Abstract
Background and Purpose:
The ketogenic diet was formulated to mimic the biochemical changes seen upon fasting, specifically the formation of ketone bodies. Recent research data suggest that the anticonvulsant efficacy of the KD may be due in part to the direct actions of ketone bodies. This study was designed to investigate the anticonvulsant effects of β-hydroxybutyrate (BHB) on pilocarpine-induced seizures in mature mice.
Methods:
Eighty-two male ICR mice at postnatal day 49 were used. All mice were pretreated with scopolamine methylbromide prior to pilocarpine injection. Experimental mice (n=42) were injected intraperitoneally with BHB (20 mmol/kg) 15 min prior to pilocarpine administration, while control animals (n=40) with normal saline. Pilocarpine (300 mg/kg) was administered intraperitoneally and mice were monitored for 2 h after pilocarpine injection.
Results:
All mice developed typical seizure behaviors. The mean (±SD) latency to the onset of seizures was significantly prolonged in the BHB-treated mice compared with controls (4.83±1.95 min vs. 3.67±1.90 min, p<0.01).
Conclusions:
This study demonstrates that treatment with BHB prolongs the latency to the onset of seizures induced by pilocarpine in mature mice and suggests that BHB, one of the ketone bodies, may have direct anticonvulsant effects.
Keywords: Ketone body, 3-hydroxybutyric acid, Pilocarpine, Mice, Seizure
Introduction
“Ketogenic diet” means a diet that generates ketone bodies, including acetoacetate (ACA), β-hydroxybutyrate (BHB), and acetone as the name indicates. Ketogenic diet is consisted of high fat, low carbohydrate and adequate protein. In spite of the several difficulties in managing the diet, the ketogenic diet is now widely used and recognized as definitely effective treatment for intractable epilepsies.1,2
Despite the scanty knowledge about the mechanisms of action of the diet, we have noticed that its success depends on the ketosis of the patients.3 It has also been reported that higher plasma BHB levels are correlated with anticonvulsant effects in mice fed a ketogenic diet4 and that ACA and acetone have been found to have an anticonvulsant action in mice.5 These data are consistent with the early hypothesis that the anticonvulsant efficacy of the ketogenic diet involves the direct actions of the ketone bodies.6 Recently, we reported that BHB, one of the ketone bodies, might be directly anticonvulsant in other seizure model7,8 and in young animal with pilocarpine-induced seizures.9
On the other hand, the ketogenic diet is so far known to be more effective in younger individuals,10 and this age-dependent efficacy is considered due to the different metabolic control.11 Our present study was designed to investigate whether exogenous injection of BHB has a direct anticonvulsant effect on pilocarpine-induced seizures in mature mice as well.
Methods
Eighty-two male ICR mice (Folas International, Korea) at postnatal day 49 were used for all the experiments. Animals were housed in a room maintained at 22±3°C with an alternating 12-h light/dark cycle, and fed normal diet. Experiments have been approved by the Institutional Animal Care and Use Committee of the Inje University and conform to the Revised Guide for the Care and Use of Laboratory Animals [NIH GUIDE, 25(28), 1996].
All mice were pretreated by intraperitoneal injection of 1 mg/kg scopolamine methylbromide (Sigma, USA) 30 min prior to pilocarpine administration. Experimental mice (n=42) were injected intraperitoneally with 20 mmol/kg (R)-3-hydroxybutyric acid sodium salt (BHB; Fluka, USA), 15 min prior to pilocarpine administration; control animals (n=40) were administered the same volume of normal saline. Blood samples of 24 mice were collected via the tail vein immediately before pilocarpine administration in order to measure blood BHB levels using a Keto-Site test kit (GDS Diagnostics, USA). Seizures were chemically induced in both BHB-treated and control mice by intraperitoneal injection of a single 300 mg/kg dose of pilocarpine (Sigma, USA).
After induction of seizure, all mice were monitored for seizure behaviors for 2 h. Seizure behaviors were categorized into five different grades according to the previously defined Racine scale.12 Twelve mice from each group were graded according to the maximum seizure behavioral response observed.
Differences between two groups were statistically analyzed using unpaired Student’s t-tests; a value of p<0.05 was considered significant. All data are presented as means (±SD).
Results
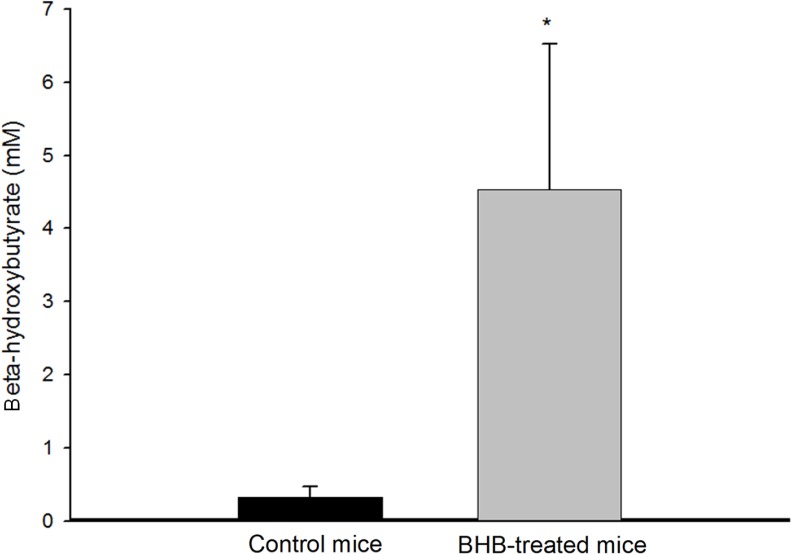
After intraperitoneal administration of BHB, we confirmed that blood BHB levels in treated mice were significantly increased compared with those in controls (4.54±1.99 mM vs. 0.32±0.15 mM, p < 0.001) (Figure 1).
Figure 1.
Blood β-hydroxybutyrate (BHB) levels in control and BHB-treated mice (each n=12) (*p<0.001, Student’s t-test).
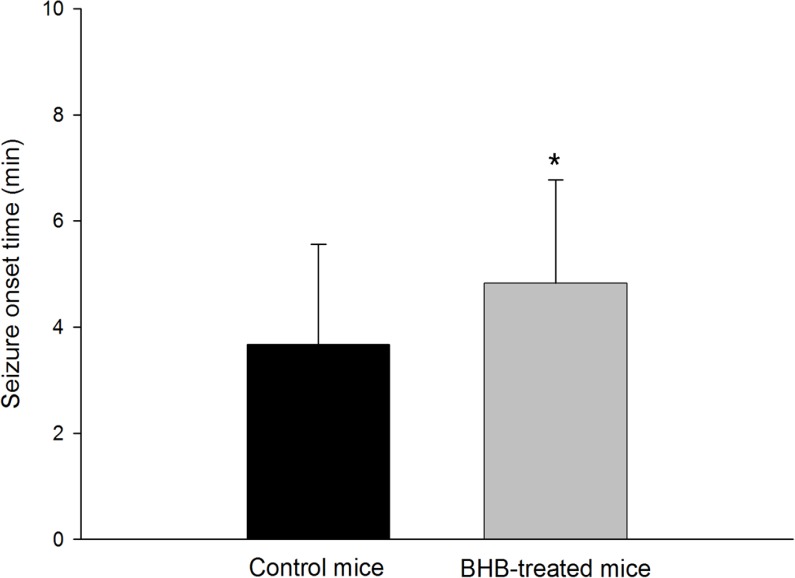
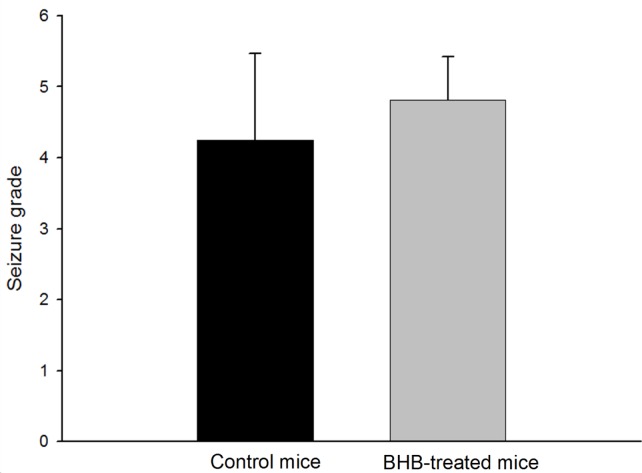
To ascertain the effect of the BHB treatment, the latency to the onset of seizures and seizure behaviors were monitored. The mean (±SD) latency to the onset of seizure after pilocarpine injection was significantly prolonged in BHB-treated mice compared with controls (4.83±1.95 min vs. 3.67±1.90 min, p<0.01) (Figure 2). During the 2 hour monitoring of seizure behaviors to determine seizure severity, all monitored mice developed typical seizure behaviors after pilocarpine administration. However, the seizure behavior grades, the seizure severity, were not significantly different between BHB- treated and control mice (4.82±0.60 vs. 4.25±1.22, Figure 3).
Figure 2.
All mice were intraperitoneally injected with pilocarpine (300 mg/kg) to induce seizure. In β-hydroxybutyrate (BHB)-treated mice (n=42), the latency to the onset of seizure was prolonged compared to that in control mice (n=40) (*p<0.01, Student’s t-test).
Figure 3.
All mice were intraperitoneally injected with pilocarpine (300 mg/kg) to induce seizures. The seizure behavior grades were not significantly different between control (n=12) and β-hydroxybutyrate (BHB)-treated mice (n=12) (p=0.18, Student’s t-test).
Discussion
There is no more controversy that the ketogenic diet is effective against refractory epilepsy. It has also been shown to be effective at increasing thresholds to flurothyl-induced seizures in several inbred strains of mice.10,13,14 The mechanism of action of the diet, however, has not been answered yet1 although several mechanisms have been hypothesized. Proposed mechanisms include direct modulation of ion channels via ketone bodies,15 activation of ATP-sensitive potassium (KATP) channels via glucose restriction,16 increased noradrenergic tone,14 and increased GABAergic inhibitory signaling.17
Here, the main hypothesis of our present study is that ketone body, a product of ketogenic diet, has direct anticonvulsant effects. We have already examined this hypothesis in previously published studies.7–9 We chose the most stable physiologic ketone body-BHB-as the representative ketone body for the present study. In our study, injection of BHB could mimic the conditions under fasting and increased blood BHB level around 4–5 mM as previously shown. And this increased BHB was accompanied by an increase in the threshold for pilocarpine-induced seizures in these mature animals. Although seizure severity, measured as seizure behavior grade, was not significantly different, the latency to the onset of seizure induced by pilocarpine was significantly prolonged in BHB-treated mice compared with controls. These results are consistent with our previous reports that treatment with BHB raises the threshold in young animals with flurothyl-induced seizures7,8 and young mice with pilocarpine-induced seizures.9 This shows that BHB has a direct anticonvulsant effect and can be a main anticonvulsant component of the ketogenic diet.
The better efficacy of ketogenic diet in a younger individual10,18,19 has been observed. In animal, age-related difference of the ketogenic diet in fluorothyl seizure susceptibility was shown before.10 For this different efficacy with age, some developmental factors such as different metabolic control of the young and mature brain were suggested. Although it has been known that mature brain does not usually burn ketone bodies for energy production unless blood glucose levels are reduced,11 additional ketone body effectively reduced the seizure threshold without lowering blood glucose level in our present study. In other words, direct supplementation of BHB effectively elevates the seizure threshold in vivo without restriction of glucose in adult age group.
The present study tested the very acute effect of the ketone body, which has no association with the long-term metabolic and genetic modification produced by the ketogenic diet. Thus, the anticonvulsant action of BHB could be an acute modulation of brain activity. For example, ketone bodies can stimulate glutamic acid decarboxylase activity, which can potentially elevate GABA content in synaptosomes,20 and ketone body-induced alterations in TCA cycle metabolites (oxaloacetate, citrate, succinyl-CoA, and α-ketoglutarate) favor the formation of glutamate over aspartate. Accordingly, an attenuation of excitatory neurotransmitters (glutamate and aspartate) together with an elevation of GABA could decrease neuronal excitation and increase inhibition.20,21 Although we have little knowledge about the mechanisms of the ketogenic diet, the ketone bodies may have acute anticonvulsant actions possibly involving multiple changes in the content and distribution of brain neurotransmitters.
While the anticonvulsant effect of ketogenic diet may depend on multiple mechanisms working synergistically, the results from this study suggest that the ketone body, BHB, has a direct anticonvulsant activity in mature mice. This finding is consistent with previous studies dealing with anticonvulsant effects of the ketone bodies.3,7–9
Acknowledgments
No funds were received in support of this work. No benefits in any form have been received from a commercial party related to this manuscript.
References
- 1.Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49(Suppl 8):3–5. doi: 10.1111/j.1528-1167.2008.01821.x. [DOI] [PubMed] [Google Scholar]
- 2.Hartman AL, Gasior M, Vining EP, Rogawski MA. The neuropharmacology of the ketogenic diet. Pediatr Neurol. 2007;36:281–92. doi: 10.1016/j.pediatrneurol.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Delft R, Lambrechts D, Verschuure P, Hulsman J, Majoie M. Blood beta-hydroxybutyrate correlates better with seizure reduction due to ketogenic diet than do ketones in the urine. Seizure. 2010;19:36–9. doi: 10.1016/j.seizure.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Samala R, Willis S, Borges K. Anticonvulsant profile of a balanced ketogenic diet in acute mouse seizure models. Epilepsy Res. 2008;81:119–27. doi: 10.1016/j.eplepsyres.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Rho JM, Anderson GD, Donevan SD, White HS. Acetoacetate, acetone, and dibenzylamine (a contaminant in l-(+)-beta-hydroxybutyrate) exhibit direct anticonvulsant actions in vivo. Epilepsia. 2002;43:358–61. doi: 10.1046/j.1528-1157.2002.47901.x. [DOI] [PubMed] [Google Scholar]
- 6.Wilder R. The effect of ketonemia on the course of epilepsy. Mayo Clin Proc. 1921;2:307–8. [Google Scholar]
- 7.Kim DW, Kim JM. Beta-Hydroxybutyrate decreases flurothyl-induced seizure susceptibility in rats. Epilepsia. 2004;45:210. [Google Scholar]
- 8.Shin CG, Kim DW. Effect of β-hydroxybutyrate on flurothyl-induced seizure susceptibility. J Korean Child Neurol Soc. 2010;18:225–9. [Google Scholar]
- 9.Yum MS, Ko TS, Kim DW. β-Hydroxybutyrate increases the pilocarpine-induced seizure threshold in young mice. Brain Dev. 2012;34:181–4. doi: 10.1016/j.braindev.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Rho JM, Kim DW, Robbins CA, Anderson GD, Schwartzkroin PA. Age-dependent differences in flurothyl seizure sensitivity in mice treated with a ketogenic diet. Epilepsy Res. 1999;37:233–40. doi: 10.1016/s0920-1211(99)00068-6. [DOI] [PubMed] [Google Scholar]
- 11.Greene AE, Todorova MT, Seyfried TN. Perspectives on the metabolic management of epilepsy through dietary reduction of glucose and elevation of ketone bodies. J Neurochem. 2003;86:529–37. doi: 10.1046/j.1471-4159.2003.01862.x. [DOI] [PubMed] [Google Scholar]
- 12.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–94. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 13.Dutton SB, Escayg A. Genetic influences on ketogenic diet efficacy. Epilepsia. 2008;49(Suppl 8):67–9. doi: 10.1111/j.1528-1167.2008.01839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szot P, Weinshenker D, Rho JM, Storey TW, Schwartzkroin PA. Nore-pinephrine is required for the anticonvulsant effect of the ketogenic diet. Brain Res Dev Brain Res. 2001;129:211–4. doi: 10.1016/s0165-3806(01)00213-9. [DOI] [PubMed] [Google Scholar]
- 15.Likhodii S, Nylen K, Burnham WM. Acetone as an anticonvulsant. Epilepsia. 2008;49(Suppl 8):83–6. doi: 10.1111/j.1528-1167.2008.01844.x. [DOI] [PubMed] [Google Scholar]
- 16.Ma W, Berg J, Yellen G. Ketogenic diet metabolites reduce firing in central neurons by opening K (ATP) channels. J Neurosci. 2007;27:3618–25. doi: 10.1523/JNEUROSCI.0132-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yudkoff M, Daikhin Y, Horyn O, Nissim I. Ketosis and brain handling of glutamate, glutamine, and GABA. Epilepsia. 2008;49(Suppl 8):73–5. doi: 10.1111/j.1528-1167.2008.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman JM, Vining EP, Pillas DJ, Pyzik PL, Casey JC, Kelly LM. The efficacy of the ketogenic diet-1998: a prospective evaluation of intervention in 150 children. Pediatrics. 1998;102:1358–63. doi: 10.1542/peds.102.6.1358. [DOI] [PubMed] [Google Scholar]
- 19.Vining EP, Freeman JM, Ballaban-Gil K, et al. A multicenter study of the efficacy of the ketogenic diet. Arch Neurol. 1998;55:1433–7. doi: 10.1001/archneur.55.11.1433. [DOI] [PubMed] [Google Scholar]
- 20.Yudkoff M, Daikhin Y, Nissim I, Lazarow A, Nissim I. Ketogenic diet, amino acid metabolism, and seizure control. J Neurosci Res. 2001;66:931–40. doi: 10.1002/jnr.10083. [DOI] [PubMed] [Google Scholar]
- 21.Veech RL, Chance B, Kashiwaya Y, Lardy HA, Cahill GF., Jr Ketone bodies, potential therapeutic uses. IUBMB Life. 2001;51:241–7. doi: 10.1080/152165401753311780. [DOI] [PubMed] [Google Scholar]