Abstract
Complex three-dimensional (3-D) heart structure is an important determinant of cardiac electrical and mechanical function. In this study, we set to develop a versatile tissue-engineered system that can promote important aspects of cardiac functional maturation and reproduce variations in myofiber directions present in native ventricular epicardium. We cultured neonatal rat cardiomyocytes within a 3-D hydrogel environment using microfabricated elastomeric molds with hexagonal posts. By varying individual post orientations along the directions derived from diffusion tensor magnetic resonance imaging (DTMRI) maps of human ventricle, we created large (2.5 × 2.5 cm2) 3-D cardiac tissue patches with cardiomyocyte alignment that replicated human epicardial fiber orientations. After 3 weeks of culture, the advanced structural and functional maturation of the engineered 3-D cardiac tissues compared to age-matched 2-D monolayers was evident from: 1) the presence of dense, aligned and electromechanically-coupled cardiomyocytes, quiescent fibroblasts, and interspersed capillary-like structures, 2) action potential propagation with near-adult conduction velocity and directional dependence on local cardiomyocyte orientation, and 3) robust formation of T-tubules aligned with Z-disks, co-localization of L-type Ca2+ channels and ryanodine receptors, and accelerated Ca2+ transient kinetics. This biomimetic tissue-engineered platform can enable systematic in vitro studies of cardiac structure-function relationships and promote the development of advanced tissue engineering strategies for cardiac repair and regeneration.
Keywords: Cardiac Tissue Engineering, Hydrogel, Calcium, Electrophysiology, T-tubules
Introduction
The complex anisotropic structure of the native myocardium, including spatially varying 3-D orientations of myocardial fibers [1, 2], governs coordinated electrical activity and efficient pumping of the heart. Conversely, abnormalities in cardiac tissue structure caused by myocardial diseases or congenital defects can severely compromise cardiac function including induction of lethal arrhythmias [3, 4]. To investigate the roles of cardiac micro- and macrostructure in action potential conduction in vitro, we previously combined high-resolution cell micropatterning and diffusion tensor magnetic resonance imaging (DTMRI) to create 2-D cultures (monolayers) of neonatal rat cardiomyocytes replicating realistic structure of ventricular tissue [5]. Electrophysiological studies in these cultures revealed that intrinsic variations in intramural cardiac fiber orientation underlie the spatial non-uniformity of action potential conduction and directly determine the likelihood, location, and spatiotemporal dynamics of conduction block [6, 7].
While these and many other studies in 2-D cardiomyocyte cultures [8–11] have provided important insights into cardiac function and pathology, the use of 2-D cell cultures is highly limited in its ability to faithfully represent the natural 3-D microenvironment of native tissue. In particular, 2-D cultured cardiomyocytes are firmly adhered to a rigid substrate which dramatically alters their shape and mechanical loading, and in turn can adversely affect their differentiation, hypertrophy, and electromechanical function [12, 13]. Furthermore, concentrations of extracellular soluble factors, oxygen content, and cellular composition (e.g. absence of microvasculature) in cultured cardiac monolayers significantly differ from those of native myocardium. The above differences from in vivo environment are believed to yield a relatively “immature” phenotype of 2-D cultured primary or pluripotent stem cell-derived cardiomyocytes which regardless of the culture duration never attain true rod shape, membrane T-tubules, or polarized intercellular junctions characteristic of adult tissue [14–17].
Over the last fifteen years, various 3-D cardiomyocyte culture systems have been utilized to better reproduce the native tissue microenvironment in vitro; however, methods to precisely control local and regional cell alignment within these engineered tissue constructs are still lacking. Furthermore, while cardiomyocytes in both 2-D and 3-D culture environments can exhibit a bi-nucleated, elongated, and striated phenotype [18–20], their ability to attain mature excitation-contraction coupling machinery, including the robust formation of T-tubules or co-localization of L-type Ca2+ channels and ryanodine receptors (RyR), have not been previously shown. Building on our previous work in 2-D cardiac monolayers [5], we set to develop a versatile fabrication approach to generate relatively large (2.5×2.5cm2) 3-D cardiac tissue patches in which cardiomyocytes are locally aligned to reproduce DTMRI-measured orientation of epicardial fibers from human ventricle. We hypothesized that compared to age-matched 2-D monolayers, this tissue-engineered cardiomimetic 3-D environment will significantly promote structural and functional maturation of neonatal rat cardiomyocytes towards the adult phenotype. To test this hypothesis, we systematically compared 2-D and 3-D cardiomyocyte cultures with respect to T-tubulation, distribution of L-type Ca2+ channels and RyRs, capillary formation, action potential propagation, and generation of Ca2+ transients.
Materials and Methods
A detailed description of experimental methods is provided in the Online Data Supplement.
Tissue Mold Fabrication
Elastomeric polydimethylsiloxane (PDMS) molds were designed to allow reproducible generation of cardiac tissue patches with anatomically accurate cell orientations based on the 3-D DTMRI fiber direction map of a human ventricle (kindly provided by Drs. Helm, Winslow, and McVeigh via http://www.ccbm.jhu.edu). Briefly, epicardial projections of DTMRI-measured fiber direction vectors were represented by 2 mm long, 200 μm wide hexagonally shaped quivers and printed on a transparence photomask (Figure 1A–1D). The high aspect-ratio soft lithography technique was employed to generate corresponding master silicon wafers [21]. Sacrificial agar templates were cast of silicon wafers and used for reproducible fabrication of intact PDMS tissue molds. The resulting PDMS molds contained 2 mm tall posts with shape and orientation corresponding to that of the DTMRI-derived photomask quivers (Figure 1E).
Figure 1. Fabrication of 3-D cardiac tissue patches with DTMRI-derived human epicardial fiber orientations.
A) A human ventricle reconstructed from DTMRI data by surface rendering. B) Schematic showing a vector projection C of a 3-D DTMRI fiber orientation vector A onto the epicardial surface (with surface normal vector B). C) Map of in-plane fiber orientation vectors from the selected epicardial region in A. D) Corresponding photomask with transparent 2-D quivers positioned along the streamlines generated from the vector map in C. E) PDMS mold with hexagonal posts corresponding to photomask quivers in D. Inset, Velcro® frame pinned within the PDMS mold. F) Resulting 3-week old tissue patch removed from the PDMS mold by a pair of forceps. The patch is attached to the frame by thin side connections that allow easy detachment by scissors.
Cell Isolation and Tissue Culture
All experiments involving animals conformed to the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, Revised 1996) and an animal protocol approved by Duke Animal Care and Use Committee. Neonatal rat ventricular cardiomyocytes (NRVMs) were isolated as previously described [7, 22, 23] and mixed at a density of 5×106 cells/ml with prepared gel solution [21] containing 2 mg/ml fibrinogen (Sigma) and 10% (vol/vol) Matrigel (BD Bioscience). One ml of cell/gel solution was injected into PDMS molds containing square Velcro frames and left to polymerize. Obtained 3-D tissue constructs were statically cultured for 3 weeks (Figure 1F). NRVMs were also plated at a density of 2×103 cells/mm2 on flat or grooved (to induce cell alignment [22]) fibronectin-coated PDMS films [24] and maintained for 3 weeks. Media in monolayer cultures was supplemented with 0.1 mM Bromodeoxyuridine (BrdU, Sigma) to inhibit non-myocyte proliferation.
Morphometry and Immunofluorescence Analysis
Local and global cardiomyocyte alignment and morphometric parameters of the tissue patches were quantified as previously described [5, 25] Cells in 3-D constructs and 2-D monolayers were immunostained [19, 20], visualized by confocal microscopy, and analyzed for the presence of fibroblasts, vascular structures, T-tubulation, and distribution of L-type Ca2+ channels and RyRs.
Mapping of Intracellular Ca2+ Transients
NRVMs in 3-D constructs and 2-D monolayers were stained with 5μM Ca2+-sensitive dye Rhod-2 (Invitrogen, Carlsbad, CA), excited by green light (520±33 nm), and imaged using CMOS (Ultima-L, SciMedia) or EMCCD (iXon3 860, Andor) cameras and custom-designed optics. Bipolar point or line electrodes were used to locally stimulate NRVMs and initiate action potential propagation. Conduction velocities were estimated from mapped Ca2+ waves with activation times assigned as times of maximum upstroke of Ca2+ transients. The shape of Ca2+ transient was characterized by applying field shock to simultaneously excite the cells in the patch followed by measurement of time-to-peak (TPT, from activation time to peak of the transient), 50% relaxation time (RT50, the time from the peak to 50% recovery), and 80% relaxation time (RT80, the time from the peak to 80% recovery) [26].
Statistical Analysis
All averaged data were expressed as mean ± SD. Data sets were analyzed using student's t-test, circular statistics, and Pearson's correlation coefficient. The p < 0.05 was considered significant.
Results
Control of Cardiomyocyte Orientation in 3-D Cardiac Tissue Patches
After 3 weeks of culture, engineered cardiac tissue patches contained uniformly distributed NRVMs (Figure 2A). Due to significant hydrogel compaction and tissue remodeling, the average thickness of the tissue patch was reduced ~9 fold, from 2 mm to 219 ± 18 μm (n = 6). Within the patch, longitudinal ends of individual hexagonal posts served as local anchor points for the hydrogel, yielding the formation of elliptical pores and compaction of inter-pore regions into tissue bundles with the average width of 340 ± 40 μm (n = 20 bundles). Cells in the 3-D patches were densely and uniformly packed throughout the entire tissue volume (as observed in both the optical coherence tomography (OCT) volumetric images (Figure 2B) and confocal cross-sections of tissue bundles (Figure 2C1), and were strongly aligned within the tissue bundles between the pores (Figure 2C3). The mean directions of cell alignment within the tissue areas around individual hexagonal posts showed minimal deviation (mean absolute angle difference 1° ± 0.3°, 10 posts per patch, n = 4 patches) from the original post directions set by the photomask and DTMRI vectors (Figure 1C). These results demonstrated the ability to reproducibly engineer large 3-D cardiac tissue patches with local cell orientations that accurately replicated in-plane directions of human epicardial fibers.
Figure 2. Morphometric assessment of cardiac tissue patches with DTMRI-derived epicardial fiber directions.
A) Representative composite image of the entire cardiac tissue patch. The mean local cardiomyocyte orientations (denoted by white arrows) in dashed rectangular regions coincide with the directions of corresponding hexagonal posts (cyan). B) A 3-D volumetric image acquired by live OCT imaging reveals uniform cellular distribution throughout the patch. C1–2) Representative confocal images of F-actin-labeled cardiomyocytes in tissue bundles (C1) and nodes (C2) formed within the patch (denoted also in A). Front view of the tissue bundle (top) in C1 shows uniform cell density throughout the tissue thickness. Cardiomyocytes were highly aligned within tissue bundles (C1) and to a less degree in nodes (C2). C3) Quantified cell alignment in tissue bundles and nodes. *, denotes statistical significance (n = 4 patches, 6 bundle and node areas analyzed per patch).
Electromechanical Coupling and Action Potential Propagation in 3-D Tissue Patches with Realistic Human Epicardial Fiber Directions
From the immunostaining analysis, the three-week old tissue patches contained dense, elongated and highly aligned cardiomyocytes that robustly expressed the gap junctional protein connexin 43 (Cx43, Figure 3A). As characteristic of neonatal cardiomyocytes, Cx43 gap junctions were uniformly distributed in cell membrane and co-localized with mechanical junctions visualized by immunostaining for N-cadherin (Figure 3B1) and desmoplakin (Figure 3B2). This robust intercellular coupling was associated with continuous action potential propagation over the entire patch area (Supplementary Movie 1 and Fig. 4A, shown during 2 Hz point pacing). Conduction velocities (CVs) measured at locations where the angle between the direction of propagating Ca2+ waves and local cardiomyocyte orientation was 0° (longitudinal), 45°, and 90° (transverse) amounted to 36.1±7.5 cm/s, 29.9 ± 7.2 cm/s and 22.9 ± 4.3 cm/s, respectively (n = 6, Figure 4B), demonstrating the expected physiological dependence of local velocity of impulse conduction on underlying direction of cardiomyocyte alignment [7, 27].
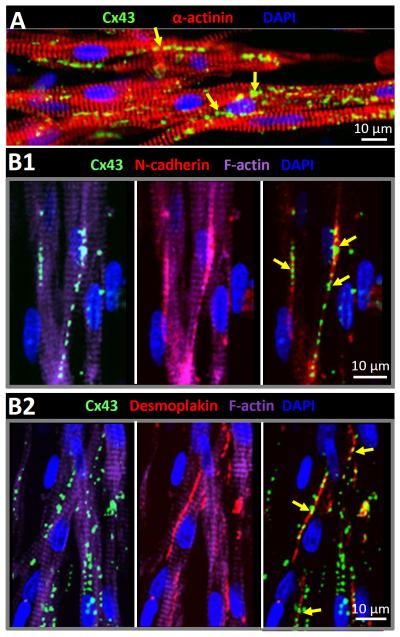
Figure 3. Distribution of electrical and mechanical junctions in 3-D cultured cardiomyocytes.
A) Uniform membrane distribution of gap junction protein connexin 43 (Cx43) in aligned cardiomyocytes within a tissue patch. B1–2) Connexin 43 protein in cardiomyocyte membrane was predominantly colocalized (yellow arrows) with cell-cell adhesion proteins, N-cadherin (B1) and desmoplakin (B2), indicating the formation of gap junctions in membrane regions where abutting cardiomyocytes also underwent mechanical coupling. Shown are representative examples from 3-week old tissue patches.
Figure 4. Electrical conduction in DTMRI-derived tissue patches with human epicardial fiber directions.
A) Representative activation map of propagating Ca2+ wave in 3-week old tissue patches, elicited by point stimuli (pulse sign) from the bottom left corner. See also Supplementary Movie 1. Red dashed lines denote the direction of long axes of elliptical pores (i..e direction of local cardiomyocyte orientation). Red arrows denote the direction of propagating wave along which local CVs were measured at 0° (longitudinal), 45°, and 90° (transverse) relative to the pore (and cardiomyocyte) orientation. B) Local CVs were increased as the propagation directions was more aligned with directio nof underlying cardiomyocytes. *, denotes statistical significance (n = 6 patches, 3 locations per patch for each CV group).
Robust T-tubulation of Cardiomyocytes in 3-D Tissue Patches
Interestingly, cell membrane stainings in 3-week old aligned tissue patches revealed deep sarcolemmal invaginations in elongated NRVMs (Figure 5A). Moreover, in these cells the two T-tubule associated proteins, caveolin-3 (Figure 5B1) and L-type Ca2+channel (Supplementary Figure S1) were distributed in a striated spatial pattern that was co-registered with the sarcomeric Z-lines. In contrast, cardiomyocytes in age-matched anisotropic NRVM monolayers cultured on PDMS microgrooves showed no or much less developed T-tubules despite having well-formed sarcomeres (Figure 5B2). The qualitatively more robust T-tubulation of NRVMs observed in the 3-D tissue patches was further confirmed by a quantitative power spectrum analysis of the caveolin-3 staining images. A peak associated with the 2 μm spacing between parallel caveolin-3-labeled T-tubules was only present in 3-week-old tissue patches, but not in age-matched anisotropic monolayers (Figure 5B3). Caveolin-3-staining was significantly better co-localized with α-actinin-stained sarcomeres in patches (Pearson's coefficient 0.64 ± 0.02) vs. monolayers (Pearson's coefficient 0.14 ± 0.03, Figure 5B4). The robust T-tubule development was also observed in randomly-oriented NRVMs in 3-week-old non-porous isotropic tissue sheets compared to age-matched isotropic monolayers cultured on PDMS films (Supplementary Figure S2) suggesting that specific 3-D environment rather than cell alignment is the main contributor to the observed T-tubulation.
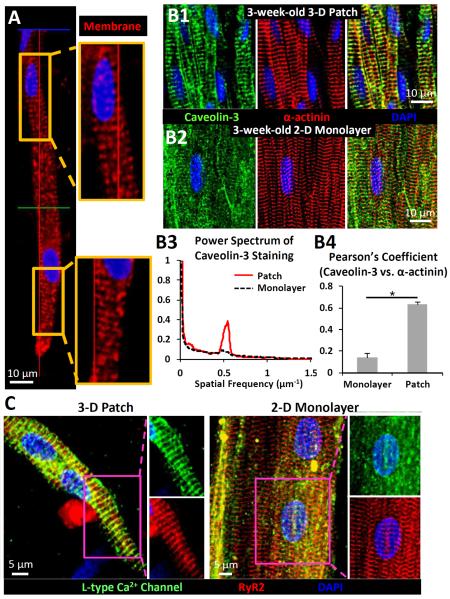
Figure 5. T-tubulation of aligned cardiomyocytes in 3-D patches vs. age-matched 2-D monolayers.
A) Representative membrane staining of aligned cardiomyocytes inside a 3-week-old patch showing parallel membrane invaginations. B1) Cardiomyocytes in aligned tissue patches show abundant T-tubules labeled by caveolin-3 which colocalize with α-actinin-labeled Z-lines. B2) No T-tubules were observed in age-matched, 3-week old anisotropic monolayers, despite the presence of parallel sarcomeres. B3) Representative power spectra of caveolin-3 stained images along the longitudinal direction of cardiomyocytes in 3-D patches and monolayers. The 0.5 μm−1 spectral peak in 3-D patches indicates a 2 μm average spacing between parallel T-tubules. B4) The degree of colocalization of caveolin-3 and α-actinin measured by Pearson's correlation coefficient. *, denotes statistical significance (n = 16 random areas from 4 patches or monolayers). C) Representative staining of cardiac ryanodine receptors (RyR2) showing cross-striated pattern in both 3-week old 3-D patches and 2-D monolayers; however, L-type Ca2+ channels co-localized with RyR2 only in 3-D patches.
Moreover, L-type Ca2+ channels were found to tightly co-localize with cardiac ryanodine receptors (RyR2) in 3-D tissue patches (Pearson's coefficient 0.82 ± 0.06) but not in age-matched monolayers (Pearson's coefficient 0.45±0.08, n = 12 random areas from 4 monolayers, Figure 5C), further demonstrating the supportive roles of patch microenvironment in both advanced T-tubulogenesis and the formation of functional E-C coupling units [28]. We also tested if the observed differences in co-localization of L-type Ca2+ channels and RyR in 3-D vs.2-D culture environment yielded functional differences in cardiomyocyte Ca2+ handling. Among the three Ca2+ transient kinetic parameters analyzed during 2 Hz pacing, TPT and RT80 were both significantly smaller in tissue patches than monolayers, while RT50 was not (Figure 6). Thus, consistent with the formation of T-tubules and change in membrane distribution of L-type Ca2+ channels, the 3-week-old NRVMs in 3-D patches exhibited faster Ca2+ transient kinetics compared to those cultured on 2-D substrates.
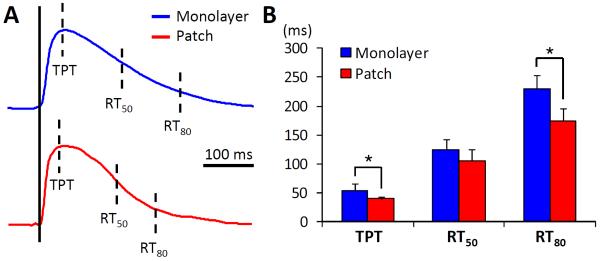
Figure 6. Ca2+ transient kinetics in cardiomyocytes from 3-D patches and age-matched 2-D monolayers.
A) Representative traces of Ca2+ transient in 3-week old aligned patches and monolayers. TPT, RT50 and RT80 denote time-to-peak, 50% and 80% relaxation time, respectively. B) Quantitative analysis demonstrates accelerated transient kinetics in patches vs. monolayers. *, denotes statistical significance (n = 8 per group).
Suppressed Proliferation of Fibroblasts and Formation of Capillary-like Structures in 3-D Tissue Patches
In the 3-week old patches, vimentin+ non-myocytes were found scattered among dense and aligned cardiomyocytes (Supplementary Figure S3). These fibroblastic cells often showed spindle-like morphology and multiple projections, distinct from the flat polymorphic shape usually observed in 2-D monolayers (not shown). Unlike in the 3-D tissue patches where percent of non-myocytes remained approximately constant during culture (<15%), the 2-D monolayers required addition of BrdU to prevent significant fibroblast overgrowth. In addition to individual vimentin+ cells, 3-D tissue patches also contained multi-cellular branching structures that stained positive for both vimentin and lectin (Figure 7A,B), suggestive of the formation of nascent capillaries. Co-staining of RECA-1, a highly specific marker for rat endothelial cells[29], and SM22-α, a protein dominantly expressed in smooth muscle cells [30], further confirmed the presence of vascular structures in which smooth muscle-like cells appeared to envelope tubules made of endothelial cells (Figure 7C). Thus, unlike 2-D cultures previously described by us and others [7, 10, 31, 32], the fibrin-based 3-D tissue patches suppressed excessive proliferation of non-myocytes and promoted the de novo formation of capillary-like structures.
Figure 7. Formation of vessel-like structures in 3-D tissue patches.
A) Lectin-positive capillary-like structures interspersed among F-actin-labeled cardiomyocytes. B) Vimentin-positive cells outline lectin-positive vessel structures. C) Independent set of vessel-like structures made of RECA-1 positive endothelial cells enveloped by SM22-α positive smooth muscle cells. Shown are representative images from 3-week old aligned patches in bundle (A) and node (B and C) area.
Application of Cardiac Patches as a Controllable Platform to Study 3-D Structure-Function Relationship in the Heart Tissue
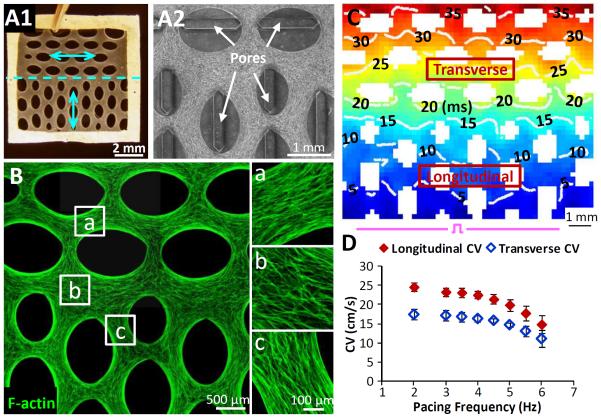
To better explore the potential effects of local alteration in cardiac fiber alignment on action potential conduction and arrhythmia induction, we fabricated proof-of-concept 7×7 mm2 tissue patches with an extreme, 90°, change in average cell orientation (Figure 8A). In these patches, the CV of planar electrical waves launched by a bipolar line electrode was significantly reduced when propagating perpendicular (transverse CV = 17.4±1.3 cm/s) vs. parallel (longitudinal CV = 25.6±1.1 cm/s) to the mean cell orientation (Figure 8C and Supplementary Movie 2). Both LCV an TCV decreased with increased pacing frequency up to 6 Hz, at which point the steady 1-to-1 capture was lost (Figure 8D). Importantly, despite multiple trials, application of rapid pacing induced no conduction block at the border between the longitudinal and transverse regions of the patch. Moreover, the partial conduction block that eventually occurred at the pacing site did not result in any episodes of transient or sustained arrhythmias.
Figure 8. Electrical conduction in 3-D tissue patches with abrupt change in cardiomyocyte orientation.
A1–2) Representative images of 7×7 mm2 tissue patches with 90° change in average cardiac fiber direction (A1, cyan arrows) induced by a 90° change in pore orientation (A2). B) F-actin labeling shows the ability to sharply change local cardiomyocyte orientation within a 3D patch by altering the orientation of surrounding elliptical pores. C) Representative map of action potential propagation initiated by a bipolar line electrode (pulse sign) at the bottom of a 90° patch. Note slower wave propagatioin perpendicular (transverse) vs. parallel (longitudinal) to the long axis of elliptical pores. D) Longitudinal and transverse CV as a function of pacing rate in 90° patches (n = 8).
Discussion
We developed a hydrogel-based 3-D culture system (“cardiomimetic tissue patch”) with the capability to precisely, at a microscopic scale, control the spatial distribution of 3-D cardiomyocyte alignment. Versatility of this method was demonstrated by generating large 3-D cardiac tissue patches that accurately replicated the natural cell orientations at the human epicardial surface as derived from high resolution DTMRI data. Previously, we combined high resolution cell micropatterning techniques with DTMRI measurements to generate 2-D cell culture replicas of transverse sections of murine ventricles[5]; however, the absence of restricted extracellular space, vascular structures, macroscopic contractions, and the inability to reliably maintain long-term cultures and promote cardiomyocyte maturation, remained some of the main limitations of this 2-D culture system. In the current study, instead of utilizing micropatterned fibronectin lines to direct cell alignment on a 2-D substrate, we locally varied orientation of PDMS posts in elastomeric 3-D tissue molds to create a spatially varying tension field imposed on the hydrogel sheet that accurately guided local cardiomyocyte alignment. This tissue fabrication method is scalable and can reproducibly generate 3-D cardiac structures of different complexity without application of external electrical or mechanical stimulation.
The optimal culture conditions for neonatal cardiomyocytes should advance their structural and functional maturation at a rate that is similar to or preferably faster than that of native postnatal development. In rodents, adherens junctions and desmosomes that are uniformly distributed in cardiomytocyte membrane at birth, start to polarize towards the opposing cell ends (nascent intercalated disks) by postnatal day 20. This is followed by the polarization of gap junctions to mature intercalated discs by postnatal day 90 [16]. In our in vitro studies, membrane polarization of intercellular junction proteins (N-cadherin, desmoplakin, connexin 43) was not observed after 3 weeks of NRVM culture in either 2-D monolayers or 3-D tissue patches (Figure 3). Previously, 24 h application of cyclic stretch in 2-D NRVM cultures was shown to increase membrane polarization of both N-cadherin and connexin 43 junctions.[33, 34] While the absence of external mechanical stimulation may be responsible for the lack of junctional polarization in our cultures, previous studies in 3-D engineered heart tissues found only a random, rather than polar, distribution of cell junctions in NRVMs despite the application of cyclic stretch [35, 36]. It is however possible that the optimal stimulation regimes for induction of junctional polarization may be significantly different between 2-D and 3-D NRVM cultures due to strong dependence of cell mechanosensing on the dimensionality of surrounding matrix [37, 38].
Despite random membrane distribution of gap junctions, aligned and elongated NRVMs in tissue patches supported near-physiological levels of electrical anisotropy (longitudinal CV/transverse CV ~ 1.6) that approached values measured in neonatal rat and dog ventricles (1.7 – 2.1) [39, 40]. Furthermore, conduction velocities measured along the direction of cardiac fibers in large epicardium-mimetic tissue patches (36.1±7.5 cm/s) are the highest reported in 3-D engineered cardiac tissues and come close to values measured in adult rat ventricles [41]. Using the same tissue fabrication technique, we further explored the effects of extreme (90°) change in cardiac fiber direction on action potential conduction during rapid excitation. The failure to induce arrhythmias despite the abrupt change in the orientation of NRVMs in these 3-D cultures can be attributed to: 1) relatively small size of the patch and 2) elliptical shape of the pores which presented a gradual, rather than sharp, increase in mismatch between local current supply and downstream sink [42] when propagating waves exited the tissue area between the pores, thereby preventing the occurrence of arrhythmogenic conduction block remote from the pacing site. In the future, ion channel and coupling properties of cardiomyocytes and/or the number of cardiac fibroblasts could be varied in this 3-D culture system to systematically study structure-function relationships present in healthy and diseased cardiac tissues.
The highly coordinated contraction of adult cardiac muscle relies on both the rapid electrical conduction [42] and presence of mature E-C coupling machinery including a well-developed T-tubule system [28]. In rodent ventricular myocytes, intense T-tubulation occurs between postnatal days 10 and 20, along with the redistribution of over 80% of L-type Ca2+ channels into T-tubular membrane [14, 15]. Co-localization of these channels with RyRs from junctional sarcoplasmic reticulum yields the formation of functional E-C coupling units, increased efficiency of calcium-induced calcium release, and faster Ca2+ transient upstroke [43]. In the current study, we assessed the structural and functional maturation of the E-C coupling apparatus in NRVMs cultured in 3-D fibrin-based patches and age-matched 2-D monolayers under both anisotropic and isotropic conditions (Figure 5, Supplementary Figures S1, S2). By immunostaining analysis, we demonstrated the robust formation of T-tubules and co-localization of L-type Ca2+ channels and RyRs in cultured cardiomyocytes, and this level of structural maturity was found only when NRVMs were cultured for 3 weeks in a 3-D patch but not 2-D monolayer environment. The time needed for T-tubule development in the 3-D cultures was comparable to that documented postnatally in vivo [14, 43] and was independent on whether the cells were aligned. Moreover, similar to the functional changes found in vivo [43], NRVMs in 3-D patches exhibited faster kinetics of Ca2+ cycling than those cultured on 2-D substrates (Figure 6). Overall, our findings suggest critical and unique roles of 3-D culture microenvironment in postnatal cardiac T-tubulation and maturation of E-C coupling in vitro, but the underlying mechanisms remain largely unknown. Specifically, the roles of extracellular matrix composition and stiffness in cardiac T-tubulation and E-C coupling warrant future studies, as these environmental cues have been shown to influence cardiomyocyte maturation in 2-D culture systems [12, 13, 44].
In addition to the described effects on cardiomyocytes, the 3-D microenvironment of cardiac tissue patch had profound effects on the behavior of non-cardiomyocytes, including the limited proliferation of fibroblasts and assembly of endothelial and smooth muscle cells into capillary-like structures (Figure 7). As previously suggested, the suppressed proliferation and altered morphology of fibroblasts in 3-D compared to 2-D culture environment could be caused by specific changes in the molecular composition, organization, and function of focal-adhesion complexes and associated integrin-mediated signaling [38, 45]. Furthermore, despite similar starting cell populations in 2-D and 3-D cultures, formation of branched vessel structures in tissue patches may have been facilitated by particular hydrogel composition (including 10% v/v matrigel) and oxygen gradients that potentially formed within dense, spontaneously contracting cardiomyocyte layers in the patch. Previous studies have shown that endothelial cells in myocardial capillaries play direct modulatory roles in growth, differentiation, and electromechanical function of adjacent cardiomyocytes through local paracrine action of various cytokines, including VEGF, neuregulin, angiotensin, and others [46–48]. These bi-directional endothelial-cardiomyocyte interactions via secreted factors would be amplified within the spatially restricted extracellular space of 3-D patches compared to large extracellular bath of 2-D monolayers. This, along with other factors, could contribute to superior maturation and function observed in 3-D vs. 2-D cultured NRVMs. Of note is also that the formed capillary structures within cardiac tissue patches are unlikely to play a significant role in oxygen and nutrient transport in vitro; however, they could promote the onset of blood perfusion and patch survival in vivo [49, 50].
Conclusions
The described 3-D cell culture system enabled the recreation of realistic human epicardial tissue architecture in vitro and simultaneously promoted the structural and functional maturation of both cardiomyocytes and non-myocytes in a developmentally-mimetic fashion. This was evidenced by the robust formation of T-tubules and E-C coupling units in cardiomyocytes, accelerated Ca2+ cycling and high impulse propagation velocities approaching those of adult myocardium, as well as the assembly of endothelial and stromal cells into capillary-like structures. We expect that this versatile and scalable methodology can allow accurate in vitro studies of complex structure-function relationships present in healthy and pathologically remodeled myocardium as well as serve to develop advanced tissue engineering strategies for human cardiac repair and regeneration.
Supplementary Material
Acknowledgements
We thank Ava Krol for assistance with NRVM isolation and Dr. George Engelmayr for critical reading of the manuscript. This work was supported by the Lew's fellowship from the Center for Biomolecular and Tissue Engineering at Duke University to W.B., American Heart Association predoctoral fellowship 07155178U to Nima Badie, and NIH-NHLBI grant R01HL104326 to Nenad Bursac. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Rohmer D, Sitek A, Gullberg GT. Reconstruction and visualization of fiber and laminar structure in the normal human heart from ex vivo diffusion tensor magnetic resonance imaging (DTMRI) data. Invest Radiol. 2007;42:777–89. doi: 10.1097/RLI.0b013e3181238330. [DOI] [PubMed] [Google Scholar]
- [2].Streeter DD, Jr., Spotnitz HM, Patel DP, Ross J, Jr., Sonnenblick EH. Fiber orientation in the canine left ventricle during diastole and systole. Circ Res. 1969;24:339–47. doi: 10.1161/01.res.24.3.339. [DOI] [PubMed] [Google Scholar]
- [3].Ripplinger CM, Li W, Hadley J, Chen J, Rothenberg F, Lombardi R, et al. Enhanced transmural fiber rotation and connexin 43 heterogeneity are associated with an increased upper limit of vulnerability in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circ Res. 2007;101:1049–57. doi: 10.1161/CIRCRESAHA.107.161240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Valderrabano M, Lee MH, Ohara T, Lai AC, Fishbein MC, Lin SF, et al. Dynamics of intramural and transmural reentry during ventricular fibrillation in isolated swine ventricles. Circ Res. 2001;88:839–48. doi: 10.1161/hh0801.089259. [DOI] [PubMed] [Google Scholar]
- [5].Badie N, Satterwhite L, Bursac N. A method to replicate the microstructure of heart tissue in vitro using DTMRI-based cell micropatterning. Ann Biomed Eng. 2009;37:2510–21. doi: 10.1007/s10439-009-9815-x. [DOI] [PubMed] [Google Scholar]
- [6].Badie N, Scull JA, Klinger RY, Krol A, Bursac N. Conduction block in micropatterned cardiomyocyte cultures replicating the structure of ventricular cross-sections. Cardiovasc Res. 2012;93:263–71. doi: 10.1093/cvr/cvr304. [DOI] [PubMed] [Google Scholar]
- [7].Badie N, Bursac N. Novel micropatterned cardiac cell cultures with realistic ventricular microstructure. Biophys J. 2009;96:3873–85. doi: 10.1016/j.bpj.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Beauchamp P, Yamada KA, Baertschi AJ, Green K, Kanter EM, Saffitz JE, et al. Relative contributions of connexins 40 and 43 to atrial impulse propagation in synthetic strands of neonatal and fetal murine cardiomyocytes. Circ Res. 2006;99:1216–24. doi: 10.1161/01.RES.0000250607.34498.b4. [DOI] [PubMed] [Google Scholar]
- [9].Hou L, Deo M, Furspan P, Pandit SV, Mironov S, Auerbach DS, et al. A major role for HERG in determining frequency of reentry in neonatal rat ventricular myocyte monolayer. Circ Res. 2010;107:1503–11. doi: 10.1161/CIRCRESAHA.110.232470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bursac N, Parker KK, Iravanian S, Tung L. Cardiomyocyte cultures with controlled macroscopic anisotropy: a model for functional electrophysiological studies of cardiac muscle. Circ Res. 2002;91:e45–54. doi: 10.1161/01.res.0000047530.88338.eb. [DOI] [PubMed] [Google Scholar]
- [11].Thompson SA, Copeland CR, Reich DH, Tung L. Mechanical coupling between myofibroblasts and cardiomyocytes slows electric conduction in fibrotic cell monolayers. Circulation. 2011;123:2083–93. doi: 10.1161/CIRCULATIONAHA.110.015057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, et al. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121:3794. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jacot JG, McCulloch AD, Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J. 2008;95:3479. doi: 10.1529/biophysj.107.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ziman AP, Gomez-Viquez NL, Bloch RJ, Lederer WJ. Excitation-contraction coupling changes during postnatal cardiac development. J Mol Cell Cardiol. 2010;48:379–86. doi: 10.1016/j.yjmcc.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Seki S, Nagashima M, Yamada Y, Tsutsuura M, Kobayashi T, Namiki A, et al. Fetal and postnatal development of Ca2+ transients and Ca2+ sparks in rat cardiomyocytes. Cardiovasc Res. 2003;58:535–48. doi: 10.1016/s0008-6363(03)00255-4. [DOI] [PubMed] [Google Scholar]
- [16].Angst BD, Khan LU, Severs NJ, Whitely K, Rothery S, Thompson RP, et al. Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium. Circ Res. 1997;80:88–94. doi: 10.1161/01.res.80.1.88. [DOI] [PubMed] [Google Scholar]
- [17].Dolnikov K, Shilkrut M, Zeevi-Levin N, Gerecht-Nir S, Amit M, Danon A, et al. Functional properties of human embryonic stem cell-derived cardiomyocytes: intracellular Ca2+ handling and the role of sarcoplasmic reticulum in the contraction. Stem Cells. 2006;24:236–45. doi: 10.1634/stemcells.2005-0036. [DOI] [PubMed] [Google Scholar]
- [18].Tiburcy M, Didie M, Boy O, Christalla P, Doker S, Naito H, et al. Terminal differentiation, advanced organotypic maturation, and modeling of hypertrophic growth in engineered heart tissue. Circ Res. 2011;109:1105–14. doi: 10.1161/CIRCRESAHA.111.251843. [DOI] [PubMed] [Google Scholar]
- [19].Liau B, Christoforou N, Leong KW, Bursac N. Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function. Biomaterials. 2011;32:9180–7. doi: 10.1016/j.biomaterials.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang D, Shadrin IY, Lam J, Xian HQ, Snodgrass HR, Bursac N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials. 2013;34:5813–20. doi: 10.1016/j.biomaterials.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bian W, Liau B, Badie N, Bursac N. Mesoscopic hydrogel molding to control the 3D geometry of bioartificial muscle tissues. Nat Protoc. 2009;4:1522–34. doi: 10.1038/nprot.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].McSpadden LC, Kirkton RD, Bursac N. Electrotonic loading of anisotropic cardiac monolayers by unexcitable cells depends on connexin type and expression level. Am J Physiol Cell Physiol. 2009;297:C339–51. doi: 10.1152/ajpcell.00024.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pedrotty DM, Klinger RY, Kirkton RD, Bursac N. Cardiac fibroblast paracrine factors alter impulse conduction and ion channel expression of neonatal rat cardiomyocytes. Cardiovasc Res. 2009;83:688–97. doi: 10.1093/cvr/cvp164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kirkton RD, Bursac N. Genetic engineering of somatic cells to study and improve cardiac function. Europace. 2012;14(Suppl 5):v40–v9. doi: 10.1093/europace/eus269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bian W, Juhas M, Pfeiler TW, Bursac N. Local tissue geometry determines contractile force generation of engineered muscle networks. Tissue Eng Part A. 2012;18:957–67. doi: 10.1089/ten.tea.2011.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bian W, Bursac N. Soluble miniagrin enhances contractile function of engineered skeletal muscle. Faseb J. 2012;26:955–65. doi: 10.1096/fj.11-187575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Valderrabano M. Influence of anisotropic conduction properties in the propagation of the cardiac action potential. Prog Biophys Mol Biol. 2007;94:144–68. doi: 10.1016/j.pbiomolbio.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- [29].Duijvestijn AM, van Goor H, Klatter F, Majoor GD, van Bussel E, van Breda Vriesman PJ. Antibodies defining rat endothelial cells: RECA-1, a pan-endothelial cell-specific monoclonal antibody. Lab Invest. 1992;66:459–66. [PubMed] [Google Scholar]
- [30].Feil S, Hofmann F, Feil R. SM22alpha modulates vascular smooth muscle cell phenotype during atherogenesis. Circ Res. 2004;94:863–5. doi: 10.1161/01.RES.0000126417.38728.F6. [DOI] [PubMed] [Google Scholar]
- [31].Munoz V, Grzeda KR, Desplantez T, Pandit SV, Mironov S, Taffet SM, et al. Adenoviral expression of IKs contributes to wavebreak and fibrillatory conduction in neonatal rat ventricular cardiomyocyte monolayers. Circ Res. 2007;101:475–83. doi: 10.1161/CIRCRESAHA.107.149617. [DOI] [PubMed] [Google Scholar]
- [32].Rohr S, Scholly DM, Kleber AG. Patterned growth of neonatal rat heart cells in culture. Morphological and electrophysiological characterization. Circ Res. 1991;68:114–30. doi: 10.1161/01.res.68.1.114. [DOI] [PubMed] [Google Scholar]
- [33].Salameh A, Wustmann A, Karl S, Blanke K, Apel D, Rojas-Gomez D, et al. Cyclic mechanical stretch induces cardiomyocyte orientation and polarization of the gap junction protein connexin43. Circ Res. 2010;106:1592–602. doi: 10.1161/CIRCRESAHA.109.214429. [DOI] [PubMed] [Google Scholar]
- [34].Matsuda T, Fujio Y, Nariai T, Ito T, Yamane M, Takatani T, et al. N-cadherin signals through Rac1 determine the localization of connexin 43 in cardiac myocytes. J Mol Cell Cardiol. 2006;40:495–502. doi: 10.1016/j.yjmcc.2005.12.010. [DOI] [PubMed] [Google Scholar]
- [35].Zimmermann WH, Didie M, Wasmeier GH, Nixdorff U, Hess A, Melnychenko I, et al. Cardiac grafting of engineered heart tissue in syngenic rats. Circulation. 2002;106:I151–7. [PubMed] [Google Scholar]
- [36].Kensah G, Gruh I, Viering J, Schumann H, Dahlmann J, Meyer H, et al. A novel miniaturized multimodal bioreactor for continuous in situ assessment of bioartificial cardiac tissue during stimulation and maturation. Tissue Eng Part C Methods. 2011;17:463–73. doi: 10.1089/ten.tec.2010.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Harunaga JS, Yamada KM. Cell-matrix adhesions in 3D. Matrix Biol. 2011;30:363–8. doi: 10.1016/j.matbio.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–12. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- [39].Litchenberg W, Norman L, Holwell A, Martin K, Hewett K, Gourdie R. The rate and anisotropy of impulse propagation in the postnatal terminal crest are correlated with remodeling of Cx43 gap junction pattern. Cardiovasc Res. 2000;45:379–87. doi: 10.1016/s0008-6363(99)00363-6. [DOI] [PubMed] [Google Scholar]
- [40].den Haan AD, Veldkamp MW, Bakker D, Boink GJ, Janssen RB, de Bakker JM, et al. Organ explant culture of neonatal rat ventricles: a new model to study gene and cell therapy. PLOS ONE. 2013;8:e59290. doi: 10.1371/journal.pone.0059290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bursac N, Papadaki M, Cohen RJ, Schoen FJ, Eisenberg SR, Carrier R, et al. Cardiac muscle tissue engineering: toward an in vitro model for electrophysiological studies. Am J Physiol Heart Circ Phys. 1999;277:H433–44. doi: 10.1152/ajpheart.1999.277.2.H433. [DOI] [PubMed] [Google Scholar]
- [42].Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–88. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- [43].Escobar AL, Ribeiro-Costa R, Villalba-Galea C, Zoghbi ME, Perez CG, Mejia-Alvarez R. Developmental changes of intracellular Ca2+ transients in beating rat hearts. Am J Physiol Heart Circ Physiol. 2004;286:H971–8. doi: 10.1152/ajpheart.00308.2003. [DOI] [PubMed] [Google Scholar]
- [44].Baharvand H, Azarnia M, Parivar K, Ashtiani SK. The effect of extracellular matrix on embryonic stem cell-derived cardiomyocytes. J Mol Cell Cardiol. 2005;38:495–503. doi: 10.1016/j.yjmcc.2004.12.011. [DOI] [PubMed] [Google Scholar]
- [45].Green JA, Yamada KM. Three-dimensional microenvironments modulate fibroblast signaling responses. Adv Drug Deliv Rev. 2007;59:1293–8. doi: 10.1016/j.addr.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Narmoneva DA, Vukmirovic R, Davis ME, Kamm RD, Lee RT. Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation. 2004;110:962–8. doi: 10.1161/01.CIR.0000140667.37070.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Brutsaert DL. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev. 2003;83:59–115. doi: 10.1152/physrev.00017.2002. [DOI] [PubMed] [Google Scholar]
- [48].Hsieh PC, Davis ME, Lisowski LK, Lee RT. Endothelial-cardiomyocyte interactions in cardiac development and repair. Annu Rev Physiol. 2006;68:51–66. doi: 10.1146/annurev.physiol.68.040104.124629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lesman A, Habib M, Caspi O, Gepstein A, Arbel G, Levenberg S, et al. Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Eng Part A. 2010;16:115–25. doi: 10.1089/ten.TEA.2009.0130. [DOI] [PubMed] [Google Scholar]
- [50].Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, Muskheli V, et al. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci U S A. 2009;106:16568–73. doi: 10.1073/pnas.0908381106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.