Abstract
Background:
Biofilms (BFs) are a potential source of highly resistant infections, frequently formed on devicesand pose problems for management.
Aim:
This study was to develop rational approach for prevention of indwelling urologic device associated biofilm colonization.
Subjects and Methods:
From randomly selected patients visiting Department of Urology of a tertiary hospital in India 150 uro catheters and 31 used ureteric stents, in-situ for > 30, were collected aseptically. The organisms were isolated and identified from washed devices dipped in broth. Evidence of bacteriuria in each case was checked by semi-quantitative method of urine culture, on day 0 and 14 of device use. The BF statuses of the device-adhered organisms were confirmed by modified method of Christensen. The antibiotic susceptibility was determined by disc diffusion method. Data were analyzed using the Graphpad Prism version 5 statistical software.
Results:
Both single and multi-species BFs were formed on catheters, whereas mono-bacterial BFs were exclusive on stents. Predominant organisms were Pseudomonas aeruginosa (30.67%,69/225,) followed by Staphylococcus aureus (15.11%, 34/225), Escherichia coli (13.78%, 31/225), Klebsiella pneumoniae (12%, 27/225), Staphylococcus epidermidis (8.44%, 19/225). Of all strains, (89.33%, 201/225) were found to be BF positive and their colonizations were early indicated by the presence of insignificant bacteriuria in follow-up urine samples. All BF isolates were resistant to at least three antibiotics.
Conclusions:
BF colonization was almost inevitable in prolonged used urinary devices and the most frequent organisms were Pseudomonas, Staphylococcus, and Escherichia spp. Their colonizations usually were indicated by insignificant bacteriuria from follow-up samples. Such BF dislodged organisms were multidrug resistant and could be a source of disseminated infection, yet were in-vitro preventable by many drugs.
Keywords: Antibiotic susceptibility, Biofilm, Catheter, Double J stent
Introduction
Among all currently used medical devices urinary catheters and stents are the most common.[1] Urinary drainage devices are often used for a term of the month or more. These provide ideal conditions for biofilm (BF) colonization.[2,3] Elimination of organisms from BFs by antimicrobial therapy is almost impossible,[4] whereas the incidence of such colonization can be lowered by preventive measures[5,6,7] and spread of infection from detached BF fragments may be reduced by using the sensitive drugs. Hence, information about the incidence of BF colonization by different microorganisms and their drug susceptibility pattern may play an important role in treatment of such infections.
Subjects and Methods
Many patients of Urology department of our tertiary care hospital are often advised to use indwelling urinary catheters with monthly changes as a temporary measure before surgery. After taking approval from Institutional Ethics Committee, random samples of 150 used Foley urinary catheters (Bardia, India) were aseptically collected from such patients with no history of urinary tract infection. Similarly, 31 used Double J (DJ) stents (Cook, India) were included in the study. The patients’ history including age, sex, hospital stay, duration of device application and underlying disease were recorded. The study was conducted in the period between October 2009 and October 2011. On day 0 and 14 of device use, urine samples were collected aseptically. Semi-quantitative method of culture was performed with urine samples by the standard laboratory procedure using a calibrated loop (0.01 ml).
Four inches of devices from the tip end were taken aseptically, and then thoroughly washed with phosphate buffer saline (pH 7.2) to remove non-adherent cells. The devices were dipped into the tubes containing 5 ml. Brain heart infusion (BHI) broths (Hi-Media, India), and then squeezed a little with the help of sterile forceps, and incubated at 37°C for 24 h. One loopful of broth from each tube was subcultured on blood agar (Hi-Media, India) plates. Isolated colonies were provisionally identified based on growth characteristics, morphology, motility, and biochemical test results.[8]
BF potential of the colonizers was assayed using the microtiter plate assay by modified Christensen's method.[9] Briefly, 2 μl of each fresh bacterial suspension (0.5 McFarland) in BHI broth was directly added to each well of sterile microtiter plates, filled with 200 μl of same broth per well. Three repetitions were performed with each isolate and were incubated at 37°C for 24 h. Planktonic bacteria were removed from each microtiter dish by briskly tapping the dish over the waste tray. The wells were washed thrice with sterile distilled water. The plates were fixed with cold methanol, and then 250 μl of 0.1% crystal violet solution was added to each well, and kept for 10 min at room temperature. Each microtiter dish was tapped briskly over the waste tray to remove the crystal violet solution and then rinsed off under running tap water. Plates were allowed to air-dry and 250 μl of 95% ethanol was added to each stained well. Dye was allowed to solubilize by covering plates and incubating for 15 min at room temperature. Based on the optical density (OD) produced by bacterial films at a wavelength of 570 nm, isolates were classified into the following categories: Non-BF producers, weak, moderate or strong BF producers as described by Stepanovic et al. 2000.[10] Briefly, the cut-off optical density (ODc) was defined as three standard deviations above the mean OD of the negative control, i.e., BHI broth. Isolates were classified as follows: OD < ODc = BF non-producer; OD > ODc, but < 2 ODc = weak BF producer; OD > 2 ODc but < 4 ODc = moderate BF producer and > 4 ODc = strong BF producer. All tests were carried out in triplicate and the average results were taken.
Antimicrobial susceptibility tests for all bacterial isolates were performed by Kirby-Bauer disk diffusion method.[11] Yeast isolates were excluded from the study.
All data were analyzed for determining central tendency and for preparing graphical representation by GraphPad Prism version 5.00 (SanDiego, California, USA).
Results
During the study period, samples were collected from both male and female patients in the ratio of 43:7 in catheters and 19:12 in DJ stents. Among catheterized patients, distribution of male: female cases between 10 years and 30 years age group was 19:3; between 31 years and 60 years age group was 33:10 and above 61 years age was 77:8. While most of the cases with stents were in 30-60 years age group (Male: Female = 17:11); only one male patient was above 61 years and one each male and female of 10-30 years age group. The most common underlying causes for catheterization were urethral obstruction (45/150, 30%,), followed by prostatitis (33/150, 22%,) and other renal pathology including calculi (30/150, 20%,). Other important causes were pathology in ureter (15/150, 10%,), hydronephrosis (9/150, 6%,), bladder pathology (6/150, 4%,), and miscellaneous (12/150, 8%,). Renal calculi were the main reason (26/31, 83.9%,) for use of stents. No correlation between formation of BF with age, sex or underlying disease was noted.
Out of 150 catheters tested, 130 were found to be culture positive and no growth was observed in 20 cases. All DJ stents showed growth of bacteria. Though all urine samples were initially sterile, bacterial growth was observed on the 14th day in follow-up urine samples of 108 catheterized cases and 20 cases with DJ stents. The organisms recovered from the devices were identical to the organisms found in the corresponding urine culture; however by colony count significant bacteriuria was detected only in 16 (12.5%) cases.
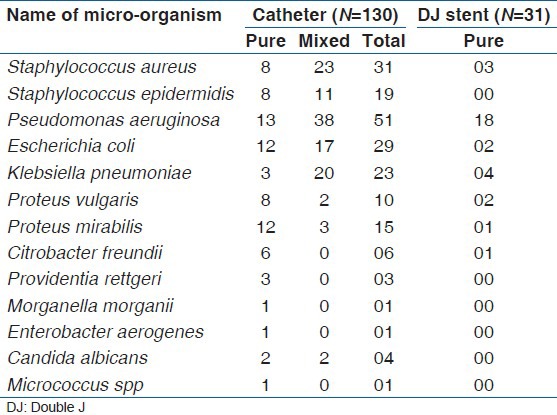
A total of 225 microbes were isolated from catheters and DJ stents. Pure isolates were grown from 78 catheters and 31 DJ stents, all the remaining devices showed polymicrobial growth [Table 1]. The predominant organisms isolated from both devices put together were Pseudomonas aeruginosa (69/225,30.67%), Staphylococcus aureus (34/225, 15.11%), Staphylococcus epidermidis (19/225, 8.44%) Klebsiella pneumoniae (27/225, 12%), Proteus mirabilis (16/225, 7.11%), Proteus vulgaris (12/225, 5.33%), Escherichia coli (31/225, 13.78%), Citrobacter freundii (7/225, 3.11%), Providentia rettgeri (3/225, 1.33%) Candida albicans (1.78%, 4/225). One strain each of Morganella morganii, Enterobacter aerogenes, and Micrococcus spp. were also recovered from catheters.
Table 1.
Organism isolated from culture positive cathetersand stents
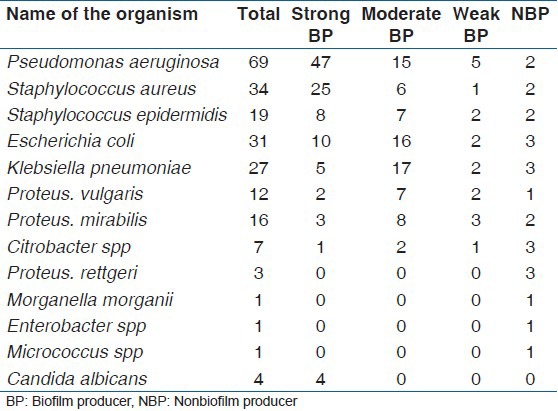
Out of the microbial isolates from both the devices 89.33% were found to be BF positive [Table 2]. Both monomicrobial and polymicrobial colonization were found in catheters. Colonization on DJ stents was monomicrobial exclusively.
Table 2.
Biofilm status of isolates (n = 225)
In-vitro antibiotic sensitivity pattern showed vancomycin and imipenem as the most sensitive drug in Gram positive and Gram negative bacteria, respectively. The other drugs showed a high incidence of resistance [Tables 3-5]. The antimicrobial resistance pattern was graphically represented in three groups. The first group includes 69 P. aeruginosa, the second group includes 54 Gram positive cocci (S. aureus, 34; S. epidermidis, 19; M. spp, 1) and the last group consists of 98 enterobacteriaceae (E. coli, 31; K. pneumoniae, 27; P. mirabilis, 16; P. vulgaris, 12; C. freundii, 7; P. rettgeri, 3; M. morganii, 1; E. aerogenes, 1).
Table 3.
Antibiotic sensitivity of Pseudomonas aeruginosa isolates (n = 69)
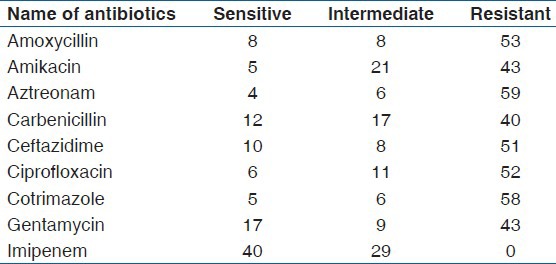
Table 5.
Antibiotic sensitivity of Enterobacteriaceae (n = 98)
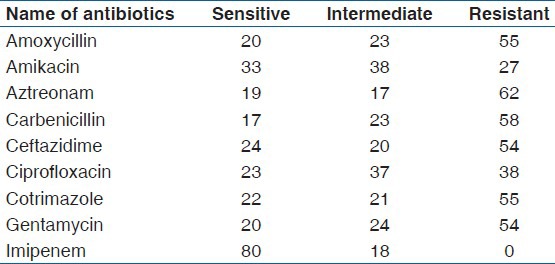
Table 4.
Antibiotic sensitivity of Gram positive cocci isolates (n = 54)
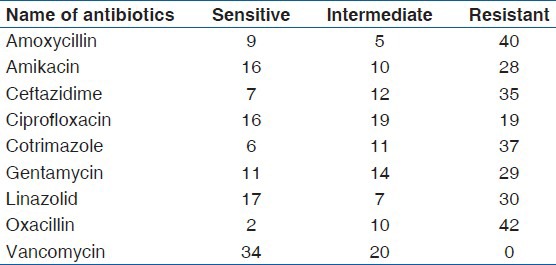
Among the four predominant organisms, which constitute 71.55% (161of 225) of total isolates, more than 50% showed resistance as described below. P. aeruginosa (n = 69) were resistant to amoxycillin 76.81% (53/69); amikacin 62.31% (43/69); aztreonam 85.5% (59/69); carbenicillin 57.97% (40/69); ciprofloxacin 75.36% (52/69); ceftazidime 73.91% (51/69); cotrimazole 84.05% (58/69); gentamycin 69.56% (48/69). S. aureus (n = 34) were resistant to amoxycillin 70.58% (24/34); amikacin 50% (17/34); ceftazidime 64.7% (22/34); cotrimazole 67.64% (23/34); gentamycin 67.64% (23/34); linezolid 58.82% (20/34); oxacillin 79.41% (27/34). E. coli (n = 31) were resistant to amoxycillin 61.29% (19/31); aztreonam 74.19% (23/31); carbenicillin 77.41% (24/31); ceftazidime 64.51% (20/31); cotrimazole 67.74% (21/31). K. pneumoniae (n = 27) were resistant to amoxycillin 55.55% (15/27); aztreonam59.25% (16/27); ciprofloxacin 59.25% (16/27) and cotrimazole 66.66% (18/27).
Discussion
BF colonization on urinary catheters is almost inevitable when devices are kept in-situ for a prolonged period. In our study, 130 catheters revealed colonization, out of which 78 were monobacterial. Twenty four of these isolates were non-BF producers. Furthermore, all 31 DJ stents (100%) showed monobacterial colonizations, each of which produced BF in-vitro. Further, all isolates of polymicrobial colonization in catheters were BF producers.
Insertion of DJ stent is usually carried out aseptically in the sterile environment and the entire device remains inside the body without access to the external environment. Colonization yet may happen mostly due to iatrogenic causes with the least possibility of ascending infection. Hence, the nature of colonization is mostly monomicrobial.
Normal bladder resists infection due to non-adherence of bacteria on the glycosoaminoglycan mucous lining, periodic voiding, and sloughing of colonized cells. However with catheter in-situ, chance of infection increases 10% each day.[12] Catheterization often promotes ascending infections by the commensals present in urethra, adjacent skin or following catheter contamination by the hand of the service provider. In bladder environment, some organisms can multiply rapidly if urine is not drained continuously. Such urinary tract infections account for more than 40% of nosocomial infections and are mostly caused by planktonic form of single bacteria amenable to treatment with susceptible drugs.[13] About 60% of urinary isolates are potential BF producers.[14] In long-term (>30 days) catheterized patient with continuous drainage, some bacteria are responsible for iatrogenic or ascending infections and may colonize on urine contact surface of the devices in the form of BF and may serve as persistent source of infections by time to time dispersion of their BF fragments. A recent report showed 100% bacteremia in urine samples of patients catheterized for more than 30 days, mostly presenting as asymptomatic and insignificant bacteriuria out of which 24% subsequently converted to symptomatic bacteriuria.[15]
Bacterial BF are often associated with long-term persistence of organisms in various environments including human systems, particularly where there are fluid flow lines and scope of microbial colonization on suitable adherent surface. The potential BF producer organisms are genetically more resistant to various antimicrobial agents due to high probability of genetic alterations during the environmental existence as mixed BF. Our results showed that all the BF producing organisms are resistant to three or more drugs, which shows concordance with earlier studies.[16] It should be kept in mind by the clinician that therapeutic use of sensitive drugs based on in-vitro susceptibility test will only be effective on planktonic form of bacteria with expected predictivity, but may not be equally effective on their BF state. Bacteria within BF exhibit several folds higher resistance to antimicrobial agents (even up to 1000 fold in some cases) for some phenotypic changes which add variable degree of drug resistance and are reversible with their planktonic conversion.[17] This high resistance is due to restricted drug permeability across BF matrix and limited drug action on starved hypoxic cells. The synergistic effect of two organisms may also result in higher resistance. In a study by Budhani and Struthers β-lactamase positive Moraxella catarrhalis reduced the susceptibility of Streptococcus pneumoniae to β-lactam antibiotics when the two organisms are grown together.[18] A similar effect was demonstrated in a fungal– bacterial BF where staphylococcal extracellular polymer protected the yeast cell from azoles and yeast cell in turn reduced the activity of vancomycin against bacterial cells.[19]
Hence, any treatment based on in-vitro drug sensitivity test for planktonic form of isolates may require up to 1000 fold higher concentration of the drug to eliminate same organisms when present inside BF. In this situation, sensitive drugs can be used temporarily to eliminate the organisms coming out as a result of biofouling until the colonized device is removed. Even after removal of a BF colonized-catheter, persistent organisms may re-colonize on the new catheter. Thus, whenever prolonged catheterization with several changes is indicated, it is better to take some effective measures to prevent BF formation than to try to eliminate organisms from BF. Periodical monitoring by urine culture in the first 14 days of catheter change may indicate the beginning of biofouling. Use of appropriate antibiotics at this stage with or without catheter change may reduce the risk of dissemination of organisms, though removal of BF colonized device is the absolute measure.
Elimination of endogenous source of infections prior to catheterization, risk reduction for iatrogenic infections, use of BF-proof devices,[20,21] frequent monitoring for insignificant bacteriuria with prompt intervention by catheter removal and flushing of bladder with sterile normal saline through a new catheter, may reduce the incidence of BF colonization and risk of disseminated infections thereof.
Conclusion
After prolonged use, BF formation on any urinary device is the rule rather than the exception. Such colonizations are mostly monobacterial with multiresistant organisms. Their planktonic form is amenable to treatment with suitable sensitive drugs, but sessile form is hardly manageable by therapeutic dose of the same drug. Because these urinary devices are temporary, their use can be continued even after BF colonization, with judicious antibiotic cover for prevention of planktonic form disseminations.
Acknowledgment
Department of Science and Technology, Government of India.
Footnotes
Source of Support: Study was conducted with a grant from Department of Science and Technology, Govt. of India under WOS-B scheme
Conflict of Interest: None declared.
References
- 1.Reid G, Habash M, Vachon D, Denstedt J, Riddell J, Beheshti M. Oral fluoroquinolone therapy results in drug adsorption on ureteral stents and prevention of biofilm formation. Int J Antimicrob Agents. 2001;17:317–9. doi: 10.1016/s0924-8579(00)00353-8. [DOI] [PubMed] [Google Scholar]
- 2.Stickler D. Urinary catheters: Ideal sites for the development of biofilm communities. Microbiol Today. 2005;32:22–5. [Google Scholar]
- 3.Stickler DJ. Bacterial biofilms in patients with indwelling urinary catheters. Nat Clin Pract Urol. 2008;5:598–608. doi: 10.1038/ncpuro1231. [DOI] [PubMed] [Google Scholar]
- 4.Allison DG, McBain AJ, Gilbert P. Microbial biofilms: problems of control. In: Allison DG, Gilbert P, Lappin-Scott H, editors. Community Structure and Cooperation in Biofilms. Reading: Society for General Microbiology Press; 2000. pp. 309–27. [Google Scholar]
- 5.O’Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2002;51(RR-10):1–29. [PubMed] [Google Scholar]
- 6.Safdar N, Maki DG. The pathogenesis of catheter-related bloodstream infection with noncuffed short-term central venous catheters. Intensive Care Med. 2004;30:62–7. doi: 10.1007/s00134-003-2045-z. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto AJ, Solomon JA, Soulen MC, Tang J, Parkinson K, Lin R, et al. Sutureless securement device reduces complications of peripherally inserted central venous catheters. J Vasc Interv Radiol. 2002;13:77–81. doi: 10.1016/s1051-0443(07)60012-8. [DOI] [PubMed] [Google Scholar]
- 8.Collee JG, Miles RS, Watt B. Tests for the identification of bacteria. In: Collee JG, Marmion BP, Fraser AG, Simmons A, editors. Practical Medical Microbiology. 14th ed. Churchill Livingstone, Imprint of Elsevier India; 2006. pp. 131–78. [Google Scholar]
- 9.Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 2000;40:175–9. doi: 10.1016/s0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 11.Vol. 27. Wayne, PA: Clinical and Laboratory Standard Institute; 2007. Clinical and Laboratory Standard Institute. Performance Standard for Antimicrobial Susceptibility Testing; Sixteenth Informational Supplement; pp. M100–S17. [Google Scholar]
- 12.Tambyah PA, Knasinski V, Maki DG. The direct costs of nosocomial catheter-associated urinary tract infection in the era of managed care. Infect Control Hosp Epidemiol. 2002;23:27–31. doi: 10.1086/501964. [DOI] [PubMed] [Google Scholar]
- 13.Kalsi J, Arya M, Wilson P, Mundy A. Hospital-acquired urinary tract infection. Int J Clin Pract. 2003;57:388–91. [PubMed] [Google Scholar]
- 14.Subramanian P, Shanmugam N, Sivaraman U, Kumar S, Selvaraj S. Antiobiotic resistance pattern of biofilm-forming uropathogens isolated from catheterised patients in Pondicherry, India. Australas Med J. 2012;5:344–8. doi: 10.4066/AMJ.2012.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siracusano S, Ciciliato S, Ollandini G, Visalli F, Nikibakhsh A. Clinical Management of Complicated Urinary Tract Infection. InTech; 2011. [Cited on 2013 Feb 21]. Catheters and infections; pp. 83–98. Available from: http://www.intechopen. com/books/clinical-management-of-complicated-urinary-tract-infection/cathetersand-infections . [Google Scholar]
- 16.Suman E, Jose J, Varghese S, Kotian MS. Study of biofilm production in Escherichia coli causing urinary tract infection. Indian J Med Microbiol. 2007;25:305–6. doi: 10.4103/0255-0857.34788. [DOI] [PubMed] [Google Scholar]
- 17.Musk DJ, Jr, Hergenrother PJ. Chemical countermeasures for the control of bacterial biofilms: Effective compounds and promising targets. Curr Med Chem. 2006;13:2163–77. doi: 10.2174/092986706777935212. [DOI] [PubMed] [Google Scholar]
- 18.Budhani RK, Struthers JK. Interaction of Streptococcus pneumoniae and Moraxella catarrhalis: Investigation of the indirect pathogenic role of beta-lactamase-producing moraxellae by use of a continuous-culture biofilm system. Antimicrob Agents Chemother. 1998;42:2521–6. doi: 10.1128/aac.42.10.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adam B, Baillie GS, Douglas LJ. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J Med Microbiol. 2002;51:344–9. doi: 10.1099/0022-1317-51-4-344. [DOI] [PubMed] [Google Scholar]
- 20.Hachem R, Reitzel R, Borne A, Jiang Y, Tinkey P, Uthamanthil R, et al. Novel antiseptic urinary catheters for prevention of urinary tract infections: Correlation of in vivo and in vitro test results. Antimicrob Agents Chemother. 2009;53:5145–9. doi: 10.1128/AAC.00718-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timsit JF, Dubois Y, Minet C, Bonadona A, Lugosi M, Ara-Somohano C, et al. New materials and devices for preventing catheter-related infections. Ann Intensive Care. 2011;1:34. doi: 10.1186/2110-5820-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]


