Abstract
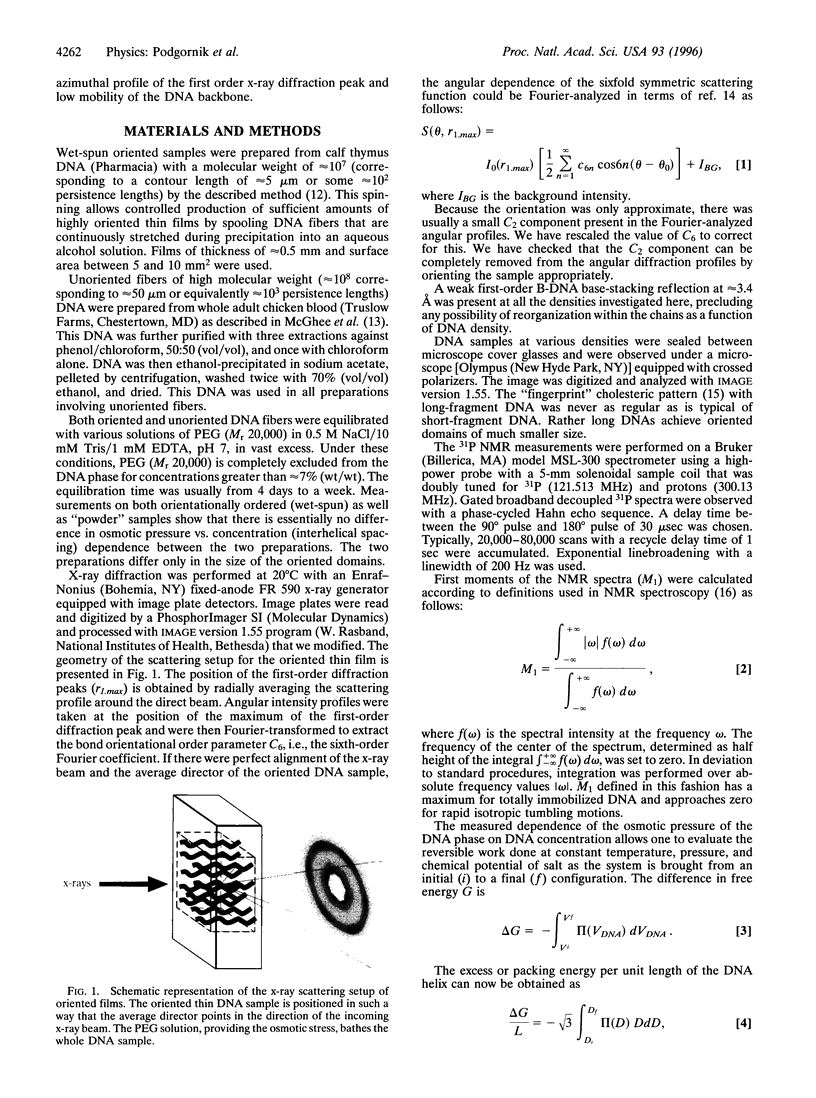
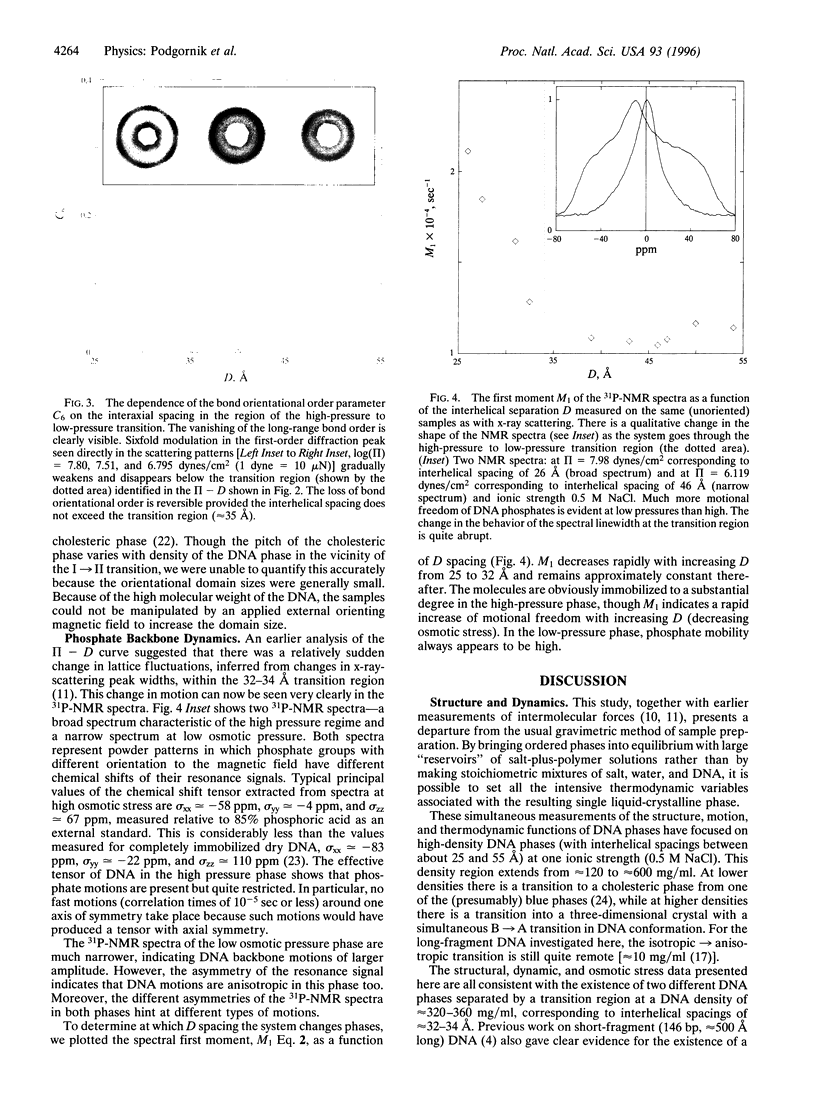
By equilibrating condensed DNA arrays against reservoirs of known osmotic stress and examining them with several structural probes, it has been possible to achieve a detailed thermodynamic and structural characterization of the change between two distinct regions on the liquid-crystalline phase diagram: (i) a higher density hexagonally packed region with long-range bond orientational order in the plane perpendicular to the average molecular direction and (ii) a lower density cholesteric region with fluid-like positional order. X-ray scattering on highly ordered DNA arrays at high density and with the helical axis oriented parallel to the incoming beam showed a sixfold azimuthal modulation of the first-order diffraction peak that reflects the macroscopic bond-orientational order. Transition to the less-dense cholesteric phase through osmotically controlled swelling shows the loss of this bond orientational order, which had been expected from the change in optical birefringence patterns and which is consistent with a rapid onset of molecular positional disorder. This change in order was previously inferred from intermolecular force measurements and is now confirmed by 31P NMR. Controlled reversible swelling and compaction under osmotic stress, spanning a range of densities between approximately 120 mg/ml to approximately 600 mg/ml, allow measurement of the free-energy changes throughout each phase and at the phase transition, essential information for theories of liquid-crystalline states.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brian A. A., Frisch H. L., Lerman L. S. Thermodynamics and equilibrium sedimentation analysis of the close approach of DNA molecules and a molecular ordering transition. Biopolymers. 1981 Jun;20(6):1305–1328. doi: 10.1002/bip.1981.360200615. [DOI] [PubMed] [Google Scholar]
- Davis J. H. The description of membrane lipid conformation, order and dynamics by 2H-NMR. Biochim Biophys Acta. 1983 Mar 21;737(1):117–171. doi: 10.1016/0304-4157(83)90015-1. [DOI] [PubMed] [Google Scholar]
- Evans E. A., Parsegian V. A. Thermal-mechanical fluctuations enhance repulsion between bimolecular layers. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7132–7136. doi: 10.1073/pnas.83.19.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leforestier A., Livolant F. Supramolecular ordering of DNA in the cholesteric liquid crystalline phase: an ultrastructural study. Biophys J. 1993 Jul;65(1):56–72. doi: 10.1016/S0006-3495(93)81063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman L. S. A transition to a compact form of DNA in polymer solutions. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1886–1890. doi: 10.1073/pnas.68.8.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti MC, Nelson DR. Dislocation loops and bond-orientational order in the Abrikosov flux-line lattice. Phys Rev B Condens Matter. 1990 Feb 1;41(4):1910–1920. doi: 10.1103/physrevb.41.1910. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Wood W. I., Dolan M., Engel J. D., Felsenfeld G. A 200 base pair region at the 5' end of the chicken adult beta-globin gene is accessible to nuclease digestion. Cell. 1981 Nov;27(1 Pt 2):45–55. doi: 10.1016/0092-8674(81)90359-7. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A., Rand R. P., Fuller N. L., Rau D. C. Osmotic stress for the direct measurement of intermolecular forces. Methods Enzymol. 1986;127:400–416. doi: 10.1016/0076-6879(86)27032-9. [DOI] [PubMed] [Google Scholar]
- Podgornik R., Rau D. C., Parsegian V. A. Parametrization of direct and soft steric-undulatory forces between DNA double helical polyelectrolytes in solutions of several different anions and cations. Biophys J. 1994 Apr;66(4):962–971. doi: 10.1016/S0006-3495(94)80877-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgornik R., Strey H. H., Rau D. C., Parsegian V. A. Watching molecules crowd: DNA double helices under osmotic stress. Biophys Chem. 1995 Dec;57(1):111–121. doi: 10.1016/0301-4622(95)00058-6. [DOI] [PubMed] [Google Scholar]
- Rau D. C., Lee B., Parsegian V. A. Measurement of the repulsive force between polyelectrolyte molecules in ionic solution: hydration forces between parallel DNA double helices. Proc Natl Acad Sci U S A. 1984 May;81(9):2621–2625. doi: 10.1073/pnas.81.9.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau D. C., Parsegian V. A. Direct measurement of temperature-dependent solvation forces between DNA double helices. Biophys J. 1992 Jan;61(1):260–271. doi: 10.1016/S0006-3495(92)81832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau D. C., Parsegian V. A. Direct measurement of the intermolecular forces between counterion-condensed DNA double helices. Evidence for long range attractive hydration forces. Biophys J. 1992 Jan;61(1):246–259. doi: 10.1016/S0006-3495(92)81831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo H. Backbone geometry of oriented DNA fibers as revealed by 31P chemical shielding anisotropy. Adv Biophys. 1985;20:39–57. doi: 10.1016/0065-227x(85)90030-9. [DOI] [PubMed] [Google Scholar]





