Abstract
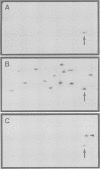
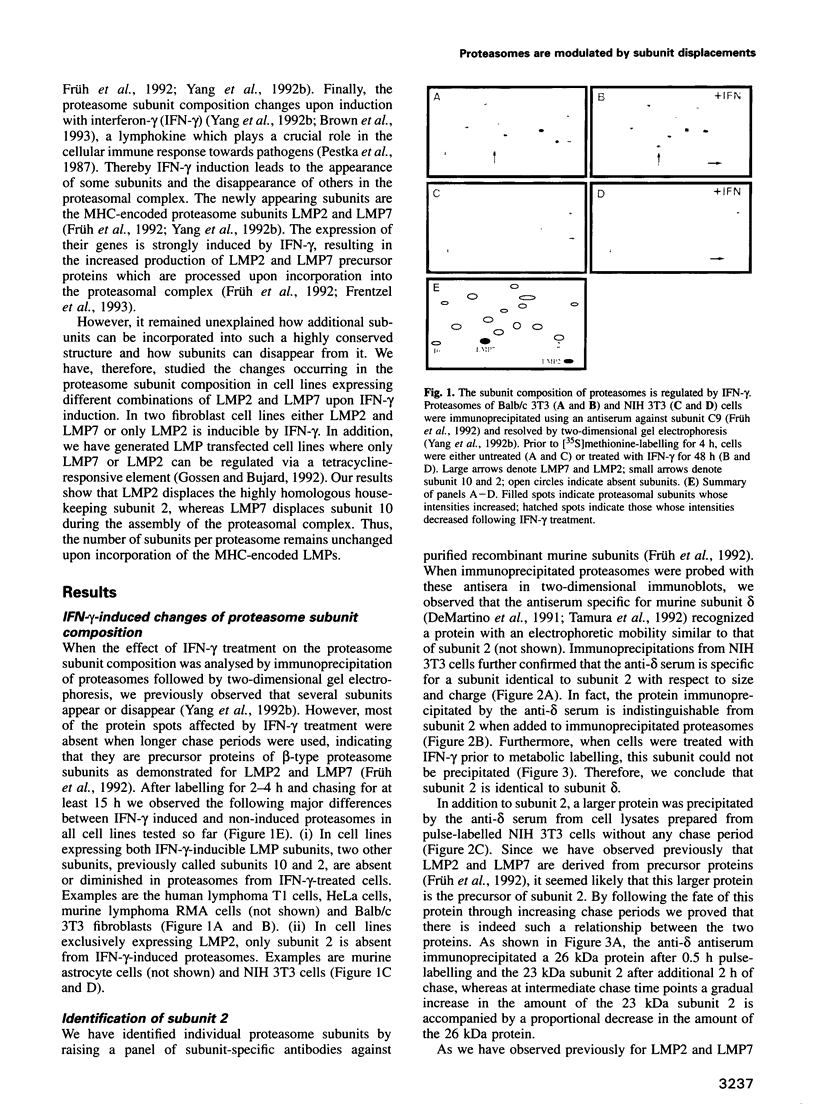
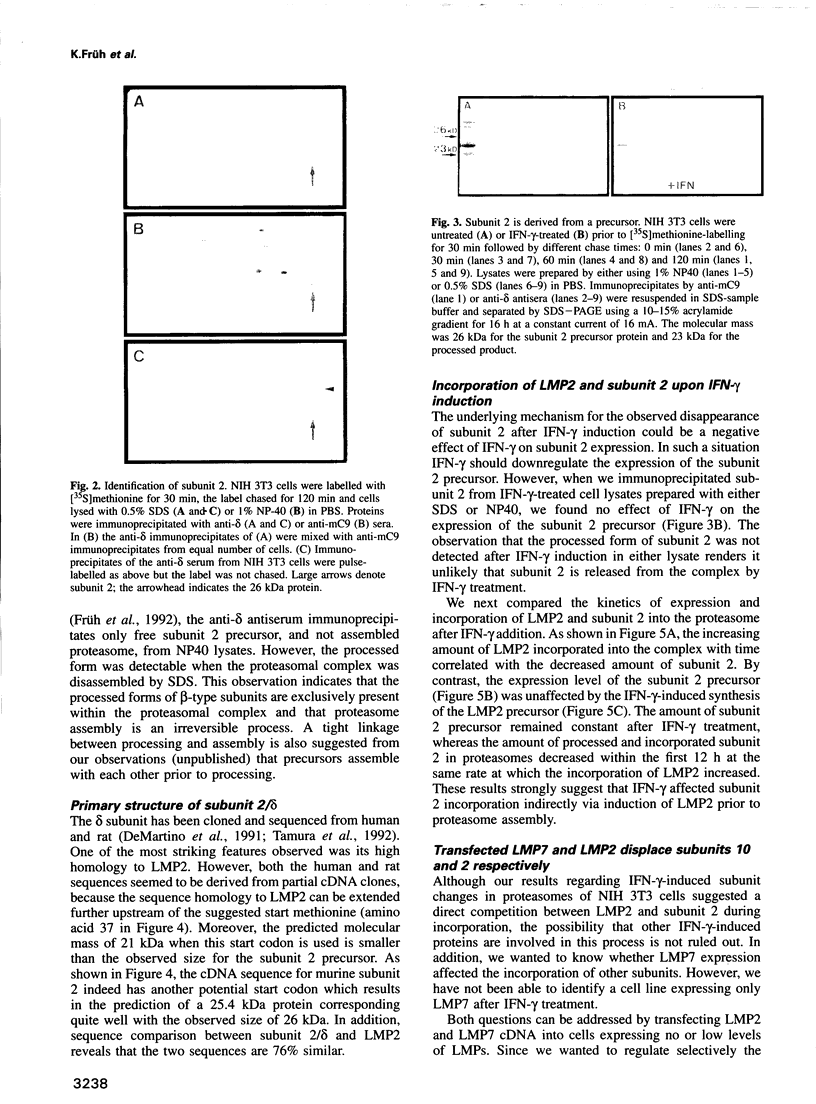
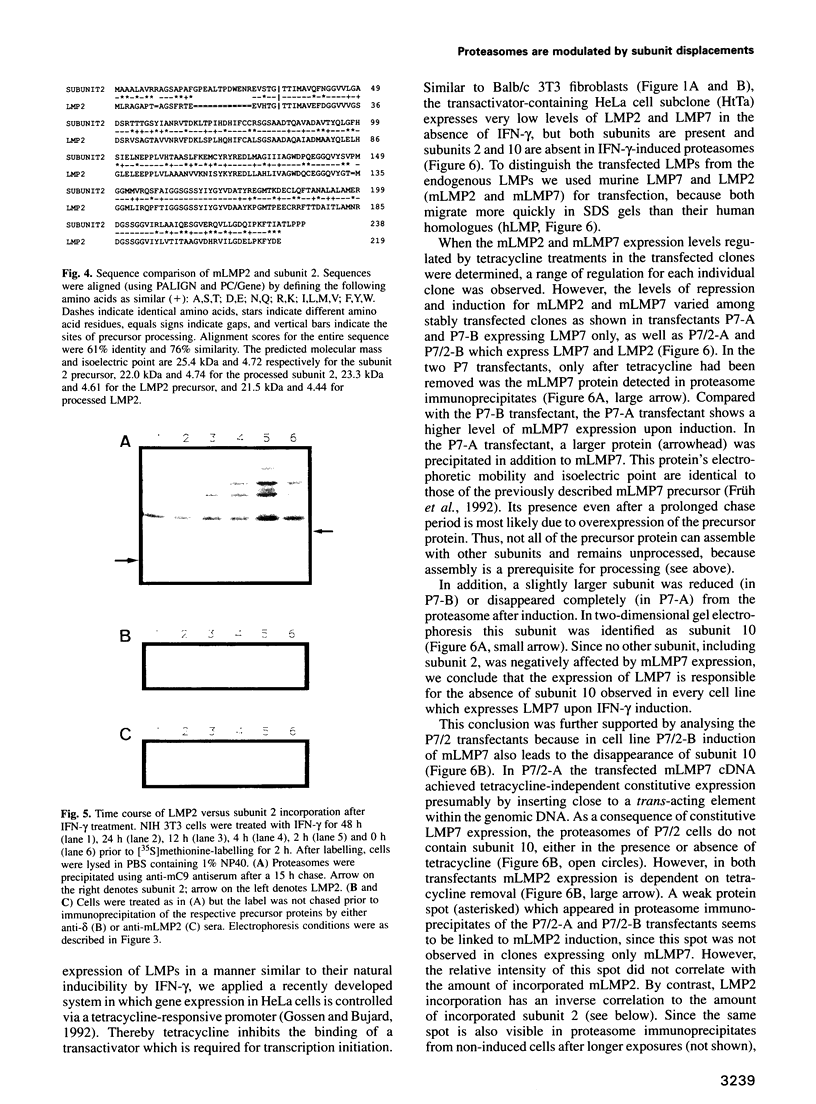
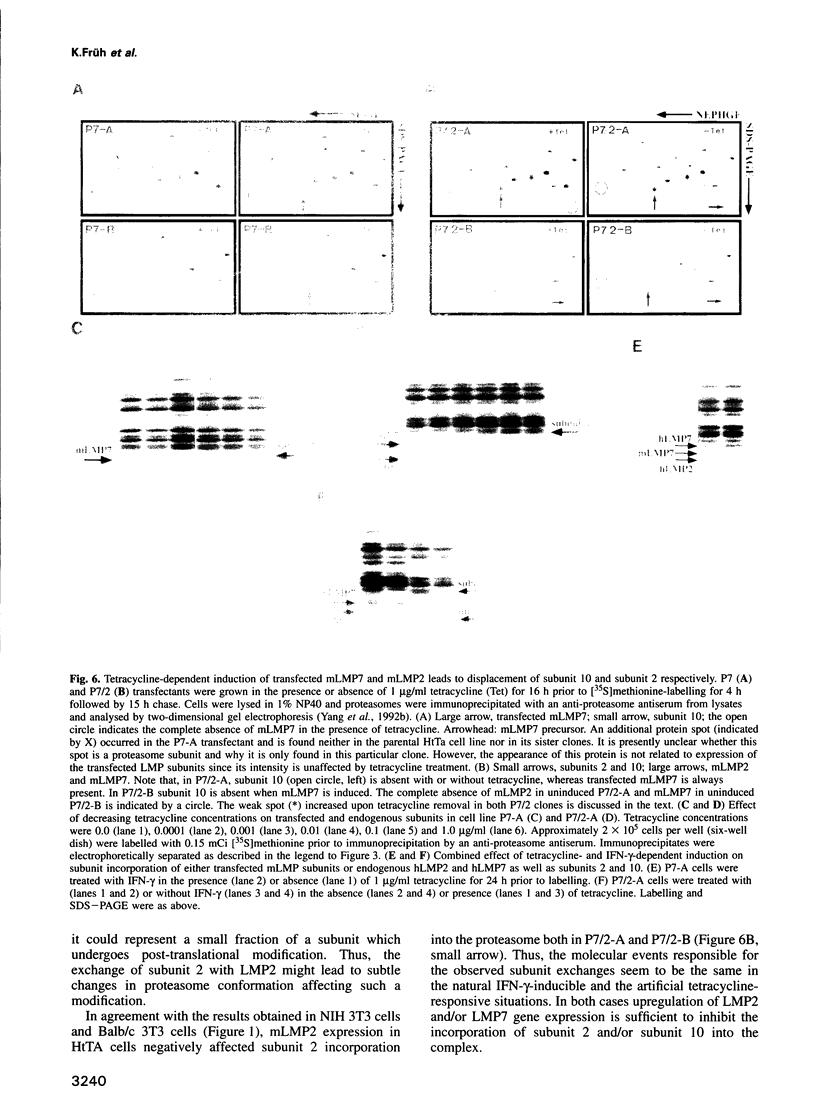
The degradation of cytoplasmic antigens to peptides presented by class I MHC molecules is thought to be mediated by the ubiquitin/proteasome pathway. Support for this view came from our observation that the subunit composition of proteasomes can be changed by interferon-gamma (IFN-gamma) treatment. Thereby two subunits, LMP2 and LMP7, which are encoded in the MHC class II region, are incorporated into the proteasomal complex, whereas other subunits disappear. In the experiments reported in this communication we studied the subunit changes occurring in cell lines where the expression of LMP2 or LMP7 can be regulated individually either by IFN-gamma induction or by applying a new system to control the expression of transfected LMPs. In both situations LMP2 induction leads exclusively to the disappearance of housekeeping subunit 2, whereas LMP7 affects only subunit 10. Subunit 2 was found to be 76% homologous to LMP2. Since incorporation of LMP2 into the proteasomal complex prevents processing of the subunit 2 precursor, we conclude that LMP2 displaces subunit 2 during assembly. Subunit displacement is most likely a general mechanism to modulate the catalytic activity of the proteasomal complex without changing its structure. Furthermore, the controlled incorporation of transfected subunits into the complex offers a new approach to study proteasome function in vivo.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold D., Driscoll J., Androlewicz M., Hughes E., Cresswell P., Spies T. Proteasome subunits encoded in the MHC are not generally required for the processing of peptides bound by MHC class I molecules. Nature. 1992 Nov 12;360(6400):171–174. doi: 10.1038/360171a0. [DOI] [PubMed] [Google Scholar]
- Brown M. G., Driscoll J., Monaco J. J. MHC-linked low-molecular mass polypeptide subunits define distinct subsets of proteasomes. Implications for divergent function among distinct proteasome subsets. J Immunol. 1993 Aug 1;151(3):1193–1204. [PubMed] [Google Scholar]
- Dahlmann B., Kopp F., Kuehn L., Niedel B., Pfeifer G., Hegerl R., Baumeister W. The multicatalytic proteinase (prosome) is ubiquitous from eukaryotes to archaebacteria. FEBS Lett. 1989 Jul 17;251(1-2):125–131. doi: 10.1016/0014-5793(89)81441-3. [DOI] [PubMed] [Google Scholar]
- Dahlmann B., Kuehn L., Grziwa A., Zwickl P., Baumeister W. Biochemical properties of the proteasome from Thermoplasma acidophilum. Eur J Biochem. 1992 Sep 15;208(3):789–797. doi: 10.1111/j.1432-1033.1992.tb17249.x. [DOI] [PubMed] [Google Scholar]
- DeMartino G. N., Orth K., McCullough M. L., Lee L. W., Munn T. Z., Moomaw C. R., Dawson P. A., Slaughter C. A. The primary structures of four subunits of the human, high-molecular-weight proteinase, macropain (proteasome), are distinct but homologous. Biochim Biophys Acta. 1991 Aug 9;1079(1):29–38. doi: 10.1016/0167-4838(91)90020-z. [DOI] [PubMed] [Google Scholar]
- Dick L. R., Moomaw C. R., DeMartino G. N., Slaughter C. A. Degradation of oxidized insulin B chain by the multiproteinase complex macropain (proteasome). Biochemistry. 1991 Mar 12;30(10):2725–2734. doi: 10.1021/bi00224a022. [DOI] [PubMed] [Google Scholar]
- Don R. H., Cox P. T., Wainwright B. J., Baker K., Mattick J. S. 'Touchdown' PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 1991 Jul 25;19(14):4008–4008. doi: 10.1093/nar/19.14.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll J., Brown M. G., Finley D., Monaco J. J. MHC-linked LMP gene products specifically alter peptidase activities of the proteasome. Nature. 1993 Sep 16;365(6443):262–264. doi: 10.1038/365262a0. [DOI] [PubMed] [Google Scholar]
- Driscoll J., Finley D. A controlled breakdown: antigen processing and the turnover of viral proteins. Cell. 1992 Mar 6;68(5):823–825. doi: 10.1016/0092-8674(92)90024-7. [DOI] [PubMed] [Google Scholar]
- Emori Y., Tsukahara T., Kawasaki H., Ishiura S., Sugita H., Suzuki K. Molecular cloning and functional analysis of three subunits of yeast proteasome. Mol Cell Biol. 1991 Jan;11(1):344–353. doi: 10.1128/mcb.11.1.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frentzel S., Gräf U., Hämmerling G. J., Kloetzel P. M. Isolation and characterization of the MHC linked beta-type proteasome subunit MC13 cDNA. FEBS Lett. 1992 May 11;302(2):121–125. doi: 10.1016/0014-5793(92)80420-l. [DOI] [PubMed] [Google Scholar]
- Frentzel S., Kuhn-Hartmann I., Gernold M., Gött P., Seelig A., Kloetzel P. M. The major-histocompatibility-complex-encoded beta-type proteasome subunits LMP2 and LMP7. Evidence that LMP2 and LMP7 are synthesized as proproteins and that cellular levels of both mRNA and LMP-containing 20S proteasomes are differentially regulated. Eur J Biochem. 1993 Aug 15;216(1):119–126. doi: 10.1111/j.1432-1033.1993.tb18123.x. [DOI] [PubMed] [Google Scholar]
- Früh K., Yang Y., Arnold D., Chambers J., Wu L., Waters J. B., Spies T., Peterson P. A. Alternative exon usage and processing of the major histocompatibility complex-encoded proteasome subunits. J Biol Chem. 1992 Nov 5;267(31):22131–22140. [PubMed] [Google Scholar]
- Fujiwara T., Tanaka K., Orino E., Yoshimura T., Kumatori A., Tamura T., Chung C. H., Nakai T., Yamaguchi K., Shin S. Proteasomes are essential for yeast proliferation. cDNA cloning and gene disruption of two major subunits. J Biol Chem. 1990 Sep 25;265(27):16604–16613. [PubMed] [Google Scholar]
- Gaczynska M., Rock K. L., Goldberg A. L. Gamma-interferon and expression of MHC genes regulate peptide hydrolysis by proteasomes. Nature. 1993 Sep 16;365(6443):264–267. doi: 10.1038/365264a0. [DOI] [PubMed] [Google Scholar]
- Glynne R., Powis S. H., Beck S., Kelly A., Kerr L. A., Trowsdale J. A proteasome-related gene between the two ABC transporter loci in the class II region of the human MHC. Nature. 1991 Sep 26;353(6342):357–360. doi: 10.1038/353357a0. [DOI] [PubMed] [Google Scholar]
- Gossen M., Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grziwa A., Baumeister W., Dahlmann B., Kopp F. Localization of subunits in proteasomes from Thermoplasma acidophilum by immunoelectron microscopy. FEBS Lett. 1991 Sep 23;290(1-2):186–190. doi: 10.1016/0014-5793(91)81256-8. [DOI] [PubMed] [Google Scholar]
- Haass C., Kloetzel P. M. The Drosophila proteasome undergoes changes in its subunit pattern during development. Exp Cell Res. 1989 Jan;180(1):243–252. doi: 10.1016/0014-4827(89)90228-0. [DOI] [PubMed] [Google Scholar]
- Heinemeyer W., Gruhler A., Möhrle V., Mahé Y., Wolf D. H. PRE2, highly homologous to the human major histocompatibility complex-linked RING10 gene, codes for a yeast proteasome subunit necessary for chrymotryptic activity and degradation of ubiquitinated proteins. J Biol Chem. 1993 Mar 5;268(7):5115–5120. [PubMed] [Google Scholar]
- Heinemeyer W., Kleinschmidt J. A., Saidowsky J., Escher C., Wolf D. H. Proteinase yscE, the yeast proteasome/multicatalytic-multifunctional proteinase: mutants unravel its function in stress induced proteolysis and uncover its necessity for cell survival. EMBO J. 1991 Mar;10(3):555–562. doi: 10.1002/j.1460-2075.1991.tb07982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. The ubiquitin system for protein degradation. Annu Rev Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- Kelly A., Powis S. H., Glynne R., Radley E., Beck S., Trowsdale J. Second proteasome-related gene in the human MHC class II region. Nature. 1991 Oct 17;353(6345):667–668. doi: 10.1038/353667a0. [DOI] [PubMed] [Google Scholar]
- Lilley K. S., Davison M. D., Rivett A. J. N-terminal sequence similarities between components of the multicatalytic proteinase complex. FEBS Lett. 1990 Mar 26;262(2):327–329. doi: 10.1016/0014-5793(90)80220-d. [DOI] [PubMed] [Google Scholar]
- Martinez C. K., Monaco J. J. Homology of proteasome subunits to a major histocompatibility complex-linked LMP gene. Nature. 1991 Oct 17;353(6345):664–667. doi: 10.1038/353664a0. [DOI] [PubMed] [Google Scholar]
- Matsumura M., Fremont D. H., Peterson P. A., Wilson I. A. Emerging principles for the recognition of peptide antigens by MHC class I molecules. Science. 1992 Aug 14;257(5072):927–934. doi: 10.1126/science.1323878. [DOI] [PubMed] [Google Scholar]
- Michalek M. T., Grant E. P., Gramm C., Goldberg A. L., Rock K. L. A role for the ubiquitin-dependent proteolytic pathway in MHC class I-restricted antigen presentation. Nature. 1993 Jun 10;363(6429):552–554. doi: 10.1038/363552a0. [DOI] [PubMed] [Google Scholar]
- Momburg F., Ortiz-Navarrete V., Neefjes J., Goulmy E., van de Wal Y., Spits H., Powis S. J., Butcher G. W., Howard J. C., Walden P. Proteasome subunits encoded by the major histocompatibility complex are not essential for antigen presentation. Nature. 1992 Nov 12;360(6400):174–177. doi: 10.1038/360174a0. [DOI] [PubMed] [Google Scholar]
- Orlowski M. The multicatalytic proteinase complex, a major extralysosomal proteolytic system. Biochemistry. 1990 Nov 13;29(45):10289–10297. doi: 10.1021/bi00497a001. [DOI] [PubMed] [Google Scholar]
- Ortiz-Navarrete V., Seelig A., Gernold M., Frentzel S., Kloetzel P. M., Hämmerling G. J. Subunit of the '20S' proteasome (multicatalytic proteinase) encoded by the major histocompatibility complex. Nature. 1991 Oct 17;353(6345):662–664. doi: 10.1038/353662a0. [DOI] [PubMed] [Google Scholar]
- Pestka S., Langer J. A., Zoon K. C., Samuel C. E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Pühler G., Weinkauf S., Bachmann L., Müller S., Engel A., Hegerl R., Baumeister W. Subunit stoichiometry and three-dimensional arrangement in proteasomes from Thermoplasma acidophilum. EMBO J. 1992 Apr;11(4):1607–1616. doi: 10.1002/j.1460-2075.1992.tb05206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechsteiner M., Hoffman L., Dubiel W. The multicatalytic and 26 S proteases. J Biol Chem. 1993 Mar 25;268(9):6065–6068. [PubMed] [Google Scholar]
- Richter-Ruoff B., Heinemeyer W., Wolf D. H. The proteasome/multicatalytic-multifunctional proteinase. In vivo function in the ubiquitin-dependent N-end rule pathway of protein degradation in eukaryotes. FEBS Lett. 1992 May 11;302(2):192–196. doi: 10.1016/0014-5793(92)80438-m. [DOI] [PubMed] [Google Scholar]
- Rivett A. J. Proteasomes: multicatalytic proteinase complexes. Biochem J. 1993 Apr 1;291(Pt 1):1–10. doi: 10.1042/bj2910001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivett A. J. The multicatalytic proteinase. Multiple proteolytic activities. J Biol Chem. 1989 Jul 25;264(21):12215–12219. [PubMed] [Google Scholar]
- Schumacher T. N., Kantesaria D. V., Heemels M. T., Ashton-Rickardt P. G., Shepherd J. C., Fruh K., Yang Y., Peterson P. A., Tonegawa S., Ploegh H. L. Peptide length and sequence specificity of the mouse TAP1/TAP2 translocator. J Exp Med. 1994 Feb 1;179(2):533–540. doi: 10.1084/jem.179.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Shimbara N., Aki M., Ishida N., Bey F., Scherrer K., Tanaka K., Ichihara A. Molecular cloning of cDNAs for rat proteasomes: deduced primary structures of four other subunits. J Biochem. 1992 Oct;112(4):530–534. doi: 10.1093/oxfordjournals.jbchem.a123933. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Tamura T., Yoshimura T., Ichihara A. Proteasomes: protein and gene structures. New Biol. 1992 Mar;4(3):173–187. [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Townsend A., Trowsdale J. The transporters associated with antigen presentation. Semin Cell Biol. 1993 Feb;4(1):53–61. doi: 10.1006/scel.1993.1007. [DOI] [PubMed] [Google Scholar]
- Yang Y., Früh K., Chambers J., Waters J. B., Wu L., Spies T., Peterson P. A. Major histocompatibility complex (MHC)-encoded HAM2 is necessary for antigenic peptide loading onto class I MHC molecules. J Biol Chem. 1992 Jun 15;267(17):11669–11672. [PubMed] [Google Scholar]
- Yang Y., Waters J. B., Früh K., Peterson P. A. Proteasomes are regulated by interferon gamma: implications for antigen processing. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4928–4932. doi: 10.1073/pnas.89.11.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R. Brefeldin A specifically inhibits presentation of protein antigens to cytotoxic T lymphocytes. Science. 1989 Jun 2;244(4908):1072–1075. doi: 10.1126/science.2471266. [DOI] [PubMed] [Google Scholar]
- Zwickl P., Grziwa A., Pühler G., Dahlmann B., Lottspeich F., Baumeister W. Primary structure of the Thermoplasma proteasome and its implications for the structure, function, and evolution of the multicatalytic proteinase. Biochemistry. 1992 Feb 4;31(4):964–972. doi: 10.1021/bi00119a004. [DOI] [PubMed] [Google Scholar]
- de la Luna S., Soria I., Pulido D., Ortín J., Jiménez A. Efficient transformation of mammalian cells with constructs containing a puromycin-resistance marker. Gene. 1988;62(1):121–126. doi: 10.1016/0378-1119(88)90585-9. [DOI] [PubMed] [Google Scholar]