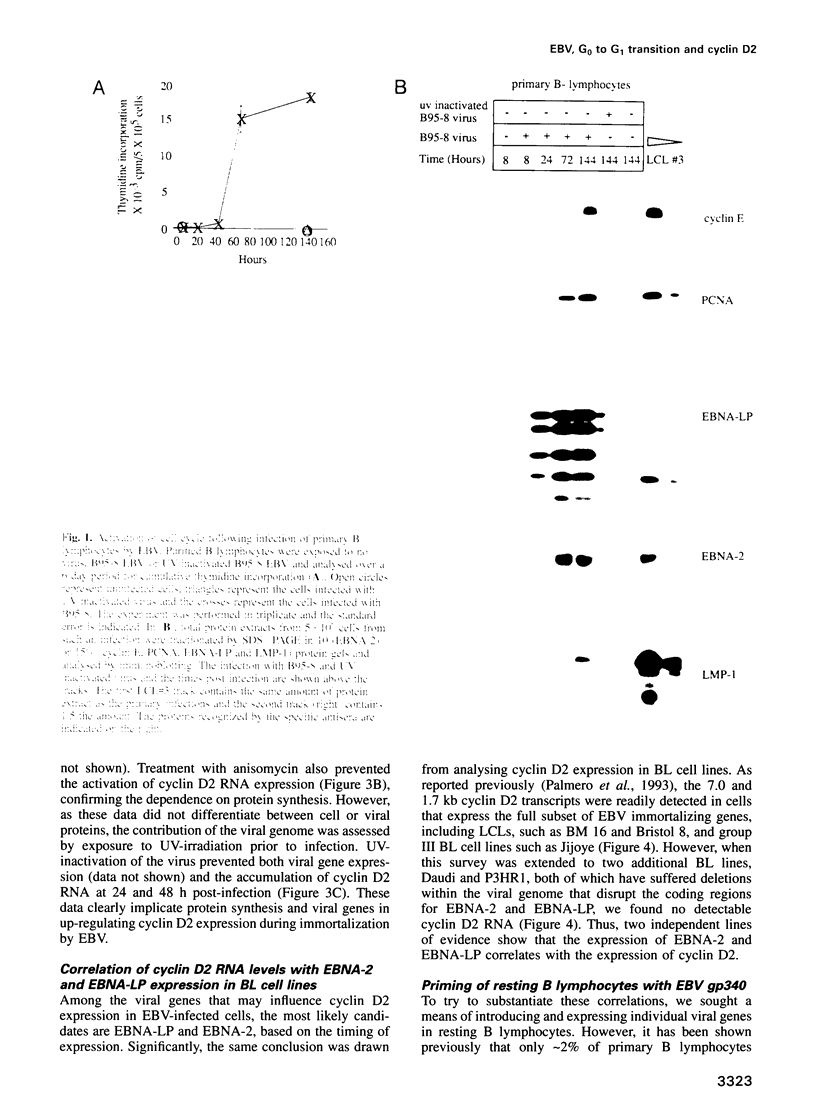
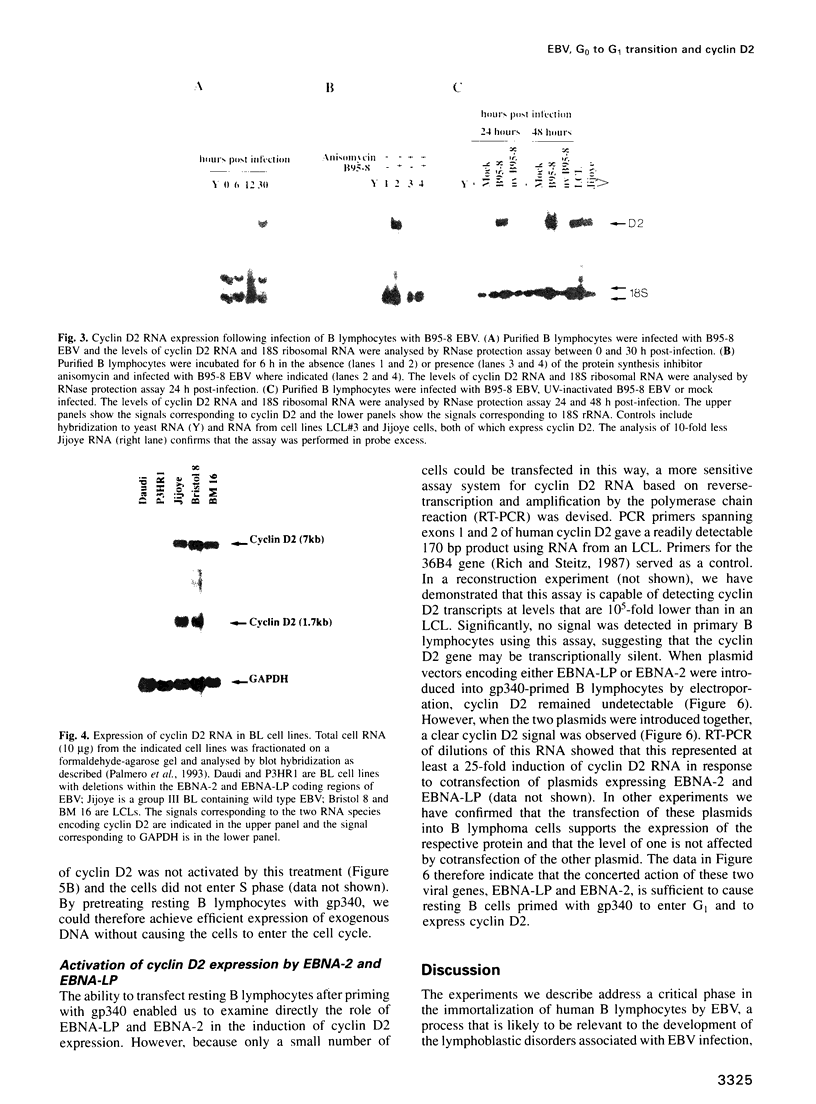
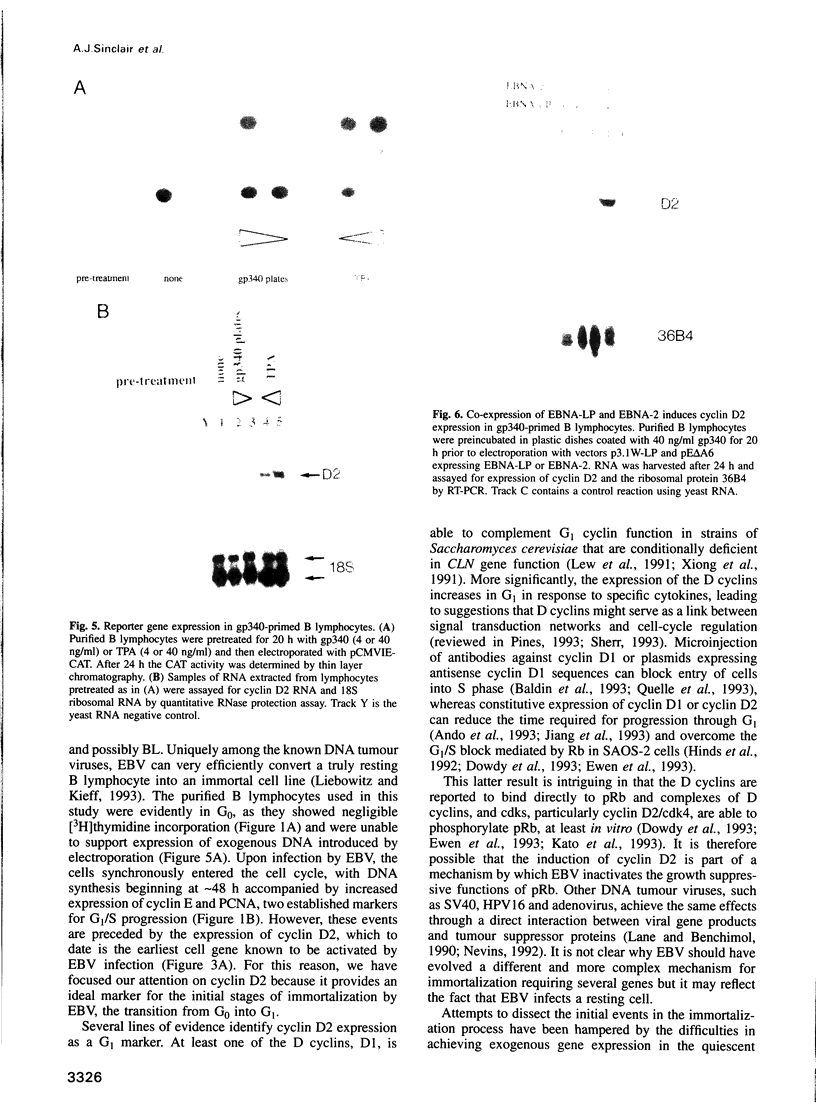
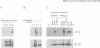Abstract
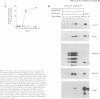
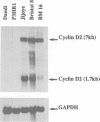
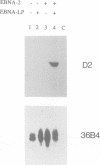
Epstein-Barr virus (EBV) is unusual among DNA tumour viruses in that the virus particle is able to infect and immortalize resting cells with very high efficiency. Mutation of the viral genome has indicated that at least six viral genes (LMP-1 and EBNAs 1, 2, 3A, 3C and LP) are essential for immortalization. We demonstrate that the activation of a G1 cyclin, cyclin D2, is an early event following infection with EBV and that cyclin D2 activation is dependent on the expression of viral genes. The different levels of cyclin D2 transcripts in Burkitt's lymphoma cell lines expressing different subsets of EBV immortalizing genes suggest an involvement of EBNA-2 or EBNA-LP in cyclin D2 regulation. By exposing resting primary B cells to a purified preparation of the EBV surface glycoprotein gp340, we have been able to achieve efficient expression of plasmid DNAs introduced by electroporation. Vectors encoding two viral genes, EBNA-2 and EBNA-LP, are sufficient to activate the expression of cyclin D2 in this system. Thus, the progression of resting B lymphocytes into the G1 phase of the cell cycle can be reconstituted in the absence of virus by the cooperation of two of the six viral genes required for immortalization.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfieri C., Birkenbach M., Kieff E. Early events in Epstein-Barr virus infection of human B lymphocytes. Virology. 1991 Apr;181(2):595–608. doi: 10.1016/0042-6822(91)90893-g. [DOI] [PubMed] [Google Scholar]
- Allan G. J., Inman G. J., Parker B. D., Rowe D. T., Farrell P. J. Cell growth effects of Epstein-Barr virus leader protein. J Gen Virol. 1992 Jun;73(Pt 6):1547–1551. doi: 10.1099/0022-1317-73-6-1547. [DOI] [PubMed] [Google Scholar]
- Allday M. J., Crawford D. H., Griffin B. E. Epstein-Barr virus latent gene expression during the initiation of B cell immortalization. J Gen Virol. 1989 Jul;70(Pt 7):1755–1764. doi: 10.1099/0022-1317-70-7-1755. [DOI] [PubMed] [Google Scholar]
- Ando K., Ajchenbaum-Cymbalista F., Griffin J. D. Regulation of G1/S transition by cyclins D2 and D3 in hematopoietic cells. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9571–9575. doi: 10.1073/pnas.90.20.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldin V., Lukas J., Marcote M. J., Pagano M., Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993 May;7(5):812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- Cordier M., Calender A., Billaud M., Zimber U., Rousselet G., Pavlish O., Banchereau J., Tursz T., Bornkamm G., Lenoir G. M. Stable transfection of Epstein-Barr virus (EBV) nuclear antigen 2 in lymphoma cells containing the EBV P3HR1 genome induces expression of B-cell activation molecules CD21 and CD23. J Virol. 1990 Mar;64(3):1002–1013. doi: 10.1128/jvi.64.3.1002-1013.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdy S. F., Hinds P. W., Louie K., Reed S. I., Arnold A., Weinberg R. A. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993 May 7;73(3):499–511. doi: 10.1016/0092-8674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Sluss H. K., Sherr C. J., Matsushime H., Kato J., Livingston D. M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993 May 7;73(3):487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- Finke J., Rowe M., Kallin B., Ernberg I., Rosén A., Dillner J., Klein G. Monoclonal and polyclonal antibodies against Epstein-Barr virus nuclear antigen 5 (EBNA-5) detect multiple protein species in Burkitt's lymphoma and lymphoblastoid cell lines. J Virol. 1987 Dec;61(12):3870–3878. doi: 10.1128/jvi.61.12.3870-3878.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderud S., Erikstein B., Asheim H. C., Nustad K., Stokke T., Blomhoff H. K., Holte H., Smeland E. B. Functional properties of CD19+ B lymphocytes positively selected from buffy coats by immunomagnetic separation. Eur J Immunol. 1990 Jan;20(1):201–206. doi: 10.1002/eji.1830200129. [DOI] [PubMed] [Google Scholar]
- Fåhraeus R., Jansson A., Ricksten A., Sjöblom A., Rymo L. Epstein-Barr virus-encoded nuclear antigen 2 activates the viral latent membrane protein promoter by modulating the activity of a negative regulatory element. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7390–7394. doi: 10.1073/pnas.87.19.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J., Walker L., Guy G., Brown G., Rowe M., Rickinson A. Control of human B-lymphocyte replication. II. Transforming Epstein-Barr virus exploits three distinct viral signals to undermine three separate control points in B-cell growth. Immunology. 1986 Aug;58(4):591–595. [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature. 1989 Aug 3;340(6232):393–397. doi: 10.1038/340393a0. [DOI] [PubMed] [Google Scholar]
- Hanna Z., Jankowski M., Tremblay P., Jiang X., Milatovich A., Francke U., Jolicoeur P. The Vin-1 gene, identified by provirus insertional mutagenesis, is the cyclin D2. Oncogene. 1993 Jun;8(6):1661–1666. [PubMed] [Google Scholar]
- Hinds P. W., Mittnacht S., Dulic V., Arnold A., Reed S. I., Weinberg R. A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992 Sep 18;70(6):993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- Hurley E. A., Thorley-Lawson D. A. B cell activation and the establishment of Epstein-Barr virus latency. J Exp Med. 1988 Dec 1;168(6):2059–2075. doi: 10.1084/jem.168.6.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba T., Matsushime H., Valentine M., Roussel M. F., Sherr C. J., Look A. T. Genomic organization, chromosomal localization, and independent expression of human cyclin D genes. Genomics. 1992 Jul;13(3):565–574. doi: 10.1016/0888-7543(92)90126-d. [DOI] [PubMed] [Google Scholar]
- Jiang W., Kahn S. M., Zhou P., Zhang Y. J., Cacace A. M., Infante A. S., Doi S., Santella R. M., Weinstein I. B. Overexpression of cyclin D1 in rat fibroblasts causes abnormalities in growth control, cell cycle progression and gene expression. Oncogene. 1993 Dec;8(12):3447–3457. [PubMed] [Google Scholar]
- Kato J., Matsushime H., Hiebert S. W., Ewen M. E., Sherr C. J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993 Mar;7(3):331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- Lammie G. A., Smith R., Silver J., Brookes S., Dickson C., Peters G. Proviral insertions near cyclin D1 in mouse lymphomas: a parallel for BCL1 translocations in human B-cell neoplasms. Oncogene. 1992 Dec;7(12):2381–2387. [PubMed] [Google Scholar]
- Lane D. P., Benchimol S. p53: oncogene or anti-oncogene? Genes Dev. 1990 Jan;4(1):1–8. doi: 10.1101/gad.4.1.1. [DOI] [PubMed] [Google Scholar]
- Lew D. J., Dulić V., Reed S. I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991 Sep 20;66(6):1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- Madej M., Conway M. J., Morgan A. J., Sweet J., Wallace L., Qualtiere L. F., Arrand J. R., Mackett M. Purification and characterization of Epstein-Barr virus gp340/220 produced by a bovine papillomavirus virus expression vector system. Vaccine. 1992;10(11):777–782. doi: 10.1016/0264-410x(92)90513-j. [DOI] [PubMed] [Google Scholar]
- Mannick J. B., Cohen J. I., Birkenbach M., Marchini A., Kieff E. The Epstein-Barr virus nuclear protein encoded by the leader of the EBNA RNAs is important in B-lymphocyte transformation. J Virol. 1991 Dec;65(12):6826–6837. doi: 10.1128/jvi.65.12.6826-6837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokura T., Arnold A. Cyclins and oncogenesis. Biochim Biophys Acta. 1993 May 25;1155(1):63–78. doi: 10.1016/0304-419x(93)90022-5. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerow G. R., Houghten R. A., Moore M. D., Cooper N. R. Identification of an epitope in the major envelope protein of Epstein-Barr virus that mediates viral binding to the B lymphocyte EBV receptor (CR2). Cell. 1989 Feb 10;56(3):369–377. doi: 10.1016/0092-8674(89)90240-7. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992 Oct 16;258(5081):424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- Nigg E. A. Targets of cyclin-dependent protein kinases. Curr Opin Cell Biol. 1993 Apr;5(2):187–193. doi: 10.1016/0955-0674(93)90101-u. [DOI] [PubMed] [Google Scholar]
- Palmero I., Holder A., Sinclair A. J., Dickson C., Peters G. Cyclins D1 and D2 are differentially expressed in human B-lymphoid cell lines. Oncogene. 1993 Apr;8(4):1049–1054. [PubMed] [Google Scholar]
- Peng M., Lundgren E. Transient expression of the Epstein-Barr virus LMP1 gene in human primary B cells induces cellular activation and DNA synthesis. Oncogene. 1992 Sep;7(9):1775–1782. [PubMed] [Google Scholar]
- Pilon M., Gullberg M., Lundgren E. Transient expression of the CD2 cell surface antigen as a sortable marker to monitor high frequency transfection of human primary B cells. J Immunol. 1991 Feb 1;146(3):1047–1051. [PubMed] [Google Scholar]
- Pines J. Cyclins and cyclin-dependent kinases: take your partners. Trends Biochem Sci. 1993 Jun;18(6):195–197. doi: 10.1016/0968-0004(93)90185-p. [DOI] [PubMed] [Google Scholar]
- Quelle D. E., Ashmun R. A., Shurtleff S. A., Kato J. Y., Bar-Sagi D., Roussel M. F., Sherr C. J. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993 Aug;7(8):1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- Rich B. E., Steitz J. A. Human acidic ribosomal phosphoproteins P0, P1, and P2: analysis of cDNA clones, in vitro synthesis, and assembly. Mol Cell Biol. 1987 Nov;7(11):4065–4074. doi: 10.1128/mcb.7.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricksten A., Svensson C., Welinder C., Rymo L. Identification of sequences in Epstein-Barr virus DNA required for the expression of the second Epstein-Barr virus-determined nuclear antigen in COS-1 cells. J Gen Virol. 1987 Sep;68(Pt 9):2407–2418. doi: 10.1099/0022-1317-68-9-2407. [DOI] [PubMed] [Google Scholar]
- Rooney C. M., Rowe D. T., Ragot T., Farrell P. J. The spliced BZLF1 gene of Epstein-Barr virus (EBV) transactivates an early EBV promoter and induces the virus productive cycle. J Virol. 1989 Jul;63(7):3109–3116. doi: 10.1128/jvi.63.7.3109-3116.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney C., Howe J. G., Speck S. H., Miller G. Influence of Burkitt's lymphoma and primary B cells on latent gene expression by the nonimmortalizing P3J-HR-1 strain of Epstein-Barr virus. J Virol. 1989 Apr;63(4):1531–1539. doi: 10.1128/jvi.63.4.1531-1539.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C. L., Wong E., Petty E. M., Bale A. E., Tsujimoto Y., Harris N. L., Arnold A. PRAD1, a candidate BCL1 oncogene: mapping and expression in centrocytic lymphoma. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9638–9642. doi: 10.1073/pnas.88.21.9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto M., Yamamoto K., Iida S., Akao Y., Utsumi K. R., Kubonishi I., Miyoshi I., Ohtsuki T., Yawata Y., Namba M. Gene rearrangement and overexpression of PRAD1 in lymphoid malignancy with t(11;14)(q13;q32) translocation. Oncogene. 1992 Jul;7(7):1401–1406. [PubMed] [Google Scholar]
- Sherr C. J. Mammalian G1 cyclins. Cell. 1993 Jun 18;73(6):1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Sinclair A. J., Brimmell M., Shanahan F., Farrell P. J. Pathways of activation of the Epstein-Barr virus productive cycle. J Virol. 1991 May;65(5):2237–2244. doi: 10.1128/jvi.65.5.2237-2244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck S. H., Pfitzner A., Strominger J. L. An Epstein-Barr virus transcript from a latently infected, growth-transformed B-cell line encodes a highly repetitive polypeptide. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9298–9302. doi: 10.1073/pnas.83.24.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J., Weis J., Fearon D., Whang Y., Kieff E. Epstein-Barr virus gp350/220 binding to the B lymphocyte C3d receptor mediates adsorption, capping, and endocytosis. Cell. 1987 Jul 17;50(2):203–213. doi: 10.1016/0092-8674(87)90216-9. [DOI] [PubMed] [Google Scholar]
- Wang F., Tsang S. F., Kurilla M. G., Cohen J. I., Kieff E. Epstein-Barr virus nuclear antigen 2 transactivates latent membrane protein LMP1. J Virol. 1990 Jul;64(7):3407–3416. doi: 10.1128/jvi.64.7.3407-3416.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers D. A., Harvey R. C., Faust J. B., Melnyk O., Carey K., Meeker T. C. Characterization of a candidate bcl-1 gene. Mol Cell Biol. 1991 Oct;11(10):4846–4853. doi: 10.1128/mcb.11.10.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Connolly T., Futcher B., Beach D. Human D-type cyclin. Cell. 1991 May 17;65(4):691–699. doi: 10.1016/0092-8674(91)90100-d. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Menninger J., Beach D., Ward D. C. Molecular cloning and chromosomal mapping of CCND genes encoding human D-type cyclins. Genomics. 1992 Jul;13(3):575–584. doi: 10.1016/0888-7543(92)90127-e. [DOI] [PubMed] [Google Scholar]