Abstract
Background
Trauma patients are often transfused allogeneic red blood cells (RBCs) in an effort to augment tissue oxygen delivery. However, the effect of RBC transfusion on microvascular perfusion in this patient population is not well understood. To this end, we investigated the effect of RBC transfusion on sublingual microvascular perfusion in trauma patients.
Methods
Sublingual microcirculation was imaged at bedside with a sidestream dark field illumination microscope before and after transfusion of one RBC unit in hemodynamically stable, anemic trauma patients. The proportion of perfused capillaries (PPC) pre- and post-transfusion was determined, and the percent change in capillary perfusion following transfusion (ΔPPC) calculated.
Results
Sublingual microcirculation was observed in 30 patients. Mean age was 47 (SD=21), mean ISS was 29 (SD=16), and mean pre-transfusion hemoglobin was 7.5 g/dL (SD=0.9). No patients had MAP < 65 mm Hg (mean 89 mm Hg, SD 17) or lactate > 2.5 mmol/L (mean 1.1 mmol/L, SD 0.3). Following transfusion, ΔPPC ranged from +68% to -36% and was found to inversely correlate significantly with pre-transfusion PPC (Spearman r= -0.63, p=0.0002).
Conclusions
Pre-transfusion PPC may be selectively deranged in otherwise stable trauma patients. Patients with relatively altered baseline PPC tend to demonstrate improvement in perfusion following transfusion, while those with relatively normal perfusion at baseline tend to demonstrate either no change or, in fact, a decline in PPC. Bedside sublingual imaging may have the potential to detect subtle perfusion defects and ultimately inform clinical decision making with respect to transfusion.
Introduction
Over the course of the last century, the management of acute hemorrhage was transformed by the practice of allogeneic blood transfusion, allowing for the correction of hemorrhagic shock physiology that had been historically fatal. Nonetheless, appreciation of the potentially deleterious effects of transfusion has grown over time. Agarwal et al. demonstrated an association between blood transfusion and infection in a trauma patient cohort in the early 1990s.1 Subsequent studies have identified post injury transfusion to be a significant predictor of pulmonary morbidity, multiorgan failure, infection, and death.2-6 Although it remains difficult to discern whether allogeneic transfusion is causally related to adverse outcomes in trauma patients, or rather simply a surrogate marker of injury severity, the relative benefit versus risk of transfusion remains a clinical concern and continues to be an area of ongoing investigation.
In clinical practice, the response to transfusion is often measured by evaluation of global indices of perfusion, such as blood pressure or base deficit, along with measurement of hemoglobin and/or hematocrit. The effect of RBC transfusion on microvascular perfusion, however, remains unclear. Until recently, evaluation of the microcirculation in clinical practice has not been possible, and was reserved for animal laboratory studies. However, the development of the orthogonal polarization spectral microscope (and its successor, the sidestream dark field microscope) has allowed for real time observation of the in vivo microcirculation.7 To date, most of the reported investigations performed with this device has concerned the evaluation of microvascular perfusion in the setting of sepsis.8-12 It has been observed that microcirculatory flow alterations occur in patients with severe sepsis,10 that persistence of such flow alterations are associated with mortality in the setting of septic shock,11 and lastly, that sublingual microvascular perfusion improves following red cell transfusion in septic patients with relatively altered microcirculation at baseline.12
Up until now, there has been relatively little if any reported investigation of the microcirculation in trauma patients. In fact, the effect of blood transfusion on microvascular perfusion in the setting of trauma remains unknown. Therefore, the object of this study was to investigate the effect of RBC transfusion on microvascular perfusion in trauma patients.
Methods
Study approval was obtained from the Institutional Review Boards of the University of Alabama at Birmingham and the University of Tennessee Health Science Center. Over the period of study, patients in the trauma intensive care unit of the University of Alabama at Birmingham University Hospital with orders to receive a RBC transfusion were screened for participation. Study inclusion criteria included admission to the trauma intensive care unit from the emergency department (directly or by way of the operating room or angiography suite) following blunt or penetrating injury. Patients who were receiving transfusion in the context of volume resuscitation were excluded from participation. Patients who were receiving vasopressors or were otherwise hemodynamically unstable were also excluded from participation. Additional exclusion criteria included pregnancy, age less than 19 years, burn injuries, and injuries to the face that precluded sublingual microscopy.
The decision to transfuse was made by the patient's attending physician prior to and independently of the patient's participation in the study. As such, there was no protocol in place related to the indication for transfusion that was specific to the study. Nonetheless, the clinical pathway at the University of Alabama at Birmingham University Hospital concerning transfusion over the time of the study was to transfuse stable anemic patients when hemoglobin levels were less than 7 g/dL or hematocrit was less than 21 %. The hemoglobin/hematocrit threshold was adjusted on clinical grounds for patients thought to be symptomatic from anemia, with cardiac comorbidities, or with anticipated blood loss from upcoming operations. During the study period, all RBC units transfused had undergone pre-storage leukoreduction. Red cells were leukoreduced within 24 hours of collection by high-efficiency filters. The storage duration (days) for each unit transfused was noted.
Enrolled patients underwent evaluation of the sublingual microvascular network with a handheld video microscope (MicroScan BV, Amsterdam, The Netherlands). This device captures video images of the sublingual microcirculation with sidestream dark field (SDF) illumination technology. The details regarding this technique have been reported elsewhere.7 In brief, the device was applied to the sublingual capillary bed, and three to five separate images of approximately 20 seconds duration each were recorded at three time points: immediately prior to transfusion of a single red blood cell unit, immediately following transfusion of the RBC unit, and one hour following completion of the transfusion.
Subsequently, all images were independently evaluated by a single observer. The observer was blinded to both study patient and image sequence (i.e. pre- vs. post-transfusion). Capillary vascular density, perfused capillary density, and perfused proportion of capillaries were determined as described by De Backer et al.10 In brief, a grid consisting of three equidistant horizontal and three equidistant vertical lines are placed over the video image. Capillary vascular density is calculated by counting the number of small vessels (< 20 Perfused proportion of capillaries (PPC, %) is the quotient of the perfused capillary density divided by capillary vascular density (multiplied by 100). Microvascular Flow Index (MFI), as described by Spronk et al.,13 was also calculated for each video image. The image is divided into four quadrants and the flow in each quadrant is scored by the observer on a scale from 0 to 3 (absent = 0, intermittent = 1, sluggish = 2, and normal = 3). The MFI score for the image is the average of the quadrant scores.
Differences between transfusion time points were evaluated statistically with nonparametric measures of comparison. Correlations between variables were evaluated by using Spearman – rho, a correlation comparison based on ranks. Correlations were calculated comparing pre-transfusion PPC to percent change in PPC immediately posttransfusion, and one hour post-transfusion. Other correlation examined included comparisons of MFI and PPC at pre-, immediate post-, and one hour post-transfusion.
Results
Between September 2009 and May 2010, 30 patients admitted to the trauma service at the University of Alabama at Birmingham University Hospital were enrolled in the study. The characteristics of the study group are demonstrated in Table 1. All patients were in the trauma intensive care unit at the time of study enrollment and had suffered severe injuries as reflected by the mean Injury Severity Score of 29. The average length of hospitalization was 39 days, with 31 ICU days and 29 ventilator support days. In-hospital mortality for the cohort was 6.7%.
Table 1.
Demographic, Injury, and Hospitalization Characteristics of Microcirculation Study Subjects (n=30).
| Demographic | |
| Mean Age, years (SD) | 47 (21) |
| Male (%) | 67 |
| Injury | |
| Mechanism (%) | |
| Blunt | 80 |
| Penetrating | 20 |
| Mean ISS (SD) | 29 (16) |
| Hospitalization | |
| Mean # Hospital Days (SD) | 39 (37) |
| Mean # ICU Days (SD) | 31 (37) |
| Mean # Ventilator support Days (SD) | 28 (37) |
| In-hospital Mortality (%) | 6.7 |
Table 2 demonstrates clinical data pertaining to the study cohort at the time of study participation. All patients were hemodynamically stable without the support of any vasopressors, and all were anemic (average hemoglobin 7.5 mm Hg). The average base excess was 2.9 mmol /L and the average serum lactate was 1.1 mmol/L. Median day of hospital stay on the day of study participation was 6 (quartiles 3,14).
Table 2.
Clinical characteristics of study cohort at time of study participation (n=30).
| Pre-transfusion Hemoglobin (g/dL) | 7.5 (0.9) |
| Serum Lactate (mmol/L) | 1.1 (0.3) |
| Base Excess (mmol/L) | 2.9 (3.3) |
| Mean Arterial Pressure (mm Hg) | 89 (17) |
Values reported as mean (SD)
MFI and PPC were calculated at three time points: pre-transfusion, immediately post-transfusion, and 1 hour post-transfusion. The median values for capillary vascular density, PPC, and capillary MFI at these time points are demonstrated in Table 3. Globally, RBC transfusion seemed to immediately improve capillary perfusion, as reflected by the significant difference between pre-transfusion and immediately post-transfusion MFI and a trend toward significance between pre-transfusion and immediately post-transfusion PPC. However, this effect was not sustained as evidenced by the insignificant differences between pre-transfusion and 1 hour post-transfusion MFI and PPC respectively.
Table 3.
Comparison of Capillary vascular density (CVD), perfused proportion of capillaries (PPC), and capillary microsvascular flow index (MFI) pre-transfusion, immediately post-transfusion, and 1 hour post-transfusion. Values are presented as median (range).
| Pre-transfusion | Immediately post-transfusion | p | 1 hour post-transfusion | p | |
|---|---|---|---|---|---|
| CVD, n/mm | 7.38 (2.53-10.90) | 7.10 (2.11-11.74) | 0.4417 | 7.14 (3.09-11.81) | 0.7796 |
| PPC, % | 0.98 (0.58-1.00) | 0.99 (0.75-1.00) | 0.0629 | 0.98 (0.64-1.05) | 0.6171 |
| MFI | 2.83 (1.50-3.00) | 3.00 (1.92-3.00) | 0.0278 | 2.96 (1.33-3.00) | 0.215 |
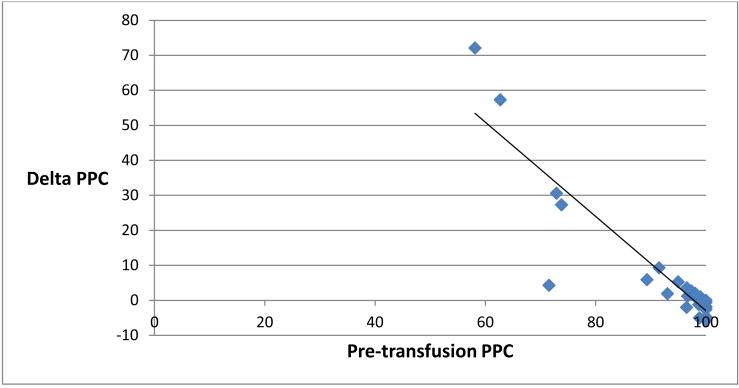
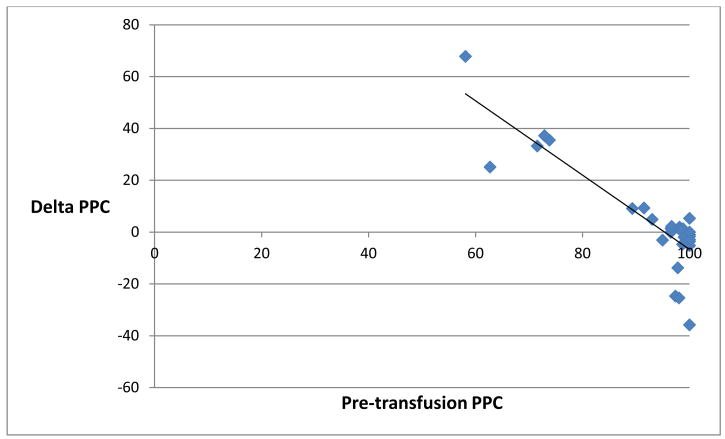
It is notable, however, that considerable variation in the response to transfusion was observed, with improvement in some patients and deterioration in others. Despite all patients appearing hemodynamically stable and well perfused by clinical and laboratory measures, some patients in the cohort had relatively altered baseline PPC compared with others in the group. While no patient in the cohort had a mean arterial blood pressure less than 65 mm Hg, serum lactate > 2.5 mmol/L or base excess < - 3 mmol/L, 17 percent (5 patients) had pre-transfusion PPC less than 75 percent, and 30 percent (9 patients) had pre-transfusion PPC less than 95 percent. Correspondingly, the relative change in PPC following transfusion was found to be significantly negatively correlated to baseline PPC. The percentage change in PPC immediately following transfusion was found to negatively correlate with pre-transfusion PPC with a Spearman-rho correlation of -0.86, p <0.0001 (Figure 1). This relationship persisted at the 1 hour post-transfusion time point, as the percentage change in PPC 1 hour following transfusion was found to negatively correlate as well with pre-transfusion PPC, with a Spearman-rho correlation of -0.63, p=0.0002 (Figure 2).
Figure 1.
Scatter plot representing pre-transfusion perfused proportion of capillaries (PPC, %, x-axis) and the relative change in PPC (%, y-axis) immediately post-transfusion. Spearman-rho = − 0.86, p< 0.0001.
Figure 2.
Scatter plot representing pre-transfusion perfused proportion of capillaries (PPC, %, x-axis) and the relative change in PPC (%, y-axis) 1 hour posttransfusion. Spearman-rho = − 0.63, p = 0.0002.
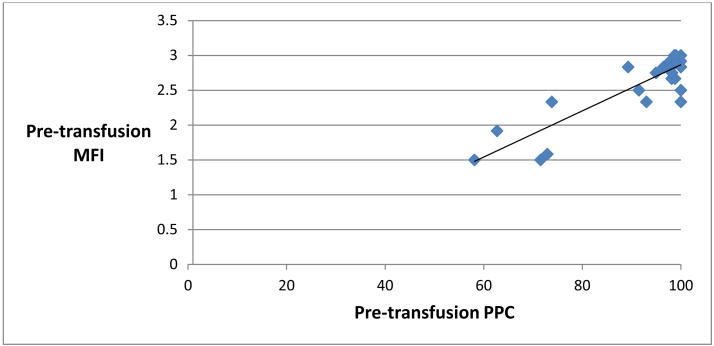
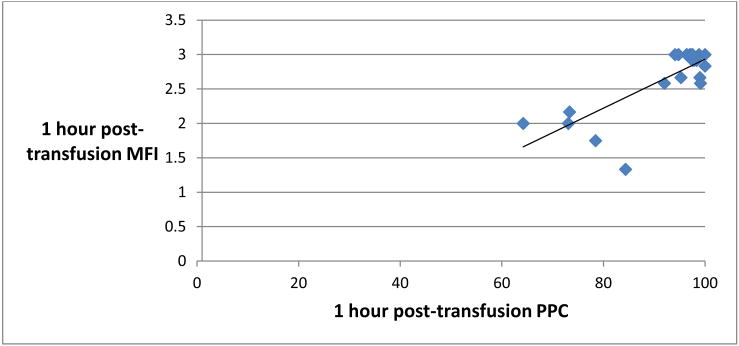
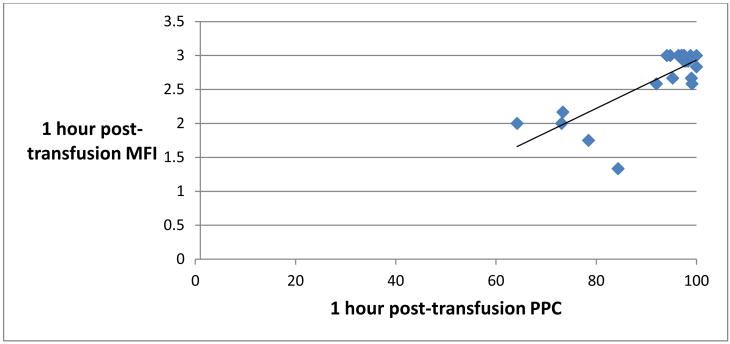
Pre-transfusion MFI was found to correlate significantly with pre-transfusion PPC, with a Spearman-rho correlation of 0.64, p < 0.0001 (Figure 3). 1 hour posttransfusion MFI also correlated significantly with 1 hour post-transfusion PPC (Figure 4). There was no significant correlation, however, between MFI and PPC at the immediately post-transfusion time point (Figure 5).
Figure 3.
Scatter plot representing pre-transfusion perfused proportion of capillaries (PPC, % x-axis) and pre-transfusion Microvascular Flow Index (MFI, y-axis). Spearman-rho = 0.64, p <0.0001.
Figure 4.
Scatter plot representing 1 hour post-transfusion perfused proportion of capillaries (PPC, % x-axis) and 1 hour post-transfusion Microvascular Flow Index (MFI, y-axis). Spearman-rho = 0.42, p <0.0209.
Figure 5.
Scatter plot representing immediately post-transfusion perfused proportion of capillaries (PPC, % x-axis) and immediately post-transfusion Microvascular Flow Index (MFI, y-axis). Spearman-rho = 0.29, p <0.1116.
The median RBC storage time was 27.5 days (quartiles 20, 34). No correlation was observed between storage time and the change in capillary perfusion observed following transfusion (immediately post-transfusion, Spearman r= -0.06, p<0.75; 1 hour post-transfusion, Spearman r=0.07, p<0.73).
Discussion
The therapeutic rationale for the transfusion of allogeneic RBCs is the augmentation of tissue oxygenation. In the past, anemia was corrected with RBC transfusion cavalierly, and the arbitrary transfusion trigger known as the “10/30 rule” (i.e., RBC transfusion indicated below a hemoglobin concentration of 10 g/dL or a hematocrit of 30% irrespective of any other clinical parameters) was commonly applied. Appreciation of the risks of transfusion has led to a culture of transfusion restraint, whereby transfusions are reserved for those patients deemed to be symptomatic from anemia or are in a compensated physiologic state that might be corrected by RBC transfusion. Nonetheless, significant anemia as evidenced by a hemoglobin in the 6-7 range usually triggers RBC transfusion, whether the patient is symptomatic or not, as anemia below this threshold has known associated risks, as demonstrated in the reported experiences with surgical patients who refuse transfusion on religious grounds.14, 15
In our study, a cohort of hemodynamically stable patients with anemia at this critical level were transfused one RBC unit. Interestingly, prior to transfusion, all patients appeared clinically similar, with none suggesting clues as to who might have relatively altered sublingual microvascular perfusion at baseline. A proportion of the cohort indeed had altered pre-transfusion sublingual perfusion, and those patients were more likely to respond favorably to transfusion (i.e. a post-transfusion improvement in perfusion), whereby those patients with relatively normal pre-transfusion sublingual perfusion tended to demonstrate either no change or, in fact, a deterioration of sublingual perfusion following RBC transfusion.
These findings are comparable to the results reported in a similar study performed by Sakr et al. concerning a cohort of 35 ICU patients with sepsis.12 While the authors found that RBC transfusion globally failed to affect sublingual microvascular perfusion, they observed that a proportion of their cohort had relatively altered pre-transfusion sublingual perfusion, and the relative change in capillary perfusion following transfusion was significantly negatively correlated to pre-transfusion capillary perfusion. They likewise found that baseline differences in sublingual perfusion were not suggested by concomitant differences in global indices of perfusion, such as serum lactate or mean arterial pressure.
The similar observations reported both herein and by Sakr et al.12 together suggest the clinical potential for bedside sidestream dark field microscopy, given the ability of this technology to identify subtle perfusion defects and monitor the effects of therapies such as RBC transfusion. At this time, however, analysis of the acquired images is relatively cumbersome and not suited to real time clinical evaluation and decision making at the bedside. The MFI, which is relatively easier to calculate then the measurements of capillary density and could feasibly be determined in the clinical setting, was found to correlate with capillary perfusion (that is, PPC) at two of the three study time points. Nonetheless, it is limited by its inability to relate any information regarding capillary density, and it remains unvalidated for use in clinical practice. It is anticipated that future developments in software-assisted image analysis will facilitate the bedside quantification of sublingual perfusion, but at this time, semiquantitative image analysis remains unsuited for real-time clinical decision making.16
Should sublingual microscopy become realized as a clinical tool from a technical standpoint, the clinical value of the evaluation of microvascular perfusion will remain uncertain. Although both in this study and in the study by Sakr et al.12, a proportion of patients had relatively altered perfusion at baseline, and this perfusion improved following transfusion, it is unclear whether this intervention contributed to favorable outcomes. Conversely, it is unclear whether those patients whose perfusion deteriorated post transfusion were exposed to a related adverse clinical outcome. Neither the current study or the Sakr et al.12 study were designed to evaluate clinical outcomes, as the contributors to outcome in these patient groups were multifactorial and longitudinal, while the transfusion event under study was cross-sectional and only one of presumably multiple interventions administered to these ICU patients. Nonetheless, others have demonstrated that persistent perfusion defects as observed with sublingual microscopy are associated with poor outcomes in the setting of sepsis, suggesting that interventions toward correcting this perfusion defect might be helpful.5, 11, 17
As to why some patients have relatively altered perfusion compared to others, endogenous RBC deformability may be a factor. It has been observed that RBC transfusions improved RBC deformability in patients with sepsis, possibly by the replacement of endogenous dysfunctionally rigid RBCs with relatively less dysfunctional allogeneic RBCs.18 It is possible that transfusions may favorably effect perfusion in patients with altered RBC deformability, and conversely may be deleterious to perfusion in patients with preserved RBC deformability. It is impossible to overlook the issue of RBC storage age when considering this concept, as older RBCs will inherently be less deformable and would be less likely to favorably affect perfusion. In this study, and in Sakr et al.12 as well, RBC storage age did not have an appreciable affect on perfusion. It may be the case, however, that given the differences in pretransfusion perfusion in patients within the cohort, that the effect of RBC age cannot be elucidated in studies of this relative size.
The current study has limitations worth noting. The microcirculation is dynamic and the perfusion would be expected to fluctuate over the course of a transfusion. Current imaging technology only allows us to take snapshots of the microcirculation, whereby continuous monitoring of the microcirculation over the course of the transfusion and a post transfusion observation would be ideal, but not possible. Additionally, it is possible that changes related to transfusion might be observable beyond our study window of 1 hour post-transfusion. Longer follow up periods however become practically challenging secondary to the inevitable changes in therapy and other patient manipulations. Additionally, the interval between injury and transfusion was variable in the patient cohort, and patient's interventions up to the study period were heterogenous with varying administration of transfusion, surgical therapy, and hemodynamic support provided prior to the day of study participation.
Conclusion
In this cohort of trauma patients, the microvascular effects of RBC transfusion were variable, and negatively correlated to baseline sublingual microvascular perfusion. Despite relative differences in baseline perfusion among patients, all patients, from the standpoint of global assessment, appeared stable. The clinical relevance of our observations regarding the microcirculation and its response to transfusion remain unknown, but the potential for sublingual microscopy to inform clinical decision making must be acknowledged, and with further upgrades in technology, could become a useful tool for assessment of perfusion and response to therapy.
Acknowledgments
This manuscript was supported by Grant Number R01HL095468 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
References
- 1.Agarwal N, Murphy JG, Cayten CG, Stahl WM. Blood transfusion increases the risk of infection after trauma. Arch Surg. 1993;128(2):171–6. doi: 10.1001/archsurg.1993.01420140048008. discussion 6-7. [DOI] [PubMed] [Google Scholar]
- 2.Claridge JA, Sawyer RG, Schulman AM, McLemore EC, Young JS. Blood transfusions correlate with infections in trauma patients in a dose-dependent manner. Am Surg. 2002;68(7):566–72. [PubMed] [Google Scholar]
- 3.Croce MA, Tolley EA, Claridge JA, Fabian TC. Transfusions result in pulmonary morbidity and death after a moderate degree of injury. J Trauma. 2005;59(1):19–23. doi: 10.1097/01.ta.0000171459.21450.dc. discussion -4. [DOI] [PubMed] [Google Scholar]
- 4.Malone DL, Dunne J, Tracy JK, Putnam AT, Scalea TM, Napolitano LM. Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. J Trauma. 2003;54(5):898–905. doi: 10.1097/01.TA.0000060261.10597.5C. discussion -7. [DOI] [PubMed] [Google Scholar]
- 5.Dunne JR, Malone DL, Tracy JK, Napolitano LM. Allogenic blood transfusion in the first 24 hours after trauma is associated with increased systemic inflammatory response syndrome (SIRS) and death. Surg Infect (Larchmt) 2004;5(4):395–404. doi: 10.1089/sur.2004.5.395. [DOI] [PubMed] [Google Scholar]
- 6.Weinberg JA, McGwin G, Jr, Vandromme MJ, Marques MB, Melton SM, Reiff DA, et al. Duration of red cell storage influences mortality after trauma. J Trauma. 2010;69(6):1427–31. doi: 10.1097/TA.0b013e3181fa0019. discussion 31-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Backer D, Hollenberg S, Boerma C, Goedhart P, Buchele G, Ospina-Tascon G, et al. How to evaluate the microcirculation: report of a round table conference. Crit Care. 2007;11(5):R101. doi: 10.1186/cc6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verdant CL, De Backer D, Bruhn A, Clausi CM, Su F, Wang Z, et al. Evaluation of sublingual and gut mucosal microcirculation in sepsis: a quantitative analysis. Crit Care Med. 2009;37(11):2875–81. doi: 10.1097/CCM.0b013e3181b029c1. [DOI] [PubMed] [Google Scholar]
- 9.Trzeciak S, Cinel I, Phillip Dellinger R, Shapiro NI, Arnold RC, Parrillo JE, et al. Resuscitating the microcirculation in sepsis: the central role of nitric oxide, emerging concepts for novel therapies, and challenges for clinical trials. Acad Emerg Med. 2008;15(5):399–413. doi: 10.1111/j.1553-2712.2008.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166(1):98–104. doi: 10.1164/rccm.200109-016oc. [DOI] [PubMed] [Google Scholar]
- 11.Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32(9):1825–31. doi: 10.1097/01.ccm.0000138558.16257.3f. [DOI] [PubMed] [Google Scholar]
- 12.Sakr Y, Chierego M, Piagnerelli M, Verdant C, Dubois MJ, Koch M, et al. Microvascular response to red blood cell transfusion in patients with severe sepsis. Crit Care Med. 2007;35(7):1639–44. doi: 10.1097/01.CCM.0000269936.73788.32. [DOI] [PubMed] [Google Scholar]
- 13.Spronk PE, Ince C, Gardien MJ, Mathura KR, Oudemans-van Straaten HM, Zandstra DF. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet. 2002;360(9343):1395–6. doi: 10.1016/s0140-6736(02)11393-6. [DOI] [PubMed] [Google Scholar]
- 14.Spence RK, Carson JA, Poses R, McCoy S, Pello M, Alexander J, et al. Elective surgery without transfusion: influence of preoperative hemoglobin level and blood loss on mortality. Am J Surg. 1990;159(3):320–4. doi: 10.1016/s0002-9610(05)81227-9. [DOI] [PubMed] [Google Scholar]
- 15.Carson JL, Poses RM, Spence RK, Bonavita G. Severity of anaemia and operative mortality and morbidity. Lancet. 1988;1(8588):727–9. doi: 10.1016/s0140-6736(88)91536-x. [DOI] [PubMed] [Google Scholar]
- 16.De Backer D, Ospina-Tascon G, Salgado D, Favory R, Creteur J, Vincent JL. Monitoring the microcirculation in the critically ill patient: current methods and future approaches. Intensive Care Med. 2010;36(11):1813–25. doi: 10.1007/s00134-010-2005-3. [DOI] [PubMed] [Google Scholar]
- 17.Ospina-Tascon G, Neves AP, Occhipinti G, Donadello K, Buchele G, Simion D, et al. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med. 2010;36(6):949–55. doi: 10.1007/s00134-010-1843-3. [DOI] [PubMed] [Google Scholar]
- 18.Friedlander MH, Simon R, Machiedo GW. The relationship of packed cell transfusion to red blood cell deformability in systemic inflammatory response syndrome patients. Shock. 1998;9(2):84–8. doi: 10.1097/00024382-199802000-00002. [DOI] [PubMed] [Google Scholar]