Abstract
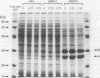
The 4.6-kb region 5'-upstream from the gene encoding a cobalt-containing and amide-induced high molecular mass-nitrile hydratase (H-NHase) from Rhodococcus rhodochrous J1 was found to be required for the expression of the H-NHase gene with a host-vector system in a Rhodococcus strain. Sequence analysis has revealed that there are at least five open reading frames (H-ORF1 approximately 5) in addition to H-NHase alpha- and beta-subunit genes. Deletion of H-ORF1 and H-ORF2 resulted in decrease of NHase activity, suggesting a positive regulatory role of both ORFs in the expression of the H-NHase gene. H-ORF1 showed significant similarity to a regulatory protein, AmiC, which is involved in regulation of amidase expression by binding an inducer amide in Pseudomonas aeruginosa. H-ORF4, which has been found to be uninvolved in regulation of H-NHase expression by enzyme assay for its deletion transformant and Northern blot analysis for R. rhodochrous J1, showed high similarity to transposases from insertion sequences of several bacteria. Determination of H-NHase activity and H-NHase mRNA levels in R. rhodochrous J1 has indicated that the expression of the H-NHase gene is regulated by an amide at the transcriptional level. These findings suggest the participation of H-ORF4 (IS1164) in the organization of the H-NHase gene cluster and the involvement of H-ORF1 in unusual induction mechanism, in which H-NHase is formed by amides (the products in the NHase reaction), but not by nitriles (the substrates).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartel B., Fink G. R. Differential regulation of an auxin-producing nitrilase gene family in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6649–6653. doi: 10.1073/pnas.91.14.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartling D., Seedorf M., Mithöfer A., Weiler E. W. Cloning and expression of an Arabidopsis nitrilase which can convert indole-3-acetonitrile to the plant hormone, indole-3-acetic acid. Eur J Biochem. 1992 Apr 1;205(1):417–424. doi: 10.1111/j.1432-1033.1992.tb16795.x. [DOI] [PubMed] [Google Scholar]
- Bartling D., Seedorf M., Schmidt R. C., Weiler E. W. Molecular characterization of two cloned nitrilases from Arabidopsis thaliana: key enzymes in biosynthesis of the plant hormone indole-3-acetic acid. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6021–6025. doi: 10.1073/pnas.91.13.6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M. E., Rouch D. A., Skurray R. A. Nucleotide sequence analysis of IS256 from the Staphylococcus aureus gentamicin-tobramycin-kanamycin-resistance transposon Tn4001. Gene. 1989 Sep 30;81(2):361–367. doi: 10.1016/0378-1119(89)90197-2. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cohen S. P., Hächler H., Levy S. B. Genetic and functional analysis of the multiple antibiotic resistance (mar) locus in Escherichia coli. J Bacteriol. 1993 Mar;175(5):1484–1492. doi: 10.1128/jb.175.5.1484-1492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D. M., Stephens D. M. Identification of an insertion sequence, IS1081, in Mycobacterium bovis. FEMS Microbiol Lett. 1991 Sep 15;67(1):11–15. doi: 10.1016/0378-1097(91)90435-d. [DOI] [PubMed] [Google Scholar]
- Crespi M., Vereecke D., Temmerman W., Van Montagu M., Desomer J. The fas operon of Rhodococcus fascians encodes new genes required for efficient fasciation of host plants. J Bacteriol. 1994 May;176(9):2492–2501. doi: 10.1128/jb.176.9.2492-2501.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran R., Nishiyama M., Horinouchi S., Beppu T. Characterization of nitrile hydratase genes cloned by DNA screening from Rhodococcus erythropolis. Biosci Biotechnol Biochem. 1993 Aug;57(8):1323–1328. doi: 10.1271/bbb.57.1323. [DOI] [PubMed] [Google Scholar]
- Finnerty W. R. The biology and genetics of the genus Rhodococcus. Annu Rev Microbiol. 1992;46:193–218. doi: 10.1146/annurev.mi.46.100192.001205. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Nishiyama M., Horinouchi S., Beppu T. Nitrile hydratase gene from Rhodococcus sp. N-774 requirement for its downstream region for efficient expression. Biosci Biotechnol Biochem. 1994 Oct;58(10):1859–1865. doi: 10.1271/bbb.58.1859. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Nishiyama M., Yu F., Watanabe I., Horinouchi S., Beppu T. Development of a host-vector system in a Rhodococcus strain and its use for expression of the cloned nitrile hydratase gene cluster. J Gen Microbiol. 1992 May;138(5):1003–1010. doi: 10.1099/00221287-138-5-1003. [DOI] [PubMed] [Google Scholar]
- Ikehata O., Nishiyama M., Horinouchi S., Beppu T. Primary structure of nitrile hydratase deduced from the nucleotide sequence of a Rhodococcus species and its expression in Escherichia coli. Eur J Biochem. 1989 May 15;181(3):563–570. doi: 10.1111/j.1432-1033.1989.tb14761.x. [DOI] [PubMed] [Google Scholar]
- KELLY M., CLARKE P. H. An inducible amidase produced by a strain of Pseudomonas aeruginosa. J Gen Microbiol. 1962 Feb;27:305–316. doi: 10.1099/00221287-27-2-305. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Izui H., Nagasawa T., Yamada H. Nitrilase in biosynthesis of the plant hormone indole-3-acetic acid from indole-3-acetonitrile: cloning of the Alcaligenes gene and site-directed mutagenesis of cysteine residues. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):247–251. doi: 10.1073/pnas.90.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Komeda H., Nagasawa T., Nishiyama M., Horinouchi S., Beppu T., Yamada H., Shimizu S. Amidase coupled with low-molecular-mass nitrile hydratase from Rhodococcus rhodochrous J1. Sequencing and expression of the gene and purification and characterization of the gene product. Eur J Biochem. 1993 Oct 1;217(1):327–336. doi: 10.1111/j.1432-1033.1993.tb18250.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Nagasawa T., Yamada H. Enzymatic synthesis of acrylamide: a success story not yet over. Trends Biotechnol. 1992 Nov;10(11):402–408. doi: 10.1016/0167-7799(92)90283-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Nishiyama M., Nagasawa T., Horinouchi S., Beppu T., Yamada H. Cloning, nucleotide sequence and expression in Escherichia coli of two cobalt-containing nitrile hydratase genes from Rhodococcus rhodochrous J1. Biochim Biophys Acta. 1991 Dec 2;1129(1):23–33. doi: 10.1016/0167-4781(91)90208-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Suzuki T., Fujita T., Masuda M., Shimizu S. Occurrence of enzymes involved in biosynthesis of indole-3-acetic acid from indole-3-acetonitrile in plant-associated bacteria, Agrobacterium and Rhizobium. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):714–718. doi: 10.1073/pnas.92.3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayaux J. F., Cerbelaud E., Soubrier F., Yeh P., Blanche F., Pétré D. Purification, cloning, and primary structure of a new enantiomer-selective amidase from a Rhodococcus strain: structural evidence for a conserved genetic coupling with nitrile hydratase. J Bacteriol. 1991 Nov;173(21):6694–6704. doi: 10.1128/jb.173.21.6694-6704.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayaux J. F., Cerebelaud E., Soubrier F., Faucher D., Pétré D. Purification, cloning, and primary structure of an enantiomer-selective amidase from Brevibacterium sp. strain R312: structural evidence for genetic coupling with nitrile hydratase. J Bacteriol. 1990 Dec;172(12):6764–6773. doi: 10.1128/jb.172.12.6764-6773.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T., Takeuchi K., Yamada H. Characterization of a new cobalt-containing nitrile hydratase purified from urea-induced cells of Rhodococcus rhodochrous J1. Eur J Biochem. 1991 Mar 28;196(3):581–589. doi: 10.1111/j.1432-1033.1991.tb15853.x. [DOI] [PubMed] [Google Scholar]
- Nagasawa T., Takeuchi K., Yamada H. Occurrence of a cobalt-induced and cobalt-containing nitrile hydratase in Rhodococcus rhodochrous J1. Biochem Biophys Res Commun. 1988 Sep 15;155(2):1008–1016. doi: 10.1016/s0006-291x(88)80597-7. [DOI] [PubMed] [Google Scholar]
- Nishiyama M., Horinouchi S., Kobayashi M., Nagasawa T., Yamada H., Beppu T. Cloning and characterization of genes responsible for metabolism of nitrile compounds from Pseudomonas chlororaphis B23. J Bacteriol. 1991 Apr;173(8):2465–2472. doi: 10.1128/jb.173.8.2465-2472.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper D. I., Fawcett T., Cooper R. A. The Escherichia coli C homoprotocatechuate degradative operon: hpc gene order, direction of transcription and control of expression. Mol Gen Genet. 1993 Feb;237(1-2):241–250. doi: 10.1007/BF00282806. [DOI] [PubMed] [Google Scholar]
- Wheatcroft R., Laberge S. Identification and nucleotide sequence of Rhizobium meliloti insertion sequence ISRm3: similarity between the putative transposase encoded by ISRm3 and those encoded by Staphylococcus aureus IS256 and Thiobacillus ferrooxidans IST2. J Bacteriol. 1991 Apr;173(8):2530–2538. doi: 10.1128/jb.173.8.2530-2538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. A., Wachira S. J., Drew R. E., Jones D., Pearl L. H. Antitermination of amidase expression in Pseudomonas aeruginosa is controlled by a novel cytoplasmic amide-binding protein. EMBO J. 1993 Sep;12(9):3637–3642. doi: 10.1002/j.1460-2075.1993.tb06037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S., Drew R. Cloning and DNA sequence of amiC, a new gene regulating expression of the Pseudomonas aeruginosa aliphatic amidase, and purification of the amiC product. J Bacteriol. 1991 Aug;173(16):4914–4921. doi: 10.1128/jb.173.16.4914-4921.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]