Abstract
Water stress adversely impacts many aspects of the physiology of plants, especially photosynthetic capacity. If the stress is prolonged, plant growth, and productivity are severely diminished. Plants have evolved complex physiological and biochemical adaptations to adjust and adapt to a variety of environmental stresses. The molecular and physiological mechanisms associated with water-stress tolerance and water-use efficiency have been extensively studied. The systems that regulate plant adaptation to water stress through a sophisticated regulatory network are the subject of the current review. Molecular mechanisms that plants use to increase stress tolerance, maintain appropriate hormone homeostasis and responses and prevent excess light damage, are also discussed. An understanding of how these systems are regulated and ameliorate the impact of water stress on plant productivity will provide the information needed to improve plant stress tolerance using biotechnology, while maintaining the yield and quality of crops.
Keywords: abiotic stress, biomass, drought stress, photosynthesis, reactive oxygen species, stomatal closure
INTRODUCTION
Plant growth and productivity are adversely affected by water stress. Therefore, the development of plants with increased survivability and growth during water stress is a major objective in the breeding crops. Water use efficiency (WUE), a parameter of crop quality and performance under water deficit is an important selection trait. In fact, plants have evolved various molecular mechanisms to reduce their consumption of resources and adjust their growth to adapt to adverse environmental conditions (Yamaguchi-Shinozaki and Shinozaki, 2006; Ahuja et al., 2010; Skirycz and Inze, 2010; Osakabe et al., 2011; Nishiyama et al., 2013; Ha et al., 2014).
Plant growth is anchored by photosynthesis; however, excess light (EL) can cause severe damage to plants. EL induces photooxidation, which results in the increased production of highly reactive oxygen intermediates that negatively affect biological molecules and, if severe, a significant decrease in plant productivity (Li et al., 2009). Water stress that induces a decrease in leaf water potential and in stomatal opening (Figure 1), leading to the down-regulation of photosynthesis-related genes and reduced availability of CO2, has been known as one of the major factors in the EL stress (Osakabe and Osakabe, 2012).
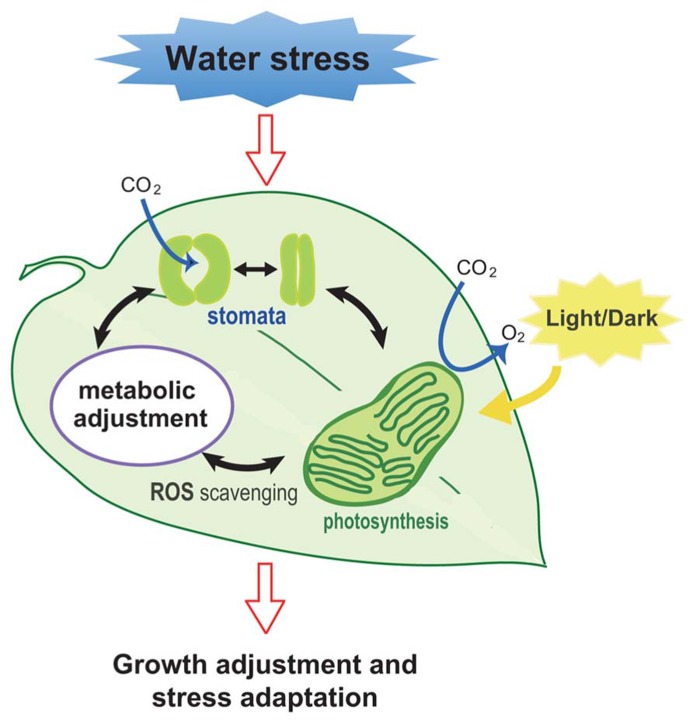
FIGURE 1.
Illustration of the response of plants to water stress. Stomatal response, ROS scavenging, metabolic changes, and photosynthesis are all affected when plants are subjected to water stress. These collective responses lead to an adjustment in the growth rate of plants as an adaptive response for survival.
Various molecular networks, including signal transduction, are involved in stress responses (Osakabe et al., 2011, 2013b; Nishiyama et al., 2013). The elucidation of these networks is essential to improve the stress tolerance of crops. In this review, plant responses to water stress are summarized, revealing that they are controlled by complex regulatory events mediated by abscisic acid (ABA) signaling, ion transport, and the activities of transcription factors (TFs) involved in the regulation of stomatal responses, all of which are integrated into orchestrated molecular networks, enabling plants to adapt and survive. Furthermore, recent findings on molecular mechanisms involved in protecting photosynthesis in order to adjust plant growth during water stress are discussed.
STOMATAL SIGNALING DURING WATER STRESS
MEMBRANE TRANSPORT AND ABA SIGNALING IN STOMATAL RESPONSES
Stomatal activity, which is affected by environmental stresses, can influence CO2 absorption and thus impact photosynthesis and plant growth. In response to a water deficit stress, ion- and water-transport systems across membranes function to control turgor pressure changes in guard cells and stimulate stomatal closure. Endogenous ABA is rapidly produced during drought, triggering a cascade of physiological responses, including stomatal closure, which is regulated by a signal transduction network. 9-cis-epoxycarotenoid dioxygenase 3 (NCED3) in Arabidopsis catalyzes a key step in ABA biosynthesis, and NCED3 expression is rapidly induced by drought stress in a vascular tissue-specific manner (Iuchi et al., 2001; Endo et al., 2008; Behnam et al., 2013; Figure 2). Mutations in nced3 reduced, while the overexpression of NCED3 enhanced drought tolerance and/or increased WUE in several plant species (Iuchi et al., 2001; Tung et al., 2008). During drought stress, the accumulated ABA in the vascular tissue is transported to guard cells via passive diffusion in response to pH changes and by specific transporters. Two members of the membrane-localized ABC transporter family, ABCG25 and ABCG40, and one member from a nitrate transporter family, AIT1/NRT1.2/NPF4.6, have been independently isolated from Arabidopsis and reported as ABA transporters (Kang et al., 2010; Kuromori et al., 2010; Kanno et al., 2012; Figure 2). ABCG25 has a role in ABA export, whereas ABCG40 and AIT1 are involved in the import of ABA. ABA-induced stomatal closure and gene expression are reduced in the atabcg40 mutation, resulting in reduced drought tolerance (Kang et al., 2010). These data indicate that the ABA transport system plays a significant role in water deficit tolerance and growth adjustment. Transcription of ABCG25 was induced by ABA and drought stress, and exhibited vascular tissue-specificity (Kuromori et al., 2010). In contrast, ABCG40 was expressed in guard cells (Kang et al., 2010), suggesting the possibility that the ABA synthesized in the vasculature during drought stress can be imported into the guard cells via these transporters. The expression pattern of AIT1/NRT1.2/NPF4.6 was similar to ABCG25 and also showed vascular tissue-specificity (Kanno et al., 2012). This finding suggests that ABA import systems in vascular tissues may also play an important role in the regulation of water stress responses.
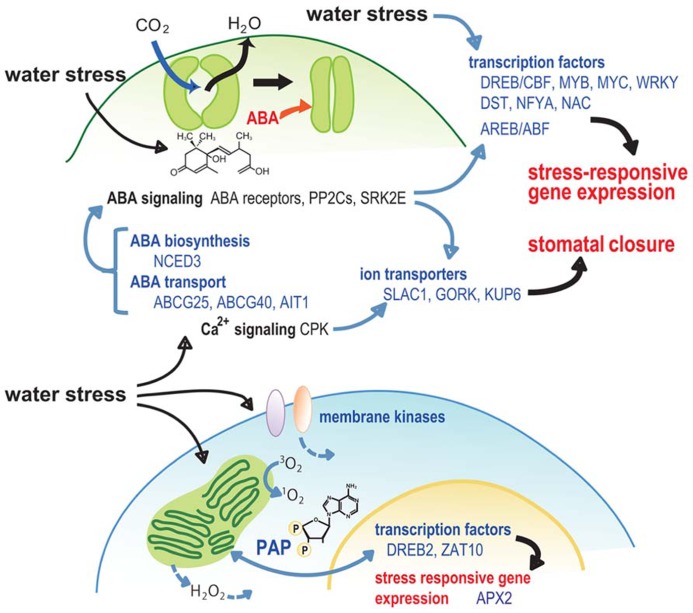
FIGURE 2.
Model for the role of signaling factors in stomatal closure and retrograde signaling during water stress.
In response to drought stress, ABA stimulates a signaling pathway that triggers the production of reactive oxygen species (ROS), which in turn induces an increase in cytosolic Ca2+. Subsequently, two distinct types of anion channels, a slow-activating sustained (S-type), and a rapid-transient (R-type), are activated and the anion efflux results in a depolarization of the plasma membrane. This leads to a decrease in the inward K+ channels (KAT1/KAT2) and H+-ATPase, which are involved in stomatal opening, and the activation of outward K+ channels, including GUARD CELL OUTWARD RECTIFYING K+ CHANNEL (GORK) that has a role in K+ efflux. The anion and K+ efflux from guard cells results in a reduction of guard cell turgor which causes stomatal closure (Schroeder and Hagiwara, 1989; Pei et al., 1997; Kwak et al., 2003; Negi et al., 2008; Vahisalu et al., 2008). SLAC1 (SLOW ANION CHANNEL-ASSOCIATED 1) functions as a major S-type anion channel in guard cells (Negi et al., 2008; Vahisalu et al., 2008), and is activated directly by a Snf1-related protein kinase 2 (SRK2E/OST1/SnRK2.6). This kinase is involved in the ABA-signaling complex of the ABA receptor, PYR family and PP2Cs (Geiger et al., 2009; Lee et al., 2009). S-type anion channels are also activated by the calcium-dependent protein kinases CPK3, CPK6, CPK21, and CPK23 (Geiger et al., 2010; Brandt et al., 2012). KAT1 has also been shown to be a direct target of regulation by ABA, since its activity is directly inhibited via phosphorylation by an ABA-activated SRK2E (Sato et al., 2009). Recently, the activity of KUP6, a KUP/HAK/KT family K+ transporter, has also been shown to be involved in the direct regulation during drought stress via phosphorylation by an ABA-activated SRK2E (Osakabe et al., 2013a). These results suggest that the complicated, but direct, control of ion transport systems by ABA may play an important role in stomatal responses that impact the tolerance of plants to water stress and influence plant growth (Figure 2).
TRANSCRIPTION FACTORS
The expression of various genes with functions in the water deficit responses, are specifically induced during the stress. Transcriptomic and proteomic analyses in various species have identified the involvement of general physiological processes associated with drought-responsive gene expression (Molina et al., 2008; Aprile et al., 2009; Walia et al., 2009; Abebe et al., 2010; Dugas et al., 2011; Jogaiah et al., 2012; Le et al., 2012; Utsumi et al., 2012). These studies have identified the conserved, as well as, species-specific regulatory and functional drought-responsive genes, including osmoprotectants and ABA biosynthesis, late embryogenesis abundant (LEA) and chaperone, ROS-related, ion homeostasis, and signaling genes. Additionally, key TFs regulating drought-responsive gene transcription have also been identified, such as MYB, MYC, DREB/CBF (drought-responsive cis-element binding protein/C-repeat-binding factor), ABF/AREB, NAC, and WRKY TFs (Stockinger et al., 1997; Sakuma et al., 2006; Tran et al., 2007b; Nakashima et al., 2009; Ishida et al., 2012; Figure 2). Corresponding cis-motifs, DRE/CRT and ABRE (ABA-responsive cis-element), have also been discovered in the promoters of many stress-responsive genes (Yamaguchi-Shinozaki and Shinozaki, 2006).
ABA-responsive cis-element-mediated transcription via ABF/AREB is directly regulated by an ABA receptor complex involving SnRK2 that activate ABF/AREBs by phosphorylation (Umezawa et al., 2010). The action of SnRK2 represents one of the important mechanisms regulating the rapid, adaptive response of plants to drought. DREB and AREB activate the transcription of various genes that are expressed in variety tissues. Additionally, novel types of TFs, with critical functions in stomatal responses, have also been identified. DST (drought and salt tolerance), a C2H2-type TF, controls the expression of genes involved in H2O2 homeostasis, and mediates ROS-induced stomatal closure and abiotic stress tolerance in rice (Huang et al., 2009). Drought-inducible nuclear TF, NFYA5, was reported to control stomatal aperture and play a role in drought tolerance in Arabidopsis (Li et al., 2008). SNAC1 (STRESS-RESPONSIVE NAC1) is expressed in rice guard cells, and overexpression of this gene enhanced ABA sensitivity, stomatal closure, and both DST in rice (Hu et al., 2006). AtMYB60 and AtMYB61 are expressed mainly in guard cells, and important TFs regulating stomatal aperture and drought tolerance in plants (Cominelli et al., 2005). AtMYB60 is a negative regulator of stomatal closure (Cominelli et al., 2005; Liang et al., 2005). Further studies to determine the molecular targets and signaling systems associated with these TFs in stomatal responses will increase our understanding of the regulatory networks controlling plant drought responses and growth adjustment.
EARLY WATER STRESS RESPONSE AND SIGNAL TRANSDUCTION PATHWAYS
Receptor and sensor proteins localized to membranes play important roles in various signaling pathways, conveying information to their cytoplasmic target proteins via catalytic processes, such as phosphorylation. Plasma membrane signaling has been hypothesized to be involved in the initial process of water status perception outside the cell (Maathuis, 2013). AHK1, an Arabidopsis histidine kinase (HK) localized to the plasma membrane mediates osmotic-stress signaling in prokaryotes and has been shown to function as an osmosensor. Overexpression of AHK1 enhanced drought tolerance in Arabidopsis (Urao et al., 1999; Tran et al., 2007a). ahk1 mutants exhibited decreased sensitivity to ABA and the downregulation of ABA- and/or stress-responsive genes, indicating that AHK1 acts as an osmosensor and functions as a positive regulator of osmotic-stress signaling (Tran et al., 2007a; Wohlbach et al., 2008). Downstream AHK1 cascades appear to be controlled by AHPs and ARRs as part of a multiple His-Asp phosphorelay. However, the factors that receive signals from AHK1, and also the precise composition of the signaling cascades, remain to be determined. In contrast, in Arabidopsis, the cytokinin (CK) receptor HKs, AHK2, AHK3, and AHK4, have been shown to negatively regulate ABA and drought signaling (Tran et al., 2007a, 2010). Multiple mutants of ahk2, ahk3, and ahk4 display increased sensitivity to ABA and enhanced tolerance to drought (Tran et al., 2007a; Jeon et al., 2010). These findings indicate the existence of crosstalk among ABA, CK, and stress-signaling pathways(Nishiyama et al., 2011; Ha et al., 2012).
In Arabidopsis, the receptor-like kinase (RLK) family includes more than 600 members, with the leucine rich-repeat (LRR)-RLKs constituting the largest subgroup (Gish and Clark, 2011). Several RLKs localized to the plasma membrane are known to be involved in the early steps of osmotic-stress signaling in a variety of plant species (Osakabe et al., 2013b). These stress-related RLKs possess a number of different extracellular domains (e.g., LRR, an extensin-like domain, or a cysteine-rich domain; Bai et al., 2009; de Lorenzo et al., 2009; Osakabe et al., 2010a; Yang et al., 2010; Tanaka et al., 2012), indicating that different environmental stimuli may activate RLK-mediated signaling pathways and convey the osmotic conditions outside of the cells. RLKs that bind to cell-walls, such as cell wall-associated kinases (WAKs), the proline-rich extensin-like receptor kinase (PERKs; Osakabe et al., 2013b), and the CrRLKs (Catharanthus roseus RLK1-like family; Schulze-Muth et al., 1996) have recently been predicted to be involved in the perception of turgor pressure (Steinwand and Kieber, 2010; Christmann et al., 2013). A potential link between the RLKs in cell-wall binding, ABA biosynthesis and water stress response could be determined by analyzing their roles in signaling systems associated with specific mechanosensing pathways activated in response to water stress. This would shed light on the early signaling system controlling water stress tolerance and growth adjustment.
PROTECTING PHOTOSYNTHESIS DURING WATER STRESS
Water stress directly affects rates of photosynthesis due to the decreased CO2 availability resulted from stomatal closure (Flexas et al., 2006; Chaves et al., 2009), and/or from changes in photosynthetic metabolism (Lawlor, 2002). EL has a negative effect on photosynthesis when the rates of photosynthesis are reduced by water stress (Li et al., 2009; Osakabe and Osakabe, 2012). A strong interconnection between the responses to EL and drought stresses has been suggested, and around 70% genes induced by EL are also induced by drought (Kimura et al., 2002; Chan et al., 2010; Estavillo et al., 2011). EL also stimulates the production of ROS, such as H2O2, superoxide (O2-) and singlet oxygen (1O2), by specific photochemical and biochemical processes, which also exerts deleterious effects on photosynthesis (Li et al., 2009). H2O2 induces the up-regulation of a variety of genes that overlap with genes up-regulated by various chemical and environmental stresses, such as methyl viologen, heat, cold, and drought (Vandenabeele et al., 2004; Vanderauwera et al., 2005). The transcription of cytosolic ascorbate peroxidase encoding genes (APXs), which have important roles in the scavenging of cytosolic H2O2, responds positively to EL stress and the redox state of plastoquinone (PQ; Karpinski et al., 1997). APX loss-of-function mutants exhibited an accumulation of degraded chloroplast proteins, indicating that APXs play a protective role as ROS scavengers for chloroplast proteins under EL conditions (Davletova et al., 2005; Li et al., 2009). AtAPX2 was also induced by drought stress and ABA (Rossel et al., 2006), suggesting that APX mediates ROS scavenging in response to both EL and water stress. A gain-of-function mutant, altered apx2 expression 8 (alx8), which has constitutively higher levels of APX2 expression, exhibited improved WUE and drought tolerance (Rossel et al., 2006; Wilson et al., 2009; Estavillo et al., 2011). In Arabidopsis, the zinc-finger TFs, ZAT10 and ZAT12, are induced in plants acclimated to EL or ROS treatment. The overexpression of ZAT10 and ZAT12 highly induced expression of various stress-related genes, including APXs (Rizhsky et al., 2004; Davletova et al., 2005; Rossel et al., 2007). Several transgenic lines that overexpressed ZAT10 exhibited enhanced drought stress tolerance (Sakamoto et al., 2004). ZAT10 and ZAT12 regulate the responses to EL and drought stresses, which are mediated by ROS (Davletova et al., 2005; Mittler et al., 2006), suggesting their potential roles in protecting photosynthesis from the injury during water stress (Figure 2).
Plants can monitor chloroplast status by plastid-to-nucleus signals, as plastid-to-nucleus retrograde signaling. This signaling system can regulate the expression of genes that function in the chloroplast. The retrograde signaling plays an important role in regulating the chloroplastic processes and also in the adaptive responses to environmental stresses (Chan et al., 2010). Chlorophyll intermediates, such as Mg-protoporphyrin IX (Mg-Proto), control the expression of nuclear genes in plants exposed to EL conditions, acting as a retrograde signal. The genomes uncoupled (gun) mutants, gun4 and gun5, exhibit impaired generation of Mg-Proto that has been shown to act as a signal to repress LHCB gene expression in Arabidopsis (Mochizuki et al., 2001; Strand et al., 2003; Pontier et al., 2007). LHCB expression is also controlled by GUN1 and ABI4 (ABSCISIC ACID-INSENSITIVE 4) that encodes a TF involved in ABA signaling (Koussevitzky et al., 2007). Collectively, these factors are thought to be involved in multiple retrograde signaling pathways. Moulin et al. (2008) re-examined the proposed role of Mg-Proto and other chlorophyll intermediates as signaling molecules and reported that none of the intermediates could be detected in ROS-induced plants under conditions where nuclear gene expression was repressed. The authors hypothesized that Mg-Proto (which accumulates in a light-dependent manner) is extremely short-lived and may generate 1O2 under EL conditions; however, a much more complex ROS signal may be generated during chloroplast degradation. There is increasing evidence for the regulation of nuclear gene expression by 1O2 (op den Camp et al., 2003) and H2O2 (Kimura et al., 2003). However, a clear role for these ROS molecules, either individually or in combination, requires further investigation.
Recently, several novel retrograde signaling pathways have been identified, including the 3′-phosphoadenosine 5′-phosphate (PAP) pathway, which is regulated by SAL1/ALX8/FRY1, and the methylerythritol cyclodiphosphate (MEcPP) pathway (Estavillo et al., 2011; Xiao et al., 2012). PAP has been described as a chloroplast to nuclear mobile signal that regulates gene expression. ALX8 encodes a phosphatase that converts PAP to AMP and regulates PAP levels (Wilson et al., 2009; Estavillo et al., 2011). alx8 mutant exhibited drought-tolerant phenotypes and constitutive upregulation of approximately 25% of the EL-regulated transcriptome, suggesting that SAL1/ALX8/FRY1 can act as a component of both EL and drought signaling networks, and that the SAL1-PAP retrograde pathway can alter nuclear gene expression during EL and drought (Rossel et al., 2006; Wilson et al., 2009; Estavillo et al., 2011; Figure 2). MEcPP is a precursor of isoprenoids generated by the methylerythritol phosphate (MEP) pathway, and can induce expression of nuclear encoded stress-responsive genes (Xiao et al., 2012). MEcPP is induced by various abiotic stresses, such as high light and wounding, and has been proposed to act as a retrograde signal in response to these stresses (Xiao et al., 2012). Evidence from the above studies suggests that metabolite signals, whose levels are influenced by environmental conditions, are used to establish an interaction between plastids and the nucleus and regulate chloroplast function to adjust plant growth in response to various stresses, including drought.
CONCLUSION AND FUTURE PERSPECTIVE
Due to the sessile life cycle, plants have evolved mechanisms to respond and adapt to adverse environmental stresses during their development and growth. Plant growth is impaired by severe drought stress due to a decrease in stomatal opening, which limits CO2 uptake and hence reduces photosynthetic activity. In order to develop strategies to maintain plant productivity, it is essential to understand the various regulatory mechanisms that control and enhance adaptive responses to stress in different plant species. In this review, we focused on the molecular mechanisms involved in the plant responses to water stress and the concomitant growth adjustment. These mechanisms include stomatal responses, ion transport, activation of stress signaling pathways, and responses to protect photosynthesis from injury. Understanding these key factors will enable us to improve plant productivity during water stress.
In parallel with the identification of the key molecular factors involved in these mechanisms, new technologies to bioengineer superior plants will also enable the development of plants with improved plant productivity. Although transgenic approaches have been effectively used to develop plant genotypes with improved stress tolerance under field conditions, estimation of the desired effects and their stability over many generations is required. Mutagenesis has also been used in plant breeding for a long time to create genetic variation; however, it takes considerable resources and effort to generate genotypes with the desired phenotype due to the random nature of the introduction of mutations. Recently, genome editing technology has made remarkable advances in the ability to modify the genome in a site-specific manner. Genome editing technology utilizes custom-designed restriction endonucleases, such as zinc finger nucleases (ZFN) or TAL-effector nucleases (TALEN; Shukla et al., 2009; Osakabe et al., 2010b; Zhang et al., 2010; Cermak et al., 2011), and more recently, the CRISPR/CAS system (Li et al., 2013; Nekrasov et al., 2013; Shan et al., 2013). Utilization of this technology will make it possible to modify the regulation of key genes that will convey improved stress tolerance while maintaining productivity. Further studies using new molecular approaches, including the identification of gene variants associated with the significant agronomic traits, will facilitate the molecular engineering of plants with increased tolerance to severe environmental stresses.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Programme for Promotion of Basic and Applied Researches for Innovations in the Bio-Oriented Industry of Japan (Yuriko Osakabe and Kazuo Shinozaki). Research in Lam-Son P. Tran’s lab was supported by the Grant (No. AP24-1-0076) from RIKEN Strategic Research Program for R & D.
REFERENCES
- Abebe T., Melmaiee K., Berg V., Wise R. P. (2010). Drought response in the spikes of barley: gene expression in the lemma, palea, awn, and seed. Funct. Integr. Genomics 10 191–205 10.1007/s10142-009-0149-4 [DOI] [PubMed] [Google Scholar]
- Ahuja I., de Vos R. C., Bones A. M., Hall R. D. (2010). Plant molecular stress responses face climate change. Trends Plant Sci. 15 664–674 10.1016/j.tplants.2010.08.002 [DOI] [PubMed] [Google Scholar]
- Aprile A., Mastrangelo A. M., De Leonardis A. M., Galiba G., Roncaglia E., Ferrari F., et al. (2009). Transcriptional profiling in response to terminal drought stress reveals differential responses along the wheat genome. BMC Genomics 10:279 10.1186/1471-2164-10-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L., Zhang G., Zhou Y., Zhang Z., Wang W., Du Y., et al. (2009). Plasma membrane-associated proline-rich extensin-like receptor kinase 4, a novel regulator of Ca signalling, is required for abscisic acid responses in Arabidopsis thaliana. Plant J. 60 314–327 10.1111/j.1365-313X.2009.03956.x [DOI] [PubMed] [Google Scholar]
- Behnam B., Iuchi S., Fujita M., Fujita Y., Takasaki H., Osakabe Y., et al. (2013). Characterization of the promoter region of an Arabidopsis gene for 9-cis-epoxycarotenoid dioxygenase involved in dehydration-inducible transcription. DNA Res. 20 315–324 10.1093/dnares/dst012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B., Brodsky D. E., Xue S., Negi J., Iba K., Kangasjärvi J., et al. (2012). Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc. Natl. Acad. Sci. U.S.A. 109 10593–10598 10.1073/pnas.1116590109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak T., Doyle E. L., Christian M., Wang L., Zhang Y., Schmidt C., et al. (2011). Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 39:e82 10.1093/nar/gkr218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K. X., Crisp P. A., Estavillo G. M., Pogson B. J. (2010). Chloroplast-to-nucleus communication: current knowledge, experimental strategies and relationship to drought stress signaling. Plant Signal. Behav. 5 1575–1582 10.4161/psb.5.12.13758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves M. M., Flexas J., Pinheiro C. (2009). Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 103 551–560 10.1093/aob/mcn125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christmann A., Grill E., Huang J. (2013). Hydraulic signals in long-distance signaling. Curr. Opin. Plant Biol. 16 293–300 10.1016/j.pbi.2013.02.011 [DOI] [PubMed] [Google Scholar]
- Cominelli E., Galbiati M., Vavasseur A., Conti L., Sala T., Vuylsteke M., et al. (2005). A guard-cell-specific MYB transcription factor regulates stomatal movements and plant drought tolerance. Curr. Biol. 15 1196–1200 10.1016/j.cub.2005.05.048 [DOI] [PubMed] [Google Scholar]
- Davletova S., Schlauch K., Coutu J., Mittler R. (2005). The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol. 139 847–856 10.1104/pp.105.068254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo L., Merchan F., Laporte P., Thompson R., Clarke J., Sousa C., et al. (2009). A novel plant leucine-rich repeat receptor kinase regulates the response of Medicago truncatula roots to salt stress. Plant Cell 21 668–680 10.1105/tpc.108.059576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas D. V., Monaco M. K., Olsen A., Klein R. R., Kumari S., Ware D., et al. (2011). Functional annotation of the transcriptome of Sorghum bicolor in response to osmotic stress and abscisic acid. BMC Genomics 12:514 10.1186/1471-2164-12-514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo A., Sawada Y., Takahashi H., Okamoto M., Ikegami K., Koiwai H., et al. (2008). Drought induction of Arabidopsis 9-cis-epoxycarotenoid dioxygenase occurs in vascular parenchyma cells. Plant Physiol. 147 1984–1993 10.1104/pp.108.116632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estavillo G. M., Crisp P. A., Pornsiriwong W., Wirtz M., Collinge D., Carrie C., et al. (2011). Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell 23 3992–4012 10.1105/tpc.111.091033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexas J., Bota J., Galmés J., Medrano H, Ribas-Carbó M. (2006). Keeping a positive carbon balance under adverse conditions: responses of photosynthesis and respiration to water stress. Physiol. Plant 127 343–352 10.1111/j.1399-3054.2006.00621.x [DOI] [Google Scholar]
- Geiger D., Scherzer S., Mumm P., Marten I., Ache P., Matschi S., et al. (2010). Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc. Natl. Acad. Sci. U.S.A. 107 8023–8028 10.1073/pnas.0912030107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D., Scherzer S., Mumm P., Stange A., Marten I., Bauer H., et al. (2009). Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc. Natl. Acad. Sci. U.S.A. 106 21425–21430 10.1073/pnas.0912021106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gish L. A., Clark S. E. (2011). The RLK/Pelle family of kinases. Plant J. 66 117–127 10.1111/j.1365-313X.2011.04518.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha C. V., Leyva-Gonzalez M. A., Osakabe Y., Tran U. T., Nishiyama R., Watanabe Y., et al. (2014). Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. U.S.A. 111 581–856 10.1073/pnas.1322135111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha S., Vankova R., Yamaguchi-Shinozaki K., Shinozaki K., Tran L. S. (2012). Cytokinins: metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci. 17 172–179 10.1016/j.tplants.2011.12.005 [DOI] [PubMed] [Google Scholar]
- Hu H., Dai M., Yao J., Xiao B., Li X., Zhang Q., et al. (2006). Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc. Natl. Acad. Sci. U.S.A. 103 12987–12992 10.1073/pnas.0604882103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X.-Y., Chao D.-Y., Gao J.-P., Zhu M.-Z., Shi M., Lin H.-X. (2009). A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes Dev. 23 1805–1817 10.1101/gad.1812409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T., Osakabe Y., Yanagisawa S. (2012). “Transcription factors: improving abiotic stress tolerance in plants,” in Improving Stress Resistance to Abiotic Stress ed. Tuteja N. (Berlin, Germany: Wiley-Blackwell) 589–619 [Google Scholar]
- Iuchi S., Kobayashi M., Taji T., Naramoto M., Seki M., Kato T., et al. (2001). Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 27 325–333 10.1046/j.1365-313x.2001.01096.x [DOI] [PubMed] [Google Scholar]
- Jeon J., Kim N. Y., Kim S., Kang N. Y., Novák O., Ku S. J., et al. (2010). A subset of cytokinin two-component signaling system plays a role in cold temperature stress response in Arabidopsis. J. Biol. Chem. 285 23371–23386 10.1074/jbc.M109.096644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jogaiah S., Govind S. R., Tran L. S. (2012). Systems biology-based approaches toward understanding drought tolerance in food crops. Crit. Rev. Biotechnol. 33 23–39 10.3109/07388551.2012.659174 [DOI] [PubMed] [Google Scholar]
- Kang J., Hwang J. U., Lee M., Kim Y. Y., Assmann S. M., Martinoia E., et al. (2010). PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc. Natl. Acad. Sci. U.S.A. 107 2355–2360 10.1073/pnas.0909222107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno Y., Hanada A., Chiba Y., Ichikawa T., Nakazawa M., Matsui M., et al. (2012). Identification of an abscisic acid transporter by functional screening using the receptor complex as a sensor. Proc. Natl. Acad. Sci. U.S.A. 109 9653–9658 10.1073/pnas.1203567109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski S., Escobar C., Karpinska B., Creissen G., Mullineaux P. M. (1997). Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 9 627–640 10.1105/tpc.9.4.627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Yamamoto Y., Seki M., Sakurai T., Sato M., Shinozaki K., et al. (2002). Identification of Arabidopsis genes regulated by high light-stress using cDNA microarray. Plant Cell Physiol. 43 S159 [DOI] [PubMed] [Google Scholar]
- Kimura M., Yamamoto Y. Y., Seki M., Sakurai T., Sato M., Abe T., et al. (2003). Identification of Arabidopsis genes regulated by high light-stress using cDNA microarray. Photochem. Photobiol. 77 226–233 [DOI] [PubMed] [Google Scholar]
- Koussevitzky S., Nott A., Mockler T. C., Hong F., Sachetto-Martins G., Surpin M., et al. (2007). Signals from chloroplasts converge to regulate nuclear gene expression. Science 316 715–719 10.1126/science.1140516 [DOI] [PubMed] [Google Scholar]
- Kuromori T., Miyaji T., Yabuuchi H., Shimizu H., Sugimoto E., Kamiya A., et al. (2010). ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proc. Natl. Acad. Sci. U.S.A. 107 2361–2366 10.1073/pnas.0912516107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak J. M., Mori I. C., Pei Z. M., Leonhardt N., Torres M. A., Dangl J. L., et al. (2003). NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22 2623–2633 10.1093/emboj/cdg277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor D. W. (2002). Limitation to photosynthesis in water-stressed leaves: stomata vs. metabolism and the role of ATP. Ann. Bot. 89 871–885 10.1093/aob/mcf110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le D. T., Nishiyama R., Watanabe Y., Tanaka M., Seki M., Ham L. H., et al. (2012). Differential gene expression in soybean leaf tissues at late developmental stages under drought stress revealed by genome-wide transcriptome analysis. PLoS ONE 7:e49522 10.1371/journal.pone.0049522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Lan W., Buchanan B. B., Luan S. (2009). A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc. Natl. Acad. Sci. U.S.A. 106 21419–21424 10.1073/pnas.0910601106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. F., Norville J. E., Aach J., McCormack M., Zhang D., Bush J., et al. (2013). Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat. Biotechnol. 31 688–691 10.1038/nbt.2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.-X., Oono Y., Zhu J., He X.-J., Wu J.-M., Iida K., et al. (2008). The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell 20 2238–2251 10.1105/tpc.108.059444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wakao S., Fischer B. B., Niyogi K. K. (2009). Sensing and responding to excess light. Annu. Rev. Plant Biol. 60 239–260 10.1146/annurev.arplant.58.032806.103844 [DOI] [PubMed] [Google Scholar]
- Liang Y. K., Dubos C., Dodd I. C., Holroyd G. H., Hetherington A. M., Campbell M. M. (2005). AtMYB61, an R2R3-MYB transcription factor controlling stomatal aperture in Arabidopsis thaliana. Curr. Biol. 15 1201–1206 10.1016/j.cub.2005.06.041 [DOI] [PubMed] [Google Scholar]
- Maathuis F. J. (2013). Sodium in plants: perception, signalling, and regulation of sodium fluxes. J. Exp. Bot. 65 846–858 10.1093/jxb/ert326 [DOI] [PubMed] [Google Scholar]
- Mittler R., Kim Y., Song L., Coutu J., Coutu A., Ciftci-Yilmaz S., et al. (2006). Gain- and loss-of-function mutations in Zat10 enhance the tolerance of plants to abiotic stress. FEBS Lett. 580 6537–6542 10.1016/j.febslet.2006.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki N., Brusslan J. A., Larkin R., Nagatani A., Chory J. (2001). Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc. Natl. Acad. Sci. U.S.A. 98 2053–2058 10.1073/pnas.98.4.2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina C., Rotter B., Horres R., Udupa S. M., Besser B., Bellarmino L., et al. (2008). SuperSAGE: the drought stress-responsive transcriptome of chickpea roots. BMC Genomics 9:553 10.1186/1471-2164-9-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin M., McCormac A. C., Terry M. J., Smith A. G. (2008). Tetrapyrrole profiling in Arabidopsis seedlings reveals that retrograde plastid nuclear signaling is not due to Mg-protoporphyrin IX accumulation. Proc. Natl. Acad. Sci. U.S.A. 105 15178–15183 10.1073/pnas.0803054105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K., Ito Y., Yamaguchi-Shinozaki K. (2009). Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol. 149 88–95 10.1104/pp.108.129791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi J., Matsuda O., Nagasawa T., Oba Y., Takahashi H., Kawai-Yamada M., et al. (2008). CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452 483–486 10.1038/nature06720 [DOI] [PubMed] [Google Scholar]
- Nekrasov V., Staskawicz B., Weigel D., Jones J. D., Kamoun S. (2013). Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31 691–693 10.1038/nbt.2655 [DOI] [PubMed] [Google Scholar]
- Nishiyama R., Watanabe Y., Fujita Y., Le D. T., Kojima M., Werner T., et al. (2011). Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 23 2169–2183 10.1105/tpc.111.087395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama R., Watanabe Y., Leyva-Gonzalez M. A., Van Ha C., Fujita Y., Tanaka M., et al. (2013). Arabidopsis AHP2, AHP3, and AHP5 histidine phosphotransfer proteins function as redundant negative regulators of drought stress response. Proc. Natl. Acad. Sci. U.S.A. 110 4840–4845 10.1073/pnas.1302265110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- op den Camp R. G., Przybyla D., Ochsenbein C., Laloi C., Kim C., Danon A., et al. (2003). Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 15 2320–2332 10.1105/tpc.014662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakabe K., Osakabe Y. (2012). “Plant light stress,” in Encyclopaedia of Life Sciences ed. Robinson S. A. (London: Nature Publishing Group) [Google Scholar]
- Osakabe Y., Arinaga N., Umezawa T., Katsura S., Nagamachi K., Tanaka H., et al. (2013a). Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell 25 609–624 10.1105/tpc.112.105700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakabe Y., Yamaguchi-Shinozaki K., Shinozaki K, Phan Tran L. S. (2013b). Sensing the environment: key roles of membrane-localized kinases in plant perception and response to abiotic stress. J. Exp. Bot. 64 445–458 10.1093/jxb/ers354 [DOI] [PubMed] [Google Scholar]
- Osakabe Y., Kajita S., Osakabe K. (2011). Genetic engineering of woody plants: current and future targets in a stressful environment. Physiol. Plant. 142 105–117 10.1111/j.1399-3054.2011.01451.x [DOI] [PubMed] [Google Scholar]
- Osakabe Y., Mizuno S., Tanaka H., Maruyama K., Osakabe K., Todaka D., et al. (2010a). Overproduction of the membrane-bound receptor-like protein kinase 1, RPK1, enhances abiotic stress tolerance in Arabidopsis. J. Biol. Chem. 285 9190–9201 10.1074/jbc.M109.051938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakabe K., Osakabe Y., Toki S. (2010b). Site-directed mutagenesis in Arabidopsis using custom-designed zinc finger nucleases. Proc. Natl. Acad. Sci. U.S.A. 107 12034–12039 10.1073/pnas.1000234107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z. M., Kuchitsu K., Ward J. M., Schwarz M., Schroeder J. I. (1997). Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell 9 409–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontier D., Albrieux C., Joyard J., Lagrange T., Block M. A. (2007). Knock-out of the magnesium protoporphyrin IX methyltransferase gene in Arabidopsis. Effects on chloroplast development and on chloroplast-to-nucleus signaling. J. Biol. Chem. 282 2297–2304 10.1074/jbc.M610286200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizhsky L., Davletova S., Liang H. J., Mittler R. (2004). The zinc finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis. J. Biol. Chem. 279 11736–11743 10.1074/jbc.M313350200 [DOI] [PubMed] [Google Scholar]
- Rossel J. B., Walter P. B., Hendrickson L., Chow W. S., Poole A., Mullineaux P. M., et al. (2006). A mutation affecting ASCORBATE PEROXIDASE 2 gene expression reveals a link between responses to high light and drought tolerance. Plant Cell Environ. 29 269–281 10.1111/j.1365-3040.2005.01419.x [DOI] [PubMed] [Google Scholar]
- Rossel J. B., Wilson P. B., Hussain D., Woo N. S., Gordon M. J., Mewett O. P., et al. (2007). Systemic and intracellular responses to photooxidative stress in Arabidopsis. Plant Cell 19 4091–4110 10.1105/tpc.106.045898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H., Maruyama K., Sakuma Y., Meshi T., Iwabuchi M., Shinozaki K., et al. (2004). Arabidopsis Cys2/His2-type zinc-finger proteins function as transcription repressors under drought, cold, and high-salinity stress conditions. Plant Physiol. 136 2734–2746 10.1104/pp.104.046599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma Y., Maruyama K., Osakabe Y., Qin F., Seki M., Shinozaki K., et al. (2006). Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell 18 1292–1309 10.1105/tpc.105.035881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A., Sato Y., Fukao Y., Fujiwara M., Umezawa T., Shinozaki K., et al. (2009). Threonine at position 306 of the KAT1 potassium channel is essential for channel activity and is a target site for ABA-activated SnRK2/OST1/SnRK2.6 protein kinase. Biochem. J. 424 439–448 10.1042/BJ20091221 [DOI] [PubMed] [Google Scholar]
- Schroeder J. I., Hagiwara S. (1989). Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338 427–430 10.1038/338427a0 [DOI] [Google Scholar]
- Schulze-Muth P., Irmler S., Schroder G., Schroder J. (1996). Novel type of receptor-like protein kinase from a higher plant (Catharanthus roseus) – cDNA, gene intramolecular autophosphorylation, and identification of a threonine important for auto- and substrate phosphorylation. J. Biol. Chem. 271 26684–26689 10.1074/jbc.271.43.26684 [DOI] [PubMed] [Google Scholar]
- Shan Q., Wang Y., Li J., Zhang Y., Chen K., Liang Z., et al. (2013). Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 31 686–688 10.1038/nbt.2650 [DOI] [PubMed] [Google Scholar]
- Shukla V. K., Doyon Y., Miller J. C., DeKelver R. C., Moehle E. A., Worden S. E., et al. (2009). Precise genome modification in the crop species Zea mays using zinc-finger nucleases. Nature 459 437–441 10.1038/nature07992 [DOI] [PubMed] [Google Scholar]
- Skirycz A., Inze D. (2010). More from less: plant growth under limited water. Curr. Opin. Biotechnol. 21 197–203 10.1016/j.copbio.2010.03.002 [DOI] [PubMed] [Google Scholar]
- Steinwand B. J., Kieber J. J. (2010). The role of receptor-like kinases in regulating cell wall function. Plant Physiol. 153 479–484 10.1104/pp.110.155887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger E. J., Gilmour S. J., Thomashow M. F. (1997). Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcription activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. U.S.A. 94 1035–1040 10.1073/pnas.94.3.1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand A., Asami T., Alonso J., Ecker J. R., Chory J. (2003). Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrin IX. Nature 421 79–83 10.1038/nature01204 [DOI] [PubMed] [Google Scholar]
- Tanaka H., Osakabe Y., Katsura S., Mizuno S., Maruyama K., Kusakabe K., et al. (2012). Abiotic stress-inducible receptor-like kinases negatively control ABA signaling in Arabidopsis. Plant J. 70 599–613 10.1111/j.1365-313X.2012.04901.x [DOI] [PubMed] [Google Scholar]
- Tran L. S., Shinozaki K., Yamaguchi-Shinozaki K. (2010). Role of cytokinin responsive two-component system in ABA and osmotic stress signalings. Plant Signal. Behav. 5 148–150 10.4161/psb.5.2.10411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L. S., Urao T., Qin F., Maruyama K., Kakimoto T., Shinozaki K., et al. (2007a). Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 104 20623–20628 10.1073/pnas.0706547105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L. S., Nakashima K., Shinozaki K., Yamaguchi-Shinozaki K. (2007b). Plant gene networks in osmotic stress response: from genes to regulatory networks. Methods Enzymol. 428 109–128 10.1016/S0076-6879(07)28006-1 [DOI] [PubMed] [Google Scholar]
- Tung S. A., Smeeton R., White C. A., Black C. R., Taylor I. B., Hilton H. W., et al. (2008). Over-expression of LeNCED1 in tomato (Solanum lycopersicum L.) with the rbcS3C promoter allows recovery of lines that accumulate very high levels of abscisic acid and exhibit severe phenotypes. Plant Cell Environ. 31 968–981 10.1111/j.1365-3040.2008.01812.x [DOI] [PubMed] [Google Scholar]
- Umezawa T., Nakashima K., Miyakawa T., Kuromori T., Tanokura M., Shinozaki K., et al. (2010). Molecular basis of the core regulatory network in ABA responses: sensing, signaling and transport. Plant Cell Physiol. 51 1821–1839 10.1093/pcp/pcq156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urao T., Yakubov B., Satoh R., Yamaguchi-Shinozaki K., Seki M., Hirayama T., et al. (1999). A transmembrane hybrid-type histidine kinase in Arabidopsis functions as an osmosensor. Plant Cell 11 1743–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi Y., Tanaka M., Morosawa T., Kurotani A., Yoshida T., Mochida K., et al. (2012). Transcriptome analysis using a high-density oligomicroarray under drought stress in various genotypes of cassava: an important tropical crop. DNA Res. 19 335–345 10.1093/dnares/dss016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T., Kollist H., Wang Y. F., Nishimura N., Chan W. Y., Valerio G., et al. (2008). SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452 487–491 10.1038/nature06608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenabeele S., Vanderauwera S., Vuylsteke M., Rombauts S., Langebartels C., Seidlitz H. K., et al. (2004). Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana. Plant J. 39 45–58 10.1111/j.1365-313X.2004.02105.x [DOI] [PubMed] [Google Scholar]
- Vanderauwera S., Zimmermann P., Rombauts S., Vandenabeele S., Langebartels C., Gruissem W., et al. (2005). Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol. 139 806–821 10.1104/pp.105.065896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walia H., Wilson C., Ismail A. M., Close T. J., Cui X. (2009). Comparing genomic expression patterns across plant species reveals highly diverged transcriptional dynamics in response to salt stress. BMC Genomics 10:398 10.1186/1471-2164-10-398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P. B., Estavillo G. M., Field K. J., Pornsiriwong W., Carroll A. J., Howell K. A., et al. (2009). The nucleotidase/phosphatase SAL1 is a negative regulator of drought tolerance in Arabidopsis. Plant J. 58 299–317 10.1111/j.1365-313X.2008.03780.x [DOI] [PubMed] [Google Scholar]
- Wohlbach D. J., Quirino B. F., Sussman M. R. (2008). Analysis of the Arabidopsis histidine kinase ATHK1 reveals a connection between vegetative osmotic stress sensing and seed maturation. Plant Cell 20 1101–1117 10.1105/tpc.107.055871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Savchenko T., Baidoo E. E., Chehab W. E., Hayden D. M., Tolstikov V., et al. (2012). Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell 149 1525–1535 10.1016/j.cell.2012.04.038 [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K., Shinozaki K. (2006). Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 57 781–803 10.1146/annurev.arplant.57.032905.105444 [DOI] [PubMed] [Google Scholar]
- Yang T., Chaudhuri S., Yang L., Du L., Poovaiah B. W. (2010). A calcium/calmodulin-regulated member of the receptor-like kinase family confers cold tolerance in plants. J. Biol. Chem. 285 7119–7126 10.1074/jbc.M109.035659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Maeder M. L., Unger-Wallace E., Hoshaw J. P., Reyon D., Christian M., et al. (2010). High frequency targeted mutagenesis in Arabidopsis thaliana using zinc finger nucleases. Proc. Natl. Acad. Sci. U.S.A. 107 12028–12033 10.1073/pnas.0914991107 [DOI] [PMC free article] [PubMed] [Google Scholar]