Abstract
Antibody fragments of moderate affinity (approximately microM) can be isolated from repertoires of approximately 10(8) immunoglobulin genes by phage display and rounds of selection with antigen, and the affinities improved by further rounds of mutation and selection. Here, as an alternative strategy, we attempted to isolate high affinity human antibodies directly from large repertoires. We first created highly diverse repertoires of heavy and light chains entirely in vitro from a bank of human V gene segments and then, by recombination of the repertoires in bacteria, generated a large (close to 6.5 x 10(10)) synthetic repertoire of Fab fragments displayed on filamentous phage. From this repertoire we isolated Fab fragments which bound to a range of different antigens and haptens, and with affinities comparable with those of antibodies from a secondary immune response in mice (up to 4 nM). Although the VH-26 (DP-47) segment was the most commonly used segment in both artificial and natural repertoires, there were also major differences in the pattern of segment usage. Such comparisons may help dissect the contributions of biological mechanisms and structural features governing V gene usage in vivo.
Full text
PDF


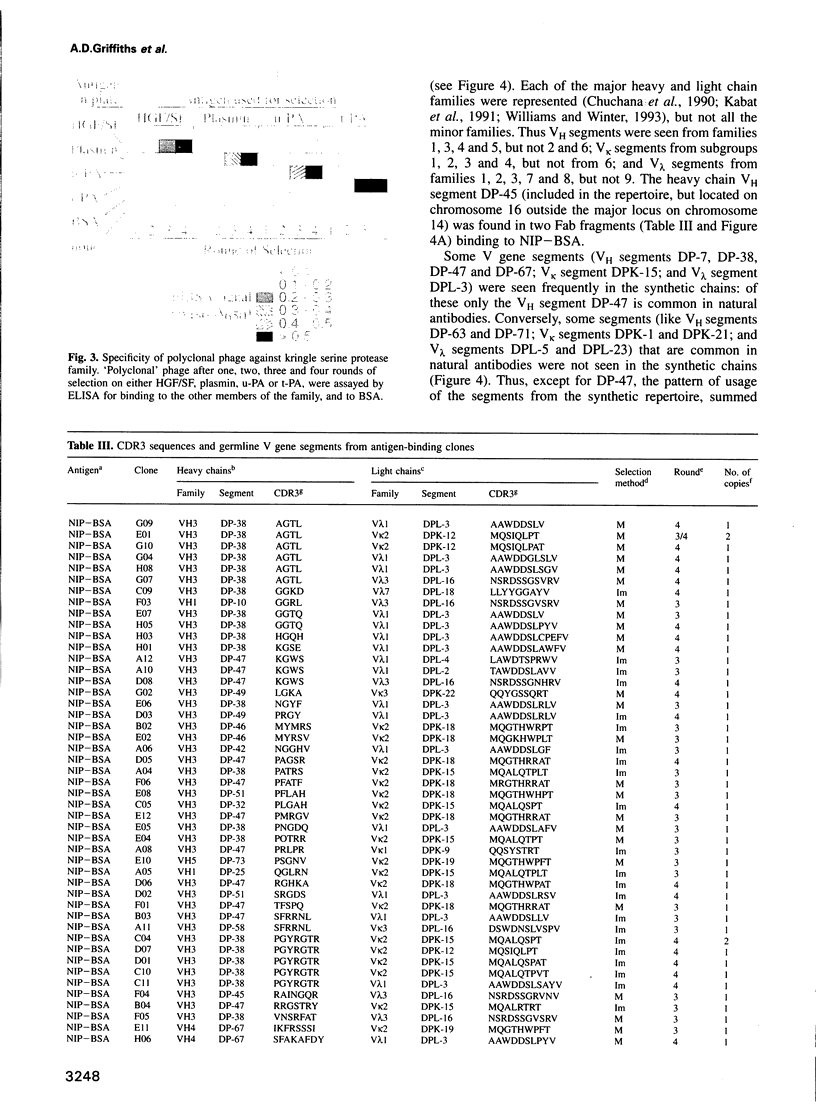
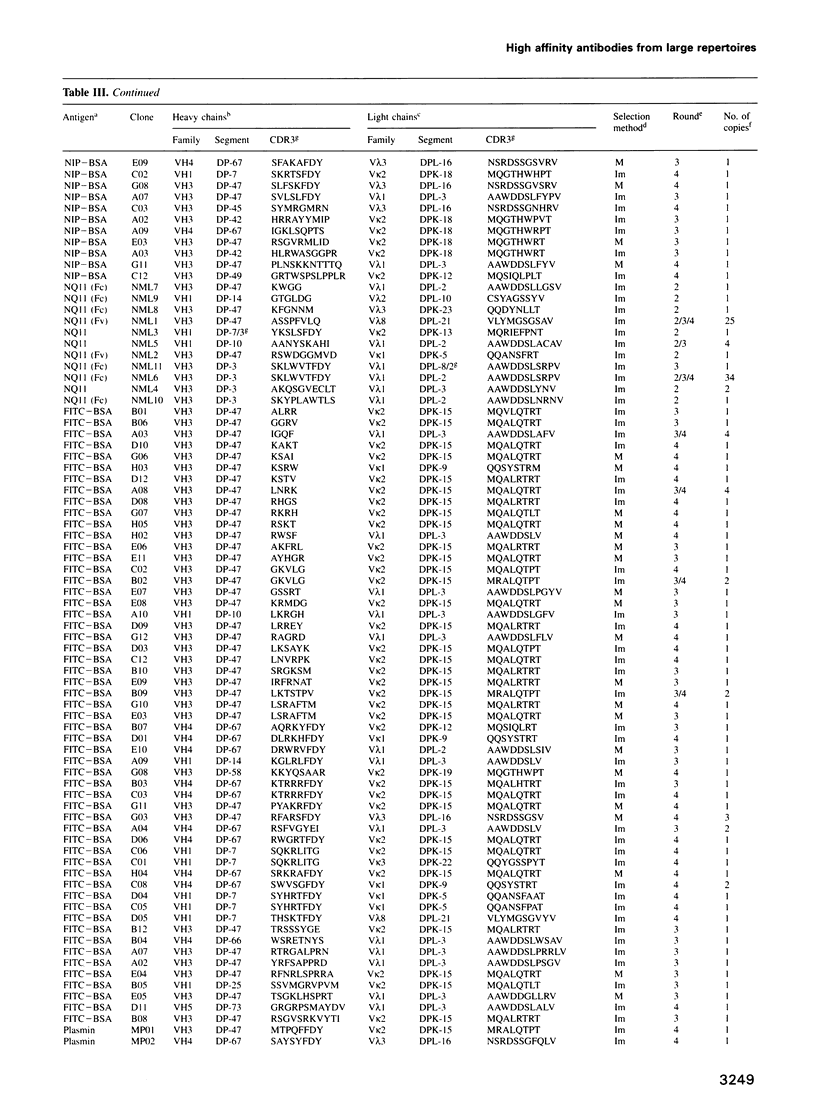
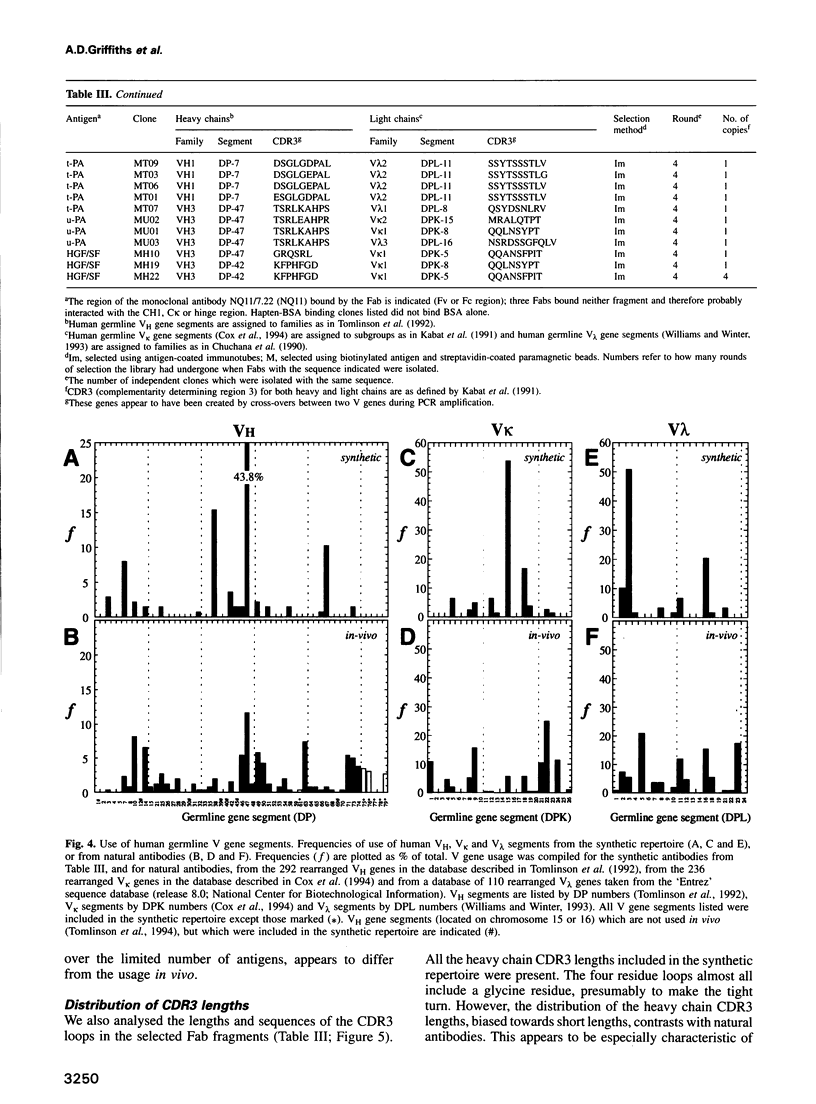
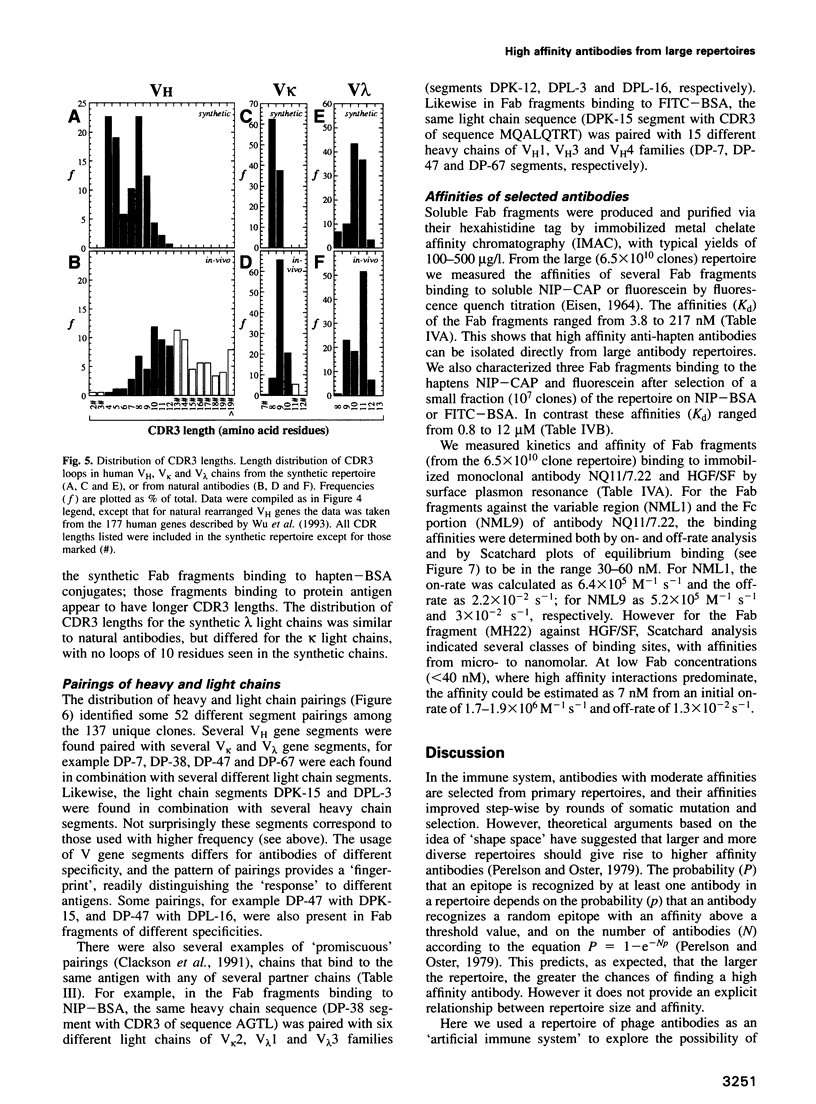





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas C. F., 3rd, Amberg W., Simoncsits A., Jones T. M., Lerner R. A. Selection of human anti-hapten antibodies from semisynthetic libraries. Gene. 1993 Dec 27;137(1):57–62. doi: 10.1016/0378-1119(93)90251-w. [DOI] [PubMed] [Google Scholar]
- Barbas C. F., 3rd, Bain J. D., Hoekstra D. M., Lerner R. A. Semisynthetic combinatorial antibody libraries: a chemical solution to the diversity problem. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4457–4461. doi: 10.1073/pnas.89.10.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas C. F., 3rd, Björling E., Chiodi F., Dunlop N., Cababa D., Jones T. M., Zebedee S. L., Persson M. A., Nara P. L., Norrby E. Recombinant human Fab fragments neutralize human type 1 immunodeficiency virus in vitro. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9339–9343. doi: 10.1073/pnas.89.19.9339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas C. F., 3rd, Crowe J. E., Jr, Cababa D., Jones T. M., Zebedee S. L., Murphy B. R., Chanock R. M., Burton D. R. Human monoclonal Fab fragments derived from a combinatorial library bind to respiratory syncytial virus F glycoprotein and neutralize infectivity. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10164–10168. doi: 10.1073/pnas.89.21.10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas C. F., 3rd, Kang A. S., Lerner R. A., Benkovic S. J. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7978–7982. doi: 10.1073/pnas.88.18.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates R. M., Ballard D. W., Voss E. W., Jr Comparative properties of monoclonal antibodies comprising a high-affinity anti-fluorescyl idiotype family. Mol Immunol. 1985 Aug;22(8):871–877. doi: 10.1016/0161-5890(85)90072-0. [DOI] [PubMed] [Google Scholar]
- Bedzyk W. D., Reinitz D. M., Voss E. W., Jr Linkage of low and high affinity anti-fluorescein idiotype families. Mol Immunol. 1986 Dec;23(12):1319–1328. doi: 10.1016/0161-5890(86)90017-9. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Breitling F., Dübel S., Seehaus T., Klewinghaus I., Little M. A surface expression vector for antibody screening. Gene. 1991 Aug 15;104(2):147–153. doi: 10.1016/0378-1119(91)90244-6. [DOI] [PubMed] [Google Scholar]
- Brownstone A., Mitchison N. A., Pitt-Rivers R. Chemical and serological studies with an iodine-containing synthetic immunological determinant 4-hydroxy-3-iodo-5-nitrophenylacetic acid (NIP) and related compounds. Immunology. 1966 May;10(5):465–479. [PMC free article] [PubMed] [Google Scholar]
- Chaiken I., Rosé S., Karlsson R. Analysis of macromolecular interactions using immobilized ligands. Anal Biochem. 1992 Mar;201(2):197–210. doi: 10.1016/0003-2697(92)90329-6. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M. Canonical structures for the hypervariable regions of immunoglobulins. J Mol Biol. 1987 Aug 20;196(4):901–917. doi: 10.1016/0022-2836(87)90412-8. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Gherardi E., Tomlinson I. M., Walter G., Marks J. D., Llewelyn M. B., Winter G. Structural repertoire of the human VH segments. J Mol Biol. 1992 Oct 5;227(3):799–817. doi: 10.1016/0022-2836(92)90224-8. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Tramontano A., Levitt M., Smith-Gill S. J., Air G., Sheriff S., Padlan E. A., Davies D., Tulip W. R. Conformations of immunoglobulin hypervariable regions. Nature. 1989 Dec 21;342(6252):877–883. doi: 10.1038/342877a0. [DOI] [PubMed] [Google Scholar]
- Chuchana P., Blancher A., Brockly F., Alexandre D., Lefranc G., Lefranc M. P. Definition of the human immunoglobulin variable lambda (IGLV) gene subgroups. Eur J Immunol. 1990 Jun;20(6):1317–1325. doi: 10.1002/eji.1830200618. [DOI] [PubMed] [Google Scholar]
- Clackson T., Hoogenboom H. R., Griffiths A. D., Winter G. Making antibody fragments using phage display libraries. Nature. 1991 Aug 15;352(6336):624–628. doi: 10.1038/352624a0. [DOI] [PubMed] [Google Scholar]
- Cox J. P., Tomlinson I. M., Winter G. A directory of human germ-line V kappa segments reveals a strong bias in their usage. Eur J Immunol. 1994 Apr;24(4):827–836. doi: 10.1002/eji.1830240409. [DOI] [PubMed] [Google Scholar]
- Cumano A., Rajewsky K. Clonal recruitment and somatic mutation in the generation of immunological memory to the hapten NP. EMBO J. 1986 Oct;5(10):2459–2468. doi: 10.1002/j.1460-2075.1986.tb04522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumano A., Rajewsky K. Structure of primary anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibodies in normal and idiotypically suppressed C57BL/6 mice. Eur J Immunol. 1985 May;15(5):512–520. doi: 10.1002/eji.1830150517. [DOI] [PubMed] [Google Scholar]
- De Bellis D., Schwartz I. Regulated expression of foreign genes fused to lac: control by glucose levels in growth medium. Nucleic Acids Res. 1990 Mar 11;18(5):1311–1311. doi: 10.1093/nar/18.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzin L. K., Voss E. W., Jr Construction, characterization, and mutagenesis of an anti-fluorescein single chain antibody idiotype family. J Biol Chem. 1992 May 5;267(13):8925–8931. [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISEN H. N. DETERMINATION OF ANTIBODY AFFINITY FOR HAPTENS AND ANTIGENS BY MEANS OF FLUORESCENCE QUENCHING. Methods Med Res. 1964;10:115–121. [PubMed] [Google Scholar]
- Foote J., Milstein C. Kinetic maturation of an immune response. Nature. 1991 Aug 8;352(6335):530–532. doi: 10.1038/352530a0. [DOI] [PubMed] [Google Scholar]
- Garrard L. J., Yang M., O'Connell M. P., Kelley R. F., Henner D. J. Fab assembly and enrichment in a monovalent phage display system. Biotechnology (N Y) 1991 Dec;9(12):1373–1377. doi: 10.1038/nbt1291-1373. [DOI] [PubMed] [Google Scholar]
- Griffiths A. D., Malmqvist M., Marks J. D., Bye J. M., Embleton M. J., McCafferty J., Baier M., Holliger K. P., Gorick B. D., Hughes-Jones N. C. Human anti-self antibodies with high specificity from phage display libraries. EMBO J. 1993 Feb;12(2):725–734. doi: 10.1002/j.1460-2075.1993.tb05706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G. M., Berek C., Kaartinen M., Milstein C. Somatic mutation and the maturation of immune response to 2-phenyl oxazolone. Nature. 1984 Nov 15;312(5991):271–275. doi: 10.1038/312271a0. [DOI] [PubMed] [Google Scholar]
- Güssow D., Clackson T. Direct clone characterization from plaques and colonies by the polymerase chain reaction. Nucleic Acids Res. 1989 May 25;17(10):4000–4000. doi: 10.1093/nar/17.10.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. E., Russell S. J., Winter G. Selection of phage antibodies by binding affinity. Mimicking affinity maturation. J Mol Biol. 1992 Aug 5;226(3):889–896. doi: 10.1016/0022-2836(92)90639-2. [DOI] [PubMed] [Google Scholar]
- Hoess R. H., Wierzbicki A., Abremski K. The role of the loxP spacer region in P1 site-specific recombination. Nucleic Acids Res. 1986 Mar 11;14(5):2287–2300. doi: 10.1093/nar/14.5.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoess R. H., Ziese M., Sternberg N. P1 site-specific recombination: nucleotide sequence of the recombining sites. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3398–3402. doi: 10.1073/pnas.79.11.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A., Roeder R. G. Purification of his-tagged proteins in non-denaturing conditions suggests a convenient method for protein interaction studies. Nucleic Acids Res. 1991 Nov 25;19(22):6337–6338. doi: 10.1093/nar/19.22.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliger P., Prospero T., Winter G. "Diabodies": small bivalent and bispecific antibody fragments. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6444–6448. doi: 10.1073/pnas.90.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenboom H. R., Griffiths A. D., Johnson K. S., Chiswell D. J., Hudson P., Winter G. Multi-subunit proteins on the surface of filamentous phage: methodologies for displaying antibody (Fab) heavy and light chains. Nucleic Acids Res. 1991 Aug 11;19(15):4133–4137. doi: 10.1093/nar/19.15.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenboom H. R., Winter G. By-passing immunisation. Human antibodies from synthetic repertoires of germline VH gene segments rearranged in vitro. J Mol Biol. 1992 Sep 20;227(2):381–388. doi: 10.1016/0022-2836(92)90894-p. [DOI] [PubMed] [Google Scholar]
- Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989 Apr 15;77(1):61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Huse W. D., Sastry L., Iverson S. A., Kang A. S., Alting-Mees M., Burton D. R., Benkovic S. J., Lerner R. A. Generation of a large combinatorial library of the immunoglobulin repertoire in phage lambda. Science. 1989 Dec 8;246(4935):1275–1281. doi: 10.1126/science.2531466. [DOI] [PubMed] [Google Scholar]
- Johnsson B., Löfås S., Lindquist G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Anal Biochem. 1991 Nov 1;198(2):268–277. doi: 10.1016/0003-2697(91)90424-r. [DOI] [PubMed] [Google Scholar]
- Karlsson R., Fägerstam L., Nilshans H., Persson B. Analysis of active antibody concentration. Separation of affinity and concentration parameters. J Immunol Methods. 1993 Nov 5;166(1):75–84. doi: 10.1016/0022-1759(93)90330-a. [DOI] [PubMed] [Google Scholar]
- Karlsson R., Michaelsson A., Mattsson L. Kinetic analysis of monoclonal antibody-antigen interactions with a new biosensor based analytical system. J Immunol Methods. 1991 Dec 15;145(1-2):229–240. doi: 10.1016/0022-1759(91)90331-9. [DOI] [PubMed] [Google Scholar]
- Kranz D. M., Herron J. N., Voss E. W., Jr Mechanisms of ligand binding by monoclonal anti-fluorescyl antibodies. J Biol Chem. 1982 Jun 25;257(12):6987–6995. [PubMed] [Google Scholar]
- Kranz D. M., Voss E. W., Jr Partial elucidation of an anti-hapten repertoire in BALB/c mice: comparative characterization of several monoclonal anti-fluorescyl antibodies. Mol Immunol. 1981 Oct;18(10):889–898. doi: 10.1016/0161-5890(81)90012-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lucisano Valim Y. M., Lachmann P. J. The effect of antibody isotype and antigenic epitope density on the complement-fixing activity of immune complexes: a systematic study using chimaeric anti-NIP antibodies with human Fc regions. Clin Exp Immunol. 1991 Apr;84(1):1–8. doi: 10.1111/j.1365-2249.1991.tb08115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariuzza R., Strand M. Chemical basis for diversity in antibody specificity analysed by hapten binding to monoclonal anti-4-hydroxy-3-nitrophenacetyl (NP) immunoglobulins. Mol Immunol. 1981 Sep;18(9):847–855. doi: 10.1016/0161-5890(81)90006-7. [DOI] [PubMed] [Google Scholar]
- Marks J. D., Griffiths A. D., Malmqvist M., Clackson T. P., Bye J. M., Winter G. By-passing immunization: building high affinity human antibodies by chain shuffling. Biotechnology (N Y) 1992 Jul;10(7):779–783. doi: 10.1038/nbt0792-779. [DOI] [PubMed] [Google Scholar]
- Marks J. D., Hoogenboom H. R., Bonnert T. P., McCafferty J., Griffiths A. D., Winter G. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J Mol Biol. 1991 Dec 5;222(3):581–597. doi: 10.1016/0022-2836(91)90498-u. [DOI] [PubMed] [Google Scholar]
- Marks J. D., Hoogenboom H. R., Griffiths A. D., Winter G. Molecular evolution of proteins on filamentous phage. Mimicking the strategy of the immune system. J Biol Chem. 1992 Aug 15;267(23):16007–16010. [PubMed] [Google Scholar]
- Marks J. D., Ouwehand W. H., Bye J. M., Finnern R., Gorick B. D., Voak D., Thorpe S. J., Hughes-Jones N. C., Winter G. Human antibody fragments specific for human blood group antigens from a phage display library. Biotechnology (N Y) 1993 Oct;11(10):1145–1149. doi: 10.1038/nbt1093-1145. [DOI] [PubMed] [Google Scholar]
- McCafferty J., Griffiths A. D., Winter G., Chiswell D. J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990 Dec 6;348(6301):552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Nissim A., Hoogenboom H. R., Tomlinson I. M., Flynn G., Midgley C., Lane D., Winter G. Antibody fragments from a 'single pot' phage display library as immunochemical reagents. EMBO J. 1994 Feb 1;13(3):692–698. doi: 10.1002/j.1460-2075.1994.tb06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J. Tolerance and ways to break it. Ann N Y Acad Sci. 1993 Aug 12;690:34–41. doi: 10.1111/j.1749-6632.1993.tb43993.x. [DOI] [PubMed] [Google Scholar]
- Näkelä O., Kaartinen M., Pelkonen J. L., Karjalainen K. Inheritance of antibody specificity V. Anti-2-phenyloxazolone in the mouse. J Exp Med. 1978 Dec 1;148(6):1644–1660. doi: 10.1084/jem.148.6.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shannessy D. J. Antibodies biotinylated via sugar moieties. Methods Enzymol. 1990;184:162–166. doi: 10.1016/0076-6879(90)84270-q. [DOI] [PubMed] [Google Scholar]
- O'Shannessy D. J., Brigham-Burke M., Peck K. Immobilization chemistries suitable for use in the BIAcore surface plasmon resonance detector. Anal Biochem. 1992 Aug 15;205(1):132–136. doi: 10.1016/0003-2697(92)90589-y. [DOI] [PubMed] [Google Scholar]
- Perelson A. S., Oster G. F. Theoretical studies of clonal selection: minimal antibody repertoire size and reliability of self-non-self discrimination. J Theor Biol. 1979 Dec 21;81(4):645–670. doi: 10.1016/0022-5193(79)90275-3. [DOI] [PubMed] [Google Scholar]
- Rathjen D. A., Furphy L. J., Aston R. Selective enhancement of the tumour necrotic activity of TNF alpha with monoclonal antibody. Br J Cancer. 1992 Jun;65(6):852–856. doi: 10.1038/bjc.1992.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinitz D. M., Voss E. W., Jr Idiotypic cross-reactivity of low-affinity anti-fluorescyl monoclonal antibodies. Mol Immunol. 1984 Sep;21(9):775–784. doi: 10.1016/0161-5890(84)90164-0. [DOI] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Schwartz R. S., Stollar B. D. Heavy-chain directed B-cell maturation: continuous clonal selection beginning at the pre-B cell stage. Immunol Today. 1994 Jan;15(1):27–32. doi: 10.1016/0167-5699(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Hamilton D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J Mol Biol. 1981 Aug 25;150(4):467–486. doi: 10.1016/0022-2836(81)90375-2. [DOI] [PubMed] [Google Scholar]
- Tomlinson I. M., Cook G. P., Carter N. P., Elaswarapu R., Smith S., Walter G., Buluwela L., Rabbitts T. H., Winter G. Human immunoglobulin VH and D segments on chromosomes 15q11.2 and 16p11.2. Hum Mol Genet. 1994 Jun;3(6):853–860. doi: 10.1093/hmg/3.6.853. [DOI] [PubMed] [Google Scholar]
- Tomlinson I. M., Walter G., Marks J. D., Llewelyn M. B., Winter G. The repertoire of human germline VH sequences reveals about fifty groups of VH segments with different hypervariable loops. J Mol Biol. 1992 Oct 5;227(3):776–798. doi: 10.1016/0022-2836(92)90223-7. [DOI] [PubMed] [Google Scholar]
- Vasicek T. J., Leder P. Structure and expression of the human immunoglobulin lambda genes. J Exp Med. 1990 Aug 1;172(2):609–620. doi: 10.1084/jem.172.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Waterhouse P., Griffiths A. D., Johnson K. S., Winter G. Combinatorial infection and in vivo recombination: a strategy for making large phage antibody repertoires. Nucleic Acids Res. 1993 May 11;21(9):2265–2266. doi: 10.1093/nar/21.9.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber P., Hof L. Introduction of a fluorescent label into the carbohydrate moiety of glycoconjugates. Biochem Biophys Res Commun. 1975 Aug 18;65(4):1298–1302. doi: 10.1016/s0006-291x(75)80371-8. [DOI] [PubMed] [Google Scholar]
- Williams S. C., Winter G. Cloning and sequencing of human immunoglobulin V lambda gene segments. Eur J Immunol. 1993 Jul;23(7):1456–1461. doi: 10.1002/eji.1830230709. [DOI] [PubMed] [Google Scholar]
- Winter G., Griffiths A. D., Hawkins R. E., Hoogenboom H. R. Making antibodies by phage display technology. Annu Rev Immunol. 1994;12:433–455. doi: 10.1146/annurev.iy.12.040194.002245. [DOI] [PubMed] [Google Scholar]
- Wu T. T., Johnson G., Kabat E. A. Length distribution of CDRH3 in antibodies. Proteins. 1993 May;16(1):1–7. doi: 10.1002/prot.340160102. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yarmolinsky M. B., Hansen E. B., Jafri S., Chattoraj D. K. Participation of the lytic replicon in bacteriophage P1 plasmid maintenance. J Bacteriol. 1989 Sep;171(9):4785–4791. doi: 10.1128/jb.171.9.4785-4791.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]





