Abstract
The quality of life for women after menopause is one of the key health issues today, and osteoporosis is a silently progressing metabolic bone disease widely prevalent in post-menopausal women in India. Rapid bone loss occurs in post-menopausal women due to hormonal factors that lead to an increased risk of fractures. Thus, the present study was undertaken to observe the serum calcium and alkaline phosphatase (ALP) levels in post-menopausal women as these substances are biochemical markers of bone metabolism. In this small-scale cross-sectional study, 100 samples were taken. Of these, 50 were taken from post-menopausal women (experimental group) and 50 were taken from pre-menopausal women (control group). Serum calcium and serum ALP were measured in the blood samples of both groups. The findings demonstrated that the serum calcium level was significantly lower in the post-menopausal group than in the pre-menopausal group, while the ALP level was slightly higher. Therefore, an increase in bone turnover accelerates bone mass reduction in post-menopausal women, whereas a decrease in bone turnover is associated with the preservation of bone mass.
Keywords: alkaline phosphatase, calcium, menopause, osteoporosis
Introduction
The word ‘menopause’ is derived from two Greek words, ‘meno’ (month) and ‘paus’ (to stop). Clinically, menopause is said to have occurred when menstruation has ceased for twelve months (1). Physiologically, menopause is defined as the permanent cessation of menses resulting from reduced ovarian hormone secretion that occurs naturally or is induced by surgery, chemotherapy, or radiation (2). The post-menopausal stage in women is essentially an oestrogen-deficient state (1). Both menopause and aging are associated with an accelerated loss of bone mass. Menopause occurs when the balance between bone formation and resorption is upset and resorption is excessive, resulting in a negative remodelling balance (3). Osteoporosis is an important public health problem in middle-aged and older women. Osteoporosis is more common in post-menopausal women and not only gives rise to morbidity but also markedly diminishes the quality of life in this population. There is lack of information regarding the risk factors of osteoporosis in developing countries (4). Serum alkaline phosphatase (ALP) is the most commonly used biomarker of bone formation. ALP is a ubiquitous enzyme that plays an important role in osteoid formation and bone mineralisation. The serum ALP pool consists of several dimeric isoforms that originate from various tissues, such as the liver, bone, intestine, spleen, kidney, and placenta (5). Thus, the aim of the present study is to evaluate the risk of accelerated bone mass loss by assessing bone markers, such as alkaline phosphatase (ALP) and serum calcium, in post-menopausal women.
Methods and Materials
This small-scale cross-sectional study was performed on a total of 100 subjects who were divided into an experimental group of 50 postmenopausal women (> 45 years old) and a control group of 50 pre-menopausal women (12–40 years old). The exclusion criteria for post-menopausal subjects included smoking, alcoholism, and calcium supplementation, whereas the exclusion criteria for pre-menopausal subjects were pregnancy, smoking, alcoholism, and oral contraceptive use. The results for continuous measurements are presented as the mean (SD) (min-max). The results were considered significant at P < 0.05. Student’s t test (two tailed, independent) was used to determine the significance of study parameters on a continuous scale between two groups. The Pearson correlation coefficient was computed in the experimental group. The software package used for statistical analysis (SPSS) 15.0 (IBM Corporation, India) was used to analyse the data.
Results
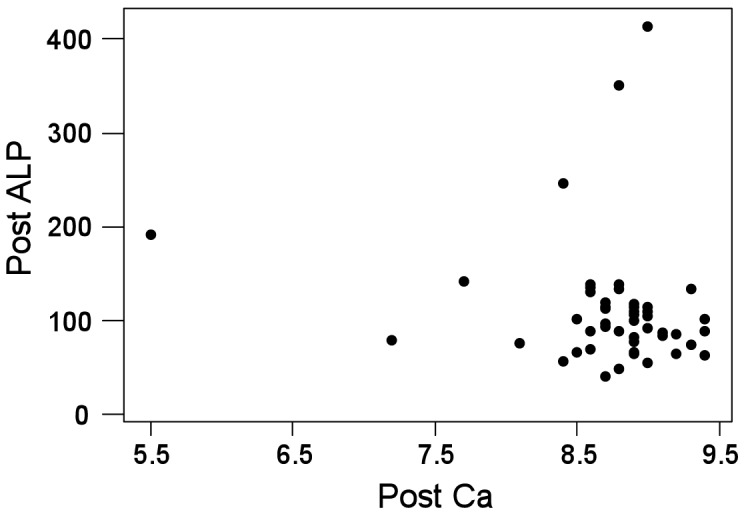
Table 1 shows that serum calcium levels were significantly reduced (P = 0.000) in the post-menopausal group 8.73 (SD 0.60) when compared to the pre-menopausal group 9.65 (SD 0.68); however, the serum calcium levels in both groups were within the normal reference range. The serum ALP levels were slightly higher in (P = 0.046) in the post-menopausal group 111.86 (SD 66.5) when compared to the pre-menopausal group 82.40 (SD 78.50). The ALP values were also in the normal reference range. Table 2 shows a significant negative correlation between calcium and ALP (r = –0.1496), wherein serum ALP levels were elevated in post-menopausal women and serum calcium levels were reduced.
Table 1.
Comparison of mean values of Ca (mg/dL) and ALP (U/L) between post and pre-menopausal women
| Variable | Post-Menopausal | Pre-Menopausal | P | t | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mini | Max | Mean | SD | Mini | Max | |||
| Calcium mg/dl | 8.73 | 0.60 | 5.50 | 9.40 | 9.65 | 0.68 | 9.00 | 13.50 | 0.000 | 7.09∗ |
| ALP U/l | 111.86 | 66.56 | 41.00 | 414.00 | 82.40 | 78.50 | 21.00 | 550.00 | 0.046 | 2.03∗ |
∗indicate the significant values at P < 0.05.
Table 2.
Pearson correlation coefficient of serum calcium and serum ALP levels in post-menopausal women
| Correlation Coefficient | Case (n = 50) |
|---|---|
| r value between calcium and ALP in post-menopausal women | –0.1496 |
Discussion
Menopause is known to be associated with numerous physiological and biochemical changes affecting bone mineral metabolism. The results of various case control studies in pre- and post-menopausal women have shown that changes in the serum calcium levels in post-menopausal women are not statistically significant (6–8); however, in the present study, we found that the serum calcium levels were significantly reduced in the post-menopausal group 8.73 (SD 0.60) when compared to the pre-menopausal group 9.65 (SD 0.68) (P = 0.000) (Table 1). Ashuma et al. reported that aging and menopause lead to a decline in oestrogen and progesterone production, which has been implicated in the increased calcium levels of post-menopausal women (6).
Conversely, the serum alkaline phosphatase (ALP) levels were significantly increased in the post-menopausal group 111.86 (SD 66.5) compared to the pre-menopausal group 82.40 (SD 78.50) (P = 0.046) (Table 1). It has also been shown that oestrogen deficiency, as occurs during menopause, induces the synthesis of cytokines by osteoblasts, monocytes, and T cells and thereby stimulates bone resorption by increasing osteoclastic activity. This action results in modification of the reabsorption, excretion, and resorption of calcium, which leads to increased circulating levels of this ion (9–12). Thus, we have reported a negative correlation between serum calcium and ALP levels in post-menopausal women (Table 2). Several studies have reported no significant correlation between serum calcium levels and ALP when assessing various years since menopause (YSM) (13–18). However, contrary to these findings, higher levels of calcium and ALP have been demonstrated in early postmenopausal women (≤ 10 YSM) compared with late menopausal women (≥ 10 YSM) (12).
Conclusion
In normal post-menopausal women, an increase in bone turnover accelerates bone mass reduction. The present study reveals that serum calcium levels are significantly reduced in postmenopausal women, whereas serum ALP levels are significantly increased. In addition, a significant negative correlation was observed between serum calcium and ALP levels in the experimental group.
Acknowledgments
We are sincerely thankful to the principal and the teaching and non-teaching staffs of Vidyasagar College for Women, University of Calcutta and obviously to the subjects who responded in this study.
Footnotes
Conflict of interest
None.
Funds
None.
Authors’ Contributions
Conception and design, analysis and interpretation of the data: TB, KB, PC, PS
Drafting of the article: TB, KB, PS
Critical revision of the article for the important intellectual content: KB, PS
Final approval of the article: TB, KB, PC, PS
Statistical expertise, collection and assembly of data: PS
Administrative, technical or logistic support: TB
References
- 1.Komaroff AL, Nicholson CR, Woo B. 14th ed. New York (NY): Mc Graw Hill; 1998. Harrison’s principle of internal medicine; 22 pp. [Google Scholar]
- 2.Nelson HD, Haney E, Humphrey L, Miller J, Nedrow A, Nicolaidis C, et al. United States of America (USA): Agency for Healthcare Research and Quality; 2005. Management of Menopause-Related Symptoms. No 120; pp. 1–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Adanna C, Uzoma C, Chinyere A. Biochemical bone turnover markers in postmenoposal women in Calabar Municipality. Asian J Biochem. 2007;2(2):130–135. [Google Scholar]
- 4.Keramat A, Patwardhan B, Larijani B, Chopra A, Mithal A, Chakravarty D, et al. The assessment of osteoporosis risk factors in Iranian women compared with Indian women. BMC Musculoskeletal Dis. 2008;9:28. doi: 10.1186/1471-2474-9-28. doi: 10.1186/1471-2474-9-28 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delmas PD, Eastell R, Garnero P, Seibe MJ. The Use of Biochemical Markers of Bone Turnover in osteoporosis. Osteoporosis Int. 2000;11(6 Suppl):S2–S17. doi: 10.1007/s001980070002. [DOI] [PubMed] [Google Scholar]
- 6.Ashuma S, Shashi S, Sachdeva S. Biochemical Markers of bone turnover: diagnostic and therapeutic principles. Osteoporosis. 2005;3:305–311. [Google Scholar]
- 7.Suresh M, Naidu DM. Influence of years since menopause on bone mineral metabolism in south Indian women. Indian J Med Sci. 2006;60(5):190–198. [PubMed] [Google Scholar]
- 8.Massé PG, Dosy J, Jougleux JL, Caissie M, Howell DS. Bone mineral density and metabolism at an early stage of menopause when oestrogen and calcium supplement are not in used and without interference of major confounding variables. J Am College Nutr. 2005;24:354–360. doi: 10.1080/07315724.2005.10719485. [DOI] [PubMed] [Google Scholar]
- 9.Esbrit P. Hyper calcemia of malignancy: New insights into an old syndrome. Clin Lab. 2001;47(1–2):67–71. [PubMed] [Google Scholar]
- 10.Riggs BL, Khosla S, Melton LJ. A unitary model of involutional osteoporosis: Oestrogen deficiency causes both type 1 and type 2 osteoporosis in postmenopausal women and contributes to bone loss in ageing men. J Bone Miner Res. 1998;13(5):763–773. doi: 10.1359/jbmr.1998.13.5.763. [DOI] [PubMed] [Google Scholar]
- 11.Kurland ES, Cosman F, McMahon DJ. Parathyroid hormone as a therapy for idiopathic osteoporosis in men. Effect on bone mineral density and bone markers. J Clin Endocinol Meta. 2000;85(9):3069–3076. doi: 10.1210/jcem.85.9.6818. [DOI] [PubMed] [Google Scholar]
- 12.Sengupta P. The Laboratory Rat: relating its age with humans. Int J Prev Med. 2013;4(6):624–630. [PMC free article] [PubMed] [Google Scholar]
- 13.Dutta S, Joshi KR, Sengupta P, Bhattacharya K. Unilateral and bilateral cryptorchidism and its effect on the testicular morphology, histology, accessory sex organs and sperm count in Laboratory Mice. J Hum Repro Sci. 2013;6(2):106–110. doi: 10.4103/0974-1208.117172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sengupta P. Potential Health Impacts of Hard Water. Int J Prev Med. 2013;4(8):866–875. [PMC free article] [PubMed] [Google Scholar]
- 15.Sengupta P, Sahoo S. A Cross Sectional Study to Evaluate the Fitness Pattern among the Young Fishermen of Coastal Orissa. Indian J Pub Health Res Dev. 2013;4(1):171–175. [Google Scholar]
- 16.Sengupta P, Chaudhuri P, Bhattacharya K. Male Reproductive Health and Yoga. Int J Yoga. 2013;6(2):87–95. doi: 10.4103/0973-6131.113391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sengupta P, Banerjee R. Environmental toxins: Alarming impacts of pesticides on male fertility. Hum Exp Toxicol. 2013;Forthcoming 2014 Feb doi: 10.1177/0960327113515504. doi: 10.1177/0960327113515504 . [DOI] [PubMed] [Google Scholar]
- 18.Sengupta P. Health Impacts of Yoga and Pranayama: An Art-of-the-state Review. Int J Prev Med. 2012;3(7):444–458. [PMC free article] [PubMed] [Google Scholar]


