Abstract
We report an interesting case involving an 81-year-old male patient who presented to our hospital with sepsis secondary to pyelonephritis and bacteremia. Blood cultures were positive for a Gram-negative bacillus, Burkholderia pseudomallei. His risk factors were diabetes, chronic obstructive pulmonary disease including travel to an endemic area. The patient received antibiotic therapy in accordance with the national guidelines. This is the first report of reactivation of latent melioidosis in the literature manifesting as pyelonephritis and bacteremia.
Keywords: Bacteremia, latent melioidosis, pyelonephritis
INTRODUCTION
Melioidosis or Whitmore disease is an anthropozoonosis caused by Burkholderia pseudomallei.[1] It is endemic mainly in Southeast Asia and northern Australia. Recently, it has been identified as a potential bioterrorism agent classified as a Category B agent by the United States Centers for Disease Control and Prevention (US CDC) and as such should be immediately reported to public health authorities by the physician.[2] The various clinical manifestations of this rare infection include acute, subacute, or chronic forms. Risk factors include diabetes, chronic lung disease and alcohol abuse. It can exist as a latent, active, or reactivated infection. The most frequent form is acute pulmonary infection and the most severe form is acute septicemic infection, with a high mortality rate, ranging from 40% in the treated cases to 90% without treatment.[3] Different organs may be involved, such as the lungs, liver, spleen, skin, brain, bones, lymph nodes, eyes and adrenal glands.[2,3] Genitourinary infection is very rare. Melioidosis occurs only sporadically in travelers returning from disease-endemic areas. We report the first case report of reactivation of latent melioidosis presenting with acute pyelonephritis and bacteremia.
CASE REPORT
The present case report is about an 81-year-old Indian male patient who presented to the emergency room with the complaining of worsening fevers, chills, dysuria and decreased appetite over a 3-week period. His medical history included diabetes mellitus, chronic obstructive pulmonary disease, hypertension, benign prostatic hyperplasia and dyslipidemia. His surgical history was unremarkable and he was taking atenolol and lovastatin as an out-patient. He reported that he had been to the emergency room 1 month ago for the treatment and management of lower gastrointestinal bleed secondary to colitis. During that admission, he developed a urinary tract infection for which he was treated with oral levofloxacin as an out-patient. He denied any history of smoking. He also denied any illicit drug or alcohol use. The patient emigrated from India to the United States of America (USA) over 40 years ago. The last time he travelled out of the USA was to India 2 years ago. His symptoms began approximately 3 weeks prior. On admission, he was febrile, with a temperature of 102°F. He was hemodynamically stable, but in obvious discomfort complaining of weakness and dizziness. On physical examination, he had suprapubic tenderness and right cost overtebral/flank tenderness, but no other physical abnormalities. His prostate exam was unremarkable. Initial laboratory work-up revealed an elevated leukocyte count of 13 × 103 with a left shift. His urine analysis revealed 10-15 red blood cells per high power field, 50 leukocytes per high-power field, positive bacteria with positive nitrates and leukocyte esterase and few hyaline casts. A chest X-ray was unremarkable, but a computed tomography scan of the abdomen and pelvis [Figure 1] revealed wedge-shaped enhancement defects in the right kidney suggestive of pyelonephritis. We drew blood and urine cultures which both grew non-lactose fermenting, aerobic and motile Gram-negative rods which were identified to be B. pseudomallei. The patient was treated with a 2-week course of imipenem/cilastatin, followed by oral trimethoprim-sulfamethoxazole for eradication therapy for 4 months. As the result of the treatment regimen, the patient clinically improved and he was asymptomatic at the 4 months follow-up.
Figure 1.
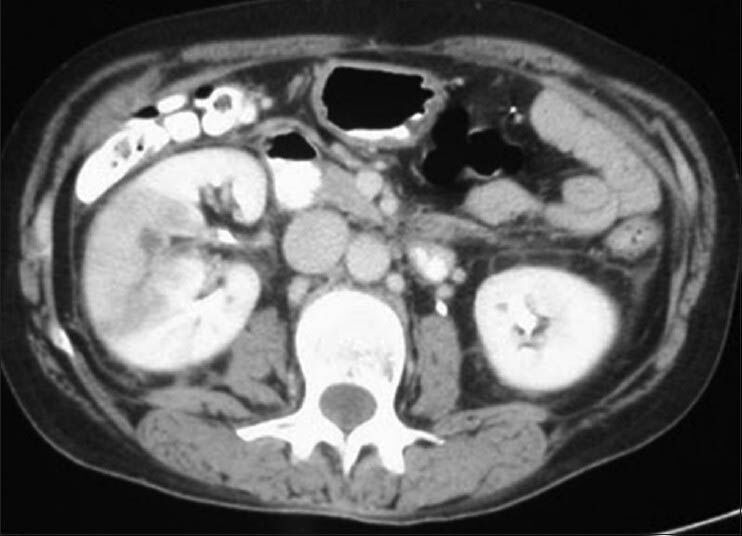
Computed tomography scan of the abdomen and pelvis revealed wedge-shaped enhancement defects in the right kidney suggestive of pyelonephritis
DISCUSSION
Our patient most likely acquired the infection when he was living in India over 40 years ago or from his recent travel there 2 years ago, since the USA is not among the countries where autochthonous cases of melioidosis have been reported.[3] Risk factors for melioidosis include diabetes mellitus, immunosuppression, kidney disease, thalassemia and occupational exposure to contaminated soil or water, none of which our patient had.[4] Latent infection with reactivation is reported in <3% of cases, but can occur as long as 19-29 years after the initial presentation.[5] B. pseudomallei appears to have the ability to demonstrate quiescence in infected individuals. As a result such people remain at risk of recrudescence of disease many years after leaving an endemic area.
This report illustrates the first case of reactivation of latent melioidosis resulting in pyelonephritis and septicemia in a US resident. Reactivation of latent disease, as with tuberculosis, refers to situations in which previously asymptomatic, presumably seropositive individuals later develop active disease at an indeterminate time after initial infection. It is important to also note that reactivation disease can be difficult to distinguish from disease that is characterized by an especially long incubation period of several years duration between exposure and illness onset.[3,4,5]
The overall mortality rate for melioidosis is about 14%, with the mortality from septic shock as high as 86%.[6] The acute phase of therapy for melioidosis involves at least 14 days of monotherapy with either ceftazidime, meropenem, or imipenem. Doxycycline with or without trimethoprim-sulfamethoxazole is the mainstay of eradication phase therapy, which lasts at least 3 months.[7] Despite serious illness with systematic involvement, our patient responded fairly quickly to the antibiotic regimen which is considered optimal for this organism.
CONCLUSION
Based on the above case report it can be concluded that reactivation of latent melioidosis is a serious opportunistic infection with a high mortality rate in immunocompromised patients. In today's age of international travel, physicians need to recognize melioidosis, especially in travelers who return from an endemic region.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Leelarasamee A, Bovornkitti S. Melioidosis: Review and update. Rev Infect Dis. 1989;11:413–25. doi: 10.1093/clinids/11.3.413. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Emergency preparedness and response, bioterrorism agents/diseases. [Last accessed on 2007 Apr 15]. Available from: http://www.bt.cdc.gov/agent/agentlist-category.asp .
- 3.Josephson J. Melioidosis: An emerging tropical health problem. Environ Sci Technol. 2001;35:454A–7. doi: 10.1021/es0125265. [DOI] [PubMed] [Google Scholar]
- 4.Suputtamongkol Y, Chaowagul W, Chetchotisakd P, Lertpatanasuwun N, Intaranongpai S, Ruchutrakool T, et al. Risk factors for melioidosis and bacteremic melioidosis. Clin Infect Dis. 1999;29:408–13. doi: 10.1086/520223. [DOI] [PubMed] [Google Scholar]
- 5.Currie BJ, Fisher DA, Anstey NM, Jacups SP. Melioidosis: Acute and chronic disease, relapse and re-activation. Trans R Soc Trop Med Hyg. 2000;94:301–4. doi: 10.1016/s0035-9203(00)90333-x. [DOI] [PubMed] [Google Scholar]
- 6.Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis. 2010;4:e900. doi: 10.1371/journal.pntd.0000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dance DA. Melioidosis. Curr Opin Infect Dis. 2002;15:127–32. doi: 10.1097/00001432-200204000-00005. [DOI] [PubMed] [Google Scholar]


