Abstract
Gene therapy has garnered significant attention as a therapeutic approach for bladder cancer but efficient delivery and gene expression remain major hurdles. The goal of this study was to determine if cationic polymers can enhance adenoviral gene expression in cells that are difficult to transduce in vitro and to subsequently investigate lead candidates for their capacity to increase adenoviral gene expression in an orthotopic in vivo model of bladder cancer. In vitro screening of linear polyamine-based and aminoglycoside-based polymer libraries identified several candidates that enhanced adenoviral reporter gene expression in vitro. The polyamine-based polymer NPGDE-1,4 Bis significantly enhanced adenoviral gene expression in the orthotopic model of bladder cancer but unfortunately further use of this polymer was limited by toxicity. In contrast, the aminoglycoside-based polymer paromomycin-BGDE, enhanced adenoviral gene expression within the bladder without adverse events. Our study demonstrates for the first time that cationic polymers can enhance adenoviral gene expression in an orthotopic model of bladder cancer, thereby providing the foundation for future studies to determine therapeutic benefits of polymer-adenovirus combination in bladder cancer gene therapy.
INTRODUCTION
Bladder cancer is the second most common cancer of the urogenital tract with an estimated 73,510 new cases diagnosed in 2012 [1]. A major problem associated with this disease is recurrence and progression, which requires close monitoring of survivors. With an estimated 585,390 survivors currently living in the United States, bladder cancer is a major burden to the healthcare system one of the costliest cancers to treat [1, 2]. Thus strategies that more effectively eliminate lesions to reduce rates of recurrence and progression of could have a significant clinical and financial impact.
Gene therapy has garnered significant attention as a therapeutic approach for bladder cancer. From a clinical point of view, this disease is an ideal target for gene therapy due to easy accessibility of the organ and the ability to locally deliver the payload thereby avoiding a systemic immune response to viral vectors. Viral vectors, including adenovirus, have been explored for bladder cancer gene therapy [3]. Adenoviral delivery of therapeutic transgenes is particularly attractive, since these vectors remain episomal thus minimizing the risk of insertional mutations. However, efficient gene delivery remains a major obstacle, particularly because Coxsackie and Adenovirus Receptor (CAR), the surface protein used for adenoviral entry, is frequently downregulated as an early event during bladder carcinogenesis [4, 5]. This decrease in CAR expression has also been associated with increased invasiveness and poor prognosis, suggesting that cells with low receptor levels, which are the most desirable to eliminate, are also the most likely to evade therapeutic targeting by adenoviral vectors [4, 5].
Cationic polymers have been evaluated for delivering plasmid DNA in non-viral delivery systems [6], and were also among the first ones to be interfaced with adenoviruses for delivery to cancer cells [7-15]. However, these polymers generally result in high cytotoxicities and thus, recent efforts have focused on the development of cationic polymers that are less toxic including those based on cyclodextrin, genetically engineered / natural polymers and biodegradable polymers [16-20]. We have recently employed combinatorial synthesis and parallel screening methods for the rapid identification of effective and biocompatible cationic polymers for improved plasmid DNA delivery to cancer cells [21, 22] and have shown that a polymer from this library can also enhance adenoviral transduction of CAR-negative TCCSUP bladder carcinoma cells in vitro [7]. The goal of this study was to identify cationic polymers, which can enhance adenoviral gene expression in cells that are difficult to transduce in vitro and to investigate adenoviral transduction in an orthotopic in vivo model of bladder cancer.
MATERIALS AND METHODS
Materials
Adenovirus constructs expressing LacZ or luciferase transgenes were purchased from VectorBiolabs. The adenovirus expressing green fluorescent protein (GFP) has been described earlier [7]. The human bladder cancer cell lines J82 and T24 were obtained from ATCC while the murine bladder cancer cell line MB49 was kindly provided by Dr. Sven Brandau (University Hospital of Essen, Germany). All cells were cultured in DMEM with 4.5 mg/ml glucose (MediaTech), supplemented with heat-inactivated 10% FBS (Hyclone) and antibiotic/antimycotic (MediaTech). 7-AAD was purchased from BD Biosciences. Kits to measure viability, luciferase activity, and β-galactosidase (β-gal) activity were purchased from Promega. Female C57BL6 mice (5-6 weeks old) were purchased from Jackson laboratories.
Diglycidyl ethers, namely, 1,4-cyclohexanedimethanol diglycidyl ether (CDDE), 1,4-butanediol diglycidyl ether (BDGE), ethyleneglycol diglycidyl ether (EDGE), neopentylglycol diglycidyl ether (NPDGE), resorcinol diglycidyl ether (RDGE), and glycerol diglycidyl ether (GDGE), as well as amines namely, 2,2 dimethyl-1,3-propanediamine, N-(2-aminoethyl)-1,3-propanediamine, ethylenediamine, triethylenetetramine, 3,3'-diamino-N-methyldipropylamine; Tris-(2-aminoethyl)amine; diethylenetriamine; 2,2'-(ethylenedioxy)bis(ethylamine);1,5-diamino-2-methylpentane, pentaethylenehexamine, 1,4-bis(3-aminopropyl) piperazine (called 1,4 Bis subsequently); and 1,3 diaminopentane were purchased from Sigma-Aldrich, and used without any further purification. Aminoglycosides namely, neomycin sulfate, kanamycin sulfate, apramycin sulfate, paromomycin sulfate, sisomicin sulfate and amikacin hydrate, were also obtained from Sigma.
Synthesis of the Linear-Polyamine based Polymer library
Poly(aminoethers) were synthesized by ring-opening polymerization reactions between amines and diglycidyl ethers as described previously [21, 22]. Briefly, diglycidyl ethers were reacted with equimolar amounts of diamines. The polymerization reaction was carried out at room temperature for 16 hours to form viscous solids, which were then dissolved in phosphate-buffered saline (PBS), pH 7.4. Synthesized polymers were thoroughly purified by extensive dialysis against nanopure water for 2 days (with two water changes) and subsequently freeze-dried resulting in colorless-to-pale yellow crystals (50-60% yields). Polymers were reconstituted in PBS before use.
Synthesis of the Aminoglycoside-based Polymer Library
Aminoglycoside-based polycations were synthesized using a ring-opening polymerization reaction [22] between amines of aminoglycosides and epoxides of diglycidyl ethers. Prior to polymerization, aminoglycosides were converted to their free amine forms by incubating with Amberlite® anion exchange resin in order to remove associated sulfates using methods previously described in the literature [23]. Sulfate-free aminoglycosides were reacted with digylcidyl ethers in 1:2 molar ratios in a mixture of water and N,N-dimethylformamide (DMF) (1.5:1) for 5 hours at 60°C. A ratio of 1:1 aminoglycoside:diglycidyl ethers was employed only in the case of amikacin, since a 1:2 ratio resulted in the formation of insoluble products. The crude reaction mixture was allowed to cool to room temperature and precipitated using acetone. The precipitated product was washed twice with acetone in order to remove unreacted diglycidyl ethers and dried. The product was further purified by dialysis using a 3500 molecular weight cutoff (MWCO) membrane to remove unreacted aminoglycoside molecules. The dialyzed material was freeze-dried to obtain the polymer product .
Determination of polymer molecular weights
Gel permeation chromatography (GPC) was employed to determine molecular weights of the NPGDE-1,4 Bis and paromomycin-BDGE (called Pa-BDGE subsequently) polymers. GPC was carried out using an Ultrahydrogel 250 column, Waters Corporation, Milford, MA with a Waters 1515 HPLC system attached to a refractive index detector (Waters 2410). The flow rate of the mobile phase was 0.5 ml/min, and the column was maintained at a temperature of 35°C. An aqueous solvent containing 0.1 M trifluoroacetic acid and 40% acetonitrile was used as the eluent. Poly (2-vinylpyridine) samples, with molecular weights (MW) of 3000, 7000, 12000, 35000, and 70000 Da, were used as standards for column calibration. Chromatograms were analyzed using Waters Millennium GPC software.
1H-NMR and FT-IR studies
1H-NMR measurements were carried out using a Varian 400 NMR instrument operating at 400 MHz in the Fourier transform mode. FT-IR measurements were carried out with a Bruker IFS 66V/S instrument using a KBr disc.
Cytotoxicity analysis
Cells were plated at 6x104 / well in a 24-well plate for the 7-AAD assay or at 1×104/well in a 96-well plate for the MTS assay. After adhering overnight, cells were incubated with or without up to 60 μg/ml polymer for 24 hours. Viability was analyzed as previously described [7, 24].
Adenoviral transduction and in vitro analysis of transgene expression
Experiments with adenovirus were conducted as described previously [7], except that virus and polymer were simultaneously added to a final volume of 500 μl DMEM media containing 2.5% FBS (Hyclone). Depending on the experiment, virus concentrations were between 20 - 100 MOI, while polymer concentrations ranged from 5-60 μg/ml. One volume of 10% FBS-containing DMEM was added 24 hours post-transduction. Reporter gene expression was analyzed at 48 hours post-transduction. GFP analysis was performed by flow cytometry as previously described [7]. Analysis of LacZ or luciferase reporter activity was performed as recommended by the manufacturers. Signals were quantified using a BMG Optima plate reader (BMG Technologies, Durham NC).
Animal experiments
Animal procedures were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee (IACUC). Implantation of MB49 cells and instillation of adenovirus were performed as previously described [25]. Briefly, eight days after implantation of MB49 cells, an adenovirus expressing the luciferase transgene under control of a CMV promoter (Ad.Luc) was instilled at the indicated concentrations in the absence or presence of polymer. At 24 hours post-instillation, bladders were harvested, weighed, and frozen at −80°C. Immediately prior to the luciferase assay, bladders were thawed and homogenized by dounce in plain DMEM at a ratio of 25 mg bladder weight/ 100 μl DMEM. Homogenates were centrifuged at 13,000 rpm in a tabletop microfuge and 50 μl of bladder homogenate were used to determine luciferase activity.
Statistical Analysis
Means across replicates with standard error bars are plotted versus MOI, concentration or condition. Comparisons of means across conditions were performed using two-sample t-tests on log-transformed values (to normalize data).
RESULTS
Selection of an in vivo model to study polymer-mediated adenoviral delivery
The goal of this study was to evaluate polymer-mediated delivery of adenovirus in vivo. For clinical relevance, we wished to use an orthotopic model of bladder cancer and, since TCCSUP cells fail to establish tumors when instilled intravesically (0/3 mice, LK unpublished results), we chose the murine bladder cancer cell line MB49, which efficiently forms tumors in the bladder [25]. To assess adenoviral infectivity in this cell line, we exposed MB49 cells to increasing concentrations of AdGFP and quantified GFP positive cells using flow cytometry. The human bladder cancer cell lines J82 and T24 were included as easily and poorly transducible controls, respectively [26]. As shown in Figure 1, the infectivity of MB49 paralleled that of T24 cells, indicating that this cell line is relatively resistant to adenoviral infection. Thus MB49 cells show two characteristics, the ability to efficiently form tumors orthotopically after intravesical instillation, and resistance to adenoviral infection that make this model ideal to explore polymerenhanced adenoviral gene therapy in vivo.
Figure 1.
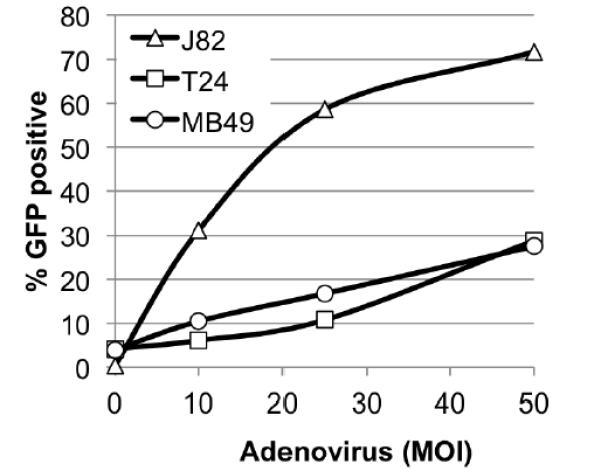
Adenoviral infectivity in bladder cancer cells. Bladder cancer cells were allowed to adhere overnight, transduced with the indicated amount of AdGFP, and GFP expression quantified by flow cytometry at 48 hours post-infection. A representative experiment is shown. Similar results were obtained in at least two additional experiments. Lines connecting the data points are for visualization alone.
Identification of NPGDE-1,4 Bis as a lead polymer
Having previously established proof-of-principle that polymers can enhance adenoviral delivery [7] several linear polyamines in order identify a lead polymer for in vivo studies. Identification of a lead polymer was done in a semi-blinded manner using a two-tier evaluation for toxicity and ability to enhance adenoviral infectivity. We initially screened six polymers for toxicity using the 7AAD assay, in which uptake of the dye is indicative of permeabilization of the plasma membrane. Results were then further validated by the MTS assay, which measures mitochondrial activity as an indicator of viability. Our results indicated that CDDE-1,4 Bis (3-aminopropyl)piperazine and NDGDE 1,4 Bis (3-aminopropyl)piperazine had little impact on viability of MB49 cells in the absence or presence of adenovirus (Figure 2A). CDDE-1,4 Bis (3-aminopropyl)piperazine, a polymer previously employed for plasmid DNA delivery leading to transgene expression [22], failed to increase the number of GFP positive cells when used in combination with adenovirus (Figure 2B). In contrast, NDGDE 1,4 Bis (3-aminopropyl)piperazine enhanced the number of GFP positive cells in a linear manner as quantified by flow cytometry (Figure 2B). These results suggested that the NPGDE-moiety is a good candidate to enhance adenoviral gene expression and led us to develop additional NPGDE-conjugated polymers. Although three additional NPDGE-conjugates (NPGDE-2,2 dimethyl-1,3-propanediamine, NPGDE-2,2’(ethylenedioxy) bis(ethylamine), and NPGDE-N-(2-aminoethyl)-1,3-propanediamine) were identified as polymers that resulted in less than 10% toxicicity in both the MTS and 7AAD assays (suppl. Fig 1), NPGDE-1,4 Bis was the only polymer that enhanced the number of GFP positive cells following viral transduction. As shown in Figure 2C a concentration of 30 μg/ml of NPGDE-1,4 Bis optimally enhanced adenoviral transduction. At higher concentrations, infectivity was adversely affected by an increase in toxicity, while at lower concentrations infectivity was lower and more variable.
Figure 2.
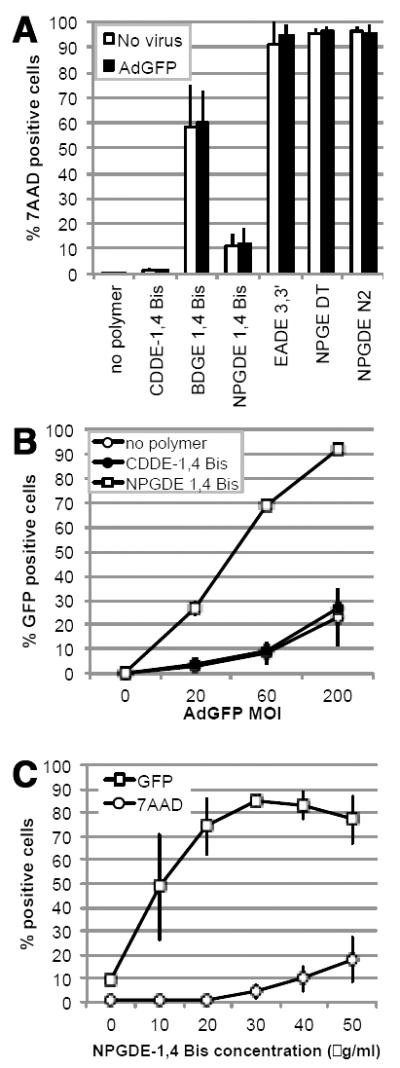
Identification of NPGDE-1,4 Bis as a lead polymer. (A) Toxicity of linear polyamines (30ug/ml) against MB49 cells in the absence and presence of 100 MOI of AdGFP. (B) Impact of 30 ug/ml CDDE-1,4 Bis and NPGDE-1,4 Bis on adenoviral GFP transgene expression. (C) Toxicity and adenoviral transgene expression as a function of NPGDE-1,4 Bis concentration at 20 MOI. Data shown are the mean ± SD from three independently performed experiments.
Characterization of the lead NPGDE-1,4 Bis polymer
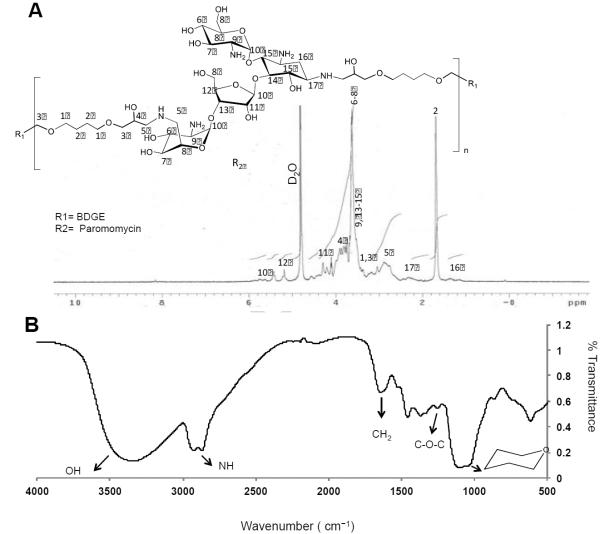
The lead NPGDE-1,4 Bis polymer was characterized using different analytical techniques, including proton NMR and FT-IR. Figure 3A shows the 1H-NMR spectrum of the polymer in D2O. The numbers in the Figure 3A represent each proton in the chemical structure of polymer, and the corresponding 1H-NMR peaks were designated accordingly. The shift in the position of the (-O-CH-) proton after opening of the epoxide ring can be seen at 3.8 ppm. Peaks corresponding to six methyl protons in the NPGDE moiety can be seen at 0.8 ppm (-CH2-C(CH3)2-CH2-). Peaks corresponding to pip erazine ring (from the polyamine) can be found between 2.2 - 3.8 ppm. The FT-IR spectrum of polymer NPGDE-1,4 Bis is shown in Figure 3B. A broad absorption band in the wavenumber range of 3200-3700 cm−1 indicates presence of large number of hydroxyl groups in the polymer. Ring-opening of epoxides in the diglycidyl ethers by primary amines from linear polyamines leads to the formation of hydro xyl groups and secondary amines. Secondary amine stretchings can be seen at 2800 cm−1. A strong absorption band at 1470 cm−1 corresponds to CH3 of NPGDE and a strong peak at 1200 cm−1 confirms the presence of ether links in the polymer. Gel permeation chromatography indicated that the molecular weight (Mw) of NPGDE-1,4 Bis polymer was 5.4 kDa and the polydispersity was 1.7.
Figure 3.
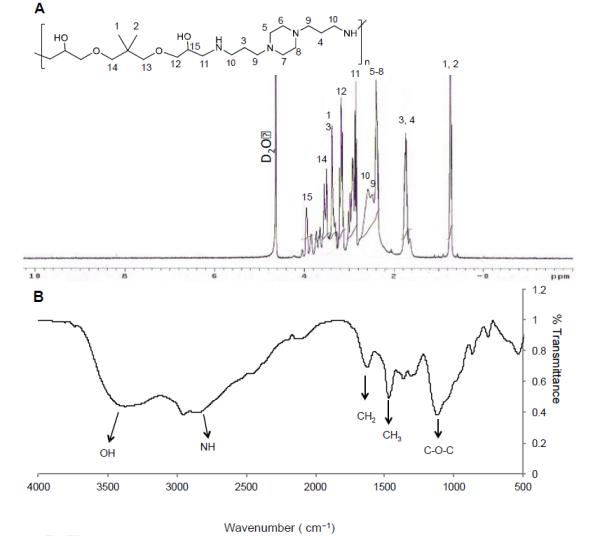
The NPGDE-1,4 Bis polymer. (A) 1H-NMR spectrum. The numbers in the figure represent each proton in the chemical structure of polymer, and the corresponding 1H-NMR peaks were designated accordingly. (B) FT-IR absorption spectrum.
In vitro evaluation of NPGDE-1,4 Bis polymer
Following identification of the NPGDE-1,4 Bis polymer as a lead, we investigated this candidate over a range of adenovirus concentrations using GFP as a reporter gene. This reporter has the advantage that both the number of transduced cells as well as transgene expression within the transduced population can be quantified by flow cytometry. As shown in Figure 4A, the number of GFP positive cells increased over a range of viral concentrations. At an MOI of 20, NPDGE-1,4 Bis increased the number of GFP positive cells nearly 6-fold (from 6% to 34%). In addition to increasing the number of GFP-positive cells, GFP intensity also increased within the GFP-positive population (Figure 4B). Figure 4C shows the total GFP signal, which was calculated by multiplying GFP-positive cells and GFP intensity.
Figure 4.
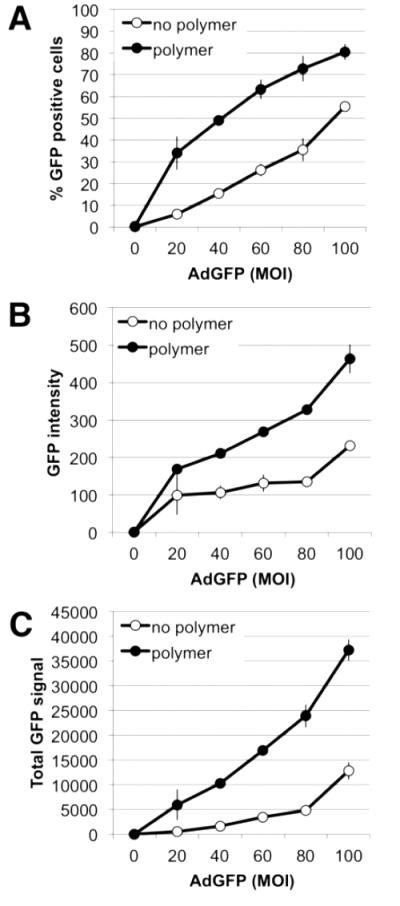
The NPGDE-1,4 Bis polymer enhances adenoviral GFP expression. (A) The number of GFP positive cells, (B) mean GFP fluorescence intensity, and (C) total GFP signal at 48 hrs post-transduction in the absence or presence of 30 μg/ml NPGDE-1,4 Bis at the indicated MOI was determined by flow cytometry. Data shown are the mean ± SD of 2 independently performed experiments. Error bars are included but may be too small to be discernable.
While the GFP reporter has advantages for in vitro analyses, in vivo use of this reporter has the disadvantage that tumor hypoxia and/or tissue processing for subsequent analysis can lead to diminished GFP fluorescence [27]. We therefore evaluated LacZ and luciferase as potential reporter genes for the in vivo studies. Figure 5A demonstrates that in the absence of NPGDE-1,4 Bis, few cells expressed the LacZ reporter gene following transduction with 100 MOI of virus. In the presence of the polymer, a virus-dose dependent increase in both the number of LacZ positive cells as well as the intensity of the staining was observed. However, quantification of the LacZ signal using an assay kit that measures β-galactosidase activity did not seem to accurately reflect the increase we observed in staining intensity at higher virus concentrations (Figure 5B). In contrast, luciferase activity increased in a near linear manner over a range of virus concentrations with high sensitivity (Figure 5C). Thus Ad.Luc was chosen for in vivo studies.
Figure 5.
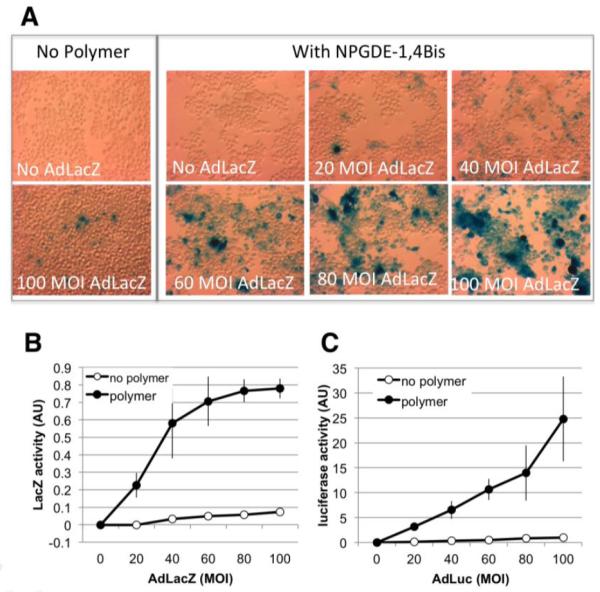
The NPGDE-1,4 Bis polymer enhances adenoviral LacZ and luciferase expression. LacZ expression was determined 48 hrs post-transduction in the absence or presence of 30 μg/ml NPGDE-1,4 Bis at the indicated MOI. (A) Staining of MB49 cells to visualize LacZ expression. A representative experiment is shown. Similar results were obtained in two additional experiments. (B) Quantification of LacZ activity. Data shown are the mean ± SD of 3 independently performed experiments. (C) Luciferase activity was determined 48 hrs post-transduction in the absence or presence of 30 μg/ml NPGDE-1,4 Bis at the indicated MOI. Data were normalized by setting signals obtained at 100 MOI in the absence of the NPGDE-1,4 Bis polymer to 1. Results shown represent the mean ± SD of 4 independently performed experiments.
In vivo evaluation of NPGDE-1,4 Bis-based adenoviral transduction
For in vivo studies, mice were orthotopically implanted with MB49 cells [25]. Eight days after implantation of tumor cells, an adenovirus expressing the luciferase transgene under control of a CMV promoter (Ad.Luc) was delivered either alone or in combination with 400 μg of NPGDE-1,4 Bis. Mice were sacrificed 24 hours after viral delivery, bladders collected, homogenized, and analyzed for luciferase activity. As shown in Figure 6, administration of the virus alone did result in detection of luciferase activity above background levels (p<0,001, PBS vs. AdLuc). However, the presence of NPGDE-1,4 Bis significantly further increased luciferase activity in vivo (p<0.001, compare AdLuc vs. AdLuc plus NPGDE-1,4 Bis). Unfortunately, NPGD resulted in an unexpected adverse reaction during anesthesia. Nearly half of the mice (5/12) receiving AdLuc plus experienced respiratory arrest within 30 minutes of instillation and did not regain consciousness, and the survivors exhibited markedly delayed recovery from anesthesia. Cardiac monitoring during treatment revealed no polymer-related cardiac toxicity, with normal heart rate and rhythm for several minutes beyond respiratory arrest, however attempts at resuscitation were unsuccessful. The polymer preparation tested negative for endotoxin contamination and further purification to remove possible remaining monomers did not alter this outcome. Therefore, no further in vivo experiments were performed with this polymer.
Figure 6.
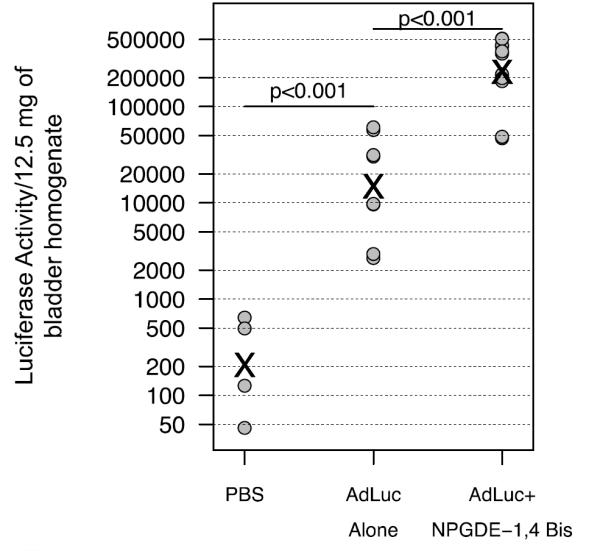
Adenoviral transgene expression in the MB49 orthotopic model in the absence and presence of the NPGDE-1,4 Bis polymer. Luciferase activity in bladder homogenates from mice 24 hours after intravesical delivery of PBS, AdLuc or AdLuc plus NPGDE-1,4 Bis polymer. AdLuc and NPGDE-1,4 Bis were given at was 1 × 109 PFU and 400 μg, respectively. Circles represent data points and “x” indicates the estimated mean. P-values were less than 0.001 in comparisons of PBS vs. AdLuc, PBS vs. AdLuc plus NPGDE-1,4 Bis polymer and AdLuc vs. AdLuc plus NPGDE-1,4 Bis polymer.
Aminoglycoside polyme adenoviral delivery in vitro and in vivo without adverse effects
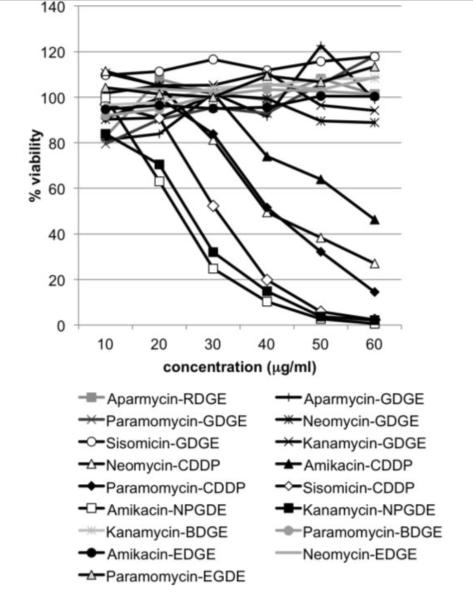
Encouraged by the observation that polymers have the potential to increase adenoviral uptake in vivo, we repeated the screening using an aminoglycoside polymer library. A total of 17 aminoglycoside polymers were analyzed for toxicity using the MTS assay (Figure 7). The highest non-toxic dose was verified by 7-AAD staining (Table 1) and further evaluated for the potential to increase adenoviral infectivity in vitro. Several polymers enhanced adenoviral transgene expression by at least two-fold although viral transduction in the presence of Aparmycin-GDGE was variable and did not reach a two-fold increase in all experiments (Fig. 8A). Interestingly, the two paromomycin (Pa)-based aminoglycosides, which were well tolerated to at least 60 μg/ml, increased infectivity over a range of polymer as well as adenovirus concentrations (Figure 8 B&C). An in vivo pilot experiment with five aminoglycoside-based polymers (Paromomycin-GDGE, Neomycin-CDDE, Amikacin-CDDE, Amikacin-NPGDE and Paromomycin-BDGE) demonstrated that none of these aminoglycoside polymers adversely impacted mice when an 80μl volume (256-400 μg) instilled intravesically. The experiment also provided initial evidence that paromomycin-BDGE is a likely candidate for enhancing adenoviral trans expression in vivo (data not shown). We subsequently expanded the in vivo analysis and confirmed that paromomycin-BDGE increased luciferase activity approximately 4-fold compared to mice receiving AdLuc alone (Figure 9, p<0.005).
Figure 7.
Impact of aminoglycoside-based polymers on viability of MB49 cells. MB49 cells were incubated with the indicated concentration of polymer for 24 hours and viability was determined by MTS assay.
Table 1.
Toxicity of aminoglycoside polymers. Toxicity (% death) was determined by 7-AAD staining at 24 hours. Data are the mean ± SD from at least 3 independent experiments.
| Polymer name | 7AAD assay | Concentration |
|---|---|---|
| No polymer | 0.65 ± 0.33 | NA |
| Aparmycin-RDGE | 2.93 ± 1.83 | 60 mg/ml |
| Aparmycin-GDGE | 1.96 ± 1.49 | 60 mg/ml |
| Paromomycin-GDGE | 3.97 ± 0.74 | 60 mg/ml |
| Neomycin-GDGE | 6.30 ± 0.99 | 40 mg/ml |
| Sisomicin-GDGE | 4.35 ± 0.38 | 60 mg/ml |
| Kanamycin-GDGE | 3.65 ± 2.74 | 30 mg/ml |
| Neomycin-CDDE | 4.19 ± 2.35 | 20 mg/ml |
| Amikacin-CDDE | 7.53 ± 6.41 | 30 mg/ml |
| Paromomycin CDDE | 5.71 ± 2.09 | 20 mg/ml |
| Sisomicin-CDDE | 3.51 ± 1.00 | 20 mg/ml |
| Amikacin-NPGDE | 3.92 ± 3.88 | 10 mg/ml |
| Kanamycin-BDGE | 5.00 ± 1.13 | 60 mg/ml |
| Paromomycin-BDGE | 1.58 ± 0.85 | 60 mg/ml |
| Amikacin-EDGE | 4.03 ± 0.56 | 60 mg/ml |
| Neomycin-EDGE | 6.55 ± 2.21 | 60 mg/ml |
| Paromomycin-EGDE | 1.65 ± 1.06 | 60 mg/ml |
Figure 8.
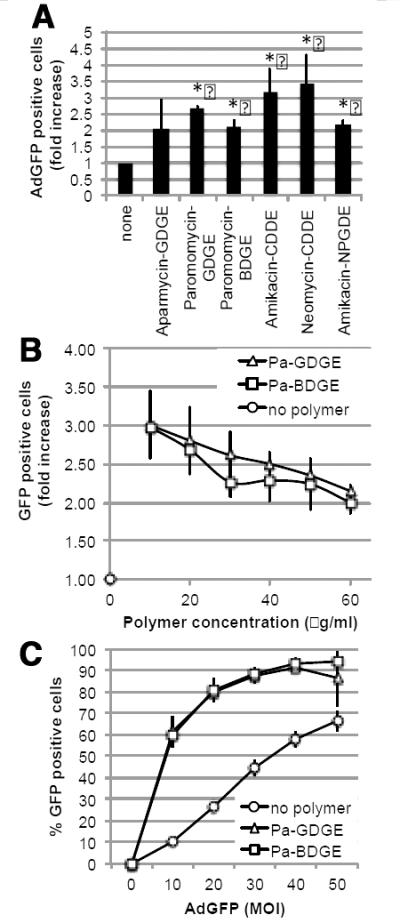
Impact of aminoglycoside-derived polymers, Pa-GDGE and Pa-BDGE, on adenoviral infectivity in vitro. (A) MB49 cells were transduced with 20 MOI in absence or presence of the highest non-toxic dose of polymer: aparmycin-GDGE (60 ug/ml), paromomycin-GDGE (60ug/ml), paromomycin-BDGE (60ug/ml), amikacin (30ug/ml), neomycin-CDDE (20ug/ml) or amicacin-NPGDE (10 ug/ml). Data shown are the mean ± SD from three independently performed experiments. (B) MB49 cells were infected with 20 MOI AdGFP in the absence or presence of increasing concentrations of paromomycin-GDGE or -BDGE. Data shown are the mean ± SD from two independently performed experiments. (C) MB49 cells were transduced with increasing concentrations of AdGFP in the absence or presence of 30 μg/ml paromomycin-GDGE or -BDGE. At 48 hours post-infection, the number of GFP positive cells was determined by flow cytometry. Data shown are the mean ± SD from three independently performed experiments. GFP expression was quantified by flow cytometry at 48 hours.
Figure 9.
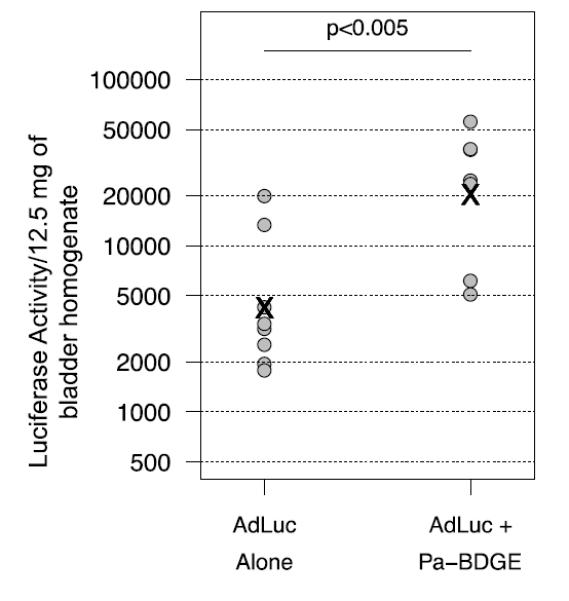
Adenoviral transgene expression in the MB49 orthotopic model in the absence and presence of Pa-BDGE. AdLuc and Pa-BDGE were given at 5 × 108 PFU and 256 μg, respectively. Circles represent data points and “x” indicates the estimated mean, p < 0.005.
Characterization of Paromomycin-BDGE polymer
The paromomycin-BDGE (Pa-BDGE) polymer was characterized using gel permeation chromatography (GPC), and 1H-NMR / FT-IR spectroscopy. Figure 10A shows 1H-NMR of the polymer in D2O. The numbers in the Figure 10A represent each proton in the chemical the chemical structure of polymer, and the corresponding 1H-NMR peaks were designated accordingly. The shift in the position of the (-O-CH-) proton following opening of the epoxide ring can be seen at 3.9 ppm. Peaks corresponding to hexose and pentose (from paromomycin) can be found between 3.4 - 3.8 ppm. The FT-IR spectrum of polymer paromomycin-BDGE is shown in Figure 10B. A broad absorption band in the wavenumber range of 3000-3685 cm-1 indicates presence of large number of hydroxyl groups.. Secondary amine stretchings can be seen at 2880 cm-1. A strong absorption band at 1650 cm-1 corresponding to CH2 groups of BDGE and stretchings corresponds to a hexose nucleus (from paromomycin) can be found at 1068 cm-1. A peak at 1269 cm-1 confirms the presence of ether links in the polymer. The molecular weight (Mw) of the Pa-BDGE polymer was 4.2 kDa, as determined from GPC and polydispersity was 1.4.
Figure 10.
Paromomycin-BDGE. (A) 1H-NMR spectrum of the Paromomycin-BDGE (Pa-BDGE) polymer in D2O and (B) FT-IR absorption spectrum of the Paromomycin-BDGE polymer.
DISCUSSION
Our goal was to investigate the potential of polymers to enhance adenoviral transduction in an orthotopic in vivo model of bladder cancer. MB49 bladder cancer cells are derived from C57BL6 mice and efficiently grow in vivo following intravesical instillation [25]. These cells are relatively resistant to adenoviral infection (Figure 1), and therefore made an excellent model to evaluate the impact of polymers on adenoviral transduction. Screening of a linear polyamine-based polymer library for toxicity and the ability to enhance adenoviral transduction in vitro, identified NPGDE-1,4 Bis as a lead candidate (Table 1, Figure 2) that significantly enhanced gene expression over a range of polymer and virus concentrations (Figures 2 and 4). NPGDE-1,4 Bis enhanced gene expression using three different reporter genes, namely GFP, β-galactosidase, and luciferase (Figure 4 and 5) and based on sensitivity and linearity of the assay, the luciferase transgene was selected for in vivo studies. In the absence of polymers, in vivo delivery of Ad.Luc did result in a significant increase in transgene expression compared to background (p<0.001, Figure 7). These results are consistent with other studies evaluating gene therapy of bladder cancer, although transgene expression without an enhancing agent is typically insufficient for effective gene therapy [28, 29]. Our results show that combining AdLuc with the NPGDE-1,4 Bis polymer prior to bladder instillation significantly further increased transgene expression (p<0.001, Figure 7). Unfortunately, this combination also caused significant in vivo toxicity resulting in respiratory arrest within minutes of delivery in nearly half the animals. Although the mechanism underlying the toxicity remains obscure, toxicity appears to be related to the structure of the polymer.
We next screened an aminoglycoside-based polymer library in an effort to discover a candidate with a better in vivo toxicity profile. While only four of 12 (33%) of NPGDE-based polymers were non-toxic in vitro, aminoglycoside-based polymers were generally better tolerated (11 of 19 polymers (58%) being non-toxic to MB49 cells) and were also tolerated better at higher concentrations than polyamine polymers (Table 3, Figure 8,). Interestingly, four of the more toxic aminoglycoside-based polymers contained CDDE while two contained NPGDE (Figure 7). None of the aminoglycosides conjugated to EGDE (3 polymers), BDGE (2 polymers), and GDGE (5 polymers) displayed toxicity towards MB49 cells. These observations suggest that polymer toxicity may be independent of the type of aminoglycoside monomer, but might depend on the type of diglycidyl ether used as cross linkers in the polymerization reactions.
Despite toxicity at higher concentrations, two CDDE conjugates enhanced adenoviral transgene expression in vitro at lower concentrations (<20 μM). Amikacin-NPGDE also enhanced adenoviral transduction in vitro, but was tolerated only up to 10 μM. Two candidates, Pa-GDGE and Pa-BDGE, both derived from paromomycin (Pa), significantly enhanced GFP transgene expression consistently and over a range of polymer and adenovirus concentrations (Table 3 and Figure 8). Interestingly, only Pa-BDGE (Figure 9) but not Pa-GDGE was effective at enhancing adenoviral transgene expression in vivo, suggesting that properties unique to the BDGE component of the conjugates may be important for enhancing adenoviral gene delivery within the bladder microenvironment. There are a number of variables, such as differences in branching or number of linkages or number hydroxyl groups that could contribute to the unique properties of Pa-BDGE.
There are multiple mechanisms by which cationic polymers are known to act to increase gene delivery by Ad5 vectors like the one used in this study. These include charge shielding, promotion of virus aggregation, and shielding from the immune system [15, 30, 31]. Shielding from the immune system is an unlikely explanation for the results reported here, since enhanced gene delivery was detected in vitro as well as in vivo, and virus-polymer mixtures were applied topically in the lumen of the bladder rather than systemically. Virus aggregation enhances gene delivery by decreasing diffusion and increasing the rate at which virus particles settle on and adsorb to cell surfaces [11, 32]. However, this effect is dependent on polymer mass and has previously been shown to require polymers 15 kDa in size or greater [11] far exceeding the masses of the polymers found to be effective for gene delivery in our system. Charge shielding is therefore the most likely explanation for the effectiveness of Pa-BDGE and Pa-GDGE in vitro. Ad5 capsids are highly negatively charged, resulting in electrostatic repulsion between virus particles and cell surfaces [33]. When infecting Coxsackie and Adenovirus Receptor (CAR)-expressing cells, the virus overcomes this repulsive force by positioning of its CAR-binding protein on a long fiber that holds it away from the negatively charged capsid surface [30]. When CAR is absent, secondary interactions between the virus and cell membrane components can result in virus uptake and infection if the negative charges are masked by cationic macromolecules [15, 30, 34, 35]. Pa-BDGE and Pa-GDGE in vivo suggests that the bladder environment, which has a reduced buffering capacity compared to tissue culture, alters the charge of Pa-GDGE such that it can no longer masks the anionic residues on the Ad5 surface. Investigation of the effect of urine on polymer candidates will be necessary if candidates are recommended for bladder cancer clinical trials.
Recent studies indicate that gene therapy of bladder cancer is a viable and safe strategy. A promising therapeutic gene is CD40 ligand, which increases the Th1 anti-tumor response. In the MB49 model, therapy with AdCD40L increased survival of the animals, cleared tumors and metastasis in part of the study cohort, and resulted in long-lived anti-tumor memory [36]. In a subsequent Phase I/IIa clinical trial AdCD40L did not cause adverse events when instilled into the bladder and was shown to boost the immune response [37]. Since the glycosamine glycan (GAG) layer, a physical barrier for protection of the underlying bladder epithelium, provides another obstacle for gene transfer within the bladder environment [38], both the preclinical and clinical studies employed chorpactin pretreatment to disrupt the GAG layer for maximized gene transfer. We have shown that polymers alone are sufficient to enhance adenoviral gene expression. Thus it will be interesting to determine how disruption the physical barrier in combination with polymer-mediated gene delivery will impact adenoviral gene expression in the bladder. To our knowledge, this study is the first to investigate the use of cationic polymers for adenoviral gene delivery in an orthotopic model of bladder cancer and provides the foundation for future in vivo studies to determine therapeutic benefits of polymer-adenovirus combinations in bladder cancer gene therapy.
CONCLUSIONS
Our study demonstrates the feasibility of using cationic polymers particularly those derived from aminoglycosides, for enhancing adenoviral transgene expression in an orthotopic model of bladder cancer. It also indicates that in vivo toxicity can be difficult to predict from in vitro studies and highlights the need for novel approaches or predictive tools that can be applied to complex systems.
Supplementary Material
ACKNOWLEDGMENTS
This project was supported by grants from the National Institutes of Health (grant R21 CA143505 from the National Cancer Institute and grant R01GM093229 from the National Institute of General Medical Sciences to CVJ and KR). The work was also supported in part by institutional grants to MUSC and the Hollings Cancer Center from the National Center for Research Resources and the Office of the Direction of National Institute of Health (C06 RR015455) and the National Cancer Institute (P30 CA138313), respectively. The authors would like to thank D’Angelo Dinkins for technical assistance with the toxicity assays and Dr. Rupak Mukherjee, director of the Multidisciplinary Murine Physiology Core in the Division of Cardiothoracic Surgery Research, for assistance with analysis of heart function in NPGDE1,4 Bis treated mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Vol. 62. a cancer journal for clinicians; CA: 2012. 2012. Cancer treatment and survivorship statistics; pp. 220–241. [DOI] [PubMed] [Google Scholar]
- 2.Avritscher EB, Cooksley CD, Grossman HB, Sabichi AL, Hamblin L, Dinney CP, Elting LS. Clinical model of lifetime cost of treating bladder cancer and associated complications. Urology. 2006;68:549–553. doi: 10.1016/j.urology.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 3.Burke J. Virus therapy for bladder cancer. Cytokine & growth factor reviews. 2010;21:99–102. doi: 10.1016/j.cytogfr.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Buscarini M, Quek ML, Gilliam-Hegarich S, Kasahara N, Bochner B. Adenoviral receptor expression of normal bladder and transitional cell carcinoma of the bladder. Urologia internationalis. 2007;78:160–166. doi: 10.1159/000098076. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto K, Shariat SF, Ayala GE, Rauen KA, Lerner SP. Loss of coxsackie and adenovirus receptor expression is associated with features of aggressive bladder cancer. Urology. 2005;66:441–446. doi: 10.1016/j.urology.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 6.Barua S, Ramos J, Potta T, Taylor D, Huang HC, Montanez G, Rege K. Discovery of cationic polymers for non-viral gene delivery using combinatorial approaches. Combinatorial chemistry & high throughput screening. 2011;14:908–924. doi: 10.2174/138620711797537076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kasman LM, Barua S, Lu P, Rege K, Voelkel-Johnson C. Polymer-enhanced adenoviral transduction of CAR-negative bladder cancer cells. Molecular pharmaceutics. 2009;6:1612–1619. doi: 10.1021/mp9000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landazuri N, Krishna D, Gupta M, Le Doux JM. Retrovirus-polymer complexes: study of the factors affecting the dose response of transduction. Biotechnology progress. 2007;23:480–487. doi: 10.1021/bp060336y. [DOI] [PubMed] [Google Scholar]
- 9.McMillin DW, Landazuri N, Gangadharan B, Hewes B, Archer DR, Spencer HT, Le Doux JM. Highly efficient transduction of repopulating bone marrow cells using rapidly concentrated polymer-complexed retrovirus. Biochemical and biophysical research communications. 2005;330:768–775. doi: 10.1016/j.bbrc.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 10.Landazuri N, Le Doux JM. Complexation of retroviruses with charged polymers enhances gene transfer by increasing the rate that viruses are delivered to cells. The journal of gene medicine. 2004;6:1304–1319. doi: 10.1002/jgm.618. [DOI] [PubMed] [Google Scholar]
- 11.Davis HE, Rosinski M, Morgan JR, Yarmush ML. Charged polymers modulate retrovirus transduction via membrane charge neutralization and virus aggregation. Biophysical journal. 2004;86:1234–1242. doi: 10.1016/S0006-3495(04)74197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Doux JM, Morgan JR, Yarmush ML. Differential inhibition of retrovirus transduction by proteoglycans and free glycosaminoglycans. Biotechnology progress. 1999;15:397–406. doi: 10.1021/bp990049c. [DOI] [PubMed] [Google Scholar]
- 13.Kim P-H, Kim T.-i., Yockman JW, Kim SW, Yun C-O. The effect of surface modification of adenovirus with an arginine-grafted bioreducible polymer on transduction efficiency and immunogenicity in cancer gene therapy. Biomaterials. 2010;31:1865–1874. doi: 10.1016/j.biomaterials.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 14.Jang J-H, Schaffer DV, Shea LD. Engineering Biomaterial Systems to Enhance Viral Vector Gene Delivery. Mol Ther. 2011;19:1407–1415. doi: 10.1038/mt.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vetter A, Virdi KS, Espenlaub S, Rodl W, Wagner E, Holm PS, Scheu C, Kreppel F, Spitzweg C, Ogris M. Adenoviral vectors coated with PAMAM dendrimer conjugates allow CAR independent virus uptake and targeting to the EGF receptor. Molecular pharmaceutics. 2013;10:606–618. doi: 10.1021/mp300366f. [DOI] [PubMed] [Google Scholar]
- 16.De Smedt SC, Demeester J, Hennink WE. Cationic polymer based gene delivery systems. Pharmaceutical research. 2000;17:113–126. doi: 10.1023/a:1007548826495. [DOI] [PubMed] [Google Scholar]
- 17.Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Advanced drug delivery reviews. 2006;58:467–486. doi: 10.1016/j.addr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Morille M, Passirani C, Vonarbourg A, Clavreul A, Benoit JP. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials. 2008;29:3477–3496. doi: 10.1016/j.biomaterials.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 19.Guo X, Huang L. Recent advances in nonviral vectors for gene delivery. Accounts of chemical research. 2012;45:971–979. doi: 10.1021/ar200151m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howarth JL, Lee YB, Uney JB. Using viral vectors as gene transfer tools (Cell Biology and Toxicology Special Issue: ETCS-UK 1 day meeting on genetic manipulation of cells) Cell biology and toxicology. 2010;26:1–20. doi: 10.1007/s10565-009-9139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vu L, Ramos J, Potta T, Rege K. Generation of a focused poly(amino ether) library: polymer-mediated transgene delivery and gold-nanorod based theranostic systems. Theranostics. 2012;2:1160–1173. doi: 10.7150/thno.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barua S, Joshi A, Banerjee A, Matthews D, Sharfstein ST, Cramer SM, Kane RS, Rege K. Parallel synthesis and screening of polymers for nonviral gene delivery. Molecular pharmaceutics. 2009;6:86–97. doi: 10.1021/mp800151j. [DOI] [PubMed] [Google Scholar]
- 23.Michael K, Wang H, Tor Y. Enhanced RNA binding of dimerized aminoglycosides. Bioorganic & Medicinal Chemistry. 1999;7:1361–1371. doi: 10.1016/s0968-0896(99)00071-1. [DOI] [PubMed] [Google Scholar]
- 24.Kelly MM, Hoel BD, Voelkel-Johnson C. Doxorubicin pretreatment sensitizes prostate cancer cell lines to TRAIL induced apoptosis which correlates with the loss of c-FLIP expression. Cancer biology & therapy. 2002;1:520–527. doi: 10.4161/cbt.1.5.169. [DOI] [PubMed] [Google Scholar]
- 25.Kasman L, Voelkel-Johnson C. An Orthotopic Bladder Cancer Model for Gene Delivery Studies Journal of visualized experiments : JoVE. 2013 doi: 10.3791/50181. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sudarshan S, Holman DH, Hyer ML, Voelkel-Johnson C, Dong JY, Norris JS. in vitro efficacy of Fas ligand gene therapy for the treatment of bladder cancer. Cancer gene therapy. 2005;12:12–18. doi: 10.1038/sj.cgt.7700746. [DOI] [PubMed] [Google Scholar]
- 27.Coralli C, Cemazar M, Kanthou C, Tozer GM, Dachs GU. Limitations of the reporter green fluorescent protein under simulated tumor conditions. Cancer research. 2001;61:4784–4790. [PubMed] [Google Scholar]
- 28.Siemens DR, Austin JC, See WA, Tartaglia J, Ratliff TL. Evaluation of gene transfer efficiency by viral vectors to murine bladder epithelium. The Journal of urology. 2001;165:667–671. doi: 10.1097/00005392-200102000-00091. [DOI] [PubMed] [Google Scholar]
- 29.Ramesh N, Memarzadeh B, Ge Y, Frey D, VanRoey M, Rojas V, Yu DC. Identification of pretreatment agents to enhance adenovirus infection of bladder epithelium. Molecular therapy : the journal of the American Society of Gene Therapy. 2004;10:697–705. doi: 10.1016/j.ymthe.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Shayakhmetov DM, Lieber A. Dependence of adenovirus infectivity on length of the fiber shaft domain. Journal of virology. 2000;74:10274–10286. doi: 10.1128/jvi.74.22.10274-10286.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang JH, Schaffer DV, Shea LD. Engineering biomaterial systems to enhance viral vector gene delivery. Molecular therapy : the journal of the American Society of Gene Therapy. 2011;19:1407–1415. doi: 10.1038/mt.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo D, Saltzman WM. Enhancement of transfection by physical concentration of DNA at the cell surface. Nature biotechnology. 2000;18:893–895. doi: 10.1038/78523. [DOI] [PubMed] [Google Scholar]
- 33.Karlin S, Brendel V. Charge configurations in viral proteins. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:9396–9400. doi: 10.1073/pnas.85.24.9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roelvink PW, Kovesdi I, Wickham TJ. Comparative analysis of adenovirus fiber-cell interaction: adenovirus type 2 (Ad2) and Ad9 utilize the same cellular fiber receptor but use different binding strategies for attachment. Journal of virology. 1996;70:7614–7621. doi: 10.1128/jvi.70.11.7614-7621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnberg N, Edlund K, Kidd AH, Wadell G. Adenovirus type 37 uses sialic acid as a cellular receptor. Journal of virology. 2000;74:42–48. [PMC free article] [PubMed] [Google Scholar]
- 36.Lindqvist C, Sandin LC, Fransson M, Loskog A. Local AdCD40L gene therapy is effective for disseminated murine experimental cancer by breaking T-cell tolerance and inducing tumor cell growth inhibition. Journal of immunotherapy. 2009;32:785–792. doi: 10.1097/CJI.0b013e3181acea69. [DOI] [PubMed] [Google Scholar]
- 37.Malmstrom PU, Loskog AS, Lindqvist CA, Mangsbo SM, Fransson M, Wanders A, Gardmark T, Totterman TH. AdCD40L immunogene therapy for bladder carcinoma--the first phase I/IIa trial. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:3279–3287. doi: 10.1158/1078-0432.CCR-10-0385. [DOI] [PubMed] [Google Scholar]
- 38.Chester JD, Kennedy W, Hall GD, Selby PJ, Knowles MA. Adenovirus-mediated gene therapy for bladder cancer: efficient gene delivery to normal and malignant human urothelial cells in vitro and ex vivo. Gene therapy. 2003;10:172–179. doi: 10.1038/sj.gt.3301851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.