Abstract
Patients with inflammatory bowel disease (IBD) have an increased risk of developing intestinal cancer. The magnitude of that increased risk as well as how best to mitigate it remain a topic of ongoing investigation in the field. It is important to quantify the risk of colorectal cancer in association with IBD. The reported risk varies widely between studies. This is partly due to the different methodologies used in the studies. Because of the limitations of surveillance strategies based on the detection of dysplasia, advanced endoscopic imaging and techniques involving the detection of alterations in mucosal antigens and genetic abnormalities are being investigated. Development of new biomarkers, predicting future occurrence of colonic neoplasia may lead to more biomarker-based surveillance. There are promising results that may lead to more efficient surveillance in IBD patients and more general acceptance of its use. A multidisciplinary approach, involving in particular endoscopists and pathologists, together with a centralized patient management, could help to optimize treatments and follow-up measures, both of which could help to reduce the IBD-associated cancer risk.
Keywords: Colorectal cancer, Crohn's disease, inflammatory bowel disease, surveillance, ulcerative colitis
Patients with inflammatory bowel disease (IBD) have an increased risk of developing intestinal cancer. The magnitude of that increased risk as well as how best to mitigate it remain a topic of ongoing investigation in the field. Although only 1% of all cases of colorectal cancer (CRC) occur in patients with ulcerative colitis (UC) or Crohn's disease (CD), patients with IBD represent one of the highest risk groups for developing this dreaded complication.[1,2] A meta-analysis of 116 studies found the mean age of UC-CRC diagnosis to be 43.2 years.[3] Lakatos and coworkers[4] found the average age of IBD-CRC diagnosis to be 10-15 years younger than sporadic CRC in Eastern Europe (50.9 years vs. 62.2 years). The prognosis for sporadic CRC and IBD-CRC is similar, with a 5-year survival of approximately 50%.[5] Identifying at-risk patients and implementing appropriate surveillance for these patients is central to managing the CRC risk in IBD.[6]
It is important to quantify the risk of CRC in association with IBD. The reported risk varies widely between studies. This is partly due to the different methodologies used in the studies.
The aim of this review is to examine the magnitude of the risk of CRC in IBD patients, quantifying the risk factors, and especially to focus on the importance of surveillance for CRC in IBD patients, paying attention to the new techniques to detect early dysplasia and cancer.
MAGNITUDE OF RISK
Data about the magnitude of the risk of CRC in IBD patients come from tertiary referral centers, district general hospitals, and population-based studies. Information from tertiary centers is likely to regard patients with severe disease who are probably at greater risk of IBD-CRC.
The early studies included patients who had already been referred with a diagnosis of CRC and those admitted to hospital with IBD, rather than gold-standard population-based studies, which have a lower proportion of patients with severe or extensive colitis, which are per se adverse prognostic factors for increased risk of CRC. The increased risk of CRC in UC has long been established.
As mentioned above, a meta-analysis of 116 studies, performed by Eaden and coworkers, including overall 54,478 UC patients, with 1698 cases of IBD-CRC, found an overall prevalence of CRC in UC of 3.7%, increasing to 5.4% in those with pancolitis. The analysis included studies from a wide variety of centers and from different geographical areas. Interestingly, the studies from the United Kingdom and the United States found a higher incidence [4 and 5 per 1000 person-years duration (pyd), respectively] than those from Scandinavia (2 per 1000 pyd).[3]
A population-based cohort study performed by Ekbom and coworkers on 3117 patients with UC, who were diagnosed between 1922 and 1983, gave a standardized incidence ratio of 5.7 [95% confidence interval (CI), 4.6-7.0] as compared with the expected incidence of CRC in the general population.[7] In another population-based study performed by Soderlund and coworkers on 7607 patients with IBD who were diagnosed between 1954 and 1989, the standardized incidence ratio was 2.3 (95% CI, 2.0-2.6) as compared with the general population.[8] Other recent population-based studies have suggested a much lower risk of IBD-CRC.
In the study performed by Jess and coworkers on 378 patients from Olmsted County with UC for a total of 5567 pyd (1940-2004), the annual crude incidence was found to be 0.10% and the cumulative risk of CRC at 30 years proved to be as low as 2%. The authors concluded that the risk of CRC is only increased in patients with extensive colitis.[9]
Bernstein and coworkers obtained data about 2672 patients with UC between 1984 and 1997, and they found an annual risk of 0.16% for colon cancer and 0.06% for rectal cancer.[10]
The prospective study by Rutter and coworkers, performed on 600 patients with extensive UC for 5932 pyd as part of a colonoscopic surveillance program (1970-2001), found a cumulative probability of IBD-CRC of 7.6% and a decreasing incidence over the period studied.[11]
A Hungarian population-based study that followed 723 UC patients (1974-2004) calculated the cumulative risks according to disease duration: 0.6% after 10 years, 5.4% after 20 years, and 7.5% after 30 years, whereas Winther and coworkers[12] followed 1160 patients with UC (1962-1987) and gave an annual crude incidence of 0.06% and a cumulative risk of 2.1% at 30 years. However, this study is from Denmark, where the colectomy rate is one of the highest in the world, a fact that may affect the results and underestimate the risk of IBD-CRC, whereas Hungary has a high rate of sporadic CRC and a low rate of colectomy for non-CRC reasons; these factors may result in a higher rate of CRC.
More recently, another study performed by Jess and coworkers on CRC risk in a nationwide cohort of 47,374 Danish patients with IBD over a 30-year period, showed a low rate of CRC. For patients with UC, the overall relative risk (RR) for CRC decreased from 1.34 (95% CI, 1.13-1.58) in 1979-1988 to 0.57 (95% CI, 0.41-0.80) in 1999-2008. Among patients with CD, the overall RR for CRC was 0.85 (95% CI, 0.67-1.07), which did not change over time. According to the authors, the decreasing risk for CRC from 1979 to 2008 might have resulted from improved therapies for patients with IBD.[13]
Interestingly, other studies have shown that Crohn's colitis carries a similar magnitude of risk for the same disease extent. A Canadian cohort study matched a population-based IBD database to a cancer registry in North America between 1984 and 1997, although a limitation of this study was the lack of definition of disease site or extent. There were 2857 cases of CD and 2672 of UC. There was an increased incidence of CRC for patients with Crohn's [risk ratio (RR), 2.64; 95% CI, 1.69-4.12] or UC (RR, 2.75; 95% CI, 1.91-3.97) as compared to the general population but no statistically significant difference between the two IBDs.[14] The authors of the study found the risk of rectal cancer to be increased in UC (RR, 1.90; 95% CI, 1.05-3.43) but not in Crohn's colitis (RR, 1.08; 95% CI, 0.43-2.70).
Ekbom and coworkers also studied the risk of CRC in CD. In their cohort study of 1655 patients performed in Sweden, patients with terminal ileal CD had the same risk of CRC as the general population but those with colonic CD had an RR of 5.6 (95% CI, 2.1-12.2).[15]
RISK FACTORS
Ulcerative colitis
Several factors have been identified that increase the risk of CRC in patients with UC, related to the disease and to the host. Among the former, the duration of disease is an important risk factor, as shown by the fact that the cumulative risk of cancer increases over time, as mentioned above.[3,7] Second, considering CRC risk as being associated with the cumulative effect of chronic inflammation, the extent of colon involvement in UC is an independent predictor of cancer risk.[7] In this connection, the study performed by Ekbom and coworkers showed that the RR of CRC was 1.7 for ulcerative proctitis, whereas the risk in left-sided colitis was 2.8 and this risk rose to 14.8 in patients with extensive colitis.[7] Third, considering the degree of mucosal inflammation, Rutter and coworkers demonstrated in their retrospective series that severity of inflammation on biopsy independently predicted risk of CRC.[16] This finding was supported by two other studies in which inflammatory activity was shown to be independently associated with CRC risk.[17,18] Additionally, it was also shown that backwash ileitis may be an independent predictor of increased CRC risk.[19]
With regard to the risk factors related to the host, a younger age at diagnosis is also associated with an elevated risk of CRC, independent of disease duration.[7] This may be because patients with an early age of diagnosis tend to have more severe inflammation.[16] Considering gender, Söderlund and coworkers[8] observed that the relative risk in males was 2.6 (95% CI, 2.2-3.1) and in females 1.9 (95% CI, 1.5-2.4) compared with the general population, whereas the cumulative incidence at 40 years after diagnosis of IBD was 8.3% in males and 3.5% in females. Similar results were found in a Swedish study performed by Ekbom and coworkers.[7]
Furthermore, a family history of CRC, independent of a family history of IBD, is associated with a higher risk of developing this neoplasia.[20,21,22,23] Finally, considering the comorbidity of UC patients, a meta-analysis by Soetikno and coworkers on the risk of CRC in UC patients with coexistent primary sclerosing cholangitis (PSC), described an odds ratio of 4.09 (95% CI, 2.89-5.76) when compared to UC patients without PSC.[24] This result has led to the recommendation of closer surveillance in this unique at-risk subset of UC patients. Furthermore, the location of colon cancer seen in PSC patients (right side predominant) should be carefully considered as this finding may help to influence the surveillance approach.
In conclusion, consistent data support the oncogenetic impact of chronic inflammation (and IBD, in particular) in colorectal mucosa. Thus the “adenoma–carcinoma” sequence of sporadic cancers becomes the “inflammation–dysplasia-carcinoma” sequence in IBD. On a clinical level, the link between inflammation and IBD-related neoplasia relies on the well-known correlation between cancer risk and disease activity, extent, and duration.[25]
Among the risk factors examined in this literature review, the severity of inflammation appears to be the only modifiable risk factor, thus underscoring the importance of chemoprevention in mitigating cancer risk, and therefore the need for a preventive approach to the early detection of CRC.
The most recent meta-analysis of population-based studies associated UC with a 2.4-fold risk of CRC, which represents an overall 1.6% CRC rate (including sporadic cases) during the first 14 years of follow-up for UC patients.[26] Indeed, the declining trend in the incidence of CRC among UC patients gives the impression that combining the control of UC-related inflammation with surveillance strategies is highly effective in CRC secondary preventions and suggests that appropriate levels of vigilance should be maintained in high-risk patient populations.[25]
Crohn's disease
The evaluation of the risk of CRC in CD poses several methodological challenges, related to the heterogeneous nature of the disease, with many patients having no colonic involvement, and considering that even among patients with Crohn's colitis it is difficult to assess the extent of colonic inflammation, given that the disease can involve any area of the colon in a patchy distribution, and that many Crohn's patients have undergone partial surgical resection of the colon, removing some of the at-risk tissue. This could justify the wide variation among the studies estimating the risk of CRC in CD.
Gyde and coworkers reported a relative risk (RR) of CRC in Crohn's colitis of 23.8, whereas the risk was 4.3 in the general CD population,[27] whereas Greenstein and coworkers calculated an RR of 6.9 for developing CRC in isolated colonic CD.[28] Another study showed an RR of CRC of 5.6 for those only with colonic involvement, as compared to an RR of 3.2 for patients with ileocolitis and 1.0 for patients with ileal involvement only.[29] These studies indicate that CD implies a higher risk of CRC, but also that this risk correlates with the extent of colonic involvement.
A meta-analysis of 12 hospital-based and population-based studies on CRC risk in CD revealed an overall RR of CRC in all the patients of 2.5 (95% CI, 1.3-4.7),[30] whereas in the subset of patients with colonic disease this risk rose to 4.5 (95% CI, 1.3-14.9), and for patients with ileal disease only the risk was not significantly different from the general population. The cumulative risk of CRC for all patients with CD, regardless of disease distribution, was 2.9% after 10 years, 5.6% after 20 years, and 8.3% after 30 years of disease. This finding is in contrast to the Jess meta-analysis, which included population studies of intestinal cancer risk in CD,[31] where the estimation of RR of CRC ranged from 0.9 to 2.2, with a pooled estimate of 1.9 (95% CI, 1.4-2.5).
Gillen and coworkers compared the risk of CRC in patients with extensive Crohn's colitis and in patients with extensive UC,[32] showing a cumulative risk of CRC of 8% at 22 years for patients with CD versus 7% at 22 years for patients with UC. With regard to the risk factors for CRC in CD, CD patients share many of the same risk factors for CRC as UC patients (ie, younger age at diagnosis, greater extent of colonic involvement, and longer duration of disease). Furthermore, bypassed segments of bowel[33] and perianal fistulae[34] in CD may also be sites at increased risk for carcinogenesis, just as bowel strictures may harbor dysplasia or cancer[35] and should be carefully biopsied and resected if a pediatric or upper endoscope cannot traverse them.
Actually it is really difficult to establish exactly the risk of CRC in CD, although it is generally accepted that patients with CD of the colon are at increased risk for dysplasia and CRC, and that this risk is related to the cumulative effect of colonic inflammation, as in UC. With the exception of strictures as mentioned above, screening and surveillance of CRC in patients with CD should be handled identically to patients with UC, matched for extent of colonic involvement.
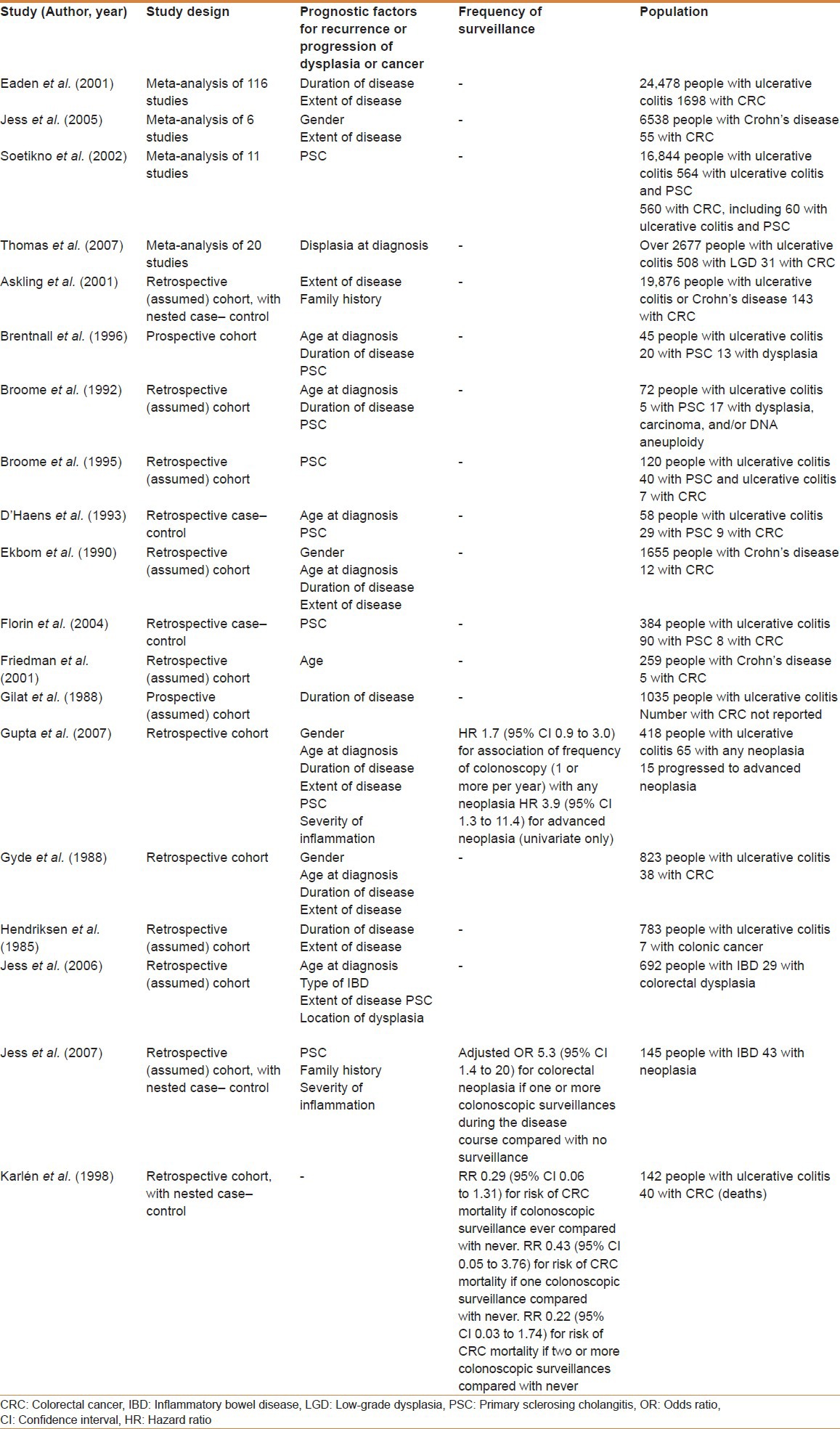
In summary, the more recent studies mentioned above, which follow a more representative proportion of all patients with IBD,[13] demonstrates that the risk of CRC is lower than previously thought. Furthermore, the influence of time of disease seems to be much less significant and more important factors such as inflammatory burden and disease extent should guide the surveillance approach. Table 1 shows the confirmed risk factors and proposed protective factors for developing CRC in IBD patients and Table 2 shows the characteristics of the studies evaluating the risk factors for CRC in IBD patients.
Table 1.
The confirmed risk factors and proposed protective factors for developing CRC in IBD patients
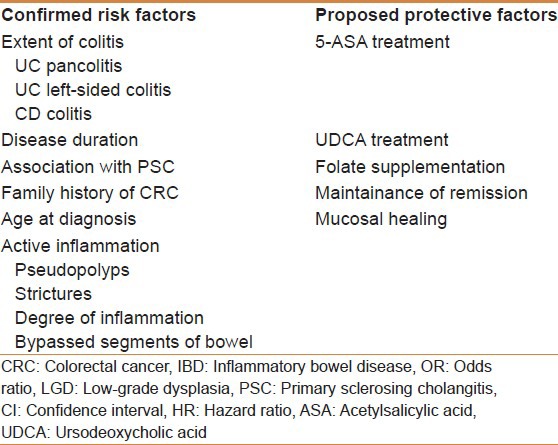
Table 2.
Summary of the characteristics of the studies evaluating the risk factors for CRC in IBD patients
SURVEILLANCE: THE PAST
Ideally, only a randomized controlled trial, with a very large number of patients and a long follow-up, could assess whether surveillance reduces CRC-related mortality. To date, only case-control studies have provided information about this topic. A Cochrane review[36] performed by Collins and coworkers revealed only three case-control studies, in the current literature, that addressed the question of whether surveillance programs reduce CRC-related mortality.[37,38,39] In the first one, Lashner and coworkers[37] found an improved overall survival but not a reduced mortality from CRC [RR, 2.09; 95% CI, 0.39-11.12]. In the second one, Karlen and coworkers[38] reported a protective effect, although not statistically significant, of surveillance colonoscopy on CRC-related mortality. In fact two of the 40 patients who died from CRC had undergone at least one surveillance colonoscopy compared with 18 of the 102 controls (RR, 0.29; 95% CI, 0.06-1.31). Only one patient who died from CRC had had at least two surveillance colonoscopies (RR, 0.22; 95% CI, 0.03-1.74), thus indicating a possible “dose”–response relationship. Finally, Choi and coworkers[39] showed that CRC in IBD patients undergoing surveillance is detected at an earlier stage (P = 0.039). The authors of the abovementioned meta-analysis concluded that there was indirect evidence for improved survival from colonoscopic surveillance.
The goal of colorectal cancer screening colonoscopy in the general population and surveillance in IBD patients is to detect premalignant changes early enough that intervention can prevent complications of invasive cancer. Dysplasia, assessed by histology, remains the most reliable marker of cancer-prone IBD patients.[25] Its detection depends on the timing of the endoscopic follow-up, on the endoscopic techniques employed during the follow-up, on the quality and the quantity of the biopsy samples obtained, on the endoscopist's and pathologist's expertise, and on the patient's compliance with the recommended follow-up.[1,25] The mandatory operation in IBD-associated dysplasia is colectomy, because of the high prevalence of synchronous or metachronous cancer, as well as the technical limitations of being able to identify confidently and completely resect dysplastic lesions in flat mucosa.[40]
In the general population, the dysplastic or premalignant lesion is the colon polyp that can be easily visualized and resected at the time of the colonoscopy before it transforms into a malignant lesion. In contrast, dysplasia in IBD can be found at sites distant from the cancer itself or before the cancer develops and is difficult to recognize on colonoscopy, as it often arises from flat, normal-appearing mucosa.[41]
IBD-related dysplasia usually presents with marked macroscopic variability. In fact it can be found, similar to flat dysplasia, in random biopsy specimens obtained from unremarkable mucosa; on the other hand, it can occur within or near plaque-like lesions or raised polypoid masses, defined as dysplasia-associated lesion or mass (DALM).
More recently, the definitions of adenoma-like mass or dysplasia (ALM or ALD) have been proposed. ALM is applied to polypoid dysplasia with no adjacent flat component, endoscopically indistinguishable from a sporadic (sessile or pedunculated) polyp and completely removable by endoscopy; in this case, histology is of little help in differentiating DALM from ALM and this distinction basically relies on endoscopy.[25]
Because IBD-CRC is preceded by dysplasia, finding dysplasia on random colon biopsies represents an increased risk of developing colorectal cancer in the near future. Dysplasia is classified as indefinite, low-grade, and high-grade.
IBD-CRC occurs in areas of chronic inflammation and can be a polypoid, ulcerated, or plaque-like lesion. Most IBD-CRCs are adenocarcinomas, but there is a higher incidence of poorly differentiated, anaplastic, and mucinous carcinomas compared with sporadic colorectal cancer.[40]
The best technique for surveillance is evolving. Currently, endoscopists are moving away from using random colonic biopsies toward targeted biopsies aimed at abnormal areas identified by newer colonoscopic techniques (chro moendoscopy, confocal microendoscopy, narrow band imaging). In this connection, although dysplasia has been shown to be highly predictive for cancer in many studies, the time-consuming process of multiple random biopsies and sometimes inconclusive pathology findings still form the basis for doubt among many gastroenterologists.
Furthermore, many studies have shown a low number of biopsies taken during surveillance, suggesting that this strategy is challenging to comply with.
The risk of IBD-CRC is estimated based on duration and extent of disease, coexistent risk factors (PSC, family history of sporadic CRC), and endoscopic and histological findings at colonoscopy. The surveillance intervals are based on this risk assessment.[42]
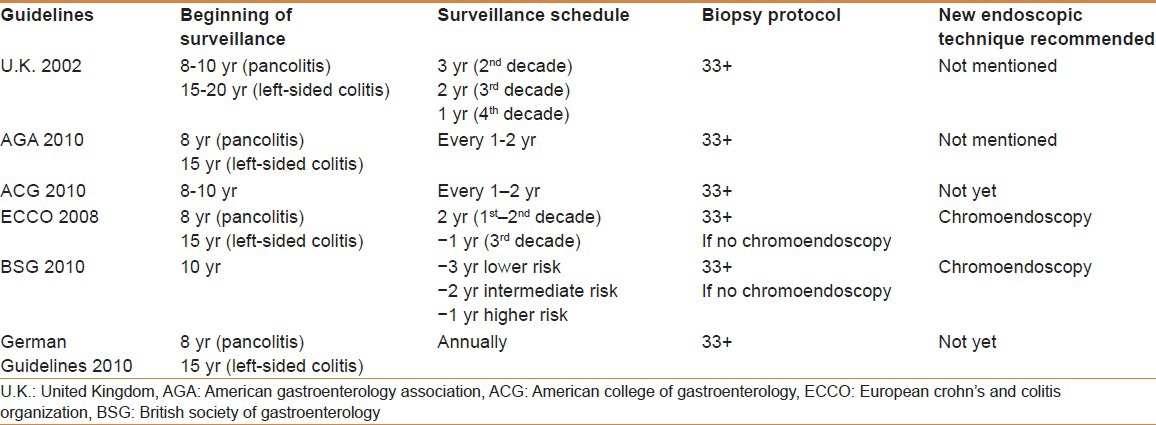
International guidelines recommend systematic surveillance in patients with a history of 8-10 years of extensive UC, or 15 years of left-sided UC, whereas earlier surveillance is not recommended. Surveillance colonoscopies should be performed when the disease is in remission. The guidelines also recommend obtaining 2-4 random colon biopsy specimens every 10 cm with additional samples of any suspicious areas.[43,44,45,46,47,48] Table 3 shows the characteristics of the main studies on surveillance for CRC in IBD patients. Table 4 shows the main differences between the recommendations, arising from the international guidelines, for colorectal cancer surveillance programs in IBD patients.
Table 3.
Characteristics of the main studies on surveillance for CRC in IBD patients

Table 4.
Summary of the main differences between the recommendations arising from the international guidelines for colorectal cancer surveillance programmes in inflammatory bowel disease patients
ROLE OF THE PATHOLOGIST IN THE DETECTION OF DYSPLASIA AND COLORECTAL CANCER IN INFLAMMATORY BOWEL DISEASE
The detection of dysplasia and colorectal cancer in IBD depends, as mentioned above, on several variables: (1) the frequency of colonoscopy and the technique used; (2) the quality and quantity of the biopsy samples obtained; (3) the endoscopist's and pathologist's expertise; and (4) the patient's compliance with the recommended follow-up.[1,25]
Histology in expert hands remain the most reliable way to identify cancer-prone cases (dysplasia patients). In fact, after delivery of paraffin-embedded biopsy samples to the pathologist, the pathologist is concerned with the microscopic examination of the biopsy samples, the determination and classification of dysplasia, and the confirmation of the suspicions of the endoscopist. The histological assessment of biopsy specimens (from wherever they were obtained) is fundamental to patient's management: It is ultimately the pathologist's interpretation that distinguishes high-risk from low-risk patients and triggers recommendations to continue surveillance or opt for colectomy.[25]
However, the detection and the characterization of dysplasia has several well-recognized limitations, including its low intra- and inter-observer variability (even among experts).[49,50]
In both prospective and retrospective studies, inter-observer consistency in the histological assessment of dysplasia has ranged between 42% and 72%.[51,52,53,54,55,56,57] Furthermore, Lim and coworkers[57] found a kappa coefficient between 10 pairings of five gastrointestinal pathologists in the range of 0.6 (acceptable) to 0.39 (unacceptable). Such agreement is naturally best when the two extremes of the histological spectrum of the lesions are considered (no dysplasia versus high-grade dysplasia), while the diagnostic consistency is worse when distinguishing between the intermediate categories.[41,58] Due to this lack of concordance, the international guidelines strongly recommended that dysplasia, as assessed prior of any surgical treatment, must be confirmed by a second, experienced gastrointestinal pathologist.[48,59,60]
SURVEILLANCE: THE PRESENT AND THE FUTURE
There have been recent advancements in optical methods and dye systems to more accurately detect dysplasia.
These techniques may allow for targeted biopsies and eliminate the need for random biopsies. The most promising technique to date and one that has been endorsed recently by the Crohn's and Colitis Foundation of America Colon Cancer in IBD Study Group[48] is chromoendoscopy. In this technique, a dye such as methylene blue or indigo carmine is applied to the colon either randomly or selectively to highlight areas of dysplasia. Methylene blue is an absorptive dye that is taken up by normal tissue but not dysplastic tissue, and indigo carmine is a contrast dye that pools in areas of abnormal dysplastic tissue.
The pioneering studies performed by Kiesslich et al.[61] and Rutter et al.[62] have shown increased dysplasia detection with chromoendoscopy compared with conventional colonoscopy. Indeed, Rutter and coworkers[62] did not find any dysplasia in 2904 random biopsies utilizing conventional colonoscopy, while nine dysplastic lesions were detected in 157 targeted biopsies with indigo carmine chromoendoscopy, seven of which were only visible with dye spraying.
Successively, several studies have shown that chrom oendoscopy increases sensitivity for the detection of dysplasia. A recently published meta-analysis calculated a pooled incremental yield of chromoendoscopy over white light for dysplasia detection. The authors were able to find that in six studies on 1277 patients, chromoendoscopy was significantly better than white light for the detection of dysplasia in colonic IBD.[63]
In the technical review and position statement published by the AGA, the authors concluded that targeted biopsies using chromoendoscopy performed by endoscopists experienced in this technique is a reasonable screening alternative to the random sampling of colon using standard white light.[64,65]
In the AGA Institute technology assessment of image-enhanced endoscopy (IEE), the authors concluded that IEE may increase the yield of detection of dysplasia and as such is recommended for patients with long-standing UC.[66]
Finally, The British Society of Gastroenterology (BSG) advises that all patients with IBD should have a screening colonoscopy approximately 10 years after symptom onset (ideally when the patient is in remission) with pancolonic dye spraying and targeted biopsies of abnormal areas.[42]
The surface guidelines for the application of chromoendoscopy in IBD patients with longstanding colitis and mucosal healing were developed to provide a better standardization of this technique.[67]
However, the use of surface dyes during chromoendoscopy in some cases has generated safety concerns. Prior in vitro biochemical studies have established that in the presence of light, methylene blue can generate single-strand breaks in DNA. As an alternative to methylene blue, indigo carmine is generally regarded as a safe dye because it is not absorbed by the colonic mucosa. To date, however, no long-term data exist that capture adverse clinical outcomes, and some groups continue to use methylene blue chromoendoscopy.[68]
Furthermore, chromoendoscopy presents multiple other potential limitations; the mucosa is not always equally sprayed, areas of colorant pooling prevent proper visualization, and, most importantly, it tends to be more time-consuming. It is also virtually impossible to perform chromoendoscopy in the context of poor colonic cleansing and in the presence of severe colonic inflammation where a neoplasia-like pit pattern is often seen, potentially producing false-positive results.[69]
With regard to the costs of the procedure the accessories needed to perform tissue staining are readily available and relatively inexpensive (208 US dollars for the Olympus Reusable kit, 67 US dollars for the Wilson-Cook Medical single-use kit, and 225 US dollars for the Hobbs medical single-use kit). However, there is no specific Current Procedural Terminology (CPT) code for billing and reimbursement for the time and effort added to the endoscopic procedure.
Other techniques that are currently under study but are not yet proved for widespread clinical use include confocal microendoscopy and narrow-band imaging.
Confocal laser endomicroscopy (CLE) allows imaging of the mucosal layer, including epithelial cells and the lamina propria. The targets of endomicroscopic examination for an accurate diagnosis can be the cells, vascular structures, and/or tissue patterns.[70] In addition, if an immediate diagnosis cannot be obtained by CLE, the same technique offers the possibility of targeted biopsies. The pathologist will receive fewer biopsy specimens but will find more specific tissue because a greater number of “intelligent” biopsy specimens containing target tissue are provided by the endoscopist.[71]
Confocal endomicroscopy has been proposed as an addition to chromoscopically detected lesions to help target biopsies and reduce their number. In a study by Kiesslich and coworkers, chromoendoscopy reduced biopsies 10-fold. The addition of confocal could have reduced the number of biopsies a further fivefold to approximately one per patient. Although there is the possibility of having to perform very few biopsies, the technique is technically demanding and the instruments currently unwieldy.[72]
CLE is possible after injecting 2.5-5 mL fluorescein 10% intravenously. A confocal miniaturized laser with a defined wavelength of 488 nm generates in vivo histology images up to a 1000-fold magnification. During ongoing endoscopy, single cellular and subcellular tissue analyses 0-250 μm in depth are visible. In patients with UC, targeted biopsies of mucosal areas suspected of harboring intra-epitelial neoplasia can be identified directly while performing the colonoscopy.
The main limitation of CLE is that this technique is only limited to a very small mucosal surface; furthermore, the high costs of CLE (90,000 US dollars for the whole system) should be evaluated in a further cost-effectiveness analysis.
An alternative technique to CLE is the miniprobe-based endomicroscopy technique. After the miniprobe is passed through a 2.8-mm working channel of any standard videoendoscope, the laser unit generates a confocal image with a high frame rate per second. Special attention toward this technique has taken place since a high-resolution miniprobe device was developed.[72]
With regard to narrow band imaging (NBI), there was great hope that NBI would be able to act as a form of “electronic chromoendoscopy” to make colitis surveillance more efficient. Unfortunately, probably due to background inflammation, dysplasia detection was no different to white light in two tandem studies and one multi-center, randomized, parallel group study.[73,74,75] Furthermore, recently a prospective, randomized crossover study was published to compare NBI and chromoendoscopy for the detection of intraepithelial neoplasia: NBI appears to be a less time-consuming and equally effective alternative to CE for the detection of intraepithelial neoplasia. However, given the NBI lesion and patient miss rates, the authors did not recommend it as the standard technique.[76] Similarly, a meta-analysis to determine whether use of NBI enhances the detection of adenomas, compared with conventional colonoscopy, was recently performed; there was no statistically significant difference in the overall adenoma detection rate with the use of NBI or WLC [36% vs. 34%; P = 0.413 (RR, 1.06; 95% CI, 0.97-1.16)], and there was no statistically significant difference in polyp detection rate by using NBI or WLC [37% vs. 35%; P = 0.289 (RR, 1.22; 95% CI, 0.85-1.76)).[77] For these reasons, it seems unlikely that NBI will be helpful for dysplasia detection in colitis.
The cost of NBI is 70,500 US dollars for the whole system (including endoscope, processor, and light source).
Endocytoscopy is a kind of reflecting light microscopy and the device is passed through a 3.7-mm working channel of any suitable endoscope. The magnification ranges from 450- to 1100-fold depending on the device and the in vivo recognition of surface cells is possible after topically applying acylcysteine for mucolysis and methylene blue for staining. So far, there are no published data on this technique in patients with IBD.[78]
Some innovative endoscopy imaging techniques are under current evaluation to prove their eligibility for clinical routine endoscopy. Optical coherence tomography is an optical analogue to endoscopic ultrasound with an imaging depth of 2 mm. The device is also used in a “mother–baby fashion” through the working channel of the endoscope as mentioned above. There are two publications on this technique in patients with IBD.[79,80]
These studies have started to show the feasibility of this method and to differentiate transmural inflammation in CD from patterns of active UC.
Fluorescence endoscopy is a technique to assess intra-epithelial neoplasia after topical or systemical sensitization with 5-aminolevulinic acid (5-ALA). After the application of 5-ALA, this substance is converted intracellularly into the fluophore protoporphyrin IX, which accumulates selectively in neoplastic tissue making it possible to detect a reddish spot while illuminated with blue monochromatic light (442 nm). The first report that evaluated fluorescence endoscopy in patients with UC was published in 2003.[81] This study examined 37 patients with UC and found a sensitivity of 87%-100% after local application and 43% after systemic application with 5-ALA. The specificity was lower with 62% after systemic and 51% after local sensitization. However, 3 years later another study could not confirm these results and evaluated 682 biopsies in 42 patients with IBD. The corresponding histology in two patients with intraepithelial neoplasia showed no correlation with the red fluorescent areas during fluorescence endoscopy.[82] However, neither optical coherence tomography nor fluorescence endoscopy is currently suitable for the detection of intraepithelial neoplasia in patients with IBD.
Finally, in vivo histology techniques are the basis for further diagnostic improvement on a single cellular level and have opened the door for molecular imaging in the gastrointestinal tract. There is one in vivo mouse model where colonic tissue samples are incubated with fluorescein-marked antibodies against epithelial growth factor receptor; in this mouse model a confocal microscope was able to detect the epithelial growth factor receptor on the surface of tissue after inducing colitis.[83] The first in vivo detection of dysplastic crypts by confocal microscopy in humans during ongoing endoscopy was possible after topical application of fluorescent-labeled heptapeptides, which were identified previously to bind to premalignant tissue as high-affinity ligands.[84]
FUTURE PERSPECTIVES
Because of the limitations of surveillance strategies based on the detection of dysplasia, further techniques involving the detection of alterations in mucosal antigens and genetic abnormalities are being investigated.[85,86] For example, it was shown that sialosyl-Tn is a mucin-associated carbohydrate antigen that may presage the development of dysplasia and CRC by several years.[87,88] Similarly, allelic deletions and point mutations of tumor suppressor genes, such as p53, Rb, mcc, and APC (adenomatous polyposis coli) have been found in dysplastic and cancerous UC.[85,87,89]
It is likely that changes in nuclear DNA content are detectable earlier than histological signs of premalignancy. As a result, detection of DNA aneuploidy by flow cytometry of mucosal specimens in UC patients may be more objective and less sensitive to assessment error than dysplasia.[86] DNA aneuploidy detected by these techniques has been correlated with dysplasia and carcinoma.[90,91,92] However, as with dysplasia, the predictive value of aneuploidy is uncertain. Neoplasms can arise without preexisting aneuploidy, and conversely, aneuploidy may exist for many years without progression to malignancy.
Furthermore, stool-based molecular tests hold a large potential for improving colorectal cancer screening. Recent advances in the development of molecular markers in fecal specimens are encouraging for its use as a screening tool. Genetic mutations and epigenetic alterations that result from the carcinogenetic process can be detected by coprocytobiology in the colonocytes exfoliated from the lesion into the fecal matter. These markers have shown promising sensitivity and specificity in the detection of both malignant and premalignant lesions and are gaining popularity as a noninvasive technique that is representative of the entire colon.[93]
CONCLUSIONS
IBD shows an increased risk for developing CRC; in this setting, surveillance colonoscopy detects early neoplastic lesions and reduces colitis-associated mortality.
However, endoscopists are moving away from using random colonic biopsies toward targeted biopsies aimed at abnormal areas identified by advanced endoscopic imaging, when high-quality bowel cleansing and inactive mucosal disease are guaranteed.
Development of new biomarkers predicting future occurrence of colonic neoplasia may lead to a more biomarker-based surveillance. These are promising results that may lead to more efficient surveillance in IBD patients and more general acceptance of its employment.
Furthermore, the clinico-pathological link between IBD and CRC is well defined and provides the rationale for a differentiated endoscopic surveillance of patients with IBD without dysplasia, lesions indefinite for dysplasia or low-grade dysplasia, considering that patients with “late-onset” IBD should be considered “more prone” to cancer development. A multidisciplinary approach, together with a centralized patient management, could help to optimize treatments and follow-up measures, both of which could help to reduce the IBD-associated cancer risk (25). Therefore, physicians should apply into routine clinical practice:
The simplification of dialogue between endoscopist and pathologist, to optimize patients’ management, together with
The application of educational measures to improve patients’ awareness, thus optimizing the acceptance to both treatments and follow-up strategies.
ACKNOWLEDGMENTS
The authors thank Dr. Ciro Marrone for helping to find the iconographic support for this work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Zisman TL, Rubin DT. Colorectal cancer and dysplasia in inflammatory bowel disease. World J Gastroenterol. 2008;14:2662–9. doi: 10.3748/wjg.14.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi PM, Zelig MP. Similarity of colorectal cancer in Crohn's disease and ulcerative colitis: Implications for carcinogenesis and prevention. Gut. 1994;35:950–4. doi: 10.1136/gut.35.7.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut. 2001;48:526–35. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakatos L, Mester G, Erdelyi Z, David G, Pandur T, Balogh M, et al. Risk factors for ulcerative colitis-associated colorectal cancer in a Hungarian cohort of patients with ulcerative colitis: Results of a population-based study. Inflamm Bowel Dis. 2006;12:205–11. doi: 10.1097/01.MIB.0000217770.21261.ce. [DOI] [PubMed] [Google Scholar]
- 5.Rhodes JM, Campbell BJ. Inflammation and colorectal cancer: IBD-associated and sporadic cancer compared. Trends Mol Med. 2002;8:10–6. doi: 10.1016/s1471-4914(01)02194-3. [DOI] [PubMed] [Google Scholar]
- 6.Dyson JK, Rutter MD. Colorectal cancer in inflammatory bowel disease: What is the real magnitude of the risk? World J Gastroenterol. 2012;18:3839–48. doi: 10.3748/wjg.v18.i29.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–33. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 8.Söderlund S, Brandt L, Lapidus A, Karlén P, Broström O, Löfberg R, et al. Decreasing time-trends of colorectal cancer in a large cohort of patients with inflammatory bowel disease. Gastroenterology. 2009;136:1561–7. doi: 10.1053/j.gastro.2009.01.064. [DOI] [PubMed] [Google Scholar]
- 9.Jess T, Loftus EV, Velayos FS, Harmsen WS, Zinsmeister AR, Smyrk TC, et al. Risk of intestinal cancer in inflammatory bowel disease: A population-based study from Olmsted county, Minnesota. Gastroenterology. 2006;130:1039–46. doi: 10.1053/j.gastro.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: A population- based study. Cancer. 2001;91:854–62. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, et al. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130:1030–8. doi: 10.1053/j.gastro.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 12.Winther KV, Jess T, Langholz E, Munkholm P, Binder V. Long-term risk of cancer in ulcerative colitis: A populationbased cohort study from Copenhagen County. Clin Gastroenterol Hepatol. 2004;2:1088–95. doi: 10.1016/s1542-3565(04)00543-9. [DOI] [PubMed] [Google Scholar]
- 13.Jess T, Simonsen J, Joergensen KT, Pedersen BV, Nielsen NM, Frisch M. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143:375–81. doi: 10.1053/j.gastro.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: A population- based study. Cancer. 2001;91:854–62. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn's disease with colonic involvement. Lancet. 1990;336:357–9. doi: 10.1016/0140-6736(90)91889-i. [DOI] [PubMed] [Google Scholar]
- 16.Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–9. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Rubin DT, Huo D, Rothe JA, Hetzel JT, Sedrak M, Yadron N, et al. Increased inflammatory activity is an independent risk factor for dysplasia and colorectal cancer in ulcerative colitis: A case-control analysis with blinded prospective pathology review. Gastroenterology. 2006;130:A2. [Google Scholar]
- 18.Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, Kornbluth A, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: A cohort study. Gastroenterology. 2007;133:1099–105. doi: 10.1053/j.gastro.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heuschen UA, Hinz U, Allemeyer EH, Stern J, Lucas M, Autschbach F, et al. Backwash ileitis is strongly associated with colorectal carcinoma in ulcerative colitis. Gastroenterology. 2001;120:841–7. doi: 10.1053/gast.2001.22434. [DOI] [PubMed] [Google Scholar]
- 20.Nuako KW, Ahlquist DA, Mahoney DW, Schaid DJ, Siems DM, Lindor NM. Familial predisposition for colorectal cancer in chronic ulcerative colitis: A case-control study. Gastroenterology. 1998;115:1079–83. doi: 10.1016/s0016-5085(98)70077-0. [DOI] [PubMed] [Google Scholar]
- 21.Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: A case control study. Aliment Pharmacol Ther. 2000;14:145–53. doi: 10.1046/j.1365-2036.2000.00698.x. [DOI] [PubMed] [Google Scholar]
- 22.Askling J, Dickman PW, Karlen P, Brostrom O, Lapidus A, Lofberg R, et al. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology. 2001;120:1356–62. doi: 10.1053/gast.2001.24052. [DOI] [PubMed] [Google Scholar]
- 23.Velayos FS, Loftus EV, Jr, Jess T, Harmsen WS, Bida J, Zinsmeister AR, et al. Predictive and protective factors associated with colorectal cancer in ulcerative colitis: A case-control study. Gastroenterology. 2006;130:1941–9. doi: 10.1053/j.gastro.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 24.Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: A metaanalysis. Gastrointest Endosc. 2002;56:48–54. doi: 10.1067/mge.2002.125367. [DOI] [PubMed] [Google Scholar]
- 25.Mescoli C, Albertoni L, D’incà R, Rugge M. Dysplasia in inflammatory bowel diseases. Dig Liv Dis. 2013;45:186–94. doi: 10.1016/j.dld.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: A meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639–45. doi: 10.1016/j.cgh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Gyde SN, Prior P, Macartney JC, Thompson H, Waterhouse JA, Allan RN. Malignancy in Crohn's disease. Gut. 1980;21:1024–9. doi: 10.1136/gut.21.12.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenstein AJ, Sachar DB, Smith H, Janowitz HD, Aufses AH., Jr A comparison of cancer risk in Crohn's disease and ulcerative colitis. Cancer. 1981;48:2742–5. doi: 10.1002/1097-0142(19811215)48:12<2742::aid-cncr2820481231>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 29.Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn's disease with colonic involvement. Lancet. 1990;336:357–9. doi: 10.1016/0140-6736(90)91889-i. [DOI] [PubMed] [Google Scholar]
- 30.Canavan C, Abrams KR, Mayberry J. Meta-analysis: Colorectal and small bowel cancer risk in patients with Crohn's disease. Aliment Pharmacol Ther. 2006;23:1097–104. doi: 10.1111/j.1365-2036.2006.02854.x. [DOI] [PubMed] [Google Scholar]
- 31.Jess T, Gamborg M, Matzen P, Munkholm P, Sorensen TI. Increased risk of intestinal cancer in Crohn's disease: A metaanalysis of population-based cohort studies. Am J Gastroenterol. 2005;100:2724–9. doi: 10.1111/j.1572-0241.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 32.Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn's disease: A comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590–2. doi: 10.1136/gut.35.11.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenstein AJ, Sachar D, Pucillo A, Kreel I, Geller S, Janowitz HD, et al. Cancer in Crohn's disease after diversionary surgery. A report of seven carcinomas occurring in excluded bowel. Am J Surg. 1978;135:86–90. doi: 10.1016/0002-9610(78)90015-6. [DOI] [PubMed] [Google Scholar]
- 34.Connell WR, Sheffield JP, Kamm MA, Ritchie JK, Hawley PR, Lennard-Jones JE. Lower gastrointestinal malignancy in Crohn's disease. Gut. 1994;35:347–52. doi: 10.1136/gut.35.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamazaki Y, Ribeiro MB, Sachar DB, Aufses AH, Jr, Greenstein AJ. Malignant colorectal strictures in Crohn's disease. Am J Gastroenterol. 1991;86:882–5. [PubMed] [Google Scholar]
- 36.Collins PD, Mpofu C, Watson AJ, Rhodes JM. Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease. Cochrane Database Syst Rev. 2006;2:CD000279. doi: 10.1002/14651858.CD000279.pub3. [DOI] [PubMed] [Google Scholar]
- 37.Lashner BA, Kane SV, Hanauer SB. Colon cancer surveillance in chronic ulcerative colitis: Historical cohort study. Am J Gastroenterol. 1990;85:1083–7. [PubMed] [Google Scholar]
- 38.Choi PM, Nugent FW, Schoetz DJ, Jr, Silverman ML, Haggitt RC. Colonoscopic surveillance reduces mortality from colorectal cancer in ulcerative colitis. Gastroenterology. 1993;105:418–24. doi: 10.1016/0016-5085(93)90715-o. [DOI] [PubMed] [Google Scholar]
- 39.Karlen P, Kornfeld D, Brostrom O, Lofberg R, Persson PG, Ekbom A. Is colonoscopic surveillance reducing colorectal cancer mortality in ulcerative colitis? A population based case control study. Gut. 1998;42:711–4. doi: 10.1136/gut.42.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mattar MC, Lough D, Pishvaian MJ, Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Cancer Res. 2011;4:53–61. [PMC free article] [PubMed] [Google Scholar]
- 41.Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634–48. doi: 10.1053/j.gastro.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 42.Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–89. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 43.Rubin DT, Kavitt RT. Surveillance for cancer and dysplasia in inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35:581–604. doi: 10.1016/j.gtc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2004;99:1371–85. doi: 10.1111/j.1572-0241.2004.40036.x. [DOI] [PubMed] [Google Scholar]
- 45.Carter MJ, Lobo AJ, Travis SP. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53(Suppl 5):V1–16. doi: 10.1136/gut.2004.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eaden JA, Mayberry JF. Guidelines for the screening and surveillance of asymptomatic colorectal cancer in patients with inflammatory bowel disease. Gut. 2002;51:10–2. doi: 10.1136/gut.51.suppl_5.v10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmiegel W, Pox C, Reinacher-Schick A, Adler G, Arnold D, Fleig W, et al. S3 guidelines for colorectal carcinoma: Results of an evidence-based consensus conference on February 6/7, 2004 and June 8/9, 2007 (for the topics IV, VI and VII) Z Gastroenterol. 2010;48:65–136. doi: 10.1055/s-0028-1109936. [DOI] [PubMed] [Google Scholar]
- 48.Itzkowitz SH, Present DH Crohn's and Colitis Foundation of America Colon Cancer in IBD Study Group. Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:314–21. doi: 10.1097/01.mib.0000160811.76729.d5. [DOI] [PubMed] [Google Scholar]
- 49.Riddell RH, Goldman H, Ransohoff DF, Appelman HD, Fenoglio CM, Haggitt RC, et al. Dysplasia in inflammatory bowel disease: Standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931–68. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- 50.Judge TA, Lewis JD, Lichtenstein GR. Colonic dysplasia and cancer in inflammatory bowel disease. Gastrointest Endosc Clin N Am. 2002;12:495–523. doi: 10.1016/s1052-5157(02)00014-4. [DOI] [PubMed] [Google Scholar]
- 51.Odze RD, Goldblum J, Noffsinger A, Alsaigh N, Rybicki LA, Fogt F. Interobserver variability in the diagnosis of ulcerative colitis-associated dysplasia by telepathology. Mod Pathol. 2002;15:379–86. doi: 10.1038/modpathol.3880534. [DOI] [PubMed] [Google Scholar]
- 52.Eaden J, Abrams K, McKay H, Denley H, Mayberry J. Inter-observer variation between general and specialist gastrointestinal pathologists when grading dysplasia in ulcerative colitis. J Pathol. 2001;194:152–7. doi: 10.1002/path.876. [DOI] [PubMed] [Google Scholar]
- 53.Dixon MF, Brown LJ, Gilmour HM, Price AB, Smeeton NC, Talbot IC, et al. Observer variation in the assessment of dysplasia in ulcerative colitis. Histopathology. 1988;13:385–97. doi: 10.1111/j.1365-2559.1988.tb02055.x. [DOI] [PubMed] [Google Scholar]
- 54.Connell WR, Lennard-Jones JE, Williams CB, Talbot IC, Price AB, Wilkinson KH. Factors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis. Gastroenterology. 1994;107:934–44. doi: 10.1016/0016-5085(94)90216-x. [DOI] [PubMed] [Google Scholar]
- 55.Riddell RH. Grading of dysplasia. Eur J Cancer. 1995;31:1169–70. doi: 10.1016/0959-8049(95)00134-5. [DOI] [PubMed] [Google Scholar]
- 56.Melville DM, Jass JR, Morson BC, Pollock DJ, Richman PI, Shepherd NA, et al. Observer study of the grading of dysplasia in ulcerative colitis: Comparison with clinical outcome. Hum Pathol. 1989;20:1008–14. doi: 10.1016/0046-8177(89)90273-6. [DOI] [PubMed] [Google Scholar]
- 57.Lim CH, Dixon MF, Vail A, Forman D, Lynch DA, Axon AT. Ten-year follow-up of ulcerative colitis patients with and without low grade dysplasia. Gut. 2003;52:1127–32. doi: 10.1136/gut.52.8.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008;14:378–89. doi: 10.3748/wjg.14.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greenson JK. Dysplasia in inflammatory bowel disease. Semin Diagn Pathol. 2002;19:31–7. [PubMed] [Google Scholar]
- 60.Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, et al. Colorectal cancer screening and surveillance: Clinical guidelines and rationale – update based on new evidence. Gastroenterology. 2003;124:544–60. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 61.Kiesslich R, Fritsch J, Holtmann M, Koehler HH, Stolte M, Kanzler S, et al. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880–8. doi: 10.1053/gast.2003.50146. [DOI] [PubMed] [Google Scholar]
- 62.Rutter MD, Saunders BP, Schofield G, Forbes A, Price AB, Talbot IC. Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut. 2004;53:256–60. doi: 10.1136/gut.2003.016386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Subramanian V, Mannath J, Ragunath K, Hawkey CJ. Meta-analysis: The diagnostic yield of chromoendoscopy for detecting dysplasia in patients with colonic inflammatory bowel disease. Alimentary Pharmacology Therapeutics. 2011;33:304–12. doi: 10.1111/j.1365-2036.2010.04525.x. [DOI] [PubMed] [Google Scholar]
- 64.Farraye FA, Odze RD, Eaden J, Itzkowitz SH, McCabe RP, Dassopoulos T, et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:738–45. doi: 10.1053/j.gastro.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 65.Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746–74.e4. doi: 10.1053/j.gastro.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 66.Kaltenbach T, Sano Y, Friedland S, Soetikno R. American gastroenterological association (AGA) institute technology assessment on image-enhanced endoscopy. Gastroenterology. 2008;134:327–40. doi: 10.1053/j.gastro.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 67.Kiesslich R, Neurath MF. Chromoendoscopy: An evolving standard in surveillance for ulcerative colitis. Inflamm Bowel Dis. 2004;10:695–6. doi: 10.1097/00054725-200409000-00031. [DOI] [PubMed] [Google Scholar]
- 68.Nass JP, Connolly SE. Current status of Chromoendoscopy and narrow band imaging. Clin Colon Rectal Surg. 2010;23:21–30. doi: 10.1055/s-0030-1247853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bessissow T, Bisschops R. Advanced endoscopic imaging for dysplasia surveillance in ulcerative colitis. Expert Rev Gastroenterol Hepatol. 2013;7:57–67. doi: 10.1586/egh.12.65. [DOI] [PubMed] [Google Scholar]
- 70.Deinert K, Kiesslich R, Vieth M, Neurath MF, Neuhaus H. In-vivo microvascular imaging of early squamous-cell cancer of the esophagus by confocal laser endomicroscopy. Endoscopy. 2007;39:366–8. doi: 10.1055/s-2007-966217. [DOI] [PubMed] [Google Scholar]
- 71.Kiesslich R, Neurath MF. Endomicroscopy is born - do we still need the pathologist? Gastrointest Endosc. 2007;66:150–3. doi: 10.1016/j.gie.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 72.Gheonea DI, Saftoiu A, Ciurea T, Popescu C, Georgescu CV, Malos A. Confocal Laser Endomicroscopy of the Colon. J Gastrointestin Liv Dis. 2010;19:207–11. [PubMed] [Google Scholar]
- 73.Dekker E, van den Broek FJ, Reitsma JB, Hardwick JC, Offerhaus GJ, van Deventer SJ, et al. Narrow-band imaging compared with conventional colonoscopy for the detection of dysplasia in patients with longstanding ulcerative colitis. Endoscopy. 2007;39:216–21. doi: 10.1055/s-2007-966214. [DOI] [PubMed] [Google Scholar]
- 74.van den Broek FJ, Fockens P, van Eeden S, Stokkers PC, Ponsioen CY, Reitsma JB, et al. Narrow-band imaging versus high-definition endoscopy for the diagnosis of neoplasia in ulcerative colitis. Endoscopy. 2011;43:108–15. doi: 10.1055/s-0030-1255956. [DOI] [PubMed] [Google Scholar]
- 75.Ignjatovic A, East JE, Subramanian V, Suzuki N, Guenther T, Palmer N, et al. Narrow band imaging for detection of dysplasia in colitis: A randomized controlled trial. Am J Gastroenterol Epub. 2012;107:885–90. doi: 10.1038/ajg.2012.67. [DOI] [PubMed] [Google Scholar]
- 76.Pellisé M, López-Cerón M, Rodríguez de Miguel C, Jimeno M, Zabalza M, Ricart E, et al. Narrow-band imaging as an alternative to chromoendoscopy for the detection of dysplasia in long-standing inflammatory bowel disease: A prospective, randomized, crossover study. Gastrointest Endosc. 2011;74:840–8. doi: 10.1016/j.gie.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 77.Dinesen L, Chua TJ, Kaffes AJ. Meta-analysis of narrow-band imaging versus conventional colonoscopy for adenoma detection. Gastrointest Endosc. 2012;75:604–11. doi: 10.1016/j.gie.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 78.Bojarski C. Malignant Transformation in Inflammatory Bowel Disease: Prevention, Surveillance and Treatment - New Techniques in Endoscopy. Dig Dis. 2009;27:571–5. doi: 10.1159/000233300. [DOI] [PubMed] [Google Scholar]
- 79.Shen B, Zuccaro G, Jr, Gramlich TL, Gladkova N, Trolli P, Kareta M, et al. In vivo colonoscopic optical coherence tomography for transmural inflammation in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2004;2:1080–7. doi: 10.1016/s1542-3565(04)00621-4. [DOI] [PubMed] [Google Scholar]
- 80.Consolo P, Strangio G, Luigiano C, Giacobbe G, Pallio S, Familiari L. Optical coherence tomography in inflammatory bowel disease: Prospective evaluation of 35 patients. Dis Colon Rectum. 2008;51:1374–80. doi: 10.1007/s10350-008-9304-6. [DOI] [PubMed] [Google Scholar]
- 81.Messmann H, Endlicher E, Freunek G, Rümmele P, Schölmerich J, Knüchel R. Fluorescence endoscopy for the detection of low and high grade dysplasia in ulcerative colitis using systemic or local 5-aminolaevulinic acid sensitisation. Gut. 2003;52:1003–7. doi: 10.1136/gut.52.7.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ochsenkühn T, Tillack C, Stepp H, Diebold J, Ott SJ, Baumgartner R, et al. Low frequency of colorectal dysplasia in patients with long-standing inflammatory bowel disease colitis: Detection by fluorescence endoscopy. Endoscopy. 2006;38:477–82. doi: 10.1055/s-2006-925165. [DOI] [PubMed] [Google Scholar]
- 83.Goetz M, Ziebart A, Vieth M, Delaney P, Galle PR, Neurath MF, et al. In vivo molecular imaging of colorectal cancer by confocal endomicroscopy. Gastroenterology. 2008;134:A48. doi: 10.1053/j.gastro.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 84.Hsiung PL, Hardy J, Friedland S, Soetikno R, Du CB, Wu AP, et al. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat Med. 2008;14:454–8. doi: 10.1038/nm1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burmer GC, Rabinovitch PS, Haggitt RC, Crispin DA, Brentnall TA, Kolli VR, et al. Neoplastic progression in ulcerative colitis: Histology, DNA content, and loss of a p53 allele. Gastroenterology. 1992;103:1602–10. doi: 10.1016/0016-5085(92)91184-6. [DOI] [PubMed] [Google Scholar]
- 86.Löfberg R, Broström O, Karlén P, Ost A, Tribukait B. DNA aneuploidy in ulcerative colitis: Reproducibility, topographic distribution, and relation to dysplasia. Gastroenterology. 1992;102:1149–54. [PubMed] [Google Scholar]
- 87.Itzkowitz SH. Inflammatory bowel disease and cancer. Gastroenterol Clin North Am. 1997;26:129–39. doi: 10.1016/s0889-8553(05)70287-9. [DOI] [PubMed] [Google Scholar]
- 88.Itzkowitz SH, Young E, Dubois D, Harpaz N, Bodian C, Chen A, et al. Sialosyl-Tn antigen is prevalent and precedes dysplasia in ulcerative colitis: A retrospective case-control study. Gastroenterology. 1996;110:694–704. doi: 10.1053/gast.1996.v110.pm8608878. [DOI] [PubMed] [Google Scholar]
- 89.Greenwald BD, Harpaz N, Yin J, Huang Y, Tong Y, Brown VL, et al. Loss of heterozygosity affecting the p53, Rb, and mcc/apc tumor suppressor gene loci in dysplastic and cancerous ulcerative colitis. Cancer Res. 1992;52:741–5. [PubMed] [Google Scholar]
- 90.Melville DM, Jass JR, Shepherd NA, Northover JM, Capellaro D, Richman PI, et al. Dysplasia and deoxyribonucleic acid aneuploidy in the assessment of precancerous changes in chronic ulcerative colitis. Observer variation and correlations. Gastroenterology. 1988;95:668–75. doi: 10.1016/s0016-5085(88)80013-1. [DOI] [PubMed] [Google Scholar]
- 91.Löfberg R, Broström O, Karlén P, Tribukait B, Ost A. Colonoscopic surveillance in long-standing total ulcerative colitis-a 15-year follow-up study. Gastroenterology. 1990;99:1021–31. doi: 10.1016/0016-5085(90)90622-8. [DOI] [PubMed] [Google Scholar]
- 92.Löfberg R, Broström O, Karlén P, Ost A, Tribukait B. Carcinoma and DNA aneuploidy in Crohn's colitis-a histological and flow cytometric study. Gut. 1991;32:900–4. doi: 10.1136/gut.32.8.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kanthan R, Senger JL, Kanthan SC. Fecal molecular markers for colorectal cancer screening. Gastroenterol Res Pract 2012. 2012 doi: 10.1155/2012/184343. 184343. [DOI] [PMC free article] [PubMed] [Google Scholar]


