Abstract
Malignant melanoma of the rectum is an extremely rare disease. It typically presents in the fifth or sixth decade of life with nonspecific complaints such as rectal bleeding or anal pain. A timely diagnosis of anal melanoma is made even more difficult by the fact that most of the lesions lack obvious pigmentation and are even histologically amelanotic. Prognosis is very poor. Anorectal malignant melanomas spread along submucosal planes and are often beyond complete resection at the time of diagnosis. We present the radiological and pathological features seen in a 43-year-old woman diagnosed with melanoma of the rectum.
Keywords: Imaging, malignant, melanoma, rectum
INTRODUCTION

Malignant melanoma of the rectum is an extremely rare and very aggressive disease.[1] This entity constitutes only 0.5-4% of all anorectal malignancies and less than 1% of all melanomas.[2,3] Patients, predominantly women, typically present with local symptoms in the fifth or sixth decade of life.[1] Malignant melanoma has a strong association with the Caucasian race. Cutaneous melanoma is 20 times more common in Caucasians than in African Americans, but there is no such evidence for anal melanomas. Patients often present with nonspecific complaints such as rectal bleeding or anal pain.[2,3] A timely diagnosis of anal melanoma is made even more difficult by the fact that up to 80% of lesions lack obvious pigmentation and up to 20% of tumors are even histologically amelanotic.[4,5] Prognosis is very poor with a median survival of 24 months and a 5-year survival of 10-15%.[1,6] Presently, there is no consensus on which surgical approach is favorable.[3] Anorectal malignant melanomas spread along submucosal planes., Therefore, they are often beyond complete resection at the time of diagnosis[7] and almost all patients die because of metastases.[8]
A 43-year-old female presented with a 5-month history of a painful mass protruding from the anus with bleeding from the rectum and alteration of bowel habit. She was a non-smoker with an unremarkable medical history. She was anemic with hemoglobin level of 6 gm% and was given five units of whole blood. Her general condition was poor with a BMI of 16.6 and a Karnofsky performance status of 60-70. (The Karnofsky score runs from 100 to 0, where 100 is “perfect” health and 0 is death. This scoring system was developed to allow physicians to evaluate a patient's ability to survive chemotherapy treatment for cancer). Digital rectal examination revealed a fleshy mass occupying almost whole of the anal lumen and reaching upto the anal verge. Her clinical examination was otherwise essentially normal.
Rigid sigmoidoscopy showed an ulcerating black-colored nodular growth starting at the anorectal junction and extending proximally for about 6 cm.
RADIOLOGICAL FEATURES
Computed tomography of the abdomen and pelvis revealed polypoidal intraluminal mass in the rectum causing near total obliteration of the lumen, extending proximally from just below the rectosigmoid junction to the anal verge distally. There was evidence of extramural extension of mass into peri-rectal fat planes with stranding. There was loco-regional, para-aortic and external iliac lymphadenopathy suggestive of nodal metastasis. There was loss of recto-vaginal fat plane with involvement of right postero-lateral wall of the vagina [Figure 1].
Figure 1.
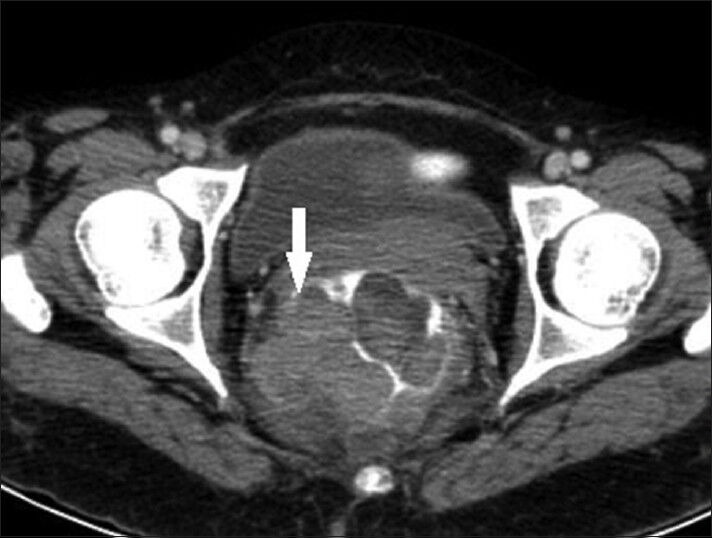
43-year-old woman with protruding anal lesion diagnosed with melanoma of the rectum. Contrast-enhanced axial CT scan images of pelvis shows heterogeneous mass lesion in rectum with extension into mesorectal fat (white solid arrow).
MRI of pelvis showed heterogeneous mass in the rectum with intra- and extra-luminal extension along with involvement of mesorectal fat and postero-lateral wall of the vagina. The mass revealed heterogeneous signal intensity [Figures 2 and 3].
Figure 2.

43-year-old woman with protruding anal lesion diagnosed with melanoma of the rectum. T2- weighted axial MR images of pelvis show irregular lobulated mass of rectum with intra- and extra-mural extension with involvement of posterolateral wall of vagina (white solid arrow).
Figure 3.
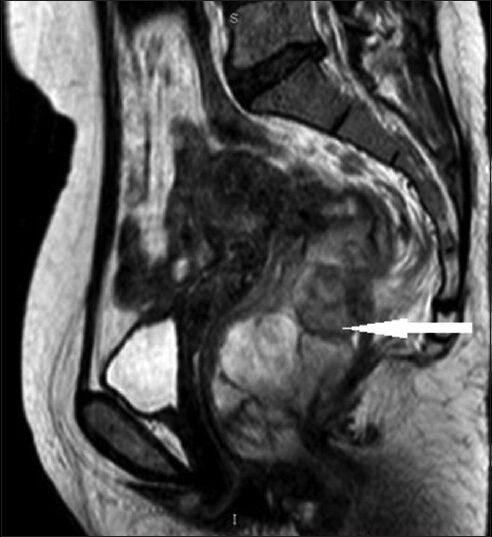
43-year-old woman with protruding anal lesion diagnosed with melanoma of the rectum. T2 weighted sagittal MR image of pelvis shows lobulated mass of rectum (white solid arrow) with variable signal intensity.
PATHOLOGICAL FEATURES
A tru-cut needle biopsy was performed. The biopsy specimens were sent for histological examination. Hematoxylin and eosin staining of the sample revealed pleomorphic lesions displaying high mitotic rate and prominent nucleoli. It showed intra- and extra-cellular melanin pigment on microscopic examination [Figure 4]. Immuno-histochemical staining with markers HMB-45 (Human Melanoma Black) and S-100 (S100 protein that is soluble in 100% ammonium sulfate), was not done in this case due to lack of availability of the tests in our institute.
Figure 4.
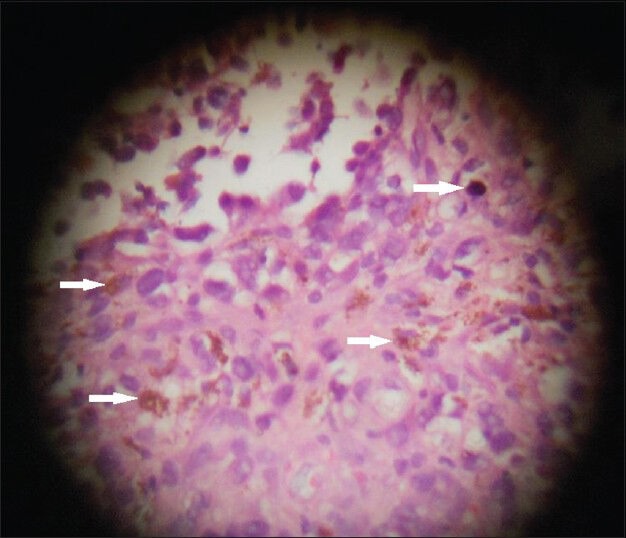
43-year-old woman with protruding anal lesion diagnosed with melanoma of the rectum. Histopathological biopsy sample stained with hematoxylin and eosin (× 100) shows pleomorphic lesions with melanin pigment (white solid arrows) suggestive of malignant melanoma.
A diversion colostomy was done. Surgery and chemotherapy were planned for her but the patient's general condition did not permit the same and she died within a few days.
DISCUSSION
All melanomas, whether cutaneous or mucosal in origin, originate from melanocytes, which are cells derived from the embryological neural crest. During fetal development, these cells migrate to many sites throughout the body, primarily to the skin. However, melanocytes also reside in the eyes (retina and uveal tract) and mucosal surfaces.[2,3] Therefore, cutaneous melanomas are by far the most common form of the disease, comprising more than 90% of all melanomas. Of the remaining (<10%) forms of melanoma, ocular melanoma accounts for 5%, melanoma of unknown origin for 2%, and mucosal melanoma for 1%.[3] Melanocytes may undergo malignant transformation when exposed to ultraviolet UVB light, which is a carcinogenic stimulus. However, this relationship is not apparent in anorectal melanoma.[7] There is a likely role of immunology in the development of anorectal melanoma as the incidence is higher in patients with Human Papilloma virus (HPV) and HIV infections.[7,9] In the rectum, melanocytes are located at the anal transition zone and squamous zone. Most anorectal melanomas arise from the dentate line and 65% are located within the anal canal or at the anal verge.[3,7] The most common presenting complaints are bleeding, anorectal discomfort or pain, an appreciable anorectal mass, or change in bowel habits. Other symptoms include pruritis, tenesmus, prolapsed hemorrhoid, change in stool habits, and diarrhea. Patients who have metastasis at the time of presentation may additionally have fatigue, weight loss, and anemia.[3,7] Lesions are most commonly found at the anorectum, followed by the anal canal and anal verge.[4] These lesions are often discounted as being benign hemorrhoids or polyps.[6,7]
A sigmoido-colonoscopy is essential both for evaluation of the cause of symptoms and obtaining a tissue biopsy from a suspicious lesion. Endoscopic endorectal ultrasound may be considered to evaluate tumor thickness and surrounding nodal status.[7]
Computed tomography (CT) scan of the abdomen and pelvis is not the preferred diagnostic modality to evaluate hematochezia or alteration in bowel habit, but is a valuable tool to assess regional disease, and is frequently utilized to determine if there is lymphadenopathy or metastasis once neoplasia is suspected.[2,6,7] Contrast-enhanced CT scan and MRI allow characterization and assessment of the extent of the tumor. On CT scans, primary rectal malignant melanomas appear as bulky intraluminal fungating masses in the distal rectum, focally expanding and obscuring the lumen without causing obstruction, with perirectal infiltration and frequently enlarged lymph nodes.[10] Rectal carcinoma or other rectal masses present as significant obstruction.
MRI shows the melanotic component as high signal intensity on T1-weighted imaging and mixed signal intensity on T2-weighted imaging.[11] Extraluminal extent of the lesion is better demonstrated by MRI imaging. Other rectal mass lesions show hypointense signal on T1-weighted imaging.
In cases of diagnostic difficulty, which is frequent even on histopathology, the presence of melanin can be helpful, but it is not easily detected in anorectal disease.[7] In our case, presence of melanin was a diagnostic marker. Melanoma antigens S-100, HMB-45, and vimentin are important immuno-histochemical markers. Polyclonal antiserum and monoclonal antibodies to carcinoembryonic antigen can help to distinguish it from a poorly differentiated epidermoid carcinoma.[2,7] Activating KIT-gene mutations, which are implicated in leukemia and gastrointestinal stromal tumors have also been associated with the pathogenesis of malignant melanoma.[3]
Malignant melanoma of the rectum is a rare and very aggressive rectal tumor.[1] Lymphatic spread to the inguinal or inferior mesenteric nodal basins is common. The most common sites for metastases are inguinal lymph nodes, mesenteric lymph nodes, hypogastric lymph nodes, para-aortic lymph nodes, liver, lung, skin, and brain.[2] The incidence rates for locoregional lymph node metastases on initial presentation are almost 60%.[1,2] At the time of diagnosis, distant metastases are identified in 26-38% of patients.[2,3]
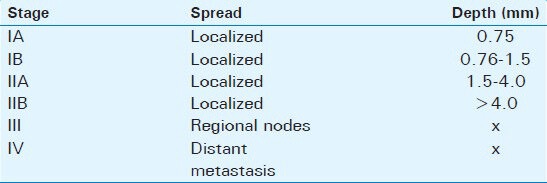
Anal melanoma is staged on a clinical basis, focusing on loco-regional and distant spread. Stage I is local disease only, Stage II is a local disease with increased thickness and ulcerations, Stage III is local disease with involvement of regional lymph nodes, and Stage IV shows distant metastatic disease [Table 1].[2,7]
Table 1.
American Joint Commission on cancer staging system for anorectal melanoma
The treatment of anal melanoma, unfortunately, is only moderately successful. Surgery remains the cornerstone of treatment.[1,2] Of all patients diagnosed with anorectal melanoma the 5-year survival rate can range from 16 to 34%. In patients who have metastasis at the time of diagnosis, the disease-free survival rate may drop to 16% from 22%.[3,7] The controversy has been whether abdominoperineal resection (APR) is needed or wide local excision (WLE) is adequate for complete treatment. APR, although a highly morbid operation, has long been thought to be the best means of control of anorectal melanoma. Lately, there has been a paradigm shift after several studies indicated that WLE may be adequate to control disease, while minimizing the morbidity of surgery because anorectal melanoma is a systemic disease at the time of diagnosis and no surgical treatment, regardless of how aggressive, will truly change the outcome.[1,2,3,7] The benefits of a WLE are quicker recovery, no need for a stoma, and minimal impact on bowel function.[7] Lymph node dissection may be indicated in clinically apparent disease or for occult disease identified with sentinel lymph node (SLN) techniques.[2,3]
There are no standards regarding systemic therapy for disseminated disease. Chemotherapy, radiation therapy, and immune therapy have a limited role. The medications used in adjuvant therapy are cisplatin, vinblastine, dacarbazine, interferon B, and Interleukins IL-2-8. Dacarbazine is the most commonly used single agent and usually initiates a partial response in 20% of patients in 4-6 months after treatment.[2,7] Recent studies show that wide local excision (WLE) combined with adjuvant loco-regional radiotherapy result in comparable loco-regional control with less loss of function compared to APR.[1]
The rarity of this disease and the limited number of patients who present with early disease, have prevented definitive trials examining the optimal treatment of curable anal melanoma.[2,3,6,7]
CONCLUSION
Malignant melanoma of the rectum is extremely rare, highly aggressive, and difficult to diagnose. Bulky fungating masses in the distal rectum obscuring the lumen without causing significant obstruction seen on CT and MRI scans raise the possibility of anorectal malignant melanoma. On MRI, the melanotic component of rectal mass can be seen as high-signal intensity on T1-weighted imaging further strengthening the diagnosis. Although biopsy and histopathological examination is essential for diagnosis, distinct radiological features on CT, and MRI may suggest possibility of malignant melanoma. Major role of CT and MRI is in preoperative staging of the tumor.
Although surgery remains the cornerstone of treatment, the exact procedure remains controversial. Role of adjuvant therapies is minimal. The diagnosis of anorectal mucosal melanoma (ARMM) portends a particularly poor prognosis and a standardized evidence-based treatment approach is not well-defined due to the rarity of this disease.[12]
The only hope of improved survival lies in early diagnosis and treatment. Since, the complaints are usually non-specific; this is only possible with a high index of suspicion followed by early sigmoidoscopy and biopsy.
Footnotes
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2014/4/1/4/126031
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.van Schaik PM, Ernst MF, Meijer HA, Bosscha K. Melanoma of the rectum: A rare entity. World J Gastroenterol. 2008;14:1633–5. doi: 10.3748/wjg.14.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer M, Mutch MG. Anal melanoma. Clin Colon Rectal Surg. 2006;19:78–87. doi: 10.1055/s-2006-942348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Row D, Weiser MR. Anorectal melanoma. Clin Colon Rectal Surg. 2009;22:120–6. doi: 10.1055/s-0029-1223844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slingluff CL, Jr, Vollmer RT, Seigler HF. Anorectal melanoma: Clinical characteristics and results of surgical management in twenty-four patients. Surgery. 1990;107:1–9. [PubMed] [Google Scholar]
- 5.Morson BC, Volkstadt H. Malignant melanoma of the anal canal. J Clin Pathol. 1963;1:126–32. doi: 10.1136/jcp.16.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liptrot S, Semeraro D, Ferguson A, Hurst N. Malignant melanoma of the rectum: A case report. J Med Case Rep. 2009;3:9318. doi: 10.1186/1752-1947-3-9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stefanou A, Nalamati SP. Anorectal Melanoma. Clin Colon Rectal Surg. 2011;24:171–6. doi: 10.1055/s-0031-1286001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maqbool A, Lintner R, Bokhari A, Habib T, Rahman I, Rao BK. Anorectal melanoma--3 case reports and a review of the literature. Cutis. 2004;73:409–13. [PubMed] [Google Scholar]
- 9.Cagir B, Whiteford MH, Topham A, Rakinic J, Fry RD. Changing epidemiology of anorectal melanoma. Dis Colon Rectum. 1999;42:1203–8. doi: 10.1007/BF02238576. [DOI] [PubMed] [Google Scholar]
- 10.Kim KW, Ha HK, Kim AY, Kim TK, Kim JS, Yu CS, et al. Primary malignant melanoma of the rectum: CT findings in eight patients. Radiology. 2004;232:181–6. doi: 10.1148/radiol.2321030909. [DOI] [PubMed] [Google Scholar]
- 11.Matsuoka H, Nakamura A, Iwamoto K, Sugiyama M, Hachiya J, Atomi Y, et al. Anorectal malignant melanoma: Preoperative usefulness of magnetic resonance imaging. J Gastroenterol. 2005;40:836–42. doi: 10.1007/s00535-005-1638-4. [DOI] [PubMed] [Google Scholar]
- 12.Carcoforo P, Raiji MT, Palini GM, Pedriali M, Maestroni U, Soliani G, et al. Primary anorectal melanoma: An update. J Cancer. 2012;3:449–53. doi: 10.7150/jca.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]


