Abstract
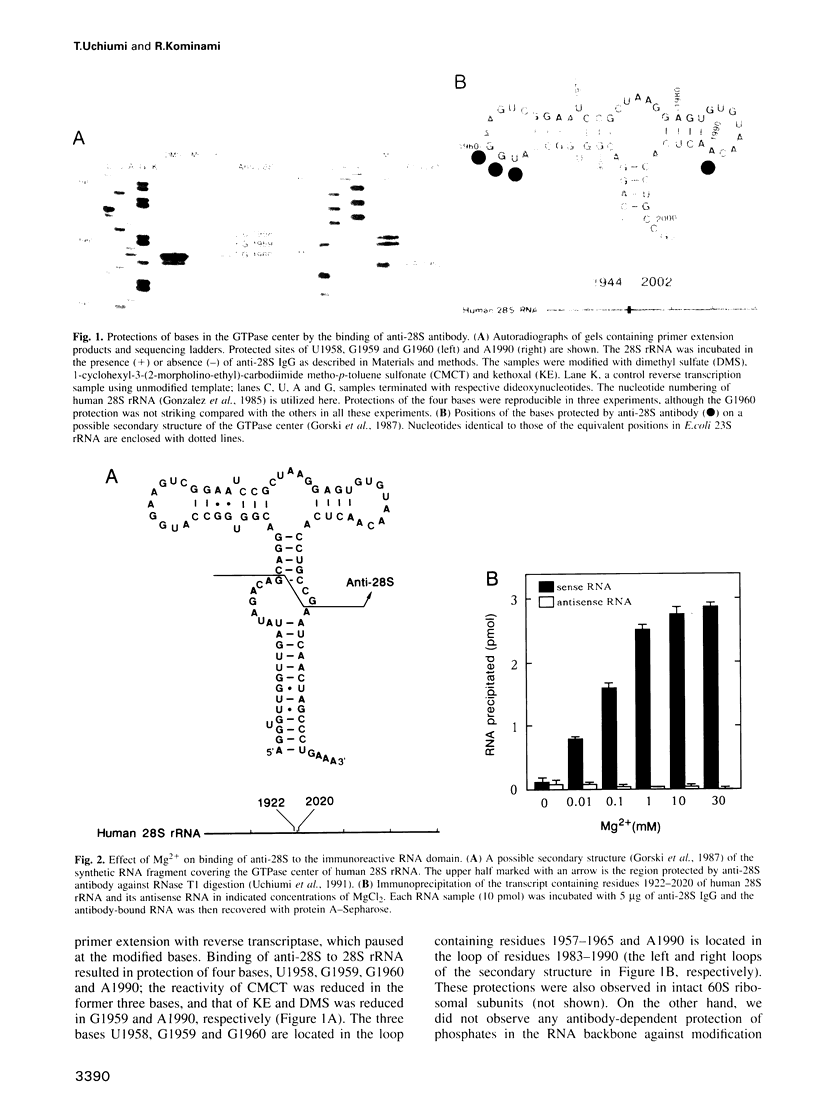
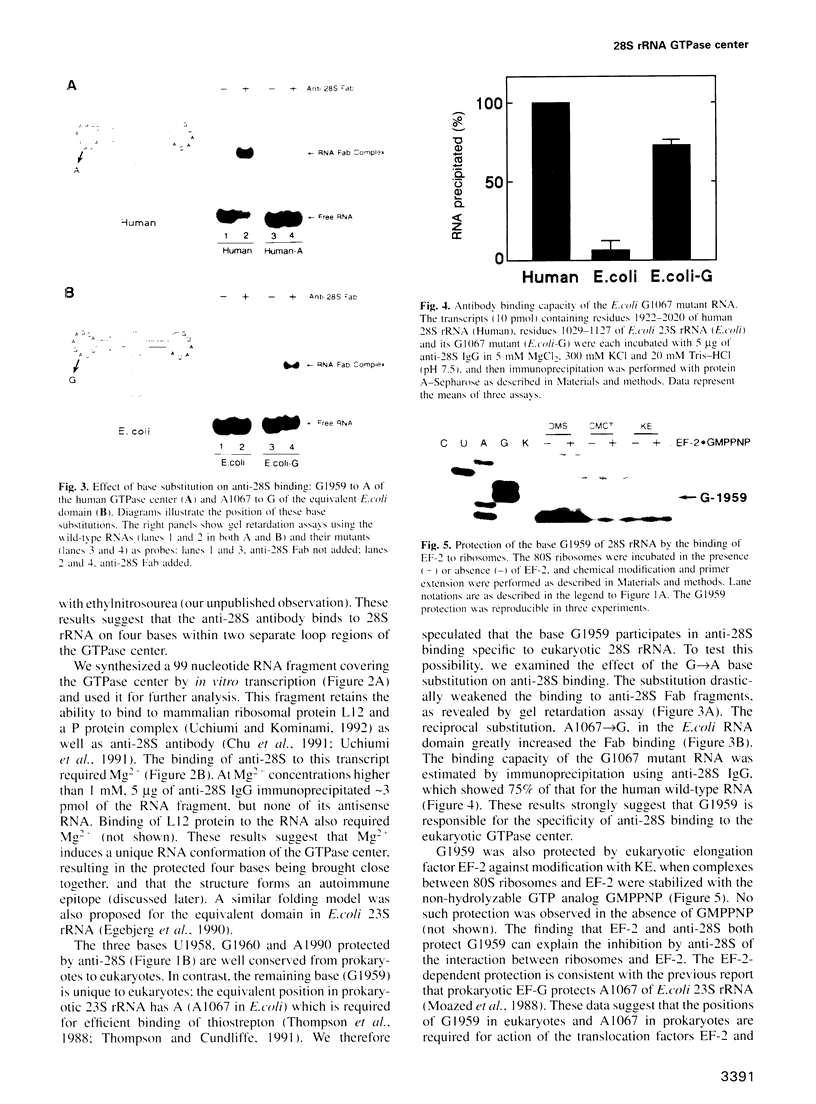
An anti-RNA autoantibody (anti-28S) was employed to identify structural and functional elements characteristic of a domain termed the 'GTPase center' in eukaryotic 28S ribosomal RNA. This antibody, an inhibitor of ribosome-associated GTP hydrolysis, has a unique property: it binds to the RNA domain of eukaryotes but not to that of prokaryotes. The antibody binding occurred in the presence of Mg2+ and protected from chemical modification three conserved bases (U1958, G1960 and A1990) and the base G1959 which is replaced by A in prokaryotic 23S rRNA (A1067 in Escherichia coli). In vitro substitution of G1959 to A drastically weakened the antibody binding, and the reciprocal substitution, A1067-->G of the E.coli domain conferred the binding ability. This suggests that the G base determines the specificity of antibody binding. The G1959 was also protected by the association of ribosomes with elongation factor EF-2. The result, together with protection of E.coli base A1067 by EFG [D.Moazed, I.M.Robertson and H.F.Noller (1988) Nature, 334, 362-364], suggests that the position of G1959 in eukaryotes and A1067 in prokaryotes constitutes at least part of the factor binding site irrespective of the base replacement during evolution.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauclerk A. A., Cundliffe E., Dijk J. The binding site for ribosomal protein complex L8 within 23 s ribosomal RNA of Escherichia coli. J Biol Chem. 1984 May 25;259(10):6559–6563. [PubMed] [Google Scholar]
- Beauclerk A. A., Hummel H., Holmes D. J., Böck A., Cundliffe E. Studies of the GTPase domain of archaebacterial ribosomes. Eur J Biochem. 1985 Sep 2;151(2):245–255. doi: 10.1111/j.1432-1033.1985.tb09095.x. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Olvera J., Wool I. G. The structure of rat 28S ribosomal ribonucleic acid inferred from the sequence of nucleotides in a gene. Nucleic Acids Res. 1983 Nov 25;11(22):7819–7831. doi: 10.1093/nar/11.22.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J. L., Brot N., Weissbach H., Elkon K. Lupus antiribosomal P antisera contain antibodies to a small fragment of 28S rRNA located in the proposed ribosomal GTPase center. J Exp Med. 1991 Sep 1;174(3):507–514. doi: 10.1084/jem.174.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egebjerg J., Douthwaite S. R., Liljas A., Garrett R. A. Characterization of the binding sites of protein L11 and the L10.(L12)4 pentameric complex in the GTPase domain of 23 S ribosomal RNA from Escherichia coli. J Mol Biol. 1990 May 20;213(2):275–288. doi: 10.1016/S0022-2836(05)80190-1. [DOI] [PubMed] [Google Scholar]
- Egebjerg J., Douthwaite S., Garrett R. A. Antibiotic interactions at the GTPase-associated centre within Escherichia coli 23S rRNA. EMBO J. 1989 Feb;8(2):607–611. doi: 10.1002/j.1460-2075.1989.tb03415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez I. L., Gorski J. L., Campen T. J., Dorney D. J., Erickson J. M., Sylvester J. E., Schmickel R. D. Variation among human 28S ribosomal RNA genes. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7666–7670. doi: 10.1073/pnas.82.22.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J. L., Gonzalez I. L., Schmickel R. D. The secondary structure of human 28S rRNA: the structure and evolution of a mosaic rRNA gene. J Mol Evol. 1987;24(3):236–251. doi: 10.1007/BF02111237. [DOI] [PubMed] [Google Scholar]
- Gutell R. R., Fox G. E. A compilation of large subunit RNA sequences presented in a structural format. Nucleic Acids Res. 1988;16 (Suppl):r175–r269. doi: 10.1093/nar/16.suppl.r175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki K., Kaziro Y. Polypeptide chain elongation factors from pig liver. Methods Enzymol. 1979;60:657–676. doi: 10.1016/s0076-6879(79)60062-9. [DOI] [PubMed] [Google Scholar]
- Maassen J. A., Möller W. Elongation factor G-dependent binding of a photoreactive GTP analogue to Escherichia coli ribosomes results in labeling of protein L11. J Biol Chem. 1978 Apr 25;253(8):2777–2783. [PubMed] [Google Scholar]
- Maassen J. A., Möller W. Identification by photo-affinity labeling of the proteins in Escherichia coli ribosomes involved in elongation factor G-dependent GDP binding. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1277–1280. doi: 10.1073/pnas.71.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacConnell W. P., Kaplan N. O. The activity of the acidic phosphoproteins from the 80 S rat liver ribosome. J Biol Chem. 1982 May 25;257(10):5359–5366. [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature. 1987 Jun 4;327(6121):389–394. doi: 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Transfer RNA shields specific nucleotides in 16S ribosomal RNA from attack by chemical probes. Cell. 1986 Dec 26;47(6):985–994. doi: 10.1016/0092-8674(86)90813-5. [DOI] [PubMed] [Google Scholar]
- Moazed D., Robertson J. M., Noller H. F. Interaction of elongation factors EF-G and EF-Tu with a conserved loop in 23S RNA. Nature. 1988 Jul 28;334(6180):362–364. doi: 10.1038/334362a0. [DOI] [PubMed] [Google Scholar]
- Musters W., Conçalves P. M., Boon K., Raué H. A., van Heerikhuizen H., Planta R. J. The conserved GTPase center and variable region V9 from Saccharomyces cerevisiae 26S rRNA can be replaced by their equivalents from other prokaryotes or eukaryotes without detectable loss of ribosomal function. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1469–1473. doi: 10.1073/pnas.88.4.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller W., Slobin L. I., Amons R., Richter D. Isolation and characterization of two acidic proteins of 60s ribosomes from Artemia salina cysts. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4744–4748. doi: 10.1073/pnas.72.12.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan P. C., Lu M., Draper D. E. Recognition of the highly conserved GTPase center of 23 S ribosomal RNA by ribosomal protein L11 and the antibiotic thiostrepton. J Mol Biol. 1991 Oct 20;221(4):1257–1268. doi: 10.1016/0022-2836(91)90932-v. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sköld S. E. Chemical crosslinking of elongation factor G to the 23S RNA in 70S ribosomes from Escherichia coli. Nucleic Acids Res. 1983 Jul 25;11(14):4923–4932. doi: 10.1093/nar/11.14.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M. J., Cundliffe E., Dijk J., Stöffler G. Functional homology between E. coli ribosomal protein L11 and B. megaterium protein BM-L11. Mol Gen Genet. 1980;180(1):11–15. doi: 10.1007/BF00267346. [DOI] [PubMed] [Google Scholar]
- Sánchez-Madrid F., Vidales F. J., Ballesta J. P. Functional role of acidic ribosomal proteins. Interchangeability of proteins from bacterial and eukaryotic cells. Biochemistry. 1981 May 26;20(11):3263–3266. doi: 10.1021/bi00514a043. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies in pathology and cell biology. Cell. 1991 Nov 29;67(5):841–842. doi: 10.1016/0092-8674(91)90356-4. [DOI] [PubMed] [Google Scholar]
- Thompson J., Cundliffe E., Dahlberg A. E. Site-directed mutagenesis of Escherichia coli 23 S ribosomal RNA at position 1067 within the GTP hydrolysis centre. J Mol Biol. 1988 Sep 20;203(2):457–465. doi: 10.1016/0022-2836(88)90012-5. [DOI] [PubMed] [Google Scholar]
- Thompson J., Cundliffe E. The binding of thiostrepton to 23S ribosomal RNA. Biochimie. 1991 Jul-Aug;73(7-8):1131–1135. doi: 10.1016/0300-9084(91)90156-u. [DOI] [PubMed] [Google Scholar]
- Thompson J., Musters W., Cundliffe E., Dahlberg A. E. Replacement of the L11 binding region within E.coli 23S ribosomal RNA with its homologue from yeast: in vivo and in vitro analysis of hybrid ribosomes altered in the GTPase centre. EMBO J. 1993 Apr;12(4):1499–1504. doi: 10.1002/j.1460-2075.1993.tb05793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Schmidt F., Cundliffe E. Site of action of a ribosomal RNA methylase conferring resistance to thiostrepton. J Biol Chem. 1982 Jul 25;257(14):7915–7917. [PubMed] [Google Scholar]
- Uchiumi T., Kikuchi M., Terao K., Iwasaki K., Ogata K. Cross-linking of elongation factor 2 to rat-liver ribosomal proteins by 2-iminothiolane. Eur J Biochem. 1986 Apr 1;156(1):37–48. doi: 10.1111/j.1432-1033.1986.tb09545.x. [DOI] [PubMed] [Google Scholar]
- Uchiumi T., Kominami R. Direct evidence for interaction of the conserved GTPase domain within 28 S RNA with mammalian ribosomal acidic phosphoproteins and L12. J Biol Chem. 1992 Sep 25;267(27):19179–19185. [PubMed] [Google Scholar]
- Uchiumi T., Traut R. R., Elkon K., Kominami R. A human autoantibody specific for a unique conserved region of 28 S ribosomal RNA inhibits the interaction of elongation factors 1 alpha and 2 with ribosomes. J Biol Chem. 1991 Feb 5;266(4):2054–2062. [PubMed] [Google Scholar]
- Uchiumi T., Traut R. R., Kominami R. Monoclonal antibodies against acidic phosphoproteins P0, P1, and P2 of eukaryotic ribosomes as functional probes. J Biol Chem. 1990 Jan 5;265(1):89–95. [PubMed] [Google Scholar]
- Woodcock J., Moazed D., Cannon M., Davies J., Noller H. F. Interaction of antibiotics with A- and P-site-specific bases in 16S ribosomal RNA. EMBO J. 1991 Oct;10(10):3099–3103. doi: 10.1002/j.1460-2075.1991.tb07863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Baradi T. T., de Regt V. C., Einerhand S. W., Teixido J., Planta R. J., Ballesta J. P., Raué H. A. Ribosomal proteins EL11 from Escherichia coli and L15 from Saccharomyces cerevisiae bind to the same site in both yeast 26 S and mouse 28 S rRNA. J Mol Biol. 1987 Jun 20;195(4):909–917. doi: 10.1016/0022-2836(87)90494-3. [DOI] [PubMed] [Google Scholar]
- van Keulen H., Gutell R. R., Campbell S. R., Erlandsen S. L., Jarroll E. L. The nucleotide sequence of the entire ribosomal DNA operon and the structure of the large subunit rRNA of Giardia muris. J Mol Evol. 1992 Oct;35(4):318–328. doi: 10.1007/BF00161169. [DOI] [PubMed] [Google Scholar]