Abstract
Background
Laboratory data suggest a role of angiotensin II in the pathogenesis of colorectal cancer (CRC). Whether angiotensin converting enzyme inhibitor (ACE-I) and/or angiotensin receptor blocker (ARB) use reduces the risk of colorectal neoplasia remains unclear. Given their widespread use, we sought to determine whether exposure to these agents would have a secondary benefit on CRC incidence.
Methods
A nested case–control study was conducted using EPIC’s General Practice Research Database (1987–2002). The study cohort consisted of hypertensive patients. Case patients were those diagnosed with CRC after the diagnosis of hypertension. Each case patient was matched to up to 10 control subjects on age, sex, and both calendar year and duration of follow-up using incidence density sampling. The association between CRC and ACE-I/ARB exposure was assessed with conditional logistic regression. All statistical tests were two-sided.
Results
Two thousand eight-hundred forty-seven case patients were matched with 28239 control subjects. The adjusted odds ratios (ORs) of CRC were 0.84 (95% confidence interval [CI] = 0.72 to 0.98; P = .03) for or more years of ACE-I/ARB therapy and 0.75 (95% CI = 0.58 to 0.97; P = .03) for 5 or more years of exposure. The strength of this association increased with high-dose exposure (OR = 0.53; 95% CI = 0.35 to 0.79; P = .003 for ≥3 years of high-dose exposure). Among patients receiving antihypertensive medications, the association with long-term therapy was no longer statistically significant for ≥5 years), but the benefit of high-dose therapy remained (OR = 0.59; 95% CI = 0.39 to 0.89; P = .01 for ≥3 years of high-dose exposure).
Conclusions
Long-term/high dose exposure to ACE-Is/ARBs may be associated with a decreased incidence of CRC.
Colorectal cancer (CRC) is the third leading cause of cancer death in the United States (1). Angiotensin-converting enzyme inhibitors (ACE-Is) and angiotensin receptor blockers (ARBs) are two classes of commonly used antihypertensive agents that mediate their effect by the renin-angiotensin system. There has been in vitro and in vivo evidence that angiotensin II is involved in promoting cancer development and that ACE-Is may play a role in cancer prevention. Angiotensin II stimulates neovascularization (2), which is a requirement for tumor growth (3) and stimulates cell proliferation (4). Renin, an enzyme that produces angiotensin, is found in cancer blood vessels (5). Angiotensin II induces cell proliferation and DNA synthesis in intestinal epithelial cells (6). In a colon cancer cell line, ACE-Is and ARBs in combination with cyclooxygenase (COX)–2 inhibitors inhibited the insulin-like growth factor I receptor (IGF-IR) pathway, leading to statistically significantly reduced tumor growth (7). Several observational studies and secondary analyses of data from clinical trials have investigated the association between ACE-I/ARB use and the risk of CRC or overall cancer risk. These studies have yielded conflicting results and might be limited by relatively small sample size, short duration of follow-up, and/or potential detection bias (8–13).
Given the widespread clinical use of ACE-Is and ARBs, elucidating their potential association with cancer risk has clinical importance. Therefore, we conducted a nested case–control study among a cohort of patients with hypertension in the General Practice Research Database (GPRD) to determine whether exposure to ACE-I/ARB therapy was associated with a decreased incidence of CRC.
Methods
A nested case–control study was conducted using a version of GPRD administered by EPIC (London, UK; recently renamed CSD Medical Research).
Data Source
The GPRD is a computerized medical records system of a selected group of general practices in the United Kingdom. Under the National Health Services, 98% of the UK population receives all forms of health care through their general practitioners. The database is broadly representative of the UK population in terms of age, sex, and geography. Information is prospectively collected in the database and includes demographic information, prescriptions, clinical diagnoses, specialty consultation notes, and hospital discharge diagnoses.
We limited our analysis to up-to-standard (UTS) data in the GPRD. An UTS date in each practice is generated based on an assessment of the completeness, continuity, and plausibility of data recording in key areas in accordance with the GPRD Recording Guidelines. Previous studies have shown that information on prescription use, diagnoses, and hospitalizations in GPRD is of excellent quality. The diagnosis of CRC has been previously validated within GPRD (14).
Study Cohort
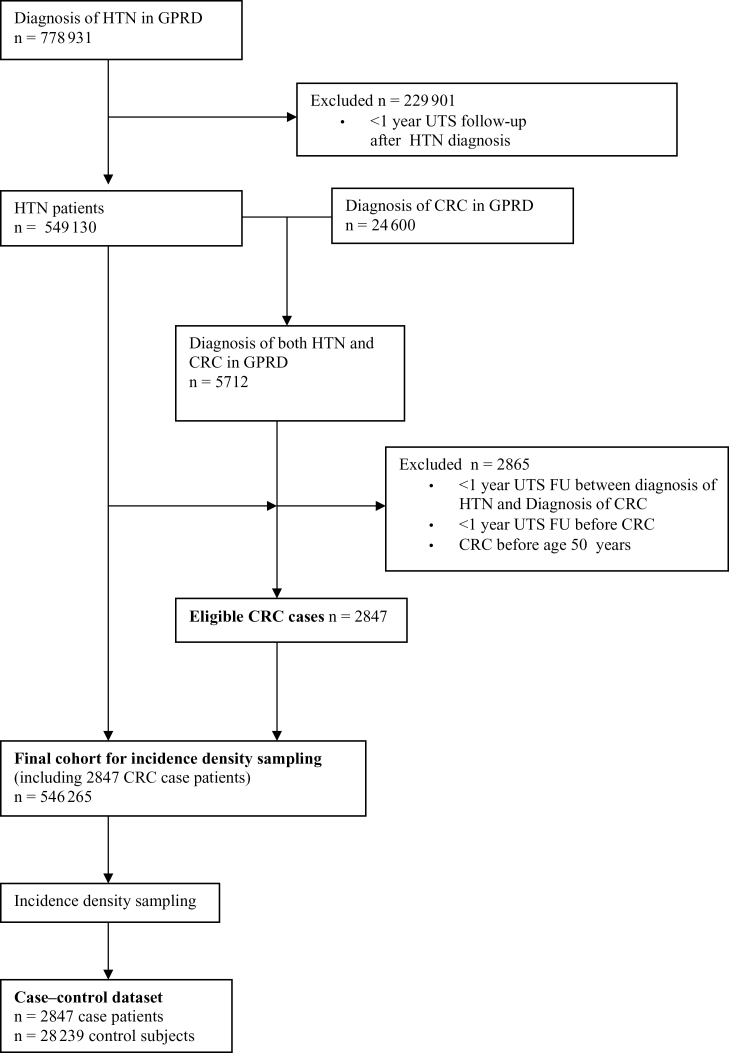
Of the 9.4 million patients followed in GPRD in the period from 1987 to 2002, the eligible study cohort included those who met the following criteria: a diagnosis of hypertension or hypertension-related complication; at least 365 days of UTS database follow-up following the first diagnosis of hypertension recorded after the UTS date; absence of CRC diagnosis on or before 365 days of UTS database follow-up after the first diagnosis of hypertension; and absence of incident CRC diagnosis before age 50 years. A total of 546265 patients met these criteria (Figure 1). Approval was obtained from the University of Pennsylvania Institutional Review Board and from the GPRD Scientific and Ethical Advisory Group.
Figure 1.
Study flow diagram. FU = follow-up; GPRD = General Practice Research Database; HTN = hypertension; UTS = up-to-standard.
Case Patients
Within the eligible hypertension cohort, there were 2847 case patients defined as having a first diagnosis of CRC 1) at least 1 year after the start of UTS database follow-up, 2) at least 1 year after the diagnosis of hypertension, and 3) after age 50 years. These criteria helped ensure the inclusion of incident sporadic CRC case patients only.
Control Subjects
Up to 10 control subjects without CRC were selected for each case from the study cohort using incidence density sampling (ie, case patients were matched to control subjects who were still at-risk for CRC at the index date) and matched on sex, year of birth (±1 year), index date (date of first diagnosis of CRC for matching case patient), and both calendar period and duration of UTS follow-up after the incident diagnosis of hypertension before the index date. For six case patients with no matching control subjects, the criterion for year of birth was expanded to match on ±2 years of year of birth so that at least one matching control subject could be identified. The incidence density sampling methodology allowed us to generate odds ratios (ORs) that are unbiased estimates of hazard ratios as in a proportional hazard analysis (15).
Validation of Hypertension
We calculated the proportion of patients in the case–control analysis who had elevated blood pressure (systolic blood pressure ≥140mm Hg or diastolic blood pressure ≥90mm Hg) recordings or prescriptions for antihypertensive medications as a measure of the validity of the hypertension diagnosis in the database.
Exposure
The exposure of interest was ACE-I or ARB therapy before index date. Based on published data (8), a beneficial effect of ACE-Is is likely only apparent after 3 years of exposure. Therefore, our primary exposure of interest was 3 or more years of exposure to ACE-Is or ARBs.
Individual periods of ACE-I and ARB exposure were determined according to the intended duration of each prescription recorded in the database. Exposure was also categorized a priori into increasing durations of cumulative exposure (no exposure, <3 years, 3–5 years, and >5 years). ARB therapy comprised only 2% of the total ACE-I/ARB prescriptions; a sensitivity analysis of ACE-Is alone was performed but could not be performed for ARB therapy alone given the small numbers of ARB prescriptions.
A dose–response effect was assessed based on average daily dose. For those patients with concurrent ACE-I and ARB therapy, the average dose was calculated by summing the dose of both ACE-I and ARB therapy.
Patients were potentially exposed to multiple medications within the same antihypertensive drug class during the follow-up period. Therefore a common metric was used: the defined daily dose (DDD), which is the assumed average maintenance dose per day (in milligrams, grams, etc) for a drug used for its main indication in adults (Supplementary Table 1, available online) (16).
Potential Confounder Variables
Data were also collected on variables potentially associated with the risk of CRC and the use of ACE-Is and ARBs. These included body mass index, smoking history, history of cholecystectomy, diabetes mellitus, other medication exposures (ie, hormone replacement therapy, statins, aspirin and nonsteroidal anti-inflammatory drugs [NSAIDs], insulin, oral hypoglycemic agents, calcium, folate), and frequency of physician contacts during the UTS follow-up time period after the incident diagnosis of hypertension.
Medication exposures were defined as none, short-term use (<3 years), and long-term use (≥3 years) and were based on cumulative exposure after the start of UTS follow-up until the index date.
Statistical Analysis
Conditional logistic regression was used to estimate the unadjusted and adjusted odds ratios (AORs) and 95% confidence intervals (CIs) for the association of ACE-I/ARB exposure and CRC. Potential confounders that led to a change of at least 10% in the crude odds ratio were chosen for inclusion in the multivariable regression model (17).
Secondary Analyses
To explore the possibility of unmeasured confounding, several secondary analyses were also performed. Odds ratios for CRC associated with increasing durations of exposure to calcium channel blockers, beta-blockers, and thiazide diuretics were calculated. The preponderance of epidemiological evidence to date argues against a substantive association between these medications and CRC risk (18–21). Evidence of notable benefit with these other drug classes in our dataset would therefore suggest the potential for unmeasured confounding. A dose–response analysis for calcium channel blockers was also performed in the same manner as for ACE-Is. This was not performed for thiazide diuretics or beta-blockers because of a high number of missing values for the medication dose variable.
Patients who are receiving active hypertensive treatment may have more frequent physician contact or healthier behaviors, which may potentially influence CRC risk. Unmatched analyses restricted to the subgroup of study patients who received antihypertensive medications during follow-up (n = 25292) were performed to assess potential bias or confounding related to these factors. In this restriction analysis, we assessed the association between the respective classes of antihypertensive medications and CRC risk. We also directly compared subjects exposed to ACE-I/ARB therapy alone vs those exposed to other antihypertensive agents exclusive of ACE-I/ARB therapy. CRC incidence associated with increasing durations of mutually exclusive exposure was assessed (<3 years and ≥3 years). Odds ratios were estimated using nonconditional logistic regression in the unmatched analyses adjusted for the original matching factorsof age, sex, and duration of follow-up besides the covariables included in the primary analysis.
All analyses were performed with Stata version 8.0 (StataCorp, College Station. TX). A P value of less than .05 was considered statistically significant for all analyses. All statistical tests were two-sided.
Results
The 2847 incident CRC case patients were matched with 28239 control subjects (Table 1). All case patients were matched with at least one control subject, with 2786 case patients matched with 10 control subjects. In the unadjusted analysis, diabetes mellitus, average number of physician visits per year, folate use for less than 3 years, and long-term (i.e., at least 3 years) use of aspirin/NSAIDs, thiazides and beta-blockers’ use for more than 3 years were the only variables statistically significantly associated with CRC risk (Table 1).
Table 1.
Characteristics of colorectacl case patients and control subjects*
| Characteristic | Case patients (n = 2847) | Control subjects (n = 28239 | Crude OR (95% CI) | P |
|---|---|---|---|---|
| Female sex, No. (%) | 1424 (50) | 14164 (50.2) | NA | |
| Age at start of follow-up, y, mean (SD) | 69.8 (9.1) | 69.5 (9.0) | NA | |
| Duration of up-to-standard database follow-up before index date, y, mean (SD) | 4.4 (2.5) | 4.4 (2.5) | NA | |
| Max body mass index, kg/m2, before index date, No. (%) | ||||
| <18.5 | 21 (0.7) | 1416(0.5) | 1.54 (0.97 to 2.47) | .07 |
| ≥18.5 and <25 | 521 (18.3) | 5588 (19.8) | 1.00 (referent) | |
| ≥25 and <30 | 890 (31.3) | 8657 (30.7) | 1.10 (0.99 to 1.24) | .09 |
| ≥30 | 472 (16.6) | 470116.7 | 1.08 (0.95 to 1.23) | .25 |
| Unknown | 943 (33.1) | 9147 (32.4) | 1.10 (0.99 to 1.24) | .08 |
| Smoking, No. (%) | ||||
| Nonsmoker | 1963 (69.0) | 19739 (69.9) | 1.00 (referent) | |
| Smoker | 403 (14.2) | 4373 (15.5) | 0.92 (0.82 to 1.03) | .17 |
| Unknown | 481 (16.9) | 4127 (14.6) | 1.18 (1.06 to 1.32) | <.01 |
| No. doctor visits/year during follow-up, mean (SD) | 8.7 (6.3) | 6.7 (5.1) | 1.05 (1.05 to 1.07) | <.01 |
| Diabetes mellitus, No. (%) | 297 (10.4) | 2545 (9.0) | 1.18 (1.04 to 1.34) | .01 |
| Cholecystectomy, No. (%) | 118 (4.1) | 1163 (4.1) | 1.01 (0.83 to 1.22) | .87 |
| Aspirin/NSAID, No. (%) | ||||
| <3years | 1414 (49.7) | 14238 (50.4) | 0.93 (0.85 to1.01) | .08 |
| ≥3 years | 253 (8.9) | 2859 (10.1) | 0.81 (0.70 to 0.94) | .01 |
| Statins, No. (%) | ||||
| <3 years | 81 (2.9) | 872 (3.1) | 0.92 (0.72 to 1.17) | .50 |
| ≥3 years | 17 (0.6) | 132 (0.5) | 1.27 (0.77 to 2.12) | .36 |
| Calcium, No. (%) | ||||
| <3 years | 74 (2.6) | 758 (2.7) | 0.96 (0.75 to 1.23) | .75 |
| ≥3 years | 3 (0.1) | 58 (0.2) | 0.50 (0.16 to 1.61) | .25 |
| Folate, No. (%) | ||||
| <3 years | 33 (1.2) | 184 (0.7) | 1.77 (1.22 to 2.58) | <.01 |
| ≥3 years | 6 (0.2) | 43 (0.2) | 1.41 (0.60 to 3.31) | .44 |
| Hormone replacement therapy, No. (%) | ||||
| <3 years | 78 (2.7) | 820 (2.9) | 0.92 (0.71 to 1.20) | .54 |
| ≥3 years | 9 (0.3) | 116 (0.4) | 0.73 (0.37 to 1.47) | .38 |
| Oral hypoglycemics, No. (%) | ||||
| <3 years | 121 (4.3) | 1061 (3.8) | 1.14 (0.94 to 1.38) | .18 |
| ≥3 years | 72 (2.5) | 655 (2.3) | 1.10 (0.86 to 1.41) | .67 |
| Insulin, No. (%) | ||||
| <3 years | 26 (0.9) | 203 (0.7) | 1.27 (0.85 to 1.92) | .25 |
| ≥3 years | 15 (0.5) | 144 (0.5) | 1.04 (0.61 to 1.77) | .88 |
| ACE-Is/ARBs, No. (%) | ||||
| <3 years | 515 (18.1) | 5195 (18.4) | 0.97 (0.88 to 1.07) | .55 |
| ≥3 years | 207 (7.3) | 2186 (7.7) | 0.92 (0.79 to 1.08) | .31 |
| Calcium channel blockers, No. (%) | ||||
| <3 years | 697 (24.5) | 6968 (24.7) | 0.99 (0.90 to 1.09) | .83 |
| ≥3 years | 351 (12.3) | 3473 (12.3) | 1.00 (0.88 to 1.13) | .96 |
| Thiazides, No. (%) | ||||
| <3 years | 933 (32.8) | 8918 (31.6) | 1.03 (0.95 to 1.13) | .47 |
| ≥3 years | 338 (11.9) | 3765 (13.3) | 0.87 (0.76 to 0.99) | .04 |
| Beta-blockers, n (%) | ||||
| (<3 years) | 852 (30.0) | 7936 (28.1) | 1.07 (0.98 to 1.17) | .15 |
| (≥3 years) | 423 (14.8) | 4784 (16.9) | 0.85 (0.75 to 0.96) | .01 |
* All statistical tests were two-sided; ACE-I = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; CI = confidence interval; NA = not applicable; NSAID = nonsteroidal anti-inflammatory drug; OR = odds ratio; SD = standard deviation.
More than 96% of the patients had either at least two documented elevated blood pressure measurements or at least one antihypertensive prescription.
Of case patients and control subjects, 7.27% and 7.74%, respectively, had 3 ore more years of exposure to ACE-Is/ARBs before the index date. In the primary analysis, the only variable associated with a greater than 10% change in the unadjusted odds ratio for ACE-I/ARB exposure was the average number of doctor visits per year. Therefore, the final adjusted model only included this variable, in addition to the matching factors as covariables. In the adjusted model, 3 or more years of cumulative exposure to ACE-Is/ARBs was associated with a lower risk of CRC (AOR = 0.84; 95% CI = 0.72 to 0.98; P = .03) and 5 or more years of cumulative exposure associated with an adjusted odds ratio of 0.75 (95% CI = 0.58 to 0.97; P = .03) (Table 2). Because visits during the last year before the index date might be related to the cancer outcome, we conducted a sensitivity analysis in which the average number of doctor visits was calculated excluding the visits in the last year before the index date. To more reliably estimate the average number of visits, we restricted this analysis to those patients who had at least 2 years of follow-up time. The effect estimates in this analysis are similar to those in our primary analyses, but the confidence interval was slightly wider because of the smaller sample size (AOR = 0.79, 95% CI = 0.61 to 1.02, P = .08 for ≥5 years of ACE-I/ARB exposure; AOR = 0.89, 95% CI = 0.77 to 1.04, P = .18 for ≥3 years of ACE-I/ARB exposure).
Table 2.
Risk of colorectal cancer associated with increasing cumulative duration of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker therapy*
| Type of OR | OR (95% CI) P | ||
|---|---|---|---|
| <3 years | ≥3 years | ≥5 years | |
| (n = 5710) | (n = 2393) | ||
| Crude | 0.97 (0.87 to 1.07) .55 | 0.92 (0.79 to 1.08) .31 | |
| Adjusted† | 0.86 (0.77 to 0.96) .01 | 0.84 (0.72 to 0.98) .03 | |
| Increasing durations of therapy, divided into 3 categories | |||
| (n = 5710) | (n = 1482) | (n = 911) | |
| Crude | 0.97 (0.88 to 1.07) .55 | 0.99 (0.82 to 1.19) .89 | 0.81 (0.63 to 1.05) .12 |
| Adjusted† | 0.86 (0.78 to 0.96) .01 | 0.89 (0.74 to 1.08) .24 | 0.75 (0.58 to 0.9) .03 |
* Results were obtained from conditional logistic regression models. All statistical tests were two-sided. CI = confidence interval OR = odds ratio.
† Adjusted for average number of doctor visits during follow-up (only variable leading to >10% change in odds ratio).
When we repeated our primary analysis for 3 or more years of ACE-I/ARB exposure after including all the potential confounders in the regression model, the adjusted odds ratio of 0.85 (95% CI = 0.73 to 0.99; P = .04) was very similar to those from our primary multivariable model. Analyses limited to ACE-I therapy without ARB therapy also produced similar findings (Supplementary Table 2, available online).
Similar analyses of increasing duration of therapy with calcium channel blockers, thiazide diuretics, and beta-blockers all demonstrated nearly statistically significant associations for 5 or more years of exposure (Table 3).
Table 3.
Risk of colorectal cancer associated with increasing cumulative duration of other antihypertensive agents*
| Antihypertensive agent | OR (95% CI) P | ||
|---|---|---|---|
| <3 years | ≥3 to <5 years | ≥5 years | |
| (n = 7665) | (n = 2276) | (n = 1548) | |
| Calcium channel blockers | |||
| Crude | 0.99 (0.90 to 1.09) .82 | 1.06 (0.91 to 1.23) .46 | 0.90 (0.74 to 1.10) .30 |
| Adjusted† | 0.89 (0.81 to 0.97) .01 | 0.99 (0.84 to 1.15) .84 | 0.85 (0.70 to 1.04) .12 |
| (n = 9851) | (n = 2551) | (n = 1552) | |
| Thiazide diuretics | |||
| Crude | 1.03 (0.95 to 1.13) .47 | 0.91 (0.78 to 1.06) .22 | 0.80 (0.65 to 0.98) .04 |
| Adjusted† | 1.00 (0.92 to 1.09) .96 | 0.93 (0.80 to 1.09) .39 | 0.85 (0.70 to 1.05) .16 |
| (n = 8788) | (n = 2848) | (n = 2359) | |
| Beta-blockers | |||
| Crude | 1.07 (0.98 to 1.17) .15 | 0.89 (0.77 to 1.03) .11 | 0.80 (0.67 to 0.95) .02 |
| Adjusted† | 1.04 (0.95 to 1.14) .44 | 0.91 (0.78 to 1.05) .20 | 0.85 (0.71 to 1.01) .09 |
† Adjusted for average number of doctor visits during follow-up
* Results were obtained from conditional logistic regression models. All statistical tests were two-sided. CI = confidence interval; OR = odds ratio.
Using the DDD system for ACE-Is/ARBs and calcium channel blockers (Supplementary Table 1, available online), both duration and average dose were calculated. Long-term (≥3 years of continuous use)/high dose (≥2 DDD) exposure to ACE-Is/ARBs was associated with an adjusted odds ratio of 0.53 (95% CI = 0.35 to 0.79; P = .003) (Table 4). A similar relationship for long-term/high-dose exposure to calcium channel blockers was not observed.
Table 4.
Colorectal cancer risk associated with increasing dose and duration of antihypertensive agents*
| Antihypertensive agent | OR (95% CI) P | OR (95% CI) P |
|---|---|---|
| ACE-I/ARB therapy | ||
| <3 years | Low dose (<2 DDD) (n = 5155) | High dose (≥2 DDD) (n = 551) |
| Crude | 0.97 (0.87 to 1.08) .59 | 0.96 (0.71 to 1.29) .80 |
| Adjusted† | 0.86 (0.77 to 0.96) .01 | 0.87 (0.64 to 1.17) .35 |
| ≥3 years | Low dose (<2 DDD) (n = 1962) | High dose (≥2 DDD) (n = 431) |
| Crude | 1.00 (0.85 to 1.17) .97 | 0.60 (0.40 to 0.90) .01 |
| Adjusted† | 0.92 (0.78 to 1.08) .29 | 0.53 (0.35 to 0.79) .003 |
| Calcium channel blocker therapy | ||
| <3 years | Low dose (<2 DDD) (n = 7396) | High dose (≥2 DDD) (n = 269) |
| Crude | 0.99 (0.90 to 1.09) .81 | 1.02 (0.67 to 1.55) .92 |
| Adjusted† | 0.88 (0.80 to 0.97) .01 | 0.91 (0.60 to 1.39) .67 |
| ≥3 years | Low dose (<2 DDD) (n = 3667) | High dose (≥2 DDD) (n = 157) |
| Crude | 1.00 (0.88 to 1.14) .99 | 0.90 (0.51 to 1.59) .71 |
| Adjusted† | 0.94 (0.82 to 1.07) .32 | 0.86 (0.49 to 1.53) .61 |
* Results were obtained from conditional logistic regression models. All statistical tests were two-sided. ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; CI = confidence interval; DDD = defined daily dose; OR = odds ratio.
† Adjusted for average number of doctor visits during follow-up (only variable leading to >10% change in odds ratio).
The unmatched analyses restricted to the subgroup of patients exposed to antihypertensive medications resulted in slight attenuation of effect for all medication classes (Table 5). However, exposure to high-dose ACE-Is/ARBs (≥ 2 DDD) for 3 or more years was associated with a reduced risk of CRC (AOR = 0.59; 95% CI = 0.39 to 0.89; P = .01) (Table 6). In contrast, long-term and high-dose calcium channel blocker use was not associated with CRC risk (AOR = 0.94; 95% CI = 0.53 to 1.67; P = .84).
Table 5.
Risk of colorectal cancer associated with increasing cumulative duration of antihypertensive agents (analysis restricted to cohort exposed to antihypertensive medication during follow-up; n = 25292)*
| Antihypertensive agent | OR (95% CI) P | ||
|---|---|---|---|
| <3 years | 3–5 years | >5 years | |
| ACE-I/ARB | n = 5710 | n = 1482 | n = 911 |
| Crude | 0.98 (0.88 to 1.09) .69 | 1.00 (0.83 to 1.20) .98 | 0.83 (0.65 to 1.07) .15 |
| Adjusted† | 0.89 (0.80 to 0.98) .03 | 0.96 (0.80 to 1.16) .66 | 0.87 (0.67 to 1.13) .30 |
| Calcium channel blockers | n = 7665 | n = 2276 | n = 1548 |
| Crude | 1.00 (0.91 to 1.10) 1.0 | 1.07 (0.92 to 1.24) .38 | 0.92 (0.77 to 1.11) .41 |
| Adjusted† | 0.90 (0.82 to 1.00) .04 | 1.05 (0.90 to 1.23) .51 | 0.99 (0.82 to 1.21) .93 |
| Thiazide diuretics | n = 9851 | n = 2551 | n = 1552 |
| Crude | 1.05 (0.95 to 1.15) .35 | 0.93 (0.80 to 1.08) .34 | 0.84 (0.69 to 1.02) .08 |
| Adjusted† | 1.03 (0.94 to 1.13) .53 | 1.01 (0.86 to 1.18) .89 | 1.01 (0.82 to 1.24) .95 |
| Beta-blockers | n = 8788 | n = 2848 | n = 2359 |
| Crude | 1.07 (0.98 to 1.18) .15 | 0.91 (0.79 to 1.06) .23 | 0.84 (0.72 to 0.99) .04 |
| Adjusted† | 1.07 (0.97 to 1.18) .16 | 0.99 (0.85 to 1.15) .90 | 1.00 (0.84 to 1.20) .97 |
* Results were obtained from unmatched analyses using nonconditional logistic regression models. All statistical tests were two-sided.ACE-I = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; CI = confidence interval; OR = odds ratio.
† Adjusted for average number of doctor visits during follow-up, age, sex, and duration of follow-up.
Table 6.
Colorectal cancer risk associated with increasing dose and duration of antihypertensive agents (analysis limited to cohort receiving antihypertensive medications; n=25292)*
| Antihypertensive agent | OR (95% CI) P | OR (95% CI) P |
|---|---|---|
| ACE-I/ARB therapy | ||
| <3 years | Low dose (<2 DDD) (n = 5155) | High dose (≥2 DDD) (n = 551) |
| Crude | 0.98 (0.88 to 1.09) .73 | 0.96 (0.71 to 1.29) .81 |
| Adjusted† | 0.89 (0.79 to 0.99) .03 | 0.87 (0.64 to 1.17) .37 |
| ≥3 years | Low dose (<2 DDD) (n = 1962) | High dose (≥2 DDD) (n = 431) |
| Crude | 1.01 (0.86 to 1.19) .91 | 0.61 (0.40 to 0.91) .02 |
| Adjusted† | 1.01 (0.85 to 1.19) .94 | 0.59 (0.39 to 0.89) .01 |
| Calcium channel blocker therapy | ||
| <3 years | Low dose (<2 DDD) (n = 7396) | High dose (≥2 DDD) (n = 269) |
| Crude | 1.00 (0.91 to 1.10) .99 | 1.02 (0.68 to 1.56) .91 |
| Adjusted† | 0.90 (0.82 to 1.00) .04 | 0.91 (0.60 to 1.38) .65 |
| ≥3 years | Low dose (<2 DDD) (n = 3667) | High dose (≥2 DDD) (n = 157) |
| Crude | 1.02 (0.89 to 1.15) .81 | 0.90 (0.51 to 1.60) .73 |
| Adjusted† | 1.03 (0.91 to 1.18) .61 | 0.94 (0.53 to 1.67) .84 |
* Results were obtained from unmatched analyses using non-conditional logistic regression models. .All statistical tests were two-sided. ACE-I = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; CI = confidence interval; DDD = defined daily dose; OR = odds ratio.
† Adjusted for average number of doctor visits during follow-up, age, sex, and duration of follow-up.
Finally, unmatched analysis comparing those exposed to ACE-Is/ARBs alone with those exposed to other antihypertensive agents exclusive of ACE-Is/ARBs suggested a duration–response effect: patients with 3 or more years of ACE-I/ARB exposure alone compared with those exposed to any other antihypertensive agents for 3 or more years exclusive of any ACE-I/ARB exposure had a decrease in CRC risk, with an adjusted odds ratio of 0.67 (95% CI = 0.47 to 0.97; P = .03). No association was observed for less than 3 years of ACE-I/ARB exposure compared with less than 3 years of other antihypertensive medications (AOR = 0.94; 95% CI = 0.75 to 1.17; P = .45).
Discussion
We found that long-term use of ACE-Is/ARBs, particularly at high-dose, was associated with reduced CRC risk in a cohort of patients with hypertension. These data support the laboratory findings demonstrating a relationship between angiotensin II inhibition and colon cancer cell growth (7).
Existing data regarding the effect of ACE-Is/ARBs on cancer risk are inconclusive. Two cohort studies yielded conflicting results regarding a benefit on reducing overall cancer risk (8,9). In a cohort study of 17897 ACE-I users followed for an average of 3.7 years, Friis et al. found a standardized incidence ratio for overall cancer of 1.07 (95% CI = 1.01 to 1.15) (9), whereas Lever et al. reported a relative risk of 0.72 (95% CI = 0.55 to 0.92) for overall cancer (8). A recent systematic review showed that ACE-I or ARB use may be associated with improved outcomes in cancer patients, including patients with metastatic CRC (10). A secondary analysis of data from randomized controlled trials did not find an association between ACE-I or ARB use and cancer risk, although the durations of follow-up were relatively short among the trials included (11). Regarding the risk of colorectal neoplasia specifically, a recent cohort study reported that long-term lisinopril use was associated with a 41% reduction in the risk of advanced colorectal adenoma (12). However, another case–control study assessing ACE-I exposure among 665 CRC case patients and an equal number of control subjects did not demonstrate a statistically significant association with CRC risk (13). Two important limitations of this study were a small sample size and a short duration of exposure (total of 106 case patients and control subjects exposed for ≥2 years to ACE-Is).
Our large population-based study extends the existing evidence. The large number of case patients allowed us to perform a series of restriction and stratified analyses to minimize the threat of potential confounding. Furthermore, the CRC diagnosis in the GPRD has been externally validated (14), and prescription medication exposure is well recorded in the database. We also found the diagnosis of hypertension to be valid in GPRD.
We chose to limit the source cohort to only patients with hypertension in an attempt to limit at least some confounding and/or bias that could be present between patients who have a chronic medical illness that requires regular physician contact and those who do not. We also adjusted for number of physician visits. Furthermore, regular follow-up with a physician may lead to greater adherence with screening guidelines that in the long-term lead to decreased CRC risk, which could have biased the results of previous studies assessing this association. However, this effect can be expected to be negligible in our study because there was no established CRC screening program in place in the United Kingdom during the study period (1987–2002). Indeed, when we repeated our primary analysis after excluding the small proportion (<2.5%) of study patients who underwent presumed screening colonoscopies or flexible sigmoidoscopies (ie, > 6 months or 1 year before the date of CRC diagnosis, respectively), the results were virtually unchanged (ie, AOR associated with ≥3 years of ACE-I/ARB exposure changed from 0.84 to 0.85).
The presence of a statistically significant association with high-dose, long-term therapy even in the analysis restricted to the subgroup exposed to antihypertensive agents suggests that the observed effect of ACE-I/ARB exposure is unlikely to be due to unmeasured confounding. Finally, the presence of a decreased rate of CRC among those exposed to 3 or more years of ACE-I/ARB therapy alone when compared with those exposed to other antihypertensive medications exclusive of ACE-Is/ARBs is further support for an association that cannot be explained by confounding from simply being on antihypertensive therapy. In total, evidence from this analysis indicates potential benefit that is more evident in those receiving long-term and high daily dose therapies.
A number of potential limitations warrant consideration in this study. Given the large number of missing data for body mass index and tobacco exposure, these variables could not be fully assessed. However, because there are no practice standards or guidelines suggesting that ACE-Is would be more or less likely to be used than other antihypertensive agents in patients who are smokers, it is less likely that smoking would be a notable source of confounding in this analysis. There have been some reports suggesting that ACE-Is may be a preferred choice in obese patients (22–24) (although a majority of these reports would have been published after the prescribing dates in this study). Given that obesity increases the risk of CRC, this would have resulted in an increased risk of CRC with ACE-I use. Our analysis restricted to those with a recorded body mass index and smoking information did not lead to a change in the association between ACE-I/ARB use and CRC incidence.
One other potential concern would be unmeasured long-term NSAID/aspirin exposure due to over-the-counter exposure. However, a prior survey of GPRD patients revealed that nearly all long-term NSAID/aspirin use was by prescription, with GPRD prescription data correctly identifying 98% of patients who were chronically exposed (≥180 days of exposure in a 1-year period) to NSAIDs or aspirin (25).
We could not capture ACE-I use before patient enrollment in the database. Therefore we could have underestimated the duration of exposure. This would have biased against observing an association with longer duration of therapy. In fact, when non-UTS ACE-I exposure was included, there was a slightly stronger inverse association with CRC risk (data not shown).
In summary, ACE-Is appear to possibly be associated with a decreased risk of CRC, although the preponderance of evidence from this analysis indicates that the potential benefit may be somewhat modest and may be more evident in those receiving higher doses of medication for a long period of time. These results suggest that the angiotensin pathway is a potential target for development of new chemopreventive agents for CRC.
Funding
This work was supported by a grant from the National Institutes of Health (T32-DK007740 to GAM).
G. A. Makar contributed to the literature search, conception of the study question, study design, data collection, data analysis, data interpretation, and writing and revision of the manuscript. Y.-X. Yang contributed to the conception of the study question, study design, data collection, data analysis, data interpretation, and writing and revision of the manuscript. J. H. Holmes contributed to study design, data collection, manipulation, and analysis; and writing of the manuscript.
The study sponsor had no role in the study design in the collection, analysis, and interpretation of data. The authors declare no conflict of interest.
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29 [DOI] [PubMed] [Google Scholar]
- 2. Fernandez LA, Twickler J, Mead A. Neovascularization produced by angiotensin II. J Lab Clin Med. 1985;105(2):141–145 [PubMed] [Google Scholar]
- 3. Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339(6219):58–61 [DOI] [PubMed] [Google Scholar]
- 4. Daemen MJ, Lombardi DM, Bosman FT, Schwartz SM. Angiotensin II induces smooth muscle cell proliferation in the normal and injured rat arterial wall. Circ Res. 1991;68(2):450–456 [DOI] [PubMed] [Google Scholar]
- 5. Taylor GM, Cook HT, Sheffield EA, Hanson C, Peart WS. Renin in blood vessels in human pulmonary tumors. An immunohistochemical and biochemical study. Am J Pathol. 1988;130(3):543–551 [PMC free article] [PubMed] [Google Scholar]
- 6. Chiu T, Santiskulvong C, Rozengurt E. ANG II stimulates PKC-dependent ERK activation, DNA synthesis, and cell division in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2003;285(1):G1–11 [DOI] [PubMed] [Google Scholar]
- 7. Yasumaru M, Tsuji S, Tsujii M, et al. Inhibition of angiotensin II activity enhanced the antitumor effect of cyclooxygenase-2 inhibitors via insulin-like growth factor I receptor pathway. Cancer Res. 2003;63(20):6726–6734 [PubMed] [Google Scholar]
- 8. Lever AF, Hole DJ, Gillis CR, et al. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet. 1998;352(9123):179–184 [DOI] [PubMed] [Google Scholar]
- 9. Friis S, Sorensen HT, Mellemkjaer L, et al. Angiotensin-converting enzyme inhibitors and the risk of cancer: a population-based cohort study in Denmark. Cancer. 2001;92(9):2462–2470 [DOI] [PubMed] [Google Scholar]
- 10. Mc Menamin UC, Murray LJ, Cantwell MM, Hughes CM. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in cancer progression and survival: a systematic review. Cancer Causes Control. 2012;23(2):221–230 [DOI] [PubMed] [Google Scholar]
- 11. Bangalore S, Kumar S, Kjeldsen SE, et al. Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol. 2011;12(1):65–82 [DOI] [PubMed] [Google Scholar]
- 12. Kedika R, Patel M, Pena Sahdala HN, Mahgoub A, Cipher D, Siddiqui AA. Long-term use of angiotensin converting enzyme inhibitors is associated with decreased incidence of advanced adenomatous colon polyps. J Clin Gastroenterol. 2011;45(2):e12–e16 [DOI] [PubMed] [Google Scholar]
- 13. Boudreau DM, Koehler E, Rulyak SJ, Haneuse S, Harrison R, Mandelson MT. Cardiovascular medication use and risk for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(11):3076–3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garcia Rodriguez LA, Huerta-Alvarez C. Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Epidemiology. 2001;12(1):88–93 [DOI] [PubMed] [Google Scholar]
- 15. Lubin JH, Gail MH. Biased selection of controls for case–control analyses of cohort studies. Biometrics. Mar 1984;40(1):63–75 [PubMed] [Google Scholar]
- 16. WHO Collaborating Centre for Drug Statistics Methodology ATC/DDD Index 2013. http://www.whocc.no/atcddd/ Accessed December 10, 2013
- 17. Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129(1):125–137 [DOI] [PubMed] [Google Scholar]
- 18. Assimes TL, Elstein E, Langleben A, Suissa S. Long-term use of antihypertensive drugs and risk of cancer. Pharmacoepidemiol Drug Saf. 2008;17(11):1039–1049 [DOI] [PubMed] [Google Scholar]
- 19. Sorensen HT, Olsen JH, Mellemkjaer L, et al. Cancer risk and mortality in users of calcium channel blockers. A cohort study. Cancer. 2000;89(1):165–170 [DOI] [PubMed] [Google Scholar]
- 20. Olsen JH, Sorensen HT, Friis S, et al. Cancer risk in users of calcium channel blockers. Hypertension. 1997;29(5):1091–1094 [DOI] [PubMed] [Google Scholar]
- 21. Zacharski LR, Moritz TE, Haakenson CM, et al. Chronic calcium antagonist use in carcinoma of the lung and colon: a retrospective cohort observational study. Cancer Invest. 1990;8(5):451–458 [DOI] [PubMed] [Google Scholar]
- 22. Scholze J, Grimm E, Herrmann D, Unger T, Kintscher U. Optimal treatment of obesity-related hypertension: the Hypertension-Obesity-Sibutramine (HOS) study. Circulation. 2007;115(15):1991–1998 [DOI] [PubMed] [Google Scholar]
- 23. Galletti F, Strazzullo P, Capaldo B, et al. Controlled study of the effect of angiotensin converting enzyme inhibition versus calcium-entry blockade on insulin sensitivity in overweight hypertensive patients: Trandolapril Italian Study (TRIS). J Hypertens. 1999;17(3):439–445 [DOI] [PubMed] [Google Scholar]
- 24. Masuo K, Mikami H, Ogihara T, Tuck ML. Weight reduction and pharmacologic treatment in obese hypertensives. Am J Hypertens. 2001;14(6 Pt 1):530–538 [DOI] [PubMed] [Google Scholar]
- 25. Yang YX, Hennessy S, Propert K, Hwang WT, Sedarat A, Lewis JD. Chronic proton pump inhibitor therapy and the risk of colorectal cancer. Gastroenterology. 2007;133(3):748–754 [DOI] [PubMed] [Google Scholar]