Abstract
The title compound, C12H8N2·2B(OH)3, is best described as a host–guest complex in which the B(OH)3 molecules form a hydrogen-bonded cyclic network of layers parallel to the ab plane into which the 1,10-phenanthroline molecules are bound. An extensive network of hydrogen bonds are responsible for the crystal stability. No π-stacking interactions occur between the 1,10-phenanthroline molecules.
Related literature
For the design and synthesis of novel systems of non-covalent hosts involving hydrogen bonds, see: Pedireddi et al. (1997 ▶). In the field of supermolecular synthesis, recognition between the complementary functional groups is a main factor for the evaluation of influence of noncovalent interactions in the formation of specific architecture, see: Lehn (1990 ▶). The ability of the –B(OH)2 functionality to form a variety of hydrogen bonds through different conformations makes it a very suitable moiety for the synthesis of novel molecular complexes, see: Lee et al. (2005 ▶). It is known to have an affinity for pyridyl N atoms, often forming O—H⋯N hydrogen bonds, as observed in some crystals of boronic acids with aza compounds (Talwelkar & Pedireddi, 2010 ▶). Non-covalent hosts are generally designed and synthesized by employing appropriate functional groups at required symmetry positions to form a cyclic network through the hydrogen bonds, see: Pedireddi (2001 ▶). This effect has been observed in simple molecular adducts such as 1,10-phenanthroline and water (Tian et al., 1995 ▶).
Experimental
Crystal data
C12H8N2·2BH3O3
M r = 303.87
Triclinic,

a = 7.1390 (13) Å
b = 9.6189 (13) Å
c = 10.4756 (15) Å
α = 93.767 (11)°
β = 101.546 (14)°
γ = 90.644 (13)°
V = 703.05 (19) Å3
Z = 2
Mo Kα radiation
μ = 0.11 mm−1
T = 295 K
0.35 × 0.16 × 0.09 mm
Data collection
Refinement
R[F 2 > 2σ(F 2)] = 0.036
wR(F 2) = 0.096
S = 1.02
2580 reflections
199 parameters
H-atom parameters constrained
Δρmax = 0.17 e Å−3
Δρmin = −0.13 e Å−3
Data collection: CrysAlis PRO (Oxford Diffraction, 2011 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis PRO; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶) and OLEX2 (Dolomanov et al., 2009 ▶); software used to prepare material for publication: publCIF (Westrip, 2010) ▶.
Supplementary Material
Crystal structure: contains datablock(s) I, New_Global_Publ_Block. DOI: 10.1107/S1600536813015134/ez2287sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813015134/ez2287Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813015134/ez2287Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1⋯N2 | 0.85 | 1.90 | 2.7360 (16) | 169 |
| O2—H2⋯N1 | 0.85 | 1.88 | 2.7132 (17) | 167 |
| O3—H3⋯O1i | 0.85 | 1.86 | 2.7076 (15) | 177 |
| O4—H4⋯O3i | 0.85 | 1.89 | 2.7286 (16) | 16 |
| O5—H5⋯O4ii | 0.85 | 1.89 | 2.7355 (18) | 179 |
| O6—H6⋯O2iii | 0.85 | 1.95 | 2.7946 (17) | 172 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
Acknowledgments
The authors acknowledge the National Science Foundation for their generous support (NSF-CAREER grant to RES, CHE-0846680).
supplementary crystallographic information
Comment
The design and synthesis of novel systems of noncovalent hosts involving hydrogen bonds is a vast research area in both molecular and supermolecular chemistry, see Pedireddi et al. (1997). In the field of supermolecular synthesis, recognition between the complementary functional groups is a main factor for the evaluation of influence of noncovalent interaction in the formation of specific architecture, see: Lehn (1990). In recent times, boric acid derivatives have been well considered to be potential co-crystal formers. In fact, the ability of –B(OH)2 functionality to form a variety of hydrogen bonds through different conformations makes it a very suitable moiety for the synthesis of novel molecular complexes, see Lee et al. (2005). The –B(OH)2 moiety is known to have an affinity for pyridyl N-atoms, often forming O—H···N hydrogen bonds, as observed in some crystals of boronic acids with aza compounds,see Talwelkar & Pedireddi (2010).
Non-covalent hosts are generally designed and synthesized by employing appropriate functional groups at required symmetry positions to form a cyclic network through the hydrogen bonds, see Pedireddi (2001). This effect has been observed vividly in simple molecular adduct such as 1,10-phenanthroline and water, see Tian et al. (1995). In this complex, a water molecule interacts with a molecule of 1,10-phenanthroline through O–H···N hydrogen bonds and an unique aza-aromatic complex is formed. In the latter, 1,10-phenanthroline could be considered as a host. Herein, we report the crystal structure of boric acid with 1,10-phenanthroline as an aza-donor compound.
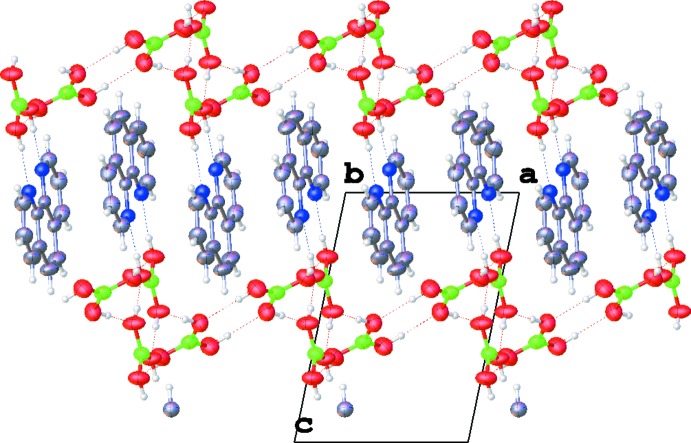
As seen in Figure 1, the phen molecule forms a H-bonded adduct via two B–O–H···N interacts from one of the included B(OH)3 moieties. A strong network of hydrogen bonds among the B(OH)3 units forms a layered structure with alternating B(OH)3 and phen layers that reside in the ab planes (Figure 2). The B(OH)3 layers alone can be described as a cyclic network formed by hydrogen bonding interactions as can be seen in Figure 3. There are not any significant π-stacking interactions between the phen molecules.
Experimental
(CH3)3NBH3 (0.73 g, 10 mmol) and iodine (2.54 g, 5 mmol) were dissolved in toluene (4 ml) and stirred for 30 min. A solution of 1,10-phenanthroline (1.98 g, 10 mmol) in toluene (4 ml) was added, and the mixture refluxed overnight. The solution was cooled to room temperature, during which process orange-brown crystals were formed. The product was recrystallized twice from CH3CN to obtain analytically pure, red-brown crystalline product.
1H NMR (DMSO-d6, 300 MHz): δH 9.22 (dd, J = 2.8, 1.6 Hz, 2H), 8.67 (dd, J = 6.3, 1.6 Hz, 2H), 8.14 (s, 2H), 7.93 (q, J = 4.4 Hz, 2H), 6.62 (br, 2H); 13C NMR (DMSO-d6, 100 MHz): δC 151.67, 146.27, 139.09, 130.58, 128.77, 125.66.
Refinement
H-atoms were placed in calculated positions and allowed to ride during subsequent refinement, with Uiso(H) = 1.2Ueq(C) and C—H distances of 0.93 Å for the aromatic H atoms and with Uiso(H) = 1.5Ueq(C) and O—H distances of 0.85 Å for hydroxyl H atoms.
Figures
Fig. 1.
The molecular structure of I, with the atom-numbering scheme. Displacement ellipsoids for non-hydrogen atoms are drawn at the 50% probability level.
Fig. 2.
A packing diagram of I viewed along the b axis.
Fig. 3.
A representation of the two-dimensional B(OH)3 layers formed via hydrogen bonding in the structure of I.
Crystal data
| C12H8N2·2BH3O3 | Z = 2 |
| Mr = 303.87 | F(000) = 316 |
| Triclinic, P1 | Dx = 1.435 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 7.1390 (13) Å | Cell parameters from 3335 reflections |
| b = 9.6189 (13) Å | θ = 3.2–25.3° |
| c = 10.4756 (15) Å | µ = 0.11 mm−1 |
| α = 93.767 (11)° | T = 295 K |
| β = 101.546 (14)° | Prism, brown |
| γ = 90.644 (13)° | 0.35 × 0.16 × 0.09 mm |
| V = 703.05 (19) Å3 |
Data collection
| Oxford Diffraction Xcalibur Eos diffractometer | 2580 independent reflections |
| Radiation source: fine-focus sealed tube | 1972 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.023 |
| Detector resolution: 16.0514 pixels mm-1 | θmax = 25.4°, θmin = 3.2° |
| ω scans | h = −8→8 |
| Absorption correction: multi-scan [CrysAlis PRO (Oxford Diffraction, 2011) based on Clark & Reid (1995)] | k = −11→11 |
| Tmin = 0.956, Tmax = 1.000 | l = −12→12 |
| 10473 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.036 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.096 | H-atom parameters constrained |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.044P)2 + 0.1204P] where P = (Fo2 + 2Fc2)/3 |
| 2580 reflections | (Δ/σ)max < 0.001 |
| 199 parameters | Δρmax = 0.17 e Å−3 |
| 0 restraints | Δρmin = −0.13 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| B1 | 0.0170 (3) | 0.65229 (18) | 0.64598 (18) | 0.0421 (4) | |
| B2 | 0.2786 (3) | −0.00132 (19) | 0.59856 (18) | 0.0439 (4) | |
| C1 | 0.1542 (2) | 0.83587 (17) | 1.04155 (17) | 0.0488 (4) | |
| H1A | 0.1058 | 0.9001 | 0.9816 | 0.059* | |
| C2 | 0.1999 (3) | 0.8822 (2) | 1.17305 (19) | 0.0573 (5) | |
| H2A | 0.1815 | 0.9743 | 1.1999 | 0.069* | |
| C3 | 0.2718 (3) | 0.7893 (2) | 1.26068 (18) | 0.0574 (5) | |
| H3A | 0.3014 | 0.8168 | 1.3493 | 0.069* | |
| C4 | 0.3018 (2) | 0.65145 (18) | 1.21789 (15) | 0.0464 (4) | |
| C5 | 0.2496 (2) | 0.61314 (16) | 1.08273 (14) | 0.0369 (4) | |
| C6 | 0.3852 (3) | 0.5520 (2) | 1.30610 (17) | 0.0587 (5) | |
| H6A | 0.4195 | 0.5784 | 1.3948 | 0.070* | |
| C7 | 0.4149 (3) | 0.4217 (2) | 1.26368 (18) | 0.0567 (5) | |
| H7 | 0.4714 | 0.3593 | 1.3231 | 0.068* | |
| C8 | 0.3612 (2) | 0.37654 (17) | 1.12795 (16) | 0.0434 (4) | |
| C9 | 0.2778 (2) | 0.47095 (15) | 1.03670 (14) | 0.0361 (3) | |
| C10 | 0.3890 (2) | 0.23972 (17) | 1.08186 (18) | 0.0521 (5) | |
| H10 | 0.4451 | 0.1755 | 1.1396 | 0.062* | |
| C11 | 0.3337 (2) | 0.20134 (17) | 0.95254 (18) | 0.0513 (4) | |
| H11 | 0.3496 | 0.1107 | 0.9206 | 0.062* | |
| C12 | 0.2522 (2) | 0.30118 (16) | 0.86861 (16) | 0.0449 (4) | |
| H12 | 0.2150 | 0.2741 | 0.7802 | 0.054* | |
| N1 | 0.17492 (17) | 0.70652 (13) | 0.99611 (12) | 0.0403 (3) | |
| N2 | 0.22495 (17) | 0.43158 (12) | 0.90711 (12) | 0.0385 (3) | |
| O1 | 0.07961 (17) | 0.52138 (11) | 0.66548 (10) | 0.0515 (3) | |
| H1 | 0.1261 | 0.5050 | 0.7441 | 0.077* | |
| O2 | 0.02929 (18) | 0.75343 (11) | 0.74334 (11) | 0.0548 (3) | |
| H2 | 0.0796 | 0.7268 | 0.8180 | 0.082* | |
| O3 | −0.06394 (19) | 0.68679 (11) | 0.52361 (11) | 0.0583 (4) | |
| H3 | −0.0642 | 0.6217 | 0.4646 | 0.087* | |
| O4 | 0.31472 (16) | 0.10158 (11) | 0.52210 (12) | 0.0539 (3) | |
| H4 | 0.2251 | 0.1595 | 0.5081 | 0.081* | |
| O5 | 0.39825 (18) | −0.11040 (12) | 0.61562 (12) | 0.0587 (3) | |
| H5 | 0.4881 | −0.1068 | 0.5734 | 0.088* | |
| O6 | 0.12843 (18) | 0.00858 (12) | 0.65864 (12) | 0.0584 (3) | |
| H6 | 0.1101 | −0.0702 | 0.6871 | 0.088* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| B1 | 0.0496 (11) | 0.0403 (10) | 0.0372 (10) | 0.0098 (8) | 0.0080 (8) | 0.0079 (8) |
| B2 | 0.0530 (12) | 0.0390 (10) | 0.0376 (10) | 0.0063 (8) | 0.0042 (9) | 0.0014 (8) |
| C1 | 0.0501 (10) | 0.0455 (10) | 0.0492 (10) | 0.0076 (7) | 0.0074 (8) | −0.0006 (8) |
| C2 | 0.0585 (11) | 0.0549 (11) | 0.0557 (11) | 0.0042 (9) | 0.0099 (9) | −0.0129 (9) |
| C3 | 0.0567 (11) | 0.0713 (13) | 0.0406 (10) | −0.0023 (9) | 0.0063 (8) | −0.0125 (9) |
| C4 | 0.0398 (9) | 0.0612 (11) | 0.0362 (9) | −0.0027 (8) | 0.0036 (7) | 0.0018 (8) |
| C5 | 0.0307 (8) | 0.0468 (9) | 0.0328 (8) | −0.0008 (6) | 0.0049 (6) | 0.0055 (7) |
| C6 | 0.0639 (12) | 0.0747 (13) | 0.0333 (9) | −0.0030 (10) | −0.0013 (8) | 0.0078 (9) |
| C7 | 0.0568 (11) | 0.0685 (13) | 0.0422 (10) | 0.0011 (9) | −0.0019 (8) | 0.0214 (9) |
| C8 | 0.0355 (9) | 0.0518 (10) | 0.0430 (9) | −0.0009 (7) | 0.0045 (7) | 0.0144 (7) |
| C9 | 0.0301 (8) | 0.0432 (9) | 0.0354 (8) | −0.0011 (6) | 0.0056 (6) | 0.0091 (7) |
| C10 | 0.0482 (10) | 0.0480 (10) | 0.0603 (12) | 0.0058 (8) | 0.0051 (9) | 0.0234 (9) |
| C11 | 0.0540 (10) | 0.0400 (9) | 0.0611 (12) | 0.0059 (7) | 0.0121 (9) | 0.0102 (8) |
| C12 | 0.0496 (10) | 0.0401 (9) | 0.0447 (9) | 0.0034 (7) | 0.0080 (8) | 0.0046 (7) |
| N1 | 0.0409 (7) | 0.0419 (7) | 0.0380 (7) | 0.0046 (6) | 0.0071 (6) | 0.0032 (6) |
| N2 | 0.0397 (7) | 0.0395 (7) | 0.0362 (7) | 0.0021 (5) | 0.0062 (6) | 0.0064 (6) |
| O1 | 0.0702 (8) | 0.0450 (6) | 0.0346 (6) | 0.0199 (5) | −0.0025 (5) | 0.0063 (5) |
| O2 | 0.0850 (9) | 0.0415 (6) | 0.0373 (6) | 0.0096 (6) | 0.0093 (6) | 0.0062 (5) |
| O3 | 0.0905 (9) | 0.0455 (7) | 0.0360 (6) | 0.0270 (6) | 0.0032 (6) | 0.0077 (5) |
| O4 | 0.0538 (7) | 0.0472 (7) | 0.0644 (8) | 0.0162 (5) | 0.0151 (6) | 0.0198 (6) |
| O5 | 0.0678 (8) | 0.0517 (7) | 0.0620 (8) | 0.0202 (6) | 0.0188 (6) | 0.0226 (6) |
| O6 | 0.0742 (9) | 0.0463 (7) | 0.0606 (8) | 0.0099 (6) | 0.0266 (7) | 0.0060 (6) |
Geometric parameters (Å, º)
| B1—O2 | 1.351 (2) | C6—H6A | 0.9300 |
| B1—O1 | 1.355 (2) | C7—C8 | 1.433 (2) |
| B1—O3 | 1.361 (2) | C7—H7 | 0.9300 |
| B2—O6 | 1.349 (2) | C8—C10 | 1.402 (2) |
| B2—O5 | 1.359 (2) | C8—C9 | 1.411 (2) |
| B2—O4 | 1.367 (2) | C9—N2 | 1.3612 (19) |
| C1—N1 | 1.323 (2) | C10—C11 | 1.358 (2) |
| C1—C2 | 1.393 (2) | C10—H10 | 0.9300 |
| C1—H1A | 0.9300 | C11—C12 | 1.397 (2) |
| C2—C3 | 1.355 (3) | C11—H11 | 0.9300 |
| C2—H2A | 0.9300 | C12—N2 | 1.3207 (19) |
| C3—C4 | 1.404 (2) | C12—H12 | 0.9300 |
| C3—H3A | 0.9300 | O1—H1 | 0.8500 |
| C4—C5 | 1.413 (2) | O2—H2 | 0.8501 |
| C4—C6 | 1.425 (2) | O3—H3 | 0.8500 |
| C5—N1 | 1.3559 (19) | O4—H4 | 0.8501 |
| C5—C9 | 1.450 (2) | O5—H5 | 0.8501 |
| C6—C7 | 1.336 (3) | O6—H6 | 0.8501 |
| O2—B1—O1 | 123.27 (15) | C6—C7—H7 | 119.5 |
| O2—B1—O3 | 116.79 (14) | C8—C7—H7 | 119.5 |
| O1—B1—O3 | 119.94 (15) | C10—C8—C9 | 118.24 (15) |
| O6—B2—O5 | 121.00 (16) | C10—C8—C7 | 121.84 (15) |
| O6—B2—O4 | 119.75 (15) | C9—C8—C7 | 119.92 (16) |
| O5—B2—O4 | 119.23 (17) | N2—C9—C8 | 121.47 (14) |
| N1—C1—C2 | 124.43 (17) | N2—C9—C5 | 119.64 (13) |
| N1—C1—H1A | 117.8 | C8—C9—C5 | 118.88 (14) |
| C2—C1—H1A | 117.8 | C11—C10—C8 | 119.70 (15) |
| C3—C2—C1 | 118.03 (16) | C11—C10—H10 | 120.2 |
| C3—C2—H2A | 121.0 | C8—C10—H10 | 120.2 |
| C1—C2—H2A | 121.0 | C10—C11—C12 | 118.49 (16) |
| C2—C3—C4 | 120.11 (16) | C10—C11—H11 | 120.8 |
| C2—C3—H3A | 119.9 | C12—C11—H11 | 120.8 |
| C4—C3—H3A | 119.9 | N2—C12—C11 | 124.05 (15) |
| C3—C4—C5 | 118.01 (16) | N2—C12—H12 | 118.0 |
| C3—C4—C6 | 121.94 (16) | C11—C12—H12 | 118.0 |
| C5—C4—C6 | 120.05 (16) | C1—N1—C5 | 118.04 (13) |
| N1—C5—C4 | 121.35 (14) | C12—N2—C9 | 118.04 (13) |
| N1—C5—C9 | 119.78 (13) | B1—O1—H1 | 115.9 |
| C4—C5—C9 | 118.86 (14) | B1—O2—H2 | 113.4 |
| C7—C6—C4 | 121.23 (16) | B1—O3—H3 | 114.0 |
| C7—C6—H6A | 119.4 | B2—O4—H4 | 113.0 |
| C4—C6—H6A | 119.4 | B2—O5—H5 | 113.6 |
| C6—C7—C8 | 121.04 (16) | B2—O6—H6 | 108.1 |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1···N2 | 0.85 | 1.90 | 2.7360 (16) | 168.9 |
| O2—H2···N1 | 0.85 | 1.88 | 2.7132 (17) | 167.4 |
| O3—H3···O1i | 0.85 | 1.86 | 2.7076 (15) | 176.8 |
| O4—H4···O3i | 0.85 | 1.89 | 2.7286 (16) | 169.1 |
| O5—H5···O4ii | 0.85 | 1.89 | 2.7355 (18) | 179.0 |
| O6—H6···O2iii | 0.85 | 1.95 | 2.7946 (17) | 171.8 |
Symmetry codes: (i) −x, −y+1, −z+1; (ii) −x+1, −y, −z+1; (iii) x, y−1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: EZ2287).
References
- Clark, R. C. & Reid, J. S. (1995). Acta Cryst. A51, 887–897.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Lee, S. O., Kariuki, B. M. & Harris, K. D. M. (2005). New. J. Chem 29, 1266–1271.
- Lehn, J. M. (1990). Angew. Chem. Int. Ed. 29, 1304-1319.
- Oxford Diffraction (2011). CrysAlis PRO Oxford Diffraction Ltd, Yarnton, England.
- Pedireddi, V. R. (2001). Cryst. Growth Des. 1, 383–385.
- Pedireddi, V. R., Chatterjee, S., Ranganathan, A. & Rao, C. N. R. (1997). J. Am. Chem. Soc. 119, 10867–10868.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Talwelkar, M. & Pedireddi, V. R. (2010). Tetrahedron Lett. 51, 6901–6905.
- Tian, Y.-P., Duan, C.-Y., Xu, X.-X. & You, X.-Z. (1995). Acta Cryst. C51, 2309–2312.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, New_Global_Publ_Block. DOI: 10.1107/S1600536813015134/ez2287sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813015134/ez2287Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813015134/ez2287Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report