detected in bats, and the other subtypes have been found in waterfowl and shore birds (2–4). The segmented nature of the influenza A genome allows for reassortment when 2 or more viruses infect the same cell at the same time, resulting in novel genotypes of influenza viruses that might have the potential to cause epidemics and/or pandemics (5, 6).
In the 20th century, 3 influenza pandemics (1918 Spanish flu, 1957 Asian flu, and 1968 Hong Kong flu) caused millions of human deaths (7). One common feature of these pandemics is the emergence of a novel, antigenically HA subtype influenza virus associated with efficient transmission among humans, resulting in greater morbidity and mortality (8, 9). In contrast, the 2009 influenza pandemic was caused by a novel, reassortant H1N1 virus. This reassortant contained NA and M gene segments derived from Eurasian avian-like and the 6 remaining gene segments from North American triple reassortant swine influenza viruses (SIVs), and it was never detected prior to the human pandemic (10, 11). The 2009 pandemic H1N1 virus (pH1N1) most likely originated from swine, according to phylogenetic analysis (10–12), and once in humans, it jumped back to swine, where it has continued to reassort with other SIVs (13–18).
Although increased surveillance and research on swine influenza have been conducted worldwide after the 2009 pandemic, to date, no swine influenza surveillance data have been available in Mexico, where the pandemic virus is presumed to have emerged. Since the progenitor virus of 2009 pandemic H1N1 virus was not detected in pigs or other species (11, 19, 20), it remains unclear when and where this virus was generated before the human pandemic. In this study, we investigated whether coinfection with two representative SIVs, a Eurasian avian-like A/Swine/Spain/53207/2004 (SP04) and a North American triple reassortant H1N1 A/Swine/Kansas/77778/2007 (KS07), would result in the generation of reassortant viruses containing a pH1N1-like genotype. Different reassortant viruses were detected in both coinfected continuous cell lines and pigs, but no 2009 pH1N1-like (NA and M genes from the Eurasian SP04 and the 6 remaining genes from the North American triple reassortant KS07) viruses were identified.
RESULTS
Genotypic analysis of KS07 and SP04 parental viruses.
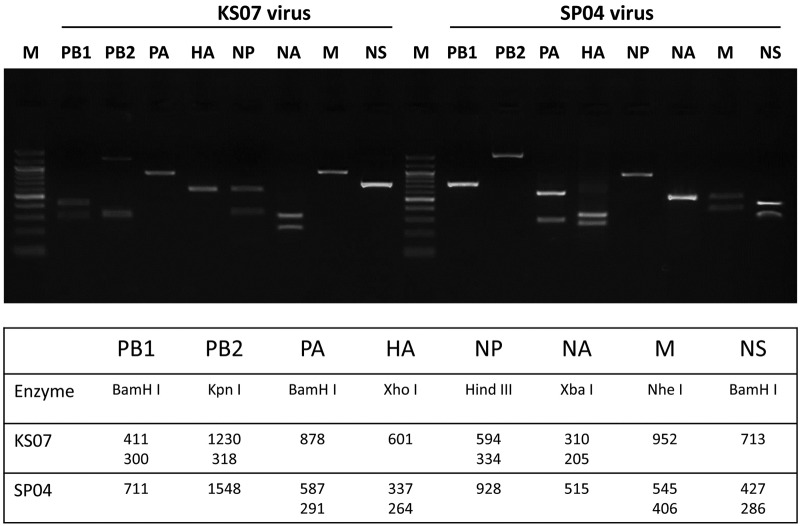
The KS07 and the SP04 virus were selected for the in vitro and in vivo infection studies because (i) they are representative North American triple reassortant and Eurasian avian-like H1N1 SIVs that were circulating in North American and European swine herds prior to the 2009 pandemic, (ii) the genetic information for both viruses is well known (21, 22), and (iii) we found that both KS07 and SP04 virus can infect and replicate efficiently in pigs and cause severe lung lesions—both viruses displayed similar virus lung replication and virulence (see Fig. S1 in the supplemental material). To investigate reassortment in cells or in pigs coinfected with KS07 and SP04 viruses, we used restriction enzyme digestion to identify each viral segment of individual viruses isolated from coinfected cells or pigs. Based on the sequences of the parental virus genes, a unique enzyme site was chosen to differentiate each segment of the SP04 and KS07 viruses from each other. The lengths of reverse transcription (RT)-PCR products and the unique enzyme for each segment of both viruses are shown in Fig. 1. The results showed that the SP04 virus could be easily differentiated from the KS07 virus by restriction enzyme digestion (Fig. 1).
FIG 1 .
Identification of origin of genes from KS07 and SP04 H1N1 viruses. The enzymes used and size of each gene after enzyme digestion are shown at the bottom.
Reassortants generated in MDCK and PK15 continuous cell lines coinfected with KS07 and SP04 viruses.
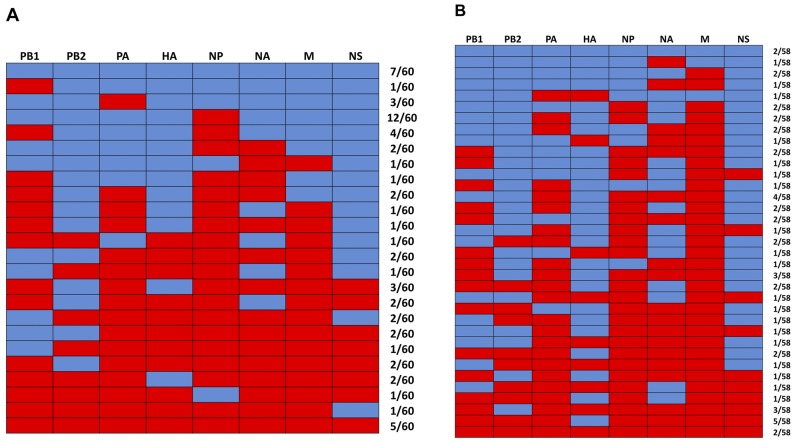
To explore reassortment between KS07 and SP04 viruses in vitro, MDCK and PK15 continuous cell lines were coinfected with both viruses at a ratio of 1:1 at a multiplicity of infection (MOI) of 1. Around 60 viruses from each coinfected continuous cell line were randomly selected and plaque purified twice. Each clonal virus population was amplified in MDCK cells, and RT-PCR was performed to amplify each gene segment (primers are available upon request). Each amplified gene segment was digested by a specific restriction enzyme, as shown in Fig. 1, to determine its origin. Different reassortants, as well as both parental viruses, were detected from coinfected MDCK cells (Fig. 2A). The majority of viruses found in coinfected MDCK cells had a genotype corresponding to a reassortant SP04 virus carrying the KS07 NP gene (SP04+KS07NP [20%, 12/60]) and to both parental KS07 (8.33%, 5/60) and SP04 (11.67%, 7/60) viruses, but no pH1N1-like (KS07+SP04NAM) virus was detected among the approximately 60 individual viral isolates in coinfected MDCK cells. Similarly, different reassortants and both parental viruses were also detected but no pH1N1-like (KS07+SP04NAM) virus was found in coinfected PK15 cells. Interestingly, the predominant virus isolated from coinfected PK15 cells was a reassortant KS07 virus with the SP04 HA gene (KS07+SP04HA [8.62%, 5/58]), and both parental viruses were only 3.44% (2/58) of the total number of viruses analyzed (Fig. 2B).
FIG 2 .
Reassortant viruses detected in continuous cell lines coinfected with KS07 and SP04 viruses. (A) Viruses found in coinfected MDCK cells. (B) Viruses found in coinfected PK15 cells. The origin of each of the eight gene segments (shown at the top of each panel) was determined; the segments from the SP04 virus are shown in blue, and those from the KS07 virus are in red. The numbers to the right of each panel show the number of viruses with the genotype/number of viruses analyzed.
Viral reassortment and transmission in coinfected pigs without preexisting immunity.
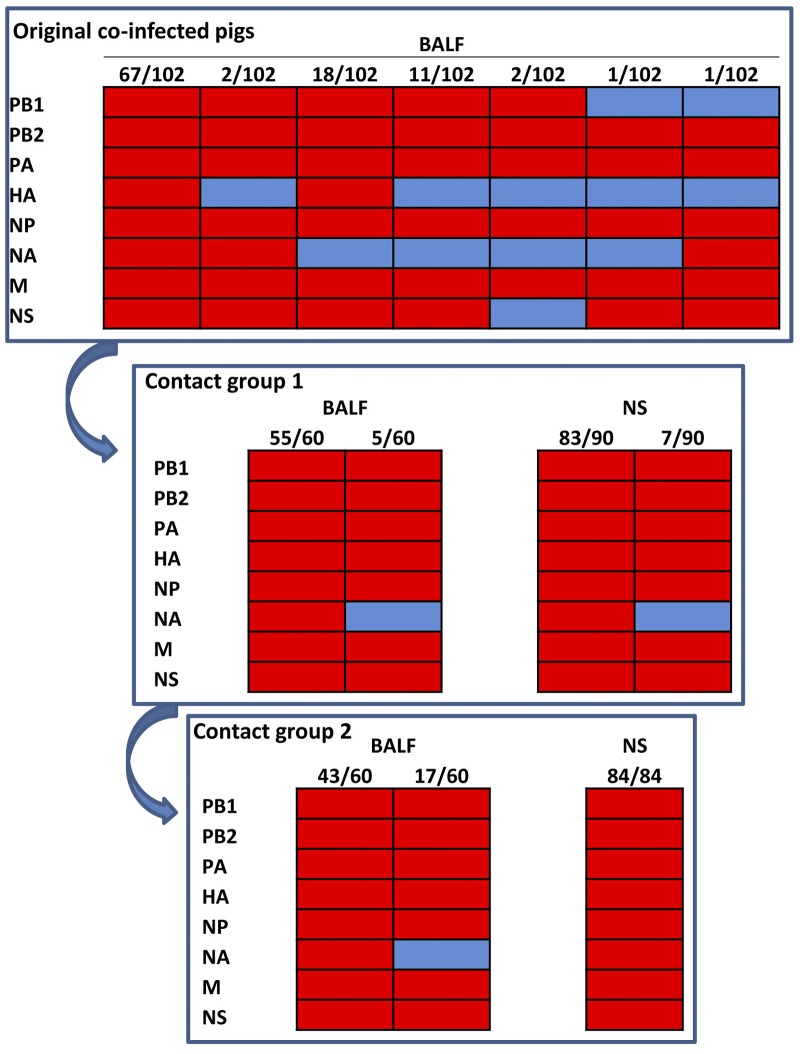
No acute respiratory clinical signs were observed in coinfected pigs with the Eurasian avian-like SP04 and North American triple-reassortant KS07 viruses. Necropsy revealed extensive severe macroscopic lung lesions (plum-colored, consolidated areas) in all inoculated pigs at 3, 5, and 7 days postinfection (d.p.i.) (Table 1). Viruses were isolated from each bronchoalveolar lavage fluid (BALF) sample collected from the six pigs that underwent necropsy at 3 or 5 d.p.i.; no virus was isolated from the three pigs necropsied at 7 d.p.i. After plaque purification was performed three times, the whole genomes of 102 single viruses (about 20 viruses per pig) were characterized by RT-PCR, enzyme digestion, and sequencing. Interestingly, only the parental KS07 virus was identified from the BALF samples from 3 pigs with primary coinfection collected at 3 d.p.i., and 6 different KS07 reassortants with 1 to 3 genes from the SP04 virus, as well as the parental KS07 virus, were found in each of 3 coinfected animals that underwent necropsy at 5 d.p.i. (Fig. 3). This result indicated that the KS07 virus seems to replicate more efficiently than the SP04 and reassortant viruses in coinfected naïve animals at early time points despite similar levels of lung replication and virulence of both parental viruses in pigs (see Fig. S1 in the supplemental material). The Eurasian SP04 NA gene was found in 4 of 6 reassortant viruses, but no Eurasian SP04 matrix gene was detected in the reassortant viruses. Significantly smaller numbers of different reassortants were found in coinfected pigs than in coinfected cells. The hemagglutination inhibition (HI) data showed that 3 pigs seroconverted for both KS07 and SP04 virus at 7 d.p.i., but the titer against the KS07 virus was 8-fold higher than that against the SP04 virus (Table 1).
TABLE 1 .
Lung lesion scores and HI titers of pigs coinfected with SP04 and KS07 viruses and of contact animals
| Group and d.p.i. or d.p.c. |
Mean lung lesion score (%) ± SEMa (no. of pigs with lesions/total no. of pigs) |
Geometric mean HI titerb against: |
|
|---|---|---|---|
| KS07 | SP04 | ||
| Coinfected | |||
| 3 | 38.57 ± 10.99 (3/3) | ND | ND |
| 5 | 24.00 ± 6.51 (3/3) | ND | ND |
| 7 | 34.69 ± 8.48 (3/3) | 403 | 50 |
| Contact 1 | |||
| 4 | 22.00 ± 5.71 (2/2) | ND | ND |
| 6 | 24.62 ± 2.36 (2/2) | ND | ND |
| 8 | 35.00 ± 15.00 (2/2) | 10 | <10 |
| Contact 2 | |||
| 4 | 16.00 ± 4.00 (2/2) | ND | ND |
| 6 | 40.29 ± 3.86 (2/2) | ND | ND |
| 8 | 14.57 ± 3.57 (2/2) | 10 | <10 |
Mean value of the percentage of gross lesions of 7 pulmonary lobes per pig. SEM, standard error of the mean.
ND, not determined.
FIG 3 .
Viruses detected in BALF and nasal swab (NS) samples from pigs coinfected with KS07 and SP04 viruses and 2 groups of contact animals. Six reassortant viruses with 1 to 3 genes from the SP04 virus as well as the parental KS07 virus were detected in lungs of coinfected pigs. Only the parental KS07 and reassortant KS07 virus containing NA from SP04 virus were found in the BALF and nasal swab samples of the first contact group of pigs and in the lungs of the second group of contact pigs; however, only the parental KS07 virus was detected in nasal swab samples from the second group of pigs. The origin of each of the eight gene segments (shown to the left of each panel) was determined; segments from the SP04 virus are shown in blue, and segments from the KS07 virus are in red. The numbers at the top of each panel show the number of viruses with the genotype/number of viruses analyzed.
Severe lesions were also observed in the lungs of each pig of both contact groups (Table 1), but no obvious clinical symptoms were noted in contact animals. Similarly, virus was only detected in the lungs of 4 pigs in both contact groups that were necropsied at early time points (4 and 6 days postcontact [d.p.c.]), and it was not detected in lungs of pigs that were necropsied at 8 d.p.c. Interestingly, only the reassortant KS07+SP04-NA and parental KS07 viruses were detected in the BALF samples of the first group of contact animals. Virus was found in nasal swabs collected from all 6 pigs at 4 d.p.c. and from 2 of 4 pigs at 6 d.p.c.; furthermore, the parental KS07 virus (about 92% of the total virus) was the predominant virus detected in the first group of contact animals, and the reassortant KS07+SP04-NA was only about 8% of the total number of viruses analyzed in either BALF or nasal swab samples (Fig. 3). In the second group of contact pigs, the same viruses were found in BALF samples collected from 4 pigs that underwent necropsy at 4 and 6 d.p.c.; however, only the parental KS07 virus was detected in the nasal swab samples collected at 4 (6 pigs) and 6 (4 pigs) d.p.c., and it was not found in the nasal swab samples collected at 8 d.p.c. (Fig. 3). An HI titer was detected only against the KS07 virus in pigs euthanized at 8 d.p.c. in both contact groups, which is consistent with the viruses detected in pigs of both contact groups (Fig. 3). These results indicate that the parental KS07 virus is more transmissible than the other viruses generated during coinfections.
Viral reassortment and transmission in coinfected pigs with preexisting immunity.
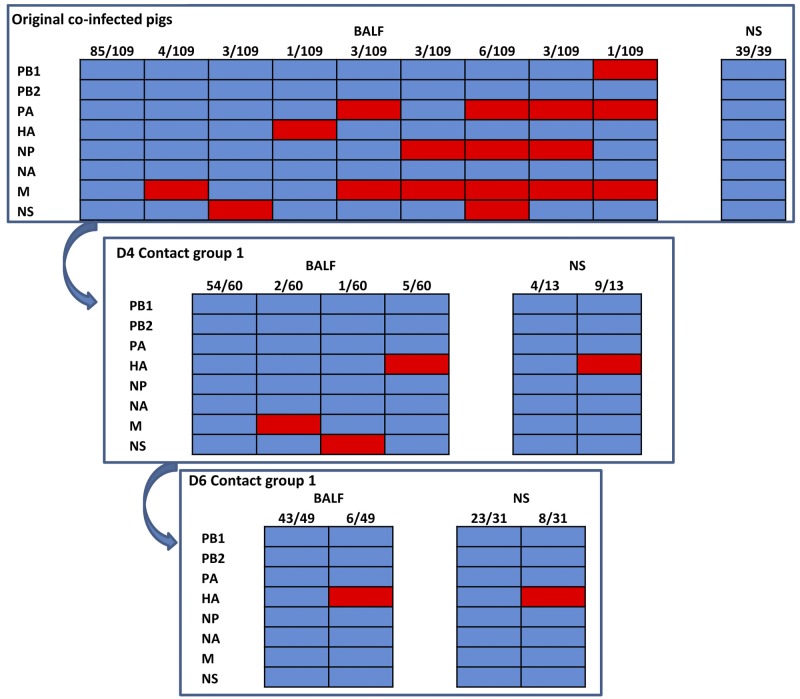
To investigate viral reassortment and transmission in pigs with preexisting immunity, nine pigs were immunized with the commercial inactivated H3N2 and H1N1 vaccine as described in Materials and Methods. After 10 days, the pigs were coinfected with SP04 and KS07 viruses. No clinical signs were noted in the coinfected pigs. Significantly fewer lung lesions were found in vaccinated pigs than in the animals without preexisting immunity (Tables 1 and 2). Virus was isolated from BALF samples from 3 coinfected pigs that underwent necropsy at 3 d.p.i. but not from those euthanized at 5 and 7 d.p.i. Eight SP04 reassortant viruses with 1 to 4 genes from the KS07 virus and the parental SP04 virus were detected from each immunized pig coinfected with both SP04 and KS07 viruses (Fig. 4), but no parental KS07 and pH1N1-like viruses were found in coinfected pig lungs; the parental SP04 virus was the predominant virus (78.0%, 85/109) detected in the pig lungs. Virus was detected from nasal swabs of only 3 coinfected pigs collected at 3 d.p.i. and 1 pig collected at 5 d.p.i. Interestingly, only 1 pig shed virus at both 3 and 5 d.p.i.; furthermore, only the parental SP04 virus was detected in the nasal swabs collected from the coinfected pigs. At 3 d.p.i., only 1 of 3 coinfected pigs had an HI titer against the SP04 virus, but all 3 coinfected pigs had equal HI titers against both SP04 and KS07 viruses at 5 d.p.i. The HI titer against the SP04 virus in coinfected pigs was significantly higher (5-fold) than that against the KS07 virus at 7 d.p.i., indicating that the parental SP04 and its reassortants were the primary viruses that replicated in immunized pigs.
TABLE 2 .
Lung lesion scores and HI titers of pigs with preexisting immunity coinfected with SP04 and KS07 viruses and of contact animals
| Group and d.p.i. or d.p.c. |
Mean lung lesion score (%) ± SEMa (no. of pigs with lesions/total no. of pigs) |
Geometric mean HI titer against: |
|
|---|---|---|---|
| KS07 | SP04 | ||
| Vaccinated and coinfected | |||
| 3 | 5.19 ± 3.39 (2/3) | 20 | 10b |
| 5 | 8.19 ± 0.87 (3/3) | 20 | 20 |
| 7 | 4.43 ± 1.05 (3/3) | 20 | 101 |
| Contact | |||
| 4 | 0.86 ± 0.58 (3/3) | 10c | <10 |
| 6 | 0.52 ± 0.25 (3/3) | 10 | <10 |
Mean value of the percentage of gross lesions of 7 pulmonary lobes per pig. SEM, standard error of the mean.
Only one pig had a detectable titer (1:10).
Two pigs had detectable titers (1:10).
FIG 4 .
Viruses detected in BALF and nasal swab (NS) samples from vaccinated pigs coinfected with KS07 and SP04 viruses and 1 group of contact animals. A total of 109 viruses, including 8 reassortant viruses and parental SP04 virus, were isolated from BALF samples of vaccinated pigs that were coinfected with KS07 and SP04 viruses (top). Although only the parental SP04 virus was found in nasal swabs from the original coinfected pigs, 3 reassortant viruses and the parental SP04 virus were detected in lungs of contact pigs that underwent necropsy 4 days postcontact (D4 Contact group 1). Only the parental SP04 virus and reassortant SP04 virus with the HA gene from the KS07 virus were found in BALF and nasal swab samples from contact animals that underwent necropsy 6 days postcontact (D6 Contact group 1). The origin of each of the eight gene segments (shown to the left of each panel) was determined; segments from the SP04 virus are shown in blue, and segments from the KS07 virus are labeled in red. The numbers at the top of each panel show the number of viruses with the genotype/number of viruses analyzed.
Minimal lung lesion scores (<1%) were observed in naïve contact animals that also did not show any clinical signs. Virus was detected in BALF samples of 6 contact pigs that underwent necropsy at 4 and 6 d.p.c., respectively. Three SP04 reassortants with a single gene from the KS07 virus (SP04+KS07-M, SP04+KS07-NS, and SP04+KS07-HA) and the parental SP04 virus were found in lungs of 3 naïve contact pigs 4 d.p.c., although no reassortant viruses were detected from nasal swabs of the coinfected animals, and the parental SP04 virus was still the predominant virus (90.0%, 54/60) detected in the lungs of contact pigs. However, only 1 of 6 pigs was found to shed virus via the nasal cavity at 4 d.p.c., and both the parental SP04 (30.7%, 4/13) and reassortant SP04+KS07-HA (69.2%, 9/13) viruses were detected from the nasal swabs. At 6 d.p.c., both the parental SP04 and reassortant SP04+KS07-HA viruses were found in all 3 contact pigs’ lungs, while no other reassortants were detected. Three of 4 pigs shed virus, and the same viruses were identified in BALF samples. Surprisingly, HI titers were detectable only against the KS07 virus (not against the SP04 virus) in 2 contact pigs at 4 d.p.c. and 3 contact animals at 6 d.p.c. (Table 2).
DISCUSSION
Reassortment plays an important role in generating pandemic influenza viruses, as has been the case for the 1957 H2N2, 1968 H3N2, and 2009 H1N1 pandemic viruses (7, 8, 11, 23–26), as well as the recent H7N9 virus (27). Different scenarios have been suggested for the generation of human pandemic influenza viruses, including direct introduction of an avian or swine influenza virus into humans and reassortment either in humans or through an intermediate mammalian host (4, 26, 28, 29). So far, there is no clear evidence to confirm any of these hypotheses. How and in which species human pandemic viruses were generated remains unknown. On the basis of phylogenic analysis, the 2009 pH1N1 virus is thought to have originated from SIVs by reassortment between North American triple reassortant and Eurasian avian-like H1N1 SIVs (11), but there is no direct evidence to support this hypothesis (10).
Natural reassortment events in pigs between Eurasian avian-like H1 and North American triple reassortant SIVs have been reported since the 2009 pandemic, and at least 8 genotypes of reassortants with different genetic constellations have been detected (18–20, 30), but no progenitor of the 2009 pH1N1 virus was found. Most of these reassortants appeared transiently in pigs, and only one reassortant has persisted in swine since 2007, which has the NS gene from the Eurasian avian-like H1 virus and the remaining 7 genes from North American triple reassortant SIV (19). In this study, we were not able to generate 2009 pH1N1-like viruses (NA and M genes from Eurasian SP04 virus and the 6 remaining genes from North American triple reassortant KS07 virus) by coinfection of MDCK and PK15 continuous cell lines or pigs with a North American triple reassortant H1N1 and a Eurasian avian-like H1N1 virus under the selected experimental conditions, although reassortant viruses and both parental viruses were detected in both coinfected continuous cell lines and pigs. In contrast to natural reassortment events in pigs in the field, 6 to 8 different genotypes of reassortant viruses were detected in lungs of coinfected pigs with or without preexisting immunity in our studies. Strikingly, only 1 or 2 viruses transmitted to contact animals in the vaccinated and naïve pig coinfection studies; this result is consistent with what we observed in our previous pig coinfection study with classical H1N1 and triple reassortant H3N2 SIVs (31). One reassortant found in coinfected pigs with preexisting immunity and transmitted to contact animals has a genetic constellation (NS gene from the Eurasian avian-like SP04 and the remaining 7 genes from North American triple reassortant KS07 virus) similar to that of the reassortant virus that emerged and persisted in the field since 2007 (19). This finding suggests that our experiments can at least partially represent the situation in the field. A previous study showed loose transmission bottlenecks in both vaccinated and naïve pigs infected with a single Eurasian SIV, and notably, the sizes of the transmission bottlenecks were not affected by vaccination (32). There was no considerable difference in the transmission bottlenecks in vaccinated and naïve pigs in our present study, but preexisting immunity in pigs resulted in completely different genotypes of reassortant viruses compared to those in naïve animals. All of these data indicate that reassortment can occur in the respiratory tract of pigs, resulting in different reassortant viruses, some of which are efficiently transmitted to sentinels. The reduced virus diversity in sentinel animals may indicate that some viruses are better fitted than others for transmission.
More reassortant viruses were found in coinfected continuous cell lines (80% of isolates in MDCK cells and 93% of isolates in PK15 cells were reassortants that belonged to 22 genotypes and 33 genotypes, respectively) than in coinfected pigs with preexisting immunity (22% of isolates were reassortants that represented 6 genotypes) or without preexisting immunity (35% of isolates were reassortants that represented 8 genotypes). The commercial H3N2 and H1N1 inactivated vaccine is at least partly efficacious against challenge from both homologous (KS07) and heterologous (SP04) viruses, as evidenced by significantly reduced lung lesions and virus replication and rapid virus clearance in vaccinated pigs compared to these parameters in naïve pigs inoculated with KS07, SP04, or both KS07 and SP04 viruses. Completely different genotypes of viruses were detected in vaccinated pigs than in naïve pigs, indicating that preexisting immunity in the host plays a critical role in driving viral reassortment in pigs. Interestingly, only the reassortant SP04 carrying the single HA from the KS07 virus continued to be detected in contact pigs in the vaccination group at later time points; this is probably due to either the genetic differences between the vaccine and KS07 virus or mutations in the HA resulting in vaccine escape; sequencing will be required to formally prove the latter hypothesis. In any case, our findings indicate that preexisting immunity of swine herds drives evolution of influenza A viruses.
Possible reasons why the 2009 pH1N1-like viruses could not be detected in our in vitro and pig studies include the following: (i) a limited number of plaques were selected and analyzed; (ii) the ratio of the viruses for infection was not ideal to favor reassortment (only one ratio, a KS07/SP04 ratio of 1:1, was investigated in this study); (iii) the order of viruses for infection was not ideal because different reassortant patterns might be observed if the pigs were first infected with the SP04 (or KS07) virus and then later infected with the KS07 (or SP04) virus; (iv) the ideal viruses for the coinfection studies were not used, because the homologies of several viral proteins (HA, NS, and NS2 from the KS07 and NA and M2 from the SP04 virus) to the corresponding proteins from 2009 pH1N1 A/CA/04/2009 virus at the amino acid level were lower than 95% (see Table S1 in the supplemental material); and (v) 2009 pH1N1 might have been generated by multiple reassortments (11, 12) and not by a single reassortment event.
Taken together, our findings indicate that it is not easy to generate a pandemic influenza virus-like genotype by mimicking the process that occurred in nature through coinfection, even though reassortments among influenza A viruses occur quite often. A reassortant virus generated in nature will have the potential to cause a pandemic if it can cross the species barrier to infect humans and efficiently transmit among humans. Although we could not detect 2009 pH1N1-like viruses, our study provides insights that host immunity plays a relevant role in driving the viral reassortment and evolution of influenza A virus.
MATERIALS AND METHODS
Viruses and cells.
Influenza A Eurasian avian-like H1N1 virus A/Swine/Spain/53207/2004 (SP04) and North American triple reassortant H1N1 A/Swine/Kansas/77778/2007 (KS07) were propagated in 10-day-old embryonated chicken eggs and used in this study. Porcine kidney PK15 and Madin-Darby canine kidney (MDCK) cells were maintained in minimum essential medium (MEM) with 5% fetal bovine serum (FBS) and antibiotics. Cells were infected with the corresponding viruses and incubated with infecting MEM medium that contained 0.3% bovine serum albumin (BSA), 1 µg/ml tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-trypsin, 1× l-glutamine (Invitrogen, Carlsbad, CA), 1× MEM vitamin solution (Invitrogen, Carlsbad, CA), and 1% antibiotics in normal MEM medium.
HI assays.
To confirm that pigs were seronegative for SIVs, sera from all experimental pigs were tested by hemagglutination inhibition (HI) assay before starting the experiment. For HI assays, sera were heat inactivated at 56°C, treated with a 20% suspension of kaolin (Sigma-Aldrich, St. Louis, MO) to eliminate nonspecific inhibitors, and adsorbed with 0.5% chicken or turkey red blood cells. An HI assay was performed to test antibodies against a panel of reference SIV strains, including A/Swine/Iowa/1973 H1N1, A/Swine/Texas/98 H3N2, A/Swine/North Carolina/2001 (a variant H1N1 representing a beta cluster triple reassortant H1N1 virus), and both KS07 and SP04 viruses.
Coinfection of cells with SP04 and KS07 viruses.
To explore reassortment between North American triple reassortant and Eurasian avian-like H1N1 SIVs in vitro, confluent MDCK or PK15 cells in a 6-well plate were coinfected with KS07 and SP04 viruses in a ratio of 1:1 at a multiplicity of infection (MOI) of 1. The infected plates were incubated at 37°C for 1 h, and then the supernatant was removed and infected cells were washed three times with fresh MEM. Infecting MEM medium (2 ml) was added to infected cells. After 72 h, the supernatants were harvested and two rounds of plaque purification were performed. Approximately 60 plaque-purified viruses from coinfected MDCK or PK15 cells were analyzed by RT-PCR and enzyme digestion to determine the genetic constellation of each virus.
Reassortment and transmission experiments in pigs.
Four-week-old crossbred pigs were obtained from a healthy herd free of SIVs and porcine reproductive and respiratory syndrome virus. All animal experiments were conducted in compliance with the Institutional Animal Care and Use Committee of Kansas State University. The inoculation protocol has been described elsewhere (33). Two pig experiments were performed to investigate viral reassortment and transmission.
In experiment 1, 9 pigs were coinoculated intratracheally with 106 50% tissue culture infective doses (TCID50)/pig of the egg-derived SP04 SIV and 106 TCID50/pig of egg-derived triple reassortant KS07 SIV. Three infected pigs were euthanized at each time point of 3, 5, and 7 days postinfection (d.p.i.). At 3 d.p.i., 6 naïve pigs (contact group 1) were cohoused with the infected pigs. The last 3 pigs from the coinfected group and 2 pigs from contact group 1 were euthanized at 7 d.p.i. (4 days postcontact [d.p.c.] for pigs from contact group 1). The remaining 4 pigs from contact group 1 were cohoused with another 6 naïve pigs (contact group 2). Two pigs from contact group 1 were euthanized at each time point of 6 and 8 d.p.c. Likewise, 2 pigs from contact group 2 were euthanized at each time point of 4, 6, and 8 d.p.c. All infected and contact pigs were bled at 0 d.p.i. and on the day of necropsy. Nasal swab samples were collected at 0, 3, 5, and 7 d.p.i. for coinfected pigs and at 0, 4, 6, and 8 d.p.c. for contact animals. Bronchoalveolar lavage fluid (BALF) samples for further analysis were obtained by flushing the pig lung with 50 ml of MEM on the day of necropsy. Macroscopic lung lesions were evaluated and scored by an experienced veterinarian.
In experiment 2, 9 pigs were intramuscularly immunized with 2 ml of a commercial swine influenza inactivated vaccine (Pfizer, New York, NY) that contained inactivated A/Swine/North Carolina/031/05 (H1N1), A/Swine/Missouri/069/05 (H3N2), and A/Swine/Iowa/110600/00 (H1N1) virus strains and Amphigen as the adjuvant. This vaccine was selected for use in this study because it was broadly employed in U.S. swine herds prior to the occurrence of the 2009 influenza pandemic. Ten days after vaccination, pigs were intratracheally coinoculated with 106 TCID50/pig of the egg-derived SP04 SIV and 106 TCID50/pig of egg-derived triple reassortant KS07 SIV. After 1 d.p.i., 6 naïve pigs were cohoused with the infected animals to investigate viral transmission. Three infected pigs were necropsied at each time point of 3, 5, and 7 d.p.i., and 3 contact animals were euthanized at each time point of 4 and 6 d.p.c. All infected and contact pigs were bled at 0 d.p.i. and on the day of necropsy. Nasal swab samples were collected at 0, 3, 5, and 7 d.p.i. for infected pigs and at 0, 4, and 6 d.p.c. for contact animals. BALF samples were collected and macroscopic lung lesions were recorded as described above.
Genotypic characterization of viruses isolated from coinfected cells, BALF, and nasal swabs.
The supernatants from coinfected cells and each BALF sample from pigs were 10-fold serially diluted and inoculated onto monolayers of MDCK cells grown in 6-well plates. Single plaques with different sizes were randomly selected (approximately 60 plaques/cell line and 18 plaques/BALF), and plaque purification was performed twice to purify the virus. The purified viruses were amplified in MDCK cells, and RNA was extracted from the supernatant for RT-PCR. All 8 viral segments of each virus were characterized by restriction enzyme digestion or by sequencing if necessary. To detect the viruses that were transmitted from the primary coinfected pigs to contact animals and among the contact animals, identification of each viral gene was performed for each virus isolated from nasal swabs from all experimental pigs and the BALF samples from contact animals. Viruses were plaque purified twice, and then each segment of the viral genome was amplified by RT-PCR and characterized by restriction enzyme digestion and sequencing. All gene segments of transmissible viruses were compared with those of the parental viruses and the viruses isolated from coinfected pigs.
Statistical analysis.
Macroscopic lung scores and virus titers were analyzed using analysis of variance (ANOVA) in GraphPad Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA); a P value of 0.05 or less was considered significant. Response variables shown to have a significant effect by treatment group were subjected to comparisons for all pairs by using the Tukey-Kramer test. Pairwise mean comparisons between inoculated and control groups were made using Student’s t test.
SUPPLEMENTAL MATERIAL
Virus titers in bronchoalveolar lavage fluid (BALF) samples and macroscopic lung lesions of pigs on day 5 postinfection with the SP04 or KS07 virus. Three 4-week-old pigs were intratracheally infected with 106 TCID50 of KS07 or SP04 virus or mock infected with serum-free MEM and euthanized on day 5 postinfection. Macroscopic lung lesions were evaluated and scored by an experienced veterinarian. Bronchoalveolar lavage fluid (BALF) samples were collected by flushing the pig lung with 50 ml of MEM on the day of necropsy for virus titration. (A) Virus titers in BALF. Values represent the log10 geometric mean BALF TCID50/ml ± SEM. (B) Lung lesion scores. Values represent the means ± standard errors of the means (SEM). Download
Comparison of sequences of 2009 pandemic H1N1 A/CA/04/2009 with those of 2009 pandemic-like H1N1 virus at nucleotide and amino acid levels.
ACKNOWLEDGMENTS
We thank Deborah Clouser, Audree Gottlob, Darlene Sheffer, and Haixia Liu for assisting with the pig study and providing technical support.
This project was partially funded by the CEIRS program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under contract numbers HHSN266200700005C and HHSN266200700010C, by the European Commission (FP7-GA258084), by the Kansas Bioscience Authority, and by Kansas State University Start-up (SRO001).
Footnotes
Citation Ma W, Liu Q, Qiao C, del Real G, García-Sastre A, Webby RJ, Richt JA. 2014. North American triple reassortant and Eurasian H1N1 swine influenza viruses do not readily reassort to generate a 2009 pandemic H1N1-like virus. mBio 5(2):e00919-13. doi:10.1128/mBio.00919-13.
REFERENCES
- 1. WHO 2009. Influenza (seasonal). Fact sheet no. 211, April 2009. World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs211/en/ [Google Scholar]
- 2. Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, Recuenco S, Ellison JA, Davis CT, York IA, Turmelle AS, Moran D, Rogers S, Shi M, Tao Y, Weil MR, Tang K, Rowe LA, Sammons S, Xu X, Frace M, Lindblade KA, Cox NJ, Anderson LJ, Rupprecht CE, Donis RO. 2012. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. U. S. A. 109:4269–4274. 10.1073/pnas.1116200109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, Yang H, Chen X, Recuenco S, Gomez J, Chen LM, Jackson A, Tao Y, Dreyfus C, Yu W, McBride R, Carney PJ, Gilbert AT, Chang J, Guo Z, Davis CT, Paulson JC, Stevens J, Rupprecht CE, Holmes EC, Wilson IA, Donis RO. 2013. New World bats harbor diverse influenza A viruses. PLoS Pathog. 9:e1003657. 10.1371/journal.ppat.1003657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. 1992. Evolution and ecology of influenza A viruses. Microbiol. Rev. 56:152–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma W, Kahn RE, Richt JA. 2009. The pig as a mixing vessel for influenza viruses: human and veterinary implications. J. Mol. Genet. Med. 3:158–166 [PMC free article] [PubMed] [Google Scholar]
- 6. Marshall N, Priyamvada L, Ende Z, Steel J, Lowen AC. 2013. Influenza virus reassortment occurs with high frequency in the absence of segment mismatch. PLoS Pathog. 9:e1003421. 10.1371/journal.ppat.1003421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith GJ, Bahl J, Vijaykrishna D, Zhang J, Poon LL, Chen H, Webster RG, Peiris JS, Guan Y. 2009. Dating the emergence of pandemic influenza viruses. Proc. Natl. Acad. Sci. U. S. A. 106:11709–11712. 10.1073/pnas.0904991106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller MA, Viboud C, Balinska M, Simonsen L. 2009. The signature features of influenza pandemics—implications for policy. N. Engl. J. Med. 360:2595–2598. 10.1056/NEJMp0903906 [DOI] [PubMed] [Google Scholar]
- 9. Simonsen L, Clarke MJ, Schonberger LB, Arden NH, Cox NJ, Fukuda K. 1998. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J. Infect. Dis. 178:53–60. 10.1086/515616 [DOI] [PubMed] [Google Scholar]
- 10. Gibbs AJ, Armstrong JS, Downie JC. 2009. From where did the 2009 “swine-origin” influenza A virus (H1N1) emerge? Virol. J. 6:207. 10.1186/1743-422X-6-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, Peiris JS, Guan Y, Rambaut A. 2009. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 459:1122–1125. 10.1038/nature08182 [DOI] [PubMed] [Google Scholar]
- 12. Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier RA, Pappas C, Alpuche-Aranda CM, López-Gatell H, Olivera H, López I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD, Jr, Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM, Smith DJ, Klimov AI, Cox NJ. 2009. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 325:197–201. 10.1126/science.1176225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ducatez MF, Hause B, Stigger-Rosser E, Darnell D, Corzo C, Juleen K, Simonson R, Brockwell-Staats C, Rubrum A, Wang D, Webb A, Crumpton JC, Lowe J, Gramer M, Webby RJ. 2011. Multiple reassortment between pandemic (H1N1) 2009 and endemic influenza viruses in pigs, United States. Emerg. Infect. Dis. 17:1624–1629. 10.3201/eid1709.110338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howard WA, Essen SC, Strugnell BW, Russell C, Barass L, Reid SM, Brown IH. 2011. Reassortant pandemic (H1N1) 2009 virus in pigs, United Kingdom. Emerg. Infect. Dis. 17:1049–1052. 10.3201/eid1706.101886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kitikoon P, Vincent AL, Gauger PC, Schlink SN, Bayles DO, Gramer MR, Darnell D, Webby RJ, Lager KM, Swenson SL, Klimov A. 2012. Pathogenicity and transmission in pigs of the novel A(H3N2)v influenza virus isolated from humans and characterization of swine H3N2 viruses isolated in 2010-2011. J. Virol. 86:6804–6814. 10.1128/JVI.00197-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Q, Ma J, Liu H, Qi W, Anderson J, Henry SC, Hesse RA, Richt JA, Ma W. 2012. Emergence of novel reassortant H3N2 swine influenza viruses with the 2009 pandemic H1N1 genes in the United States. Arch. Virol. 157:555–562. 10.1007/s00705-011-1203-9 [DOI] [PubMed] [Google Scholar]
- 17. Moreno A, Di Trani L, Faccini S, Vaccari G, Nigrelli D, Boniotti MB, Falcone E, Boni A, Chiapponi C, Sozzi E, Cordioli P. 2011. Novel H1N2 swine influenza reassortant strain in pigs derived from the pandemic H1N1/2009 virus. Vet. Microbiol. 149:472–477. 10.1016/j.vetmic.2010.12.011 [DOI] [PubMed] [Google Scholar]
- 18. Zhu H, Zhou B, Fan X, Lam TT, Wang J, Chen A, Chen X, Chen H, Webster RG, Webby R, Peiris JS, Smith DK, Guan Y. 2011. Novel reassortment of Eurasian avian-like and pandemic/2009 influenza viruses in swine: infectious potential for humans. J. Virol. 85:10432–10439. 10.1128/JVI.05352-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lam TT, Zhu H, Wang J, Smith DK, Holmes EC, Webster RG, Webby R, Peiris JM, Guan Y. 2011. Reassortment events among swine influenza A viruses in China: implications for the origin of the 2009 influenza pandemic. J. Virol. 85:10279–10285. 10.1128/JVI.05262-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vijaykrishna D, Poon LL, Zhu HC, Ma SK, Li OT, Cheung CL, Smith GJ, Peiris JS, Guan Y. 2010. Reassortment of pandemic H1N1/2009 influenza A virus in swine. Science 328:1529. 10.1126/science.1189132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Busquets N, Segalés J, Córdoba L, Mussá T, Crisci E, Martín-Valls GE, Simon-Grifé M, Pérez-Simó M, Pérez-Maíllo M, Núñez JI, Abad FX, Fraile L, Pina S, Majó N, Bensaid A, Domingo M, Montoya M. 2010. Experimental infection with H1N1 European swine influenza virus protects pigs from an infection with the 2009 pandemic H1N1 human influenza virus. Vet. Res. 41:74. 10.1051/vetres/2010046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma W, Vincent AL, Lager KM, Janke BH, Henry SC, Rowland RR, Hesse RA, Richt JA. 2010. Identification and characterization of a highly virulent triple reassortant H1N1 swine influenza virus in the United States. Virus Genes 40:28–36. 10.1007/s11262-009-0413-7 [DOI] [PubMed] [Google Scholar]
- 23. Fang R, Min Jou W, Huylebroeck D, Devos R, Fiers W. 1981. Complete structure of A/duck/Ukraine/63 influenza hemagglutinin gene: animal virus as progenitor of human H3 Hong Kong 1968 influenza hemagglutinin. Cell 25:315–323. 10.1016/0092-8674(81)90049-0 [DOI] [PubMed] [Google Scholar]
- 24. Gething MJ, Bye J, Skehel J, Waterfield M. 1980. Cloning and DNA sequence of double-stranded copies of haemagglutinin genes from H2 and H3 strains elucidates antigenic shift and drift in human influenza virus. Nature 287:301–306. 10.1038/287301a0 [DOI] [PubMed] [Google Scholar]
- 25. Kilbourne ED. 2006. Influenza pandemics of the 20th century. Emerg. Infect. Dis. 12:9–14. 10.3201/eid1201.051254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scholtissek C. 1994. Source for influenza pandemics. Eur. J. Epidemiol. 10:455–458. 10.1007/BF01719674 [DOI] [PubMed] [Google Scholar]
- 27. Liu D, Shi W, Shi Y, Wang D, Xiao H, Li W, Bi Y, Wu Y, Li X, Yan J, Liu W, Zhao G, Yang W, Wang Y, Ma J, Shu Y, Lei F, Gao GF. 2013. Origin and diversity of novel avian influenza A H7N9 viruses causing human infection: phylogenetic, structural, and coalescent analyses. Lancet 381:1926–1932. 10.1016/S0140-6736(13)60938-1 [DOI] [PubMed] [Google Scholar]
- 28. Kawaoka Y, Krauss S, Webster RG. 1989. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J. Virol. 63:4603–4608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scholtissek C, Rohde W, Von Hoyningen V, Rott R. 1978. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology 87:13–20. 10.1016/0042-6822(78)90153-8 [DOI] [PubMed] [Google Scholar]
- 30. Xu M, Huang Y, Chen J, Huang Z, Zhang J, Zhu Y, Xie S, Chen Q, Wei W, Yang D, Huang X, Xuan H, Xiang H. 2011. Isolation and genetic analysis of a novel triple-reassortant H1N1 influenza virus from a pig in China. Vet. Microbiol. 147:403–409. 10.1016/j.vetmic.2010.07.012 [DOI] [PubMed] [Google Scholar]
- 31. Ma W, Lager KM, Lekcharoensuk P, Ulery ES, Janke BH, Solórzano A, Webby RJ, García-Sastre A, Richt JA. 2010. Viral reassortment and transmission after co-infection of pigs with classical H1N1 and triple-reassortant H3N2 swine influenza viruses. J. Gen. Virol. 91:2314–2321. 10.1099/vir.0.021402-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murcia PR, Hughes J, Battista P, Lloyd L, Baillie GJ, Ramirez-Gonzalez RH, Ormond D, Oliver K, Elton D, Mumford JA, Caccamo M, Kellam P, Grenfell BT, Holmes EC, Wood JL. 2012. Evolution of an Eurasian avian-like influenza virus in naive and vaccinated pigs. PLoS Pathog. 8:e1002730. 10.1371/journal.ppat.1002730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Richt JA, Lager KM, Janke BH, Woods RD, Webster RG, Webby RJ. 2003. Pathogenic and antigenic properties of phylogenetically distinct reassortant H3N2 swine influenza viruses cocirculating in the United States. J. Clin. Microbiol. 41:3198–3205. 10.1128/JCM.41.7.3198-3205.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Virus titers in bronchoalveolar lavage fluid (BALF) samples and macroscopic lung lesions of pigs on day 5 postinfection with the SP04 or KS07 virus. Three 4-week-old pigs were intratracheally infected with 106 TCID50 of KS07 or SP04 virus or mock infected with serum-free MEM and euthanized on day 5 postinfection. Macroscopic lung lesions were evaluated and scored by an experienced veterinarian. Bronchoalveolar lavage fluid (BALF) samples were collected by flushing the pig lung with 50 ml of MEM on the day of necropsy for virus titration. (A) Virus titers in BALF. Values represent the log10 geometric mean BALF TCID50/ml ± SEM. (B) Lung lesion scores. Values represent the means ± standard errors of the means (SEM). Download
Comparison of sequences of 2009 pandemic H1N1 A/CA/04/2009 with those of 2009 pandemic-like H1N1 virus at nucleotide and amino acid levels.