Abstract
Nitric oxide (NO) is an endogenous vasodilator as well as natural inhibitor of platelet adhesion/activation. Nitric oxide releasing (NOrel) materials can be prepared by doping an NO donor species, such as diazeniumdiolated dibutylhexanediamine (DBHD/N2O2), within a polymer coating. The inherent hemocompatibility properties of the base polymer can also influence the efficiency of such NO release coatings. In this study, four biomedical grade polymers were evaluated in a 4 h rabbit model of thrombogenicity for their effects on extracorporeal circuit thrombus formation and circulating platelet count. At the end of 4 h, Elast-Eon E2As was found to preserve 58% of baseline platelets versus 48, 40, and 47% for PVC/DOS, Tecophilic SP-60D-60, and Tecoflex SG80A, respectively. Elast-Eon also had significantly lower clot area of 5.2 cm2 compared to 6.7, 6.1, and 6.9 cm2 for PVC/DOS, SP-60D-60, and SG80A, respectively. Based on the results obtained for the base polymer comparison study, DBHD/N2O2-doped E2As was evaluated in short-term (4 h) rabbit studies to observe the NO effects on prevention of clotting and preservation of platelet function. Platelet preservation for this optimal NO release formulation was 97% of baseline after 4 h, and clot area was 0.9 cm2 compared to 5.2 cm2 for controls, demonstrating that combining E2As with NO release provides a truly advanced hemocompatible polymer coating for extracorporeal circuits and potentially other blood contacting applications.
Keywords: Diazeniumdiolate, Extracorporeal Circulation, Nitric Oxide, Platelets, PLGA
1. Introduction
The hemocompatibility of blood-contacting medical devices (e.g., extracorporeal circuits, catheters, stents, grafts, etc.) is still a challenge, despite decades of research.1-3 Extracorporeal circulation (ECC) includes a wide variety of devices, from short-term hemodialysis and cardiopulmonary bypass (several hours), to extracorporeal life support (ECLS) (days to weeks).4 The most common complications with ECLS devices are bleeding (7-34%) and thrombosis (8-17%).5 In a clinical setting, these extracorporeal devices require the use of systematic anticoagulation (e.g., heparin) to avoid device failure.6 Systemic infusion of anticoagulants, such as heparin, is known to be the cause of hemorrhage, thrombocytopenia, and thrombosis.7 Despite these complications, heparin is still used as the standard in anticoagulation therapy for patients on ECC. Biomaterial related thrombosis is a complex process, where the initial biological response when blood comes in contact with a foreign surface is protein adsorption, which is followed by platelet adhesion and activation, leading to thrombus formation. One approach to improve the hemocompatibility of a surface is to prevent platelet adhesion, which is desirable for improving clinical outcomes. Nitric oxide (NO) is a well-known inhibitor of platelet activation and adhesion that is released from the endothelium lining of blood vessels. The amount of NO released from normal and stimulated endothelium has been estimated to between 0.5-4.0 ×10−10 mol cm−2 min−1.8 Hence, one potential strategy to decrease the level or completely avoid systemic heparinization is to develop coatings that mimic the endothelium with respect to NO release at physiological levels.
Due to the short lifetime of NO under physiological conditions, a wide number of NO donor molecules have been studied, including S-nitrosothiols9-11 and N-diazeniumdiolates,12-15 and these species have been used to develop polymeric materials that mimic this NO release from normal endothelium. Diazeniumdiolates release NO through thermal16 and proton17 driven mechanisms. Diazeniumdiolated dibutylhexanediamine (DBHD/N2O2) has proven to be an excellent NO donor when incorporated into polymer materials, however the release of NO creates lipophilic amines which raises the pH within the polymer matrix and effectively turns off the NO release.14, 15 Prior work demonstrated that tetrakis-(p-chlorophenyl)-borate could be used as an additive to prolong the NO release up to a few days from the DBHD/N2O2-based coatings.12, 14 However, this additive did not enable the release of the entire payload of NO from the polymer coatings and was also found to be cytotoxic towards endothelial and smooth muscle cells.18 Recently, it was shown that ester-capped poly(lactic-co-glycolic acid) (PLGA), a bioabsorbable polymer, can be used as a small additive to DBHD/N2O2-based coatings and greatly extends the NO release profile of these coatings.15 PLGA hydrolyzes and continuously generates lactic and glycolic acid, which can compensate for the pH elevation from the organoammonium hydroxide (resulting from the reaction between the lipophilic amines and water), thereby controlling the pH within the organic polymer phase and sustaining the NO release up to 14 d.15, 19
Previously, our group has observed preservation of platelets and reduced thrombus area in a rabbit model of ECC with NO release coatings using DBHD/N2O2 doped poly(vinyl chloride)/dioctyl sebacate (PVC/DOS) as the base polymer.12, 15 However, the inherent hemocompatiblity properties of the base polymers used in combination with NO release can have a direct effect on the ultimate efficacy in preventing thrombus formation. In this study, we evaluate the degree of platelet consumption and thrombus formation of four biomedical grade polymers (poly(vinyl chloride) (PVC)/dioctyl sebacate (DOS), Tecophillic SP-60D-60, Elast-Eon E2As, and Tecoflex SG80A) in a rabbit thrombogenicity model using an arterio-venous (A-V) shunt. The polymer yielding the best inherent hemocompatible properties was then used for the NO release studies by doping with DBHD/N2O2 and ester-capped PLGA additive, which was then evaluated in the 4 h rabbit model for effects on platelet consumption and thrombus formation.
2. Materials and Methods
2.1. Materials
TygonTM poly(vinyl chloride) (PVC) tubing was purchased from Fisher Healthcare (Houston, TX). Tecophilic SP-60D-60 and Tecoflex SG-80A were obtained from Lubrizol Advanced Materials Inc. (Cleveland, OH). Elast-EonTM E2As was a product of AorTech International, plc (Scoresby, Victoria, Australia). Anhydrous tetrahydrofuran (THF), anhydrous acetonitrile, sodium chloride, potassium chloride, sodium phosphate dibasic, and potassium phosphate monobasic were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). Poly(D,L-lactide-co-glycolide) 5050DLG7E was obtained from SurModics Pharmaceuticals Inc. (Birmingham, AL). Human P-selectin glycoprotein (CD62P) PE and IgG1 PE were obtained from AbD Serotec (Raleigh, NC). N,N’-Dibutyl-1,6-hexanediamine (DBHD) was purchased from Alfa Aesar (Ward Hill, MA). DBHD/N2O2 was synthesized by treating DBHD with 80 psi NO gas purchased from Cryogenic Gases (Detroit, MI) at room temperature for 48 h, as previously described.14 Phosphate buffered saline (PBS), pH 7.4, containing 138 mM NaCl, 2.7 mM KCl, 10 mM sodium phosphate, was used for all in vitro experiments.
2.2. Preparation of coated ECC loops
The ECC configuration employed in the in vivo rabbit study was previously described.9, 12, 15 Briefly, the ECC consisted of a 16-gauge and 14-gauge IV polyurethane angiocatheters (Kendall Monoject Tyco Healthcare Mansfield, MA), two 16 cm in length ¼ inch inner diameter (ID) Tygon™ tubing and an 8 cm length of 3/8 inch ID Tygon™ tubing that created a thrombogenicity chamber where thrombus could form more easily due to more turbulent blood flow.
Base polymer coated control ECCs
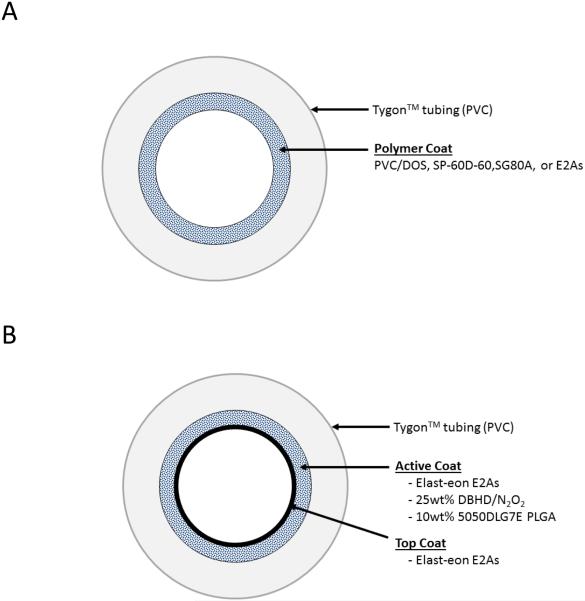
Polymer control loops were coated with E2As, SG80A, SP60D60, or PVC/DOS solutions, as shown in Fig. 1A. All the control loops contained 2 coats of the polymer/THF solution (2500 mg in 15 mL).
Fig. 1.
Diagram of the extracorporeal circuit (ECC) tubing coated with (A) base polymers PVC/DOS, SP-60D-60, SG80A, or E2As, and (B) E2As doped with a lipophilic DBHD/N2O2 and the PLGA additive with a top coat of E2As.
NOrel coated ECCs
NOrel loops were prepared with E2As coating containing 25 wt% DBHD/N2O2 as the NO donor, and 10 wt% 5050DLG7E PLGA additive, as shown in Fig. 1B. The NOrel solution was prepared by dissolving 1600 mg E2As and 250 mg PLGA in 15 mL THF. DBHD/N2O2 (625 mg) was then dispersed within the polymer cocktail by sonication for 30 min to obtain a slightly cloudy dispersion of the diazeniumdiolate in the solution. The PVC tubing was first coated with 2 layers of the NOrel solution, followed by 1 coat of the E2As control solution (2500 mg in 15 mL).
All ECC loops were allowed to air dry for 1 h between each coat. The completely coated ECC was assembled together using THF, starting with the 16-gauge angiocatheter, one 15 cm length ¼ inch ID tubing, the 8 cm length thrombogenicity chamber, the second 15 cm length ¼ inch ID tubing and finally the 14-gauge angiocatheter. The angiocatheters were interfaced with tubing using two luer-lock PVC connectors. The assembled ECC loops were dried for 1 d under ambient conditions and then under vacuum for 48 h.
2.5. NO release measurements
Nitric oxide released from the samples was measured using a Sievers chemiluminescence Nitric Oxide Analyzer (NOA), model 280 (Boulder, CO).9, 12, 14, 15 A sample was placed in 4 mL PBS buffer at 37°C. Nitric oxide liberated from the sample was continuously swept from the headspace of the sample cell and purged from the buffer with a nitrogen sweep gas and bubbler into the chemiluminescence detection chamber. The flow rate was set to 200 mL/min with a chamber pressure of 5.6 Torr and an oxygen pressure of 6.0 psi.
2.6. The 4 h rabbit thrombogenicity model
Rabbit thrombogenicity model protocol
The animal handling and surgical procedures were approved by the University Committee on the Use and Care of Animals in accordance with university and federal regulations. A total of 22 New Zealand white rabbits (Myrtle’s Rabbitry, Thompson’s Station, TN) were used in this study. All rabbits (2.5-3.5 kg) were initially anesthetized with intramuscular injections of 5 mg/kg xylazine injectable (AnaSed® Lloyd Laboratories Shenandoah, Iowa) and 30 mg/kg ketamine hydrochloride (Hospira, Inc. Lake Forest, IL).
Maintenance anesthesia was administered via isoflurane gas inhalation at a rate of 1.5-3% via mechanical ventilation which was done via a tracheotomy and using an A.D.S. 2000 Ventilator (Engler Engineering Corp. Hialeah, FL). Peek inspiratory pressure was set to 15 cm of H2O and the ventilator flow rate set to 8 L/min. In order to aid in maintenance of blood pressure stability, IV fluids of Lactated Ringer’s were given at a rate of 10 mL/kg/h. For monitoring blood pressure and collecting blood samples, the rabbits’ right carotid artery was cannulated using a 16-gauge IV angiocatheter (Jelco®, Johnson & Johnson, Cincinnati, OH). Blood pressure and derived heart rate were monitored with a Series 7000 Monitor (Marquette Electronics Milwaukee, WI). Body temperature was monitored with a rectal probe and maintained at 37°C using a water-jacketed heating blanket. Prior to placement of the arteriovenous (AV) custom-built extracorporeal circuits, the rabbit left carotid artery and right external jugular vein were isolated and baseline hemodynamics as well as arterial blood pH, pCO2, pO2, and total hemoglobin were measured using an ABL 825 blood-gas analyzer. In addition, baseline blood samples were collected for platelet and total white blood cell (WBC) counts which were measured on a Coulter Counter Z1 (Coulter Electronics Hialeah, FL). Activated clotting times (ACT) were monitored using a Hemochron Blood Coagulation System Model 801 (International Technidyne Corp. Edison, NJ), platelet function was assessed using a Chrono-Log optical aggregometer model 490 (Havertown, PA).
After baseline blood measurements, the custom-built ECC was placed into position by cannulating the left carotid artery for ECC inflow and the right external jugular vein for ECC outflow. The flow through the ECC was initiated by unclamping the arterial and venous sides of ECC and blood flow in circuit was monitored with an ultrasonic flow probe and flow meter (Transonic HT207 Ithaca, NY). Animals were not systemically anticoagulated during the experiments.
Blood sampling
Rabbit whole blood samples were collected in non-anticoagulated 1 mL syringes for ACT, 3.2% sodium citrate vacutainers (Becton, Dickinson. Franklin Lakes, NJ) in 3 mL volumes for cell counts, aggregometry, and 1 mL syringes containing 40 U/mL of sodium heparin (APP Pharmaceuticals, LLC Schaumburg, IL) for blood-gas analysis. Following the initiation of ECC blood flow, blood samples were collected every hour for 4 h for ex vivo measurements. Samples were used within 2 h of collection to avoid any activation of platelets, monocytes or plasma fibrinogen.
Platelet aggregometry
Rabbit platelet aggregation was assayed based on the Born’s turbidimetric method using a Chrono-Log optical aggregometer. Briefly, citrated blood (1:10 blood to 3.8% sodium citrate) was collected (6 mL) and platelet-rich plasma (PRP) was obtained by centrifugation at 110 × g for 15 min. Platelet-poor plasma (PPP) was obtained by another centrifugation of the PRP-removed blood sample at 2730 × g for 15 min and was used as the blank for aggregation. PRP was incubated for 10 min at 37°C and then 25 μg/mL collagen (Chrono-PAR #385 Havertown, PA) was added. The percentage of aggregation was determined 3 min after the addition of collagen using Chrono-Log Aggrolink software.
Flow Cytometery for P-selectin expression
To determine platelet P-selectin (CD62P) expression, 100 μL diluted blood aliquots (1:100 dilution of blood to Hank’s Balanced Salt Solution (HBSS) without CaCl2 and MgCl2) were directly prepared for cell surface staining of P-selectin. In four 12 × 75 polypropylene tubes containing 100 μL of diluted blood, 40 μg/mL collagen is added to two tubes and 4 μL saline is added to the other two tubes. At this point, saturating concentrations (10 μL) of monoclonal antihuman CD62P PE antibody was added to one collagen and one saline treated tube and incubated for 15 min at room temperature (RT) in the dark. In the other two tubes containing collagen and saline, 10 μL of IgG1 PE was added as nonbinding isotype control and also incubated for 15 min at RT in the dark. After the antibody incubation, each tube received 700 μL of freshly prepared 1% formaldehyde buffer (in dPBS) and stored at 4°C until ready for flow cytometric analysis. A FACSCalibur flow cytometer (Becton Dickinson San Jose, CA) was used for the acquisition of flow data and the CellQuest software used for data analysis. Cell populations were identified for data collection by their forward scatter (FSC) and side scatter (SSC) light profiles. For each sample, 30,000 total events were collected. Fluorescence intensity of immunostaining was quantitated by histogram log plot analysis. Mean fluorescent intensity (MFI) was expressed as the geometric mean channel fluorescence minus the appropriate isotype control.
Determination of thrombus area
After 4 h on ECC, the circuits were clamped, removed from animal, rinsed with 60 mL of saline, and drained. Any residual thrombus in the larger tubing of ECC (i.e., thrombogenicity chamber) was photographed and the degree of thrombus image was quantitated using Image J imaging software from National Institutes of Health (Bethesda, MD). The animals were euthanized using a dose of Fatal Plus (130 mg/kg sodium pentobarbital) (Vortech Pharmaceuticals Dearborn, MI).
2.7. Statistical analysis
Data are expressed as mean ± SEM (standard error of the mean). Comparison of ECC results between the various control polymer groups and NOrel and the control polymer groups were analyzed by a one-way ANOVA with a multiple comparison of means using Student's t-test. Values of p < 0.05 were considered statistically significant for all tests.
3. Results and Discussion
3.1 Comparison of hemodynamic effects of four biomedical grade polymers in ECC rabbit model
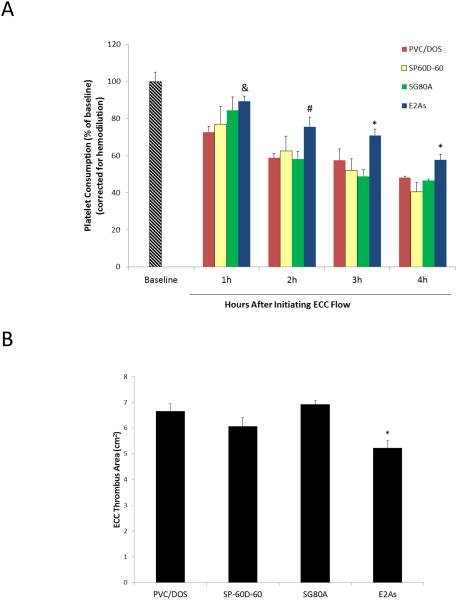
The hemocompatibility of Tecophillic SP-60D-60, Tecoflex SG80A, Elast-Eon E2As, poly(vinyl chloride) (PVC)) polymer coatings was compared using the 4 h rabbit thrombogenicity model. The goal of this comparison was to choose the polymer with the best hemocompatible properties to be combined with the NO release chemistry for optimizing NOrel coatings for extracorporeal circulation testing in rabbits. Polymers were coated on the ECC tubings as described in Section 2.2. Platelet preservation during exposure of the coated ECC surfaces to flowing blood was assessed by measuring the platelet count every hour. Platelet count was used as one of the parameters to assess the hemocompatibility of the surface because the decrease in the platelet count over time indicates the activation of platelets on the tubing surface.9, 12, 15 Platelet count was corrected for any hemodilution due to any IV infusion of fluids into the rabbits. Only 1 out of 5 loops coated with E2As clotted before the end of the 4 h experiment, whereas, 2-3 of the SP-60D-60, SG80A, and PVC/DOS loops clotted (4 loops were tested for each polymer). As shown in Fig. 2A, at the end of 4 h, all polymer coatings exhibited a time dependent loss in platelets, however, 58 ± 3% of platelets were preserved for E2As ECCs whereas, animals tested with PVC/DOS, SG80A, and SP-60D-60 exhibited a more significant loss in platelets count (46 ± 3%, 44 ± 4%, 41 ± 5%, respectively). To ascertain the differential of the thrombus in the thrombogenicity chambers (i.e., the 3/8” Tygon 8 cm in length within the ECC loop) of the coated ECCs, a two dimensional analysis was performed after 4 h of blood exposure. NIH Image J imaging software was used to calculate the representative 2-D thrombus area (cm2) in each tubing chamber.12, 15 The thrombus area of the E2As polymer coated ECC was significantly lower than the other polymers tested. These polymers had clot areas of 5.2 ± 0.3, 6.7 ± 0.3, 6.1 ± 0.4, and 6.9 ± 0.2 cm2 for E2As, PVC/DOS, SP-60D-60, and SG80A, respectively (see Fig. 2B). Based on the platelet count and clot area, the E2As polymer was found to have enhanced intrinsic hemocompatible properties compared to the other polymers. The preservation of platelet count and reduced clot area can be attributed to the fact that the E2As polymer binds to albumin more strongly than fibrinogen, which likely aids in passivating the surface.20 It is widely accepted fact that protein adsorption is the first event that occurs upon surface-blood contact.21 Fibrinogen is a key protein in the coagulation cascade that rapidly adsorbs to foreign surfaces and binds to activated platelets. Fibrinogen contains multiple binding sites for platelet integrin αIIbβ3 (GPIIbIIIa).22, 23 These fibrinogen-αIIbβ3 interactions play a significant role in platelet adhesion, activation and aggregation that ultimately leads to a clot formation.23 Platelets can bind to both albumin and fibrinogen, however albumin can significantly reduce the platelet adhesion in comparison to fibrinogen coated surfaces.24 The fact that E2As polymer adsorbs albumin more strongly than fibrinogen indicates its potential in passivating the blood contacting surfaces though this mechanism.20 This study provided the opportunity to compare various biomedical grade polymers in order to choose the best candidate material for incorporating NO donors. Our hypothesis is that by combining the E2As polymer (with excellent intrinsic hemocompatibility properties) with NO release will further improve the overall hemocompatibility of such coatings than can be achieved by use of either approach alone.
Fig. 2.
(A) Comparison of time-dependent effects of 4 base polymers coated ECC loops on platelet consumption. * = p < 0.05, E2As vs. other polymers; # = p < 0.05, E2As vs. PVC/DOS and SG80A; & = p < 0.05, E2As vs. PVC/DOS. (B) Quantitation of thrombus area as calculated with NIH ImageJ software using a 2D representation of thrombus. The data are means ± SEM. * = p < 0.05, E2As vs. other polymers.
3.2 Nitric oxide release measurements from E2As-based NOrel coatings and their effects on rabbit hemodynamics
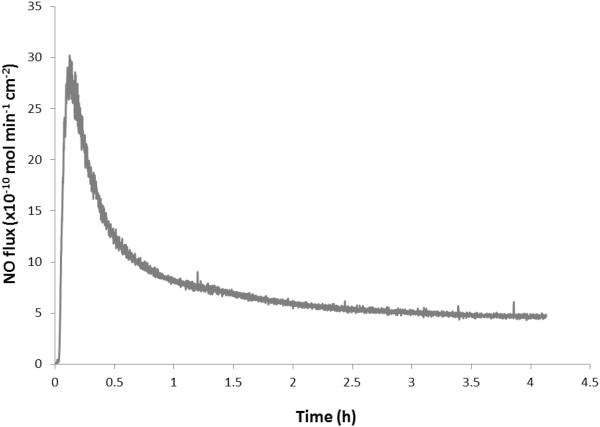
ECC circuits were coated with the DBHD/N2O2-doped E2As polymer as described in Section 2.2 above. The NOrel loops were prepared with E2As coating containing 25 wt% DBHD/N2O2, and 10 wt% 5050DLG7E PLGA additive. This ratio of DBHD/N2O2 and 5050DLG7E PLGA additive was found to be optimal in our previous work with PVC coatings.15 The combined E2As-based NOrel coating material continuously releases NO under physiological conditions for greater than 4 h at levels that exceeds the physiological NO flux levels from endothelial cells (0.5-4 × 10−10 mol cm−2 min−1).8 The NO release, as measured using chemiluminescence NO analyzer, shows an average sustained NO flux of approximately 6 × 10−10 mol cm−2 min−1 for 4 h (Fig. 3). The NOrel coated ECCs exhibited a slight burst of NO upon initial exposure to the PBS and 37°C that lasted ~ 30 min. Therefore, the ECC loops were first soaked with PBS for 30 min prior to the rabbit experiments in order to reduce the effects of this burst. The NO flux from the surface of the ECC circuit does not decrease significantly after exposure to the flowing blood. Indeed, after 4 h of blood flow, the NO flux was found to be 5 × 10−10 mol cm−2 min−1. The fact that the blood environment does not alter the kinetics of the NO release from the coating agrees well with the previously reported data for various NO release ECC circuits.12, 15
Fig. 3.
Representative NO surface flux profiles from E2As base NOrel polymer ECC containing DBHD/N2O2 at 37 °C and pH 7.4 obtained via chemiluminescence detection method.
No significant difference in the mean arterial pressure (MAP) of the animals on the NOrel vs. control circuits was noted, with pressures averaging 45±5 mm Hg for both types of circuits. Heart rates for the NOrel and control ECC groups were unchanged over the 4 h time period. The ECC blood flow was maintained at approximately 110 mL/min for the NOrel circuits over the 4 h animal test period. However, the blood flow dropped from the initial 110 mL/min to approximately 75 mL/min in the first one hour for the control circuits, and then further dropped to 60 mL/min over the next 3 h period. Intravascular fluids were maintained at 10 mL/kg/min to maintain the blood flow in both NOrel and control circuits. The activation clotting time for blood obtained from the test animals increases over the 4 h period for both NOrel and control coated circuits. As noted in previous studies,12 this behavior can be attributed to the increase in intravascular fluids and concomitant hemodilution effect.
3.3. Effects of E2As-based NOrel coating on rabbit platelet function and thrombus formation in a short-term (4 h) application
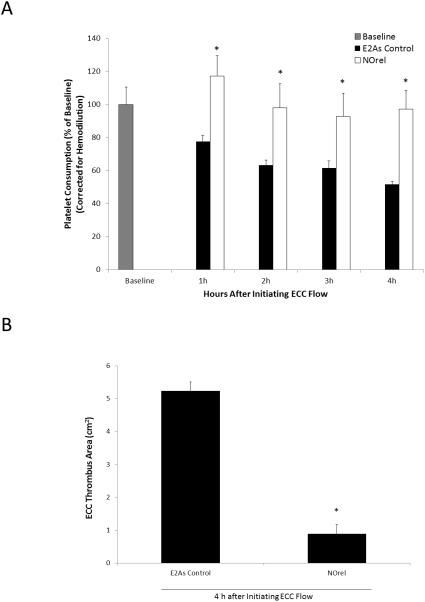
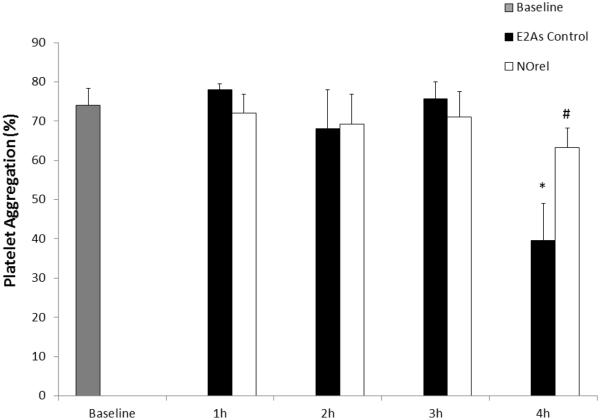
The short-term 4 h rabbit ECC model was used to observe the effects of the NOrel coating on platelet count, platelet function, and clotting. As described in Section 2.6, platelet activation and function throughout the 4 h ECC was assessed by recording the platelet count and platelet aggregation, which were both corrected for hemodilution due to the added IV fluids. For NOrel coated ECC loops, all 5 survived the 4 h experiment, whereas for E2As control only 4 out of 5 loops survived the 4 h ECC run. For the NOrel circuits, the platelet count rose slightly above the baseline value during the first hour, likely due to the release of reserve platelets from the spleen. The platelet count returned to the baseline level during the second hour and was maintained at an average of 97 ± 10% of baseline levels at the end of the 4 h experiments. The platelet count for E2As control circuits showed a time-dependent loss in platelets dropping to 58 ± 3% of baseline after 4 h (Fig. 4A). As shown in Fig. 4B, a significant reduction of clot area was observed on NOrel (0.9 ± 0.3 cm2) as compared to E2As controls (5.2 ± 0.3 cm2).
Fig. 4.
(A) Time dependent effects of NOrel ECC (25 wt% DBHD/N2O2 + 10 wt% 5050DLG7E PLGA in E2As) as compared to control ECC on rabbit platelet count (i.e. consumption) as measured via Coulter counter. (B) Quantitation of thrombus area as calculated with NIH ImageJ software using a 2D representation of thrombus. The data are means ± SEM. * = p < 0.05, control vs. NOrel ECC circuits.
Platelet function during exposure to NOrel and E2As control polymer coated ECCs was also assessed by percentage of aggregation, as determined by ex vivo collagen-stimulated of PRP and measured by optical turbidity12, 15 over the course of the 4 h ECC experiment (Fig. 5). The NOrel polymer ECC showed a greater preservation of platelet aggregation over the course of 4 h blood exposure, while the E2As control polymer showed a significant loss in ability to aggregate upon collagen stimulation at the 4 h time point. The ability to aggregate upon exogenous collagen stimulation was maintained with NOrel ECCs at 91% of baseline values after 4 h, whereas the platelets from control ECCs had only 49% ability to aggregate. This data is in agreement with the observation by Major et al., who previously showed that NOrel materials have the ability to preserve platelet function and their ability to aggregate.12
Fig. 5.
Time-dependent effects of NOrel vs. control polymer ECC on platelet function, as measured via aggregometry. The data are means ± SEM. * = p < 0.05, control vs. baseline; # = p < 0.05, control vs. NOrel ECC circuits.
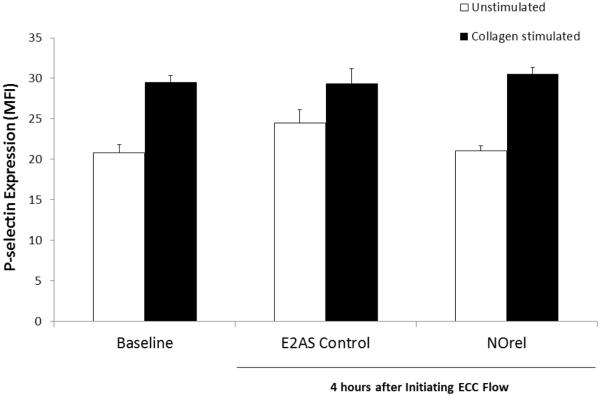
To further evaluate the effects of the ECC loops, platelet P-selectin (CD62P) expression was used as an early activation marker to test the hemocompatibility of the ECC loops. In order to ascertain the differential expression of the platelet adhesion glycoprotein, P-selectin, between the NOrel and E2As control polymer coated ECCs, fluorescence-activated cell sorting (FACS) analysis was performed on rabbit circulating platelets. A gated region is chosen on forward scatter (FSC) vs. side scatter (SSC) dot plot which is representative of the circulating platelet population and the same gate was used in all subsequent FACS analysis for CD62P expression. When platelets become activated, the surface expression of P-selectin increases.25 The platelet P-selectin surface expression data is shown via mean fluorescence intensity (MFI) in Fig. 6. P-selectin expression was considerably elevated on unstimulated control ECC platelets in comparison to the NOrel ECC platelets with respect to the baseline values. This indicates higher platelet activation in the case of control vs. NOrel surfaces after 4 h ECC blood exposure. Addition of collagen (40 μg/mL) significantly stimulated an increase in P-selectin expression at baseline and after 4 h on control and NORel ECCs, indicating proper functioning of the platelets after NO exposure. This data indicates that NO flux of 6 × 10−10 mol cm−2 min−1 was sufficient to keep the protein markers of platelet activation quiescent for the 4 h period without adversely affecting their functionality.
Fig. 6.
Platelet P-selecting mean fluorescent intensity after 4 h on ECC with or without 40 μg/mL collagen stimulation in E2As-based NOrel and E2As control coatings.
In the previous work it was shown that using DBHD/N2O2 chemistry in PVC/DOS polymer an NO flux of approximately 10 – 15 × 10−10 mol cm−2 min−1 is needed to prevent clotting and maintain 80% of baseline platelet count in extracorporeal circulation.12, 15 Here we report that with the appropriate polymer, such as E2As, an NO flux of only 6 × 10−10 mol cm−2 min−1 is sufficient to prevent platelet activation, reduce clot area, and is able to maintain 97% of baseline platelet count at the end of 4 h, showing a significant improvement over our previous work.
5. Conclusions
This study reports on a comparison of four biomedical polymers with respect to their inherent ability to resist platelet consumption and thrombus formation in 4 h ECC rabbit model. Elast-Eon E2As was found to be superior to SP-60D-60, SG80A, and PVC/DOS, in prevention of thrombus formation in the short-term rabbit model. Nitric oxide release from optimal E2As coatings containing a diazeniumdioloate NO donor and PLGA additive on the inner walls of the ECC circuits was able to further attenuate the activation of the platelets while maintaining their functionality in a 4 h ECC rabbit model and reduce clot area. We were also able to demonstrate that a lower flux of NO is sufficient to maintain platelet count and reduce clotting when using circuits prepared with a more hemocompatible base polymer coating such as E2As as compared to our previous work where other polymers such as PVC/DOS was employed as the base polymer. These encouraging results demonstrate that E2As-based NOrel materials have great potential to improve the hemocompatibility of both short and potentially long-term blood contacting devices such as catheters, vascular grafts, stents and other extracorporeal life support devices. These E2As based NOrel coatings are currently under investigation for longer-term catheter applications.
Acknowledgements
The authors declare this work is supported by the National Institutes of Health, Grants K25HL111213, EB000783, R21EB016236, and HL015434. The authors wish to thank AorTech International for the gift of Elast-Eon E2As polymer.
The authors, except Dr. Robert Bartlett, confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. Dr. Robert Bartlett has an equity interest in MC3, Inc. but no financial support from this company was utilized in any aspect of the studies reported in this manuscript.
References
- 1.Ratner BD. Biomaterials. 2007;28:5144–5147. doi: 10.1016/j.biomaterials.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sin DC, Kei HL, Miao X. Expert.Rev.Med.Devices. 2009;6:51–60. doi: 10.1586/17434440.6.1.51. [DOI] [PubMed] [Google Scholar]
- 3.Ranucci M, Balduini A, Ditta A, Boncilli A, Brozzi S. Ann.Thorac.Surg. 2009;87:1311–1319. doi: 10.1016/j.athoracsur.2008.09.076. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds MM, Annich GM. Organogenesis. 2011;7:42–49. doi: 10.4161/org.7.1.14029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Extracorporeal Life Support Organization (ELSO) Extracorporeal Life Support (ECLS) registry report international summary. 2010 [Google Scholar]
- 6.Gaffney AM, Wildhirt SM, Griffin MJ, Annich GM, Radomski MW. BMJ. 2010;341 doi: 10.1136/bmj.c5317. [DOI] [PubMed] [Google Scholar]
- 7.Robinson TM, Kickler TS, Walker LK, Ness P, Bell W. Critical Care Medicine. 1993;21:1029–1034. doi: 10.1097/00003246-199307000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Vaughn MW, Kuo L, Liao JC. Am J Physiol. 1998;274:H2163–H2176. doi: 10.1152/ajpheart.1998.274.6.H2163. [DOI] [PubMed] [Google Scholar]
- 9.Brisbois EJ, Handa H, Major TC, Bartlett RH, Meyerhoff, ME. Biomaterials. 2013 doi: 10.1016/j.biomaterials.2013.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Souza GFP, Yokoyama-Yasunaka JKU, Seabra AB, Miguel DC, de Oliveira MG, Uliana SRB. Nitric Oxide-Biology and Chemistry. 2006;15:209–216. doi: 10.1016/j.niox.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Seabra AB, de Souza GFP, da Rocha LL, Eberlin MN, de Oliveira MG. Nitric Oxide-Biology and Chemistry. 2004;11:263–272. doi: 10.1016/j.niox.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Major TC, Brant DO, Reynolds MM, Bartlett RH, Meyerhoff ME, Handa H, Annich GM. Biomaterials. 2010;31:2736–2745. doi: 10.1016/j.biomaterials.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoenfisch MH, Mowery KA, Rader MV, Baliga N, Wahr JA, Meyerhoff ME. Anal.Chem. 2000;72:1119–1126. doi: 10.1021/ac991370c. [DOI] [PubMed] [Google Scholar]
- 14.Batchelor MM, Reoma SL, Fleser PS, Nuthakki VK, Callahan RE, Shanley CJ, Politis JK, Elmore J, Merz SI, Meyerhoff ME. J Med.Chem. 2003;46:5153–5161. doi: 10.1021/jm030286t. [DOI] [PubMed] [Google Scholar]
- 15.Handa H, Brisbois EJ, Major TC, Refahiyat L, Amoako KA, Annich GM, Bartlett RH, Meyerhoff ME. Journal of Materials Chemistry B. 2013;1:3578–3587. doi: 10.1039/C3TB20277A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Annich GM, Miskulin J, Osterholzer K, Merz SI, Bartlett RH, Meyerhoff ME. Biomaterials. 2002;23:1485–1494. doi: 10.1016/s0142-9612(01)00274-5. [DOI] [PubMed] [Google Scholar]
- 17.Davies KM, Wink DA, Saavedra JE, Keefer LK. J Am Chem.Soc. 2001;123:5473–5481. doi: 10.1021/ja002899q. [DOI] [PubMed] [Google Scholar]
- 18.Wu B. PhD Dissertation, University of Michigan, Ann Arbor, MI. 2009 [Google Scholar]
- 19.Cai W, Wu J, Xi C, Meyerhoff ME. Biomaterials. 2012;33:7933–7944. doi: 10.1016/j.biomaterials.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cozzens D, Luk A, Ojha U, Ruths M, Faust R. Langmuir. 2011;27:14160–14168. doi: 10.1021/la202586j. [DOI] [PubMed] [Google Scholar]
- 21.Xu L-C, Siedlecki CA. Langmuir. 2009;25:3675–3681. doi: 10.1021/la803258h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agnihotri A, Soman P, Siedlecki CA. Colloids Surf B Biointerfaces. 2009;71:138–147. doi: 10.1016/j.colsurfb.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 23.Hussain MA, Agnihotri A, Siedlecki CA. Langmuir. 2005;21:6979–6986. doi: 10.1021/la046943h. [DOI] [PubMed] [Google Scholar]
- 24.Sivaraman B, Latour RA. Biomaterials. 2011;32:5365–5370. doi: 10.1016/j.biomaterials.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrews RK, Berndt MC. Thrombosis Research. 2004;114:447–453. doi: 10.1016/j.thromres.2004.07.020. [DOI] [PubMed] [Google Scholar]